Abstract
Nitrate (NO3−) supplementation via beetroot juice (BR) preferentially improves vascular conductance and O2 delivery to contracting skeletal muscles comprised predominantly of type IIb + d/x (i.e. highly glycolytic) fibers following its reduction to nitrite and nitric oxide (NO). To address the mechanistic basis for NO3− to improve metabolic control we tested the hypothesis that increased NO bioavailability via BR supplementation would elevate microvascular PO2 (PO2mv) in fast twitch but not slow twitch muscle. Twelve young adult male Sprague-Dawley rats were administered BR ([NO3−] 1 mmol/kg/day, n=6) or water (control, n=6) for 5 days. PO2mv (phosphorescence quenching) was measured at rest and during 180s of electrically induced 1-Hz twitch contractions (6–8 V) of the soleus (9% type IIb +d/x) and mixed portion of the gastrocnemius (MG, 91% type IIb + d/x) muscles. In the MG, but not the soleus, BR elevated contracting steady state PO2mv by ~43% (control: 13.7 ± 0.5, BR: 19 ± 1.6 mmHg, (P<0.05). This higher PO2mv represents a greater blood-myocyte O2 driving force during muscle contractions thus providing a potential mechanism by which NO3− supplementation via BR improves metabolic control in fast twitch muscle. Recruitment of higher order type II muscle fibers is thought to play a role in the development of the slow component which is inextricably linked to the fatigue process. These data therefore provide a putative mechanism for the BR-induced improvements in high-intensity exercise performance seen in humans.
Keywords: exercise, blood flow, nitrite, nitric oxide, metabolic control, oxygen flux
1. Introduction
At exercise onset, the immediate increase in ATP turnover within contracting skeletal muscle mandates an elevated rate of O2 delivery (QO2), such that capillary blood flow is rapidly increased to meet the rising O2 demand of contracting myocytes (i.e. O2 uptake; ) [1]. This augmented capillary flow is accomplished via elevated cardiac output and blood flow redistribution (neurohumoral activation) as well as local mechanical and vasomotor mechanisms [reviewed by 2]. Of the local controllers, the powerful signaling molecule nitric oxide (NO) promotes vasodilation of terminal arterioles within skeletal muscle, helping to facilitate this hyperemic response and better match QO2 to the elevated demands [3; 4; 5].
It is now understood that nitrate (NO3−) and nitrite (NO2−) can be converted to NO and other reactive nitrogen species in vivo following a stepwise reduction [reviewed by 6]. In humans, dietary NO3− supplementation has been shown to enhance muscle contractile [7] and mitochondrial [P/O ratio; 8] efficiency, both of which are associated with a reduction in the O2 cost of submaximal exercise [7; 8; 9; 10; 11; 12; 13; 14] and improvements in tolerance to high intensity exercise [7; 9; 11; 13; 15; 16; 17; 18].
What is particularly interesting is that the improvements in performance have been seen predominantly during severe-intensity exercise [15; 19] rather than long term endurance exercise [20; 21]. Recent studies performed in murine models suggest that this phenomenon may be due to a fiber type selective enhancement in skeletal muscle vascular and metabolic control following NO3− ingestion [22; 23]. Specifically, our laboratory has demonstrated that rats supplemented with BR (NO3− concentration 1 mmol/kg/day) for five days had higher exercising blood flow and vascular conductance in muscles comprised principally of type II muscle fibers [22]. BR also raised the pressure head for capillary-myocyte O2 flux during the crucial transition period from rest to muscle contractions (i.e., ~20–60 s) in the rat spinotrapezius muscle, which is composed of approximately 50% Type IIb+d/x muscle fibers [24; 25]. Moreover, Hernandez et al. [23] demonstrated improved calcium handling and rate of force development in type II but not type I muscles of NO3− supplemented mice.
Given the lower contracting PO2mv reported in fast twitch muscles [26] and the evidence that NO2− reduction to NO is potentiated in environments with low PO2 [27] the physiological effects of BR on the PO2mv profile may be intensified in fast twitch muscles. Therefore the purpose of the present investigation was to examine the effects of 5 days of NO3− supplementation via BR (NO3− concentration 1 mmol/kg/day) on the PO2mv profile of rat muscles comprised of predominantly type I (slow twitch) and type IIb+d/x (fast twitch) muscle fibers. We tested the hypothesis that BR would attenuate the fall in PO2mv in the fast twitch mixed portion of the gastrocnemius (MG) across the rest-contraction transition with either a lesser or no effect in the slow twitch soleus muscle.
2. Methods
2.1 Animal selection and care
Twelve young adult male Sprague-Dawley rats (average body mass = 521±20 g, Charles River Laboratories, Wilmington, MA) were used in this investigation. Rats were maintained in accredited animal facilities at Kansas State University on a 12/12 hr light-dark cycle with food and water provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University and conducted according to National Institutes of Health guidelines.
2.2 Supplementation protocol
Rats were randomly assigned to receive 5 days of BR supplementation with a NO3− dose of 1 mmol/kg/day (BR; n=6, Beet it™, James White Drinks, Ipswich UK, diluted with 100 ml of tap water) or NO3− depleted BR (control; n=6, Beet it™ placebo, diluted with 100 ml of tap water) with consumption monitored. This NO3− dose (1 mmol/kg/day) represents a NO3− concentration similar to that used in humans by Jones and colleagues [9; 11; 14; 18] after accounting for the resting metabolic rate of rats [~7× that of humans, 28]. In addition, this dose was used in our laboratory previously with significant vascular effects observed following supplementation [22; 25].
2.3 Surgical instrumentation
Rats were anaesthetized with a 5% isoflurane-O2 mixture and maintained subsequently on 3% isoflurane-O2 mixture. The carotid artery was cannulated and a catheter (PE-10 connected to PE-50, Intra-Medic polyethylene tubing, Clay Adams Brand, Becton, Dickinson and Company, Sparks, MD) inserted into carotid artery catheter for measurement of mean arterial pressure (MAP) and heart rate (HR), arterial blood sampling (Nova State Profile M, Waltham, MA, USA) and, infusion of the phosphorescent probe (see below). A second catheter was also placed in the caudal artery. Incisions were then closed and the rats were transitioned to pentobarbital sodium anesthesia (administered to effect and subsequently maintained via the caudal artery catheter) with level of anesthesia monitored continuously via the toe pinch and blink reflexes. If indicated, additional pentobarbital sodium was administered in supplemental dosage (5–10 mg/kg) as needed. Rats were then transferred onto a heating pad to maintain core body temperature at ~38°C (measured via rectal probe thermometer) and the carotid artery catheter was connected to a pressure transducer (Digi-Med BPA model 200, Louisville, KY, USA) for measurement of MAP and HR.
The muscles chosen for the present experiment (soleus and mixed portion of the gastrocnemius, MG) were selected based on their fiber type composition [24] and represent the spectrum of slow twitch (type I/IIa) and fast twitch (type IIb+d/x) muscle fiber types. The highly oxidative soleus [84% type I, 7% type IIa and 9% type IIb+d/x, 24] serves as a postural muscle whose primary functions are ankle stabilization and plantar flexion while the MG functions in plantar flexion and is comprised predominantly of highly glycolytic fast-twitch muscle fibers [3% type I, 6% type IIa, 91% type IIb+d/x, 24]. Each muscle was exposed for PO2mv experiments in the following manner. Overlaying skin and fascia along the sagittal plane on the right hindlimb were reflected carefully to expose the muscles of the ‘calf’. For measurements made in the MG, silver wire electrodes were sutured (6–0 silk) to the proximal (cathode) and distal (anode) portions of the muscle. Following measurements of PO2mv in the MG the soleus muscle was exposed by carefully reflecting overlaying tissue covering the peroneal muscle group (cornal plane) and silver wire electrodes were sutured (6–0 silk) in the same manner as for the MG. Measurement order was randomized to avoid the potential influences of an ordering effect caused by NO3− metabolism. The exposed muscles were continuously superfused with warmed (38°C) Krebs–Henseleit bicarbonate buffered solution equilibrated with 5% CO2–95% N2 and surrounding exposed tissue was covered with Saran wrap (Dow Brands, Indianapolis, IN). This method has been used previously in our laboratory and facilitates access to the MG and soleus muscles whilst minimizing perturbation caused by surgery [29].
2.4 Experimental protocol
The phosphorescent probe palladium meso-tetra (4 carboxyphenyl)tetrabenzoporphyrin-dendrimer (G2: 1–5 mg/kg dissolved in 0.4 ml saline) was infused via the carotid artery catheter. After a brief stabilization period (~10 min), the common end of the light guide of a frequency domain phosphorimeter (PMOD 5000, Oxygen Enterprises, Philadelphia, PA) was positioned ~2–4 mm superficial to the lateral surface of the exposed muscle (either MG or soleus) of the right hindlimb over a randomly selected muscle field absent of large vessels thus ensuring that the region contained principally capillary blood. PO2mv was measured via phosphorescence quenching (see below) and reported at 2 s intervals throughout the duration of the 180 s contraction protocol (1 Hz, ~6 V, 2 ms pulse duration) elicited via a Grass stimulator (model S88, Quincy, MA). As an indicator of preserved vasomotor function, it was ensured that PO2mv returned to baseline values following the contraction period. Rats were euthanized via pentobarbital sodium overdose (≥50 mg/kg administered into the carotid artery catheter). Power analysis based on a known sample variability of PO2mv and anticipated supplementation effects [22; 25] indicate that six rats per group would be sufficient to demonstrate a statistical difference, if present.
2.5 PO2mv measurement and curve-fitting
The Stern-Volmer relationship allows the calculation of PO2mv through the direct measurement of a phosphorescence lifetime via the following equation [30]:
Where kQ is the quenching constant and τ° and τ are the phosphorescence lifetimes in the absence of O2 and the ambient O2 concentration, respectively. For G2, kQ is 273 mmHg/s and τ° is 251 μs at 38°C [31] and these characteristics do not change over the physiological range of pH and temperature in the rat in vivo and, therefore, the phosphorescence lifetime is determined directly by the O2 pressure [30; 31].
Curve-fitting of the measured PO2mv responses was performed with commercially available software (SigmaPlot 11.01, Systat Software, San Jose, CA) and the data were fit with either a one- or two-component model as described below:
where PO2mv(t) represents the PO2mv at any given time t PO2mv (BL) corresponds to the pre-contracting resting baseline PO2mv, Δ1 and Δ2 are the amplitudes for the first and second component, respectively, TD1 and TD2 are the time delays for each component, and τ1 and τ2 are the time constants (i.e., time to 63% of the final response value) for each component. The two component model was only used when the PO2mv increased above its initial nadir during contractions. Goodness of fit was determined using the following criteria: 1) the coefficient of determination; 2) sum of the squared residuals; and 3) visual inspection and analysis of the model fits to the data and the residuals. The mean response time (MRT) of the kinetics response was calculated for the first component in order to provide an index of the overall principal kinetics response according to the following equation:
where TD1 and τ1 are as described above. The delta of the initial PO2mv fall following contractions onset was normalized to τ1 (Δ1 PO2mv/τ1) to provide an index of the relative rate of fall. Additionally, the time taken to reach 63% of the initial PO2mv fall was determined independently from and prior to the modeling procedures (T63) to ensure appropriateness of the model fits. Specifically, the raw PO2mv data were interpolated, and the time coinciding with 63% of the total amplitude (Δtotal PO2mv) was determined.
2.6 Blood sampling and measurement of plasma [NO3−] and [NO2−]
Post-supplementation blood samples were collected following the experiment via the caudal artery catheter to assess: 1) plasma [NO3−] and [NO2−]; and 2) pH; PO2; and %O2 saturation. For measurements of plasma [NO3−] and [NO2−], ~0.8 ml of blood was drawn into heparinized tubes and rapidly centrifuged at 6000 g at 4°C for 6 minutes. Plasma was then extracted and frozen immediately at −80°C for later analysis. A second ~0.3 ml blood sample was drawn and analyzed for blood [lactate], pH, PO2, and %O2 saturation (Nova Stat Profile M, Nova Biomedical, Waltham, MA, USA).
All measurements of plasma NO3− and NO2− were performed within 30 minutes of thawing via chemiluminescence with an Ionic/Sievers NO analyzer (NOA 280i, Sievers Instruments, Boulder, CO, USA). In order to obtain plasma NO2− levels and to avoid potential reduction of NO3−, potassium iodide in acetic acid was used as a reductant. This reductant possesses the ability to reduce NO2− to NO but is incapable of reducing higher oxides of nitrogen (i.e. NO3−) thus increasing the specificity for NO2−. Plasma NO3− concentrations were then obtained using the same apparatus with the stronger reductant vanadium chloride in hydrochloric acid at a temperature of 95°C. This stronger reductant reduces the sum of all nitrogen oxides with an oxidation state of +2 or higher (predominantly NO3− [μM]) but also includes NO2− and nitrosothiols [nM]. Signals obtained using potassium iodide were then subtracted from those with vanadium chloride to provide a clearer representation of the NO3− concentrations.
2.7 Statistical analysis
Data are presented as mean ± SEM. Results were compared within and between groups using mixed 2-way ANOVAs (MAP and HR) with Student-Newman-Keuls post hoc tests where appropriate or unpaired student’s t-test (PO2mv kinetics parameters, blood gasses, [NO3−], [NO2−]). Significance was accepted at P<0.05.
3. Results
3.1 Plasma [NO3−] and [NO2−]
BR rats had significantly higher plasma [NO3−] compared to control (control: 29 ± 6, BR: 79 ± 17 μmol/l, P=0.02).). Relative to control, plasma [NO2−] tended to be higher in BR rats, however these changes did not reach significance (control: 156 ± 66, BR: 216 ± 46 nmol/l, P=0.22).
3.2 MAP, HR, and arterial blood gases
There were no between group differences in arterial PO2, PCO2, or pH (data not shown, P>0.05 for all). Resting (control: 1.6 ± 0.1, BR: 1.1 ± 0.2 mmol/l, P>0.05) arterial blood [lactate] was not different between groups. There were no differences in resting or contracting steady-state MAP for the soleus (resting control: 122 ± 7, resting BR: 114 ± 6, contracting control: 124 ± 6, contracting BR: 117 ± 8 mmHg, P>0.05 for all) or MG (resting control: 114 ± 5, resting BR: 106 ± 6, contracting control: 116 ± 6, contracting BR: 110 ± 6 mmHg, P>0.05 for all). Furthermore, there were no differences in resting or contracting steady-state HR for the soleus (resting control: 364 ± 16, resting BR: 345 ± 25, contracting control: 366 ± 17, contracting BR 381 ± 45 mmHg, P>0.05 for all) or MG (resting control: 377 ± 16, resting BR: 372 ± 35, contracting control: 391 ± 19, contracting BR: 354 ± 52 mmHg, P<0.05 for all).
3.3 PO2mv baseline, contracting steady-state and kinetics parameters
Baseline and contracting steady-state
As expected control PO2mv(base line) and contracting PO2mv(steady-state) (evident over the final ~30 s of contractions) were higher in the soleus compared to MG (Table 1, Figure 1). However, during contractions BR supplemented rats demonstrated an elevated PO2mv(steady-state) in the MG when compared to control (Table 1, P=0.01) with no significant difference evident in the soleus (Table 1, P=0.31). It was noteworthy that BR raised PO2mv(steady-state) in the MG to that found in the control soleus.
Table 1.
Microvascular O2 partial pressure (PO2mv) kinetics parameters during soleus and MG contractions in control and BR rats.
| Soleus | MG | |||
|---|---|---|---|---|
| Control | BR | Control | BR | |
| PO2mv(Base line) (mmHg) | 32 ± 3 | 33 ± 3 | 24 ± 2† | 25 ± 2† |
| Δ1PO2mv (mmHg) | 11 ± 1 | 9 ± 2 | 11 ± 2 | 10 ± 1 |
| Δ2 PO2mv (mmHg) | 1 ± 0 | 4 ± 1 | ||
| Δtotal PO2mv (mmHg) | 11 ± 1 | 9 ± 2 | 9 ± 1 | 5 ± 1 |
| PO2mv(steady-state) (mmHg) | 20 ± 3 | 24 ± 2 | 14 ± 1† | 20 ± 2* |
| Time delay 1 (s) | 12 ± 1 | 8 ± 2 | 6 ± 2† | 2 ± 1† |
| Time delay 2 (s) | 51 ± 14 | |||
| Time constant 1 (s) | 25 ±5 | 26 ± 6 | 13 ± 2† | 15 ± 4† |
| Time constant 2 (s) | 58 ± 12 | |||
| Mean response time (s) | 37 ± 4 | 34 ± 6 | 19 ± 3† | 17 ± 3† |
| T63 (s) | 39 ± 5 | 34 ± 6 | 19 ± 3† | 13 ± 2† |
Values are mean ± SEM. Where second component model averages are shown the value reflects only those rats where a two-component model was applied to describe the PO2mv data (control: n=1 of 6 BR: n=6 of 6 MG profiles).
P<0.05 vs. control.
P<0.05 vs. soleus.
Figure 1.
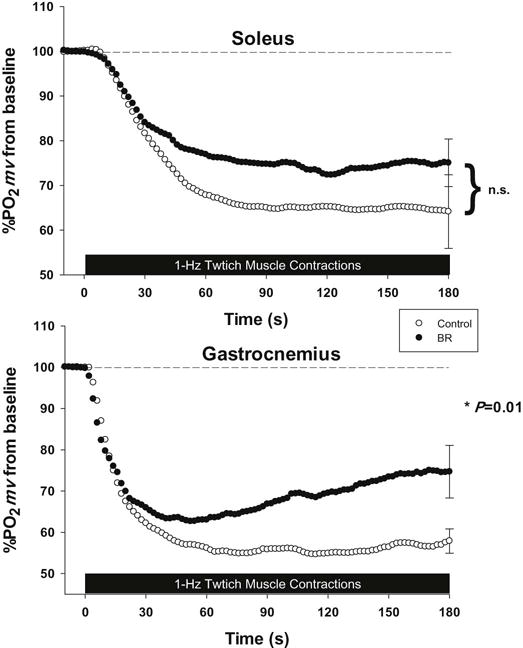
Mean percent delta PO2mv profiles for the soleus (top panel) and MG (bottom panel) muscles of control and BR rats. Time “0” represents the onset of contractions. *P<0.05 versus control.
PO2mv kinetics parameters
The kinetics following the onset of contractions for the soleus were adequately fit by a one-component model in all control and BR rats. In contrast, for the MG, the more complex two-component model was necessary for 1 of 6 control and all BR rats. Indeed, ~80% of the increased PO2mv(steady-state) for the BR group resulted from the secondary component increase of PO2mv (Table 1). The coefficient of determination (r2) for soleus and MG (≥0.98 for control and BR) and low sum of squared residuals (RSS <20) for both groups suggested the appropriateness of the respective model fits.
Table 1 presents the mean PO2mv kinetics parameters. The time delay and time constant for the first component as well as the mean response time and T63 were greater in the soleus versus the MG (for both control and BR, P<0.05 for all, Table 1). There were no between-group differences in any kinetic parameter following the onset of contractions for the soleus or MG (Table 1, P>0.05 for all). Importantly, within the control and BR groups the model-dependent MRT1 and model-independent T63 were not different (Table 1) providing additional confidence in the model parameters.
4. Discussion
The present investigation provides the first demonstration that NO3− supplementation-induced (via BR) elevation of PO2mv occurs preferentially in muscles comprised of fast twitch (MG) rather than slow twitch (soleus) fibers. This finding coheres with the presence of lower PO2mv levels in fast twitch muscles [26; 29] and a physicochemical milieu (lower pH, higher lactate) that favors the reduction of NO2− to NO [27]. It is also true that the low PO2 environment extant in these muscles which favors NO2− reduction will suppress the endogenous production of NO from the neuronal and endothelial nitric oxide synthase (nNOS, eNOS) pathways; of which nNOS is the most important in fast twitch muscles [32; 33; 34; 35]. NO3− supplementation (BR) raises PO2mv (i.e., QO2/ ratio) by simultaneously increasing O2 delivery [QO2, 22] and reducing the O2 cost ( ) of exercise via changes in mitochondrial and contractile function [6; 8; 12; 23]. Increases in PO2mv in-and-of themselves have the capacity to improve blood-myocyte O2 flux, increase intramyocyte PO2 and consequently enhance mitochondrial oxidative phosphorylation whilst suppressing glycolysis. This behavior may underlie the reduced arterial [lactate] levels found during heavy intensity exercise in running rats [22].
4.1 Effects of BR on the PO2mv Profile
As also shown in the present investigation, previous studies have reported pronounced fiber type differences in both kinetics parameters and the magnitude of the overall change in PO2mv following the onset of contractions. Specifically, Behnke et al. [29] found a longer time delay, MRT, slower rate of PO2mv fall, and a lower overall amplitude of PO2mv fall (e.g. Δ PO2mv) in the soleus (slow-twitch) vs. peroneal (fast-twitch) muscles during electrically induced contractions. Additionally, McDonough and colleagues [26] reported a higher PO2mv(steady-state) in soleus versus MG muscles. In the present investigation, despite BR raising the MG PO2mv(steady-state) to levels commensurate with those observed in the soleus muscle of control rats herein (PO2mv(steady-state) ↑43%, Table 1) the kinetics profile was unchanged. This situation does not mean that the kinetics profile is intransigent. Indeed, changes in the PO2mv kinetics profile can be driven by both NOS-dependent and independent mechanisms invoked by 6–8 weeks of exercise training [33]. Interestingly, the training-induced adaptation of the PO2mv kinetics profile occurred in the absence of an elevated PO2mv(steady-state). Thus, exercise training raises PO2mv across the dynamic transition at a time when is increasing most rapidly. In marked contrast, the elevation of PO2mv in the MG after BR seen in the present investigation is delayed and appears to consist of a secondary effect that only becomes apparent after ~60 s of contractions (Figure 1). Such an augmented PO2mv (steady-state) is expected to facilitate fatigue resistance consequent to decreased metabolic perturbations during exercise [e.g.↓PCr breakdown, 36; 37].
4.2 Relationship to existing literature
BR supplementation elevates QO2 preferentially in muscles comprised of ≥66% type IIb+d/x muscle fibers [22]. Those fiber type selective elevations in QO2 observed during treadmill exercise, if present herein, would help explain the greater PO2mv seen in the MG (Figure 1). As mentioned above, one potential explanation for the fiber type specific elevations in QO2 (and thus PO2mv) is that NO2− reduction is facilitated to a greater extent in fast twitch muscles as a result of lower contracting PO2mv in type II vs. type I fibers [26; 29; 38]. This is supported by Cosby et al. [27] who demonstrated that NO2− reduction is potentiated in environments with low PO2 and pH, such that may exist in skeletal muscle during exercise. Furthermore, activity of the nitric oxide synthase family of enzymes (nNOS and eNOS rather than iNOS being relevant here) may be reduced under such conditions [39] allowing the NO3− NO2−-NO pathway to serve a complimentary role in the local regulation of NO bioavailability. In this respect also, there is a fiber type specificity: The Michaelis-Menten constant (Km) for nNOS (350 μ m) is over 15-fold greater than eNOS (23 μ m) [40]. Thus, the sensitivity of nNOS to the changes in PO2 (and PO2mv) in the MG evoked by BR may potentiate the overall NO bioavailability by allowing nNOS, the predominant NOS in fast twitch fibers, to function more effectively.
In addition to the beneficial vascular impacts of NO3− supplementation, there may be fiber type specific improvements in metabolic control brought about via improvements in contractile function. For example the improvements in rate of force development and tetanic contractile force reported by Hernandez et al. [23], consequent to improvements in intracellular calcium handling (↑ expression of calsequestrin 1 and the dihydropyridine receptor), may serve to reduce in the face of elevated QO2, further raising the QO2/ ratio. Furthermore, NO3− supplementation reportedly increases mitochondrial efficiency in the human vastus lateralis muscle [comprised of ~58% Type IIa/IIb+dx fibers, 41] in proportion to the reduced pulmonary response to submaximal exercise [8]. Collectively, these studies support that simultaneous increases in muscle(s) QO2 combined with reductions in may account for the observed fiber type-specific elevations in contracting PO2mv(steady-state).
With regards to performance, the fiber type-selective impacts of BR supplementation may delay the onset of fatigue given that phosphocreatine and glycogen degradation is greater in Type II vs. Type I muscles during maximal exercise [42]. Therefore, an elevated PO2mv(steady-state) has the means to contribute to BR-induced improvements in intense intermittent exercise [19] especially when considering that type II muscle fibers are heavily recruited during the transition from low to high metabolic rates [43; 44]. In addition, higher exercise intensities result in a greater muscle /QO2 ratio exacerbating intramyocyte hypoxia and accumulation of [ADP], [Pi], [K+] and [H+] each of which may play a role in the fatigue process [45; 47]. That these perturbations may be ameliorated, at least in part, by BR-induced elevations in PO2mv(steady-state) carries significant implications for individuals suffering from diseases where derangements in skeletal muscle O2 delivery/utilization balance (e.g. chronic heart failure, peripheral artery disease) expedite fatigue.
4.3 Experimental considerations
That the elevated PO2mv(steady-state) occurred in the absence of elevated plasma [NO2−] suggests that alternate pools of NO2−, perhaps within muscle tissue, contributed to this effect. None-the-less, an improved QO2/ ratio in the highly glycolytic muscles and muscle parts would presumably delay the onset of fatigue and thus, may be the mechanism responsible for the improvements in high intensity exercise seen in humans following BR supplementation [15; 19]. Contrary to our original hypothesis, BR did not impact PO2mv during the immediate rest-contraction transition (i.e. rapid PO2mv kinetics) as shown previously in the mixed fiber type spinotrapezius muscle [25]. This effect (or lack thereof) may be due to the length of the experimental procedure utilized herein which, given the relatively short half-life of plasma NO2− [~45 minutes in humans, 18; 48] may have allowed any significant elevation of circulating plasma [NO2−] to subside reducing/abolishing any effect on PO2mv kinetics. In this regard, it is important to note that muscle [NO2−] can remain elevated after plasma [NO2−] has returned to normal (see Calvert et al. [49]). The robust changes in PO2mv(steady-state) seen in BR supplemented rats herein combined with the results reported by Calvert et al. [49] suggests that high plasma [NO2−] may not be obligatory to elicit beneficial physiological responses. Elevated tissue [NO2−] may be the consequence of prolonged periods (i.e., several days to weeks) of high exposure for example with BR supplementation or exercise training.
It is important to note that PO2mv(steady-state) was numerically (~20%), but not significantly, higher in in the contracting soleus of BR supplemented rats (Table 1). This finding may be due to the very small proportion of fast twitch fibers in the soleus (~10%) and/or the reduced effect of BR on slow twitch fibers. Post-hoc power analysis revealed that 19 additional animals would be needed to achieve significance.
4.4 Potential limitations, future directions, and conclusions
Five days of BR-induced NO3− supplementation raised substantially the contracting PO2mv(steady-state) in the fast twitch MG with no significant changes evoked in the predominantly slow twitch soleus. That this effect occurred in the presence of unchanged plasma [NO2−] seen in BR supplemented rats highlights the complex nature of NO3−/NO2− bioactivation and suggest that potentially other storage pools of NO2− (i.e., within skeletal muscle) may impact skeletal muscle vascular and metabolic function. Importantly, the elevated PO2mv(steady-state) in the MG reflects an improved ability to maintain QO2 relative to and thus, is expected to ameliorate fatigue during high intensity exercise as demonstrated in humans [15; 19]. Future measurements of QO2 and calculation of will provide valuable insight into the relative contribution of vascular versus intramyocyte (mitochondrial, contractile machinery) mechanisms responsible for this effect.
In addition, while the lower PO2mv in the gastrocnemius offers one putative mechanism for NO2− reduction to NO in vivo (i.e. ↓PO2 ↑NO2− reduction) experiments in which tissue pH and/or mitochondrial function are manipulated may offer further insight into the precise mechanism(s) responsible for the fiber type preferential effect observed herein. Indeed, fiber type differences in tissue pH may afford enhanced bioactivation of NO2− particularly if tissue, rather than plasma, [NO2−] is responsible for the effects on PO2mv. In this regard, investigations into the impacts of tissue pH on NO2− bioactivation are warranted.
Considering the preponderance of fast twitch fibers in human locomotory muscles and the increased reliance on these fibers in diseased states (e.g. heart failure) NO3− supplementation via BR may constitute a novel and powerful “bench-to-bedside” therapeutic modality [47; 50]. Furthermore, considering that traditional organic NO3− therapies employing isosorbide mononitrate or nitroglycerine eventually lead to tachyphylaxis [51] it is plausible that utilizing the NO3−-NO2−-NO pathway will provide a viable NO-based treatment strategy for various disease conditions.
Highlights.
Dietary nitrate (NO3−) increases skeletal muscle function during exercise.
Improvements were seen in muscles comprised predominantly of fast twitch fibers.
Improved vascular function would be expected to raise microvascular PO2 (PO2mv).
The impacts of NO3− on the PO2mv profile of fast vs. slow twitch muscles is unknown.
NO3− supplementation preferentially elevates PO2mv in fast twitch muscle.
Acknowledgments
The authors would like to thank Ms. K. Sue Hageman, Dr. Steven W. Copp, and Dr. Daniel M. Hirai for their excellent technical assistance and support. These experiments were funded by a Kansas State University SMILE award to TIM, and American Heart Association Midwest Affiliate (10GRNT4350011) and NIH (HL-108328) awards to DCP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol (1985) 2002;92:2513–20. doi: 10.1152/japplphysiol.01222.2001. [DOI] [PubMed] [Google Scholar]
- 2.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol. 2007;583:855–60. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol (1985) 1994;77:1288–93. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- 4.Joyner MJ, Tschakovsky ME. Nitric oxide and physiologic vasodilation in human limbs: where do we go from here? Can J Appl Physiol. 2003;28:475–90. doi: 10.1139/h03-035. [DOI] [PubMed] [Google Scholar]
- 5.King CE, Melinyshyn MJ, Mewburn JD, Curtis SE, Winn MJ, Cain SM, Chapler CK. Canine hindlimb blood flow and O2 uptake after inhibition of EDRF/NO synthesis. J Appl Physiol (1985) 1994;76:1166–71. doi: 10.1152/jappl.1994.76.3.1166. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg JO, Weitzberg E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch Pharm Res. 2009;32:1119–26. doi: 10.1007/s12272-009-1803-z. [DOI] [PubMed] [Google Scholar]
- 7.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 2010;109:135–48. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 8.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–59. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 2009;107:1144–55. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 10.Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22:64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol (1985) 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 12.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–7. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–31. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 15.Breese BC, McNarry MA, Marwood S, Blackwell JR, Bailey SJ, Jones AM. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1441–50. doi: 10.1152/ajpregu.00295.2013. [DOI] [PubMed] [Google Scholar]
- 16.Kelly J, Vanhatalo A, Wilkerson DP, Wylie LJ, Jones AM. Effects of nitrate on the power-duration relationship for severe-intensity exercise. Med Sci Sports Exerc. 2013;45:1798–806. doi: 10.1249/MSS.0b013e31828e885c. [DOI] [PubMed] [Google Scholar]
- 17.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. The effects of a single dose of concentrated beetroot juice on performance in trained flatwater kayakers. Int J Sport Nutr Exerc Metab. 2013;23:498–506. doi: 10.1123/ijsnem.23.5.498. [DOI] [PubMed] [Google Scholar]
- 18.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 2013;115:325–36. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 19.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermiotadis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol. 2013;113:1673–84. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]
- 20.Cermak NM, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon LJ. No improvement in endurance performance after a single dose of beetroot juice. Int J Sport Nutr Exerc Metab. 2012;22:470–8. doi: 10.1123/ijsnem.22.6.470. [DOI] [PubMed] [Google Scholar]
- 21.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol. 2012;112:4127–34. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol. 2013;591:547–57. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol. 2012;590:3575–83. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol (1985) 1996;80:261–70. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir Physiol Neurobiol. 2013;187:250–5. doi: 10.1016/j.resp.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–13. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 28.Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol (1985) 1988;65:964–70. doi: 10.1152/jappl.1988.65.2.964. [DOI] [PubMed] [Google Scholar]
- 29.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988;241:1649–51. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- 31.Lo LW, Vinogradov SA, Koch CJ, Wilson DF. A new, water soluble, phosphor for oxygen measurements in vivo. Adv Exp Med Biol. 1997;428:651–6. doi: 10.1007/978-1-4615-5399-1_91. [DOI] [PubMed] [Google Scholar]
- 32.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol. 2013;591:2885–96. doi: 10.1113/jphysiol.2013.251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Exercise training and muscle microvascular oxygenation: functional role of nitric oxide. J Appl Physiol (1985) 2012;113:557–65. doi: 10.1152/japplphysiol.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–8. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 35.Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–9. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- 36.Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol (1985) 1998;85:1457–63. doi: 10.1152/jappl.1998.85.4.1457. [DOI] [PubMed] [Google Scholar]
- 37.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol. 2011;589:5517–28. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira LF, McDonough P, Behnke BJ, Musch TI, Poole DC. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fiber types. Respir Physiol Neurobiol. 2006;153:237–49. doi: 10.1016/j.resp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 40.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–70. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 41.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–9. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 42.Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, Hultman E. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol. 1994;478(Pt 1):149–55. doi: 10.1113/jphysiol.1994.sp020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krustrup P, Soderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004;447:855–66. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- 44.Krustrup P, Soderlund K, Relu MU, Ferguson RA, Bangsbo J. Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: effect of thigh occlusion. Scand J Med Sci Sports. 2009;19:576–84. doi: 10.1111/j.1600-0838.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 45.Krustrup P, Mohr M, Amstrup T, Rysgaard T, Johansen J, Steensberg A, Pedersen PK, Bangsbo J. The yo-yo intermittent recovery test: physiological response, reliability, and validity. Med Sci Sports Exerc. 2003;35:697–705. doi: 10.1249/01.MSS.0000058441.94520.32. [DOI] [PubMed] [Google Scholar]
- 46.Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, Bangsbo J. Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflugers Arch. 2004;448:452–6. doi: 10.1007/s00424-004-1257-6. [DOI] [PubMed] [Google Scholar]
- 47.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol (1985) 2011;110:1582–91. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pluta RM, Oldfield EH, Bakhtian KD, Fathi AR, Smith RK, Devroom HL, Nahavandi M, Woo S, Figg WD, Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS One. 2011;6:e14504. doi: 10.1371/journal.pone.0014504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–58. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sperandio PA, Oliveira MF, Rodrigues MK, Berton DC, Treptow E, Nery LE, Almeida DR, Neder JA. Sildenafil improves microvascular O2 delivery-to-utilization matching and accelerates exercise O2 uptake kinetics in chronic heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H1474–80. doi: 10.1152/ajpheart.00435.2012. [DOI] [PubMed] [Google Scholar]
- 51.Elkayam U, Kulick D, McIntosh N, Roth A, Hsueh W, Rahimtoola SH. Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure. Circulation. 1987;76:577–84. doi: 10.1161/01.cir.76.3.577. [DOI] [PubMed] [Google Scholar]


