Abstract
The proteins alpha-synuclein (αSyn) and LRRK2 are both key players in the pathogenesis of the neurodegenerative disorder Parkinson’s disease (PD), but establishing a functional link between the two proteins has proven elusive. Research studies for these two proteins have traditionally and justifiably focused in neuronal cells, but recent studies indicate that each protein could play a greater pathological role elsewhere. αSyn is expressed at high levels within neurons, but they also secrete the protein into the extracellular milieu, where it can have broad ranging effects in the nervous system and relevance to disease etiology. Similarly, low neuronal LRRK2 expression and activity suggests that LRRK2-related functions could be more relevant in cells with higher expression, such as brain-resident microglia. Microglia are monocytic immune cells that protect neurons from noxious stimuli, including pathological αSyn species, and microglial activation is believed to contribute to neuroinflammation and neuronal death in PD. Interestingly, both αSyn and LRRK2 can be linked to microglial function. Secreted αSyn can directly activate microglia, and can be taken up by microglia for clearance, while LRRK2 has been implicated in the intrinsic regulation of microglial activation and of lysosomal degradation processes. Based on these observations, the present review will focus on how PD-associated mutations in LRRK2 could potentially alter microglial biology with respect to neuronally-secreted αSyn, resulting in cell dysfunction and neurodegeneration.
Keywords: synuclein, LRRK2, neurodegeneration, microglia, neuroinflammation, autophagy
Introduction
Leucine rich repeat kinase 2 (LRRK2) is the most commonly mutated gene in both idiopathic and familial Parkinson’s disease (PD) (Healy et al., 2008; Paisán-Ruíz et al., 2008; Kumari and Tan, 2009; Lesage et al., 2010). The gene product is a large protein of 260 kDa with multiple enzymatic and protein-binding domains, which may imply a complex role in its cellular function. A consensus for a bona-fide substrate or canonical cellular function of LRRK2 has remained elusive. This is likely due to the complex nature of its architecture or perhaps its weak kinase activity or expression in neurons, which has represented the major areas of investigation in the field (Galter et al., 2006; Mandemakers et al., 2012; Schapansky et al., 2014; West et al., 2014). Recent observations confirm the relative high expression of LRRK2 in certain cells of the immune system, which might indicate that the contribution of LRRK2 signaling to human neurologic disease is more complicated than initially assumed.
Expression of LRRK2 has been detected in most immune cells, including T cells, B cells, and various subtypes of monocytes (Gardet et al., 2010; Hakimi et al., 2011; Thévenet et al., 2011; Schapansky et al., 2014). Consistent with a functional role within these cells, genomic studies have implicated LRRK2 in the development of autoimmune disorders including Crohn’s disease, colitis, and leprosy (Cardoso et al., 2011; Umeno et al., 2011). Among the immune cell types, however, the highest expression is observed in monocytes (Gardet et al., 2010), which implicates LRRK2 as a significant player in the innate immune system. One of many monocytic populations that express LRRK2 is microglia, the resident immune cells of the brain. Microglia are responsible for homeostatic and trophic support of the nervous system, phagocytosis of extracellular debris, and the initiation of inflammation. This review will explore the complex biologically and pathologically relevant interactions between microglia and α-synuclein (αSyn), a protein intimately linked to PD, and the multiple opportunities for disease-linked mutations in LRRK2 to perturb these associations.
The biochemical properties of LRRK2 in non-neuronal tissues
The observation that LRRK2 is expressed in cells of the immune system was the first evidence that it could play a broad role in human biology with functions outside of the nervous system. Higher expression in both B cells and monocytes as compared to T cells (Gardet et al., 2010; Thévenet et al., 2011) suggests a specific role in the innate immune system, or the first line of defense against infection. Furthermore, monocytes have greater basal LRRK2 expression than neurons (Schapansky et al., 2014), raising the possibility that normal monocytic function may more heavily involve LRRK2-dependent processes and signaling than neurons. Monocyte activation also results in biochemical changes to LRRK2 that have not been formally investigated in neurons, including alterations in expression, phosphorylation state, and subcellular distribution. Treatment of monocytes with interferon gamma, a cytokine responsible for priming monocyte activation, significantly increases LRRK2 expression (Gardet et al., 2010; Kuss et al., 2014). In addition, LPS-induced monocyte activation through stimulation of toll-like receptor 4 (TLR4) also elevates its expression (Moehle et al., 2012) and results in rapid and sustained (minutes to hours) LRRK2 phosphorylation at serine residue 935 (Ser935) (Dzamko et al., 2012; Schapansky et al., 2014). This particular biochemical event was once thought to be an early indicator of LRRK2 activation state (Nichols et al., 2010; Deng et al., 2011), but current data now question whether the status of this phosphorylation site is truly related to intrinsic kinase activity. Nonetheless, it is still believed that LRRK2 phosphorylation correlates well with conditions of increased LRRK2 function (Dzamko et al., 2012; Schapansky et al., 2014; Vancraenenbroeck et al., 2014). In our hands, stimulation induced phosphorylation of LRRK2 at Ser935 preceded the dimerization and translocation of LRRK2 to membranous organelles (Schapansky et al., 2014). These findings may shed light on a previous study which showed that disruption of LRRK2 phosphorylation results in accumulation of the protein within the cytosol (Dzamko et al., 2010). LRRK2 phosphorylation has also been shown to regulate its association with 14-3-3 adapter proteins (Nichols et al., 2010; Li et al., 2011), and a disruption of 14-3-3/LRRK2 interactions can alter LRRK2 localization in the cell (Nichols et al., 2010; Mamais et al., 2014) and potentially impact membrane-related functions, such as exosome release (Fraser et al., 2013). Lastly, multiple PD-associated missense mutations in LRRK2 at position R1441 have been shown to reduce LRRK2 phosphorylation and prevent 14-3-3 binding (Muda et al., 2014), suggesting that mutations in LRRK2 could affect LRRK2 phosphorylation and activation upstream of its cellular function. Thus, the upregulation of LRRK2 expression and phosphorylation observed early in monocyte activation is likely an initial requirement for downstream changes in its kinase activity or interaction with cofactors, important events to its role within the innate immune system.
Emerging clues and consensus on LRRK2 function
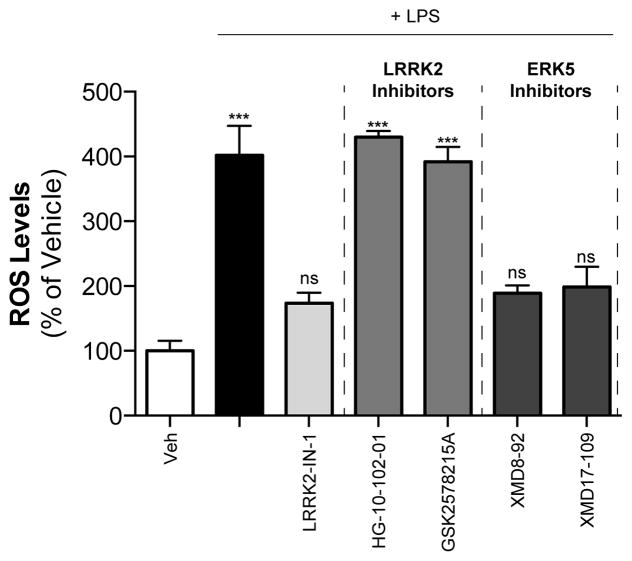
A number of functions across multiple cellular systems have been implicated for LRRK2. These include rather diverse processes such as cell migration (Caesar et al., 2013), phagocytosis (Marker et al., 2012), and neuronal excitability (Cirnaru et al., 2014). Other proposed functions include an involvement in mitochondrial fission (Wang et al., 2012), synaptic vesicle endocytosis (Matta et al., 2012), and modulation of the expression of cell-surface markers in monocytes (Thévenet et al., 2011). Many of these observations were made by employing compounds that inhibit the kinase activity of LRRK2. Several inhibitors have now been generated for use as both tool compounds and potential therapeutic agents, but the significant off-target effects of earlier compounds likely complicate interpretability. Early research relied on broad range ROCK kinase inhibitors that also affected LRRK2, preventing its phosphorylation of a pseudo-substrate (Nichols et al., 2009). The first inhibitor developed specifically for LRRK2, named LRRK2-IN-1, was a highly potent agent with an IC50 in the low nanomolar range (Deng et al., 2011). However, the inhibitor also strongly inhibits the kinase ERK5 (Deng et al., 2011) (http://graylab.dfci.harvard.edu/index.php?id=157). This begs the question as to whether the effects of LRRK2-IN-1 on ERK5 better explain early results originally ascribed to LRRK2. The first off-targets effects were suggested in a study that found an increase in neurite outgrowth by LRRK2-IN-1 in LRRK2 knockout (KO) neurons, while a structurally distinct LRRK2 inhibitor had no effect (Luerman et al., 2013). Data from our lab revealed that LRRK2-IN-1 could suppress LPS-induced phagocytosis, but two newer, structurally-distinct inhibitors could not (Schapansky et al., 2014). Importantly, in our study the effects of LRRK2-IN-1 persisted in cells where LRRK2 expression had been silenced. We have since extended these findings and now show that LPS-induced reactive oxygen species generation can be prevented by selective ERK5 inhibitors and LRRK2-IN-1, but not other LRRK2 kinase inhibitors (Figure 1). Further complicating the issue, a direct interaction between ERK5 and LRRK2 has been discovered, with the ERK5 inhibitor XMD-98 preventing LRRK2 phosphorylation at Ser935 (Kuss et al., 2014). Thus, the promiscuity of pharmacological kinase inhibitors requires that care be taken to not rely exclusively on a single compound. Ideally, a panel of reagents in conjunction with LRRK2-null cells would provide an opportunity for a more precise determination of the contribution of LRRK2 protein and/or kinase activity to a cellular function.
Figure 1. LRRK2-IN-1 prevents ROS generation in a similar manner to ERK5 inhibitors, but not other LRRK2 inhibitors.
RAW264.7 macrophage cells were treated with LPS (100 ng/ml) with and without LRRK2-IN-1 (1 μM), newer LRRK2 inhibitors (1 μM), or ERK5 inhibitors (2.5 μM) for 16 hours. Media was removed, and cells were treated with the fluorescent dye DCFDA (10 μM, in PBS) for 30 minutes. Cells were washed once, lifted in 500 μl PBS containing 10 mM EDTA and transferred to round bottomed tubes. Cytosolic ROS was measured using FACScalibur flow cytometer under the FL1 channel, counting 30,000 cells/treatment. Results are from 3 independent experiments. ***, p<0.001, n=3, ANOVA followed by Dunnett’s post-hoc analysis.
Cytokine production, a key feature of activated monocytes (Shi and Pamer, 2011), was suggested to be regulated by LRRK2. However, a summative analysis from numerous studies on both sides of the debate indicates that a direct link is unlikely. Bone marrow-derived macrophages from LRRK2 KO animals have increased IL-12p40 and IL-6 secretion in one study (Liu et al., 2011), but two other studies found secreted cytokine levels in LRRK2 KO macrophages were the same as those seen from wild-type mice (Hakimi et al., 2011; Dzamko et al., 2012). Similar contradictions have been observed in transgenic mice expressing disease-linked mutations in LRRK2. Microglial cultures from R1441G knock-in (KI) mice demonstrate increased cytokine expression (Gillardon et al., 2012), but macrophages from R1441C KI mice have similar IL-6 secretion compared to wild-type cells (Hakimi et al., 2011). Data obtained from the use of LRRK2-IN-1 also supported a LRRK2 kinase-dependent regulation of cytokines with reduced cytokine production and secretion in primary cultured microglia (Marker et al., 2012; Moehle et al., 2012) that is fully in agreement with some KO data mentioned above. However, this is likely due to aforementioned off-target effects, as LRRK2-IN-1 and the specific ERK5 inhibitor XMD-98 had a very similar dose-dependent effects on cytokine production (Luerman et al., 2013). The opposing nature of these results brings into question the true effect of LRRK2 on cytokine secretion, and demonstrates the need for orthogonal methods when addressing LRRK2 function.
Future efforts to address the cellular role of LRRK2 activity will require careful and appropriate use of multiple inhibitors as well as KO animals, resources that the field only now has available. The tools employed may not be the only considerations for future investigation. LRRK2 inhibition, resulting in reduced LRRK2 phosphorylation in an acute manner, occurs within minutes of treatment. Thus, it may seem reasonable to consider short incubation periods of an inhibitor, with the expectation that modulation of a subsequent LRRK2 function will occur within this acute time frame. However, we and others have shown that changes in LRRK2 biochemistry, including homodimerization and membrane translocation, can take several hours following cell activation (Mamais et al., 2014; Schapansky et al., 2014), and may suggest that the consequences of LRRK2 inhibition occur hours after stimulation. This indicates the potential requirement for extended inhibitor treatment to adequately interrogate LRRK2-dependent biology. For example, regulatory changes in the autophagy-lysosome pathway, to which LRRK2 has been functionally linked by many labs (see summary below), can take several hours to evolve. In addition, LRRK2 phosphorylation at key serine residues seems to be less dependent on an autophosphorylation event than originally thought (Doggett et al., 2011; Li et al., 2011; Dzamko et al., 2012), and thus the removal of these phosphate groups by phosphatase enzymes may contribute great complexity to the effects of LRRK2 and LRRK2 inhibitors in intact cells (Lobbestael et al., 2013; Vancraenenbroeck et al., 2014). In these regards, the future identification and validation of bona fide LRRK2 kinase substrates will dramatically clarify the experimental use of LRRK2 kinase inhibitors, and remains one of the greatest challenges in the field.
LRRK2 and the autophagosome/lysosome pathway
The autophagy/lysosomal degradation pathway (ALP) is common to virtually all cells, and involves the cytoplasmic engulfment of cellular organelles, protein aggregates, or foreign bodies by a lipid bilayer encapsulated organelle prior to digestion by proteases provided by lysosomal fusion. In monocytes, autophagy is often relied upon to degrade internalized single cell organisms or other toxins as part of their immune function. LRRK2 has been shown to be involved at both sides of the pathway, functionally and physically associating with autophagosomes and lysosomes. Autophagic regulation by LRRK2 was originally suggested when the protein was detected on autophagosome vesicles via immuno-electron microscopy (Alegre-Abarrategui et al., 2009; Gómez-Suaga et al., 2011), and later to be shown present on autophagosomal membranes biochemically isolated from activated macrophage cells (Schapansky et al., 2014). The interaction of LRRK2 with numerous organelles suggests that migration of cytoplasmic LRRK2 to membranes is likely part of its normal function across many cell types (Biskup et al., 2006; Berger et al., 2010; Schapansky et al., 2014). Some of these functions could include the formation or functioning of synaptic vesicles (Matta et al., 2012), secretory exosomes (Fraser et al., 2013), and centrosomes, (Mamais et al., 2014). Thus, the redistribution of LRRK2 to autophagosomes is likely not part of its degradation, but rather a necessary component of their maturation. However, direct association of LRRK2 with LAMP2A during chaperone-mediated autophagy, and with the late-endosomal marker Rab7, a regulator of lysosome biogenesis, are indications that LRRK2 may alter lysosomal biology directly (Higashi et al., 2009; Dodson et al., 2012; MacLeod et al., 2013; Orenstein et al., 2013; Gómez-Suaga et al., 2014). It remains unclear with which organelles LRRK2 can play a definitive role, but its involvement at various points in the autophagy/lysosomal axis indicates a direct function in critical homeostatic degradation pathways.
Phenotypes observed in the kidneys and lungs of LRRK2 KO animals also imply a significant role for LRRK2 in ALP biology. LRRK2 KO mice have deformed kidneys and protein accumulation indicative of defective autophagic clearance, with increased levels of autophagy substrates (Tong et al., 2010; Herzig et al., 2011; Hinkle et al., 2012). In addition, the lungs of LRRK2 KO mice also have increased size and number of lamellar bodies (secretory lysosomes). These animals display the classic foamy cytoplasm and poor surfactant production/secretion in Type II pneumocytes that are typical of lamellar body defects (Herzig et al., 2011; Miklavc et al., 2014). LRRK2 KO rats have a similar phenotype, with age-dependent increases in lysosomal size and lamellar body number in the kidney and lung, respectively (Baptista et al., 2013). These rats also have increased levels of lipofuscin, a lipid-based by-product indicative of poor lysosomal turnover, which further indicts LRRK2’s involvement in ALP (Baptista et al., 2013). The kinase activity of LRRK2 has also been directly implicated in autophagosome and lysosome function, as LRRK2-kinase dead KI mice develop abnormal kidneys with elevated lysosomal numbers as well (Herzig et al., 2011). The convergent nature of these in vivo data demonstrate that either reduced expression or dysregulation of endogenous LRRK2 activity can result in severe tissue pathology related to the ALP.
Do PD-Linked Mutations in LRRK2 Affect ALP Function?
The G2019S mutation within the LRRK2 kinase domain results in a multi-fold increase in LRRK2 kinase activity (West et al., 2005; Greggio et al., 2006; Jaleel et al., 2007), and is the most common causal mutation in LRRK2 PD patients (Healy et al., 2008). The mechanism by which LRRK2-G2019S induces PD pathology remains unclear, but multiple studies have implicated this mutation as a dysregulator of autophagic function. Given that loss of LRRK2 expression results in impaired autophagy, one might expect the gain-of-kinase function G2019S mutation to promote protein degradation. However, the dysregulation of LRRK2 kinase function through an autonomous increase in activity may result in complex and unexpected behaviors. Cells expressing G2019S LRRK2 consistently demonstrate increased autophagic vesicles or markers when compared to wild-type LRRK2 (Plowey et al., 2008; Bravo-San Pedro et al., 2012; Sánchez-Danés et al., 2012), which may be a result of increased autophagic flux (Plowey et al., 2008; Bravo-San Pedro et al., 2012), leading to neuritic loss in neurons (MacLeod et al., 2006; Plowey et al., 2008; Lin et al., 2010; Chan et al., 2011; Winner et al., 2011). Yet these increased vesicle numbers could also be an indication of an arrested autophagy/lysosomal network, following the inability of autophagosomes to fuse with lysosomes (Sánchez-Danés et al., 2012). Increases in lysosomal size and clustering in G2019S expressing cells, which can be rescued by the lysosomal marker Rab7, would further support the interpretation of a defect in the degradation pathway rather than an accelerated autophagic flux (MacLeod et al., 2006; Dodson et al., 2012; MacLeod et al., 2013). A model of insufficient lysosomal clearance would certainly explain Lewy body pathology in PD, which can be viewed as a selective proteinopathy involving disrupted proteostasis of the αSyn protein (Bosco et al., 2011; Lee et al., 2011). Yet G2019S knock-in (KI) mice do not demonstrate the same pathology in lung or kidney as observed in LRRK2 KO mice (Herzig et al., 2011), and thus the mutant LRRK2-directed defects in protein clearance are likely more subtle than the complete loss-of-function observed in the KO animals.
Arrested fusion at various time points during autophagolysosome formation may underlie the various reported G2019S-induced cellular phenotypes, such as increased autophagosomes versus enlarged lysosomes (MacLeod et al., 2006; Sánchez-Danés et al., 2012). Difficulties in the interpretation of autophagy data could partially explain previous confusing and contradictory results. The fastidious application of appropriate controls could provide additional clarity (for review, see (Mizushima et al., 2010; Klionsky et al., 2012)). Further work will be required to understand the precise mechanism by which PD-linked LRRK2 mutations, G2019S and otherwise, affect ALP biology and direct the development of the disease. We propose that the nature of LRRK2-dependent deficits along the ALP continuum may manifest itself in unique ways that are likely to be highly dependent on cell type. The foamy cytoplasm phenotype in the Type II pneumocytes of LRRK2 KO animals suggests a predominant LRRK2-lysosome dynamic in these cells, for example, while the emphasis in monocytes seems to indicate an important role in the autophagolysosome function. Differential expression of LRRK2 splice variants in cells of the brain would support a diversity or alteration of LRRK2 function (Giesert et al., 2013). The nature of LRRK2’s association with the ALP may be questionable, but it is important to note that any disruption to normal LRRK2 signaling could result in profound changes to ALP/lysosomal function that have deleterious effects to the cell.
Influence of autophagy in the immune system
The uptake and digestion of pro-inflammatory molecules is an integral part of immune cell activation. A recent review describes how normal autophagic degradation can limit inflammation, and details the importance of lysosomal function to this process (Deretic et al., 2013). Firstly, cytokine production and secretion appears to be affected through either activation or inhibition of the autophagolysosomal pathway. Stimulation of autophagy in macrophages with the mTOR inhibitor rapamycin resulted in a degradation of IL-1β that prevented its release (Harris et al., 2011). Conversely, a blockade of autophagy increased secretion of the cytokine IL-23 from macrophage cells (Peral de Castro et al., 2012). Another group observed a reduction in TNF-α production and secretion from peripheral blood mononuclear cells when autophagy is prevented, but an increase in IL-1β secretion (Kleinnijenhuis et al., 2011). These actions are likely related to an inhibitory role on the activation of inflammasomes, autophagy-sensitive cytosolic protein complexes responsible for the proteolytic cleavage of pro-cytokines prior to secretion (Deretic et al., 2013).
Secondly, monocyte phagocytosis can also be driven by autophagy, to promote clearance of apoptotic cells, for example (Zang et al., 2012). However, macrophages deficient in autophagy demonstrate increased phagocytosis of bacteria (Bonilla et al., 2013). In addition, LC3-associated phagocytosis, a hybrid autophagy-phagocytosis process that is LC3-dependent, would also would be affected by the availability of autophagy machinery, including proteins such as beclin-1, ATG5, and ATG7 (Martinez et al., 2011; Vernon and Tang, 2013). Hence, the ALP system can influence the uptake of extracellular material in monocytes in addition to their subsequent degradation.
Finally, and perhaps most importantly, the processing of self-antigens for MHC class II presentation is potentially regulated through lysosomal proteolysis (Münz, 2009). This is required to permit immune tolerance, and the inability for adaptive immune cells to recognize these self-antigens can result in an autoimmune response that promotes inflammation and cell death. Therefore LRRK2-dependent ALP deficits could result in autoimmune dysregulation, as suggested for inflammatory disorders such as Crohn’s disease, colitis, and leprosy (Wellcome Trust Case Control Consortium, 2007; Henckaerts et al., 2011; Jostins et al., 2012; Yang et al., 2014). Indeed, co-morbidity between Parkinson’s disease and Crohn’s disease indicates that people diagnosed with PD could suffer from this dysregulation within the periphery, evidence for the potential involvement of the immune system as a player in vulnerability of PD, as well (Nalls et al., 2014). A recent review assesses the role of monocytes of the innate immune system in PD, including the promotion of neuroinflammation and the infiltration of peripheral macrophages, and supports a hypothesis of broad monocyte dysfunction in PD (Kannarkat et al., 2013). Thus, the etiology of PD could be partially rooted in an improper reactivity of monocytes such as microglia in response to native stimuli, including the protein αSyn, a central player in the pathogenesis and neuropathology of PD.
αSyn and Microglia Activation
Microglial activation is a normal biological process in which microglia respond to changes in their local environment, dynamically modifying their contributions to central nervous system function accordingly. These functional changes often are accompanied by conspicuous alterations in cellular morphology, where quiescent microglia exist in a ramified state and occupy minimal cytoplasmic volume. Upon activation, there is expansion of the cytoplasmic volume and contraction of processes and further ramification (hyper-ramified), or complete loss of processes and emergence of anamoeboid state (Perry et al., 1985; Giulian and Baker, 1986; Ransohoff and Cardona, 2010). These adaptations are further accompanied by changes in the expression of various cell-surface markers including but not limited to CD14, major histocompatibility complex (MHC) molecules, and chemokine and fractalkine receptors (Cho et al., 2006; Sheridan and Murphy, 2013). Regulated glial activation (a.k.a reactive gliosis) can be homeostatic, as both astrocytes and microglia have been implicated in the regulation of blood brain barrier integrity (Banerjee and Bhat, 2007; Ryu and McLarnon, 2009), synaptic plasticity (Fourgeaud and Boulanger, 2010; Schafer and Stevens, 2013), and the trophic support of neurons through the regulated release of growth factors (Nakajima and Kohsaka, 2004). In contrast, aberrant prolonged gliosis can lead to a harmful neuro-inflammatory state (Smith et al., 2012; Suzumura, 2013) that could contribute to neuropathology.
Lewy bodies are the pathological hallmark of PD (Spillantini et al., 1998) and αSyn is the major protein component of these insoluble inclusions. Aberrant accumulation of αSyn can promote both neuronal dysfunction and neuroinflammation. Missense mutations in αSyn associated with familial PD are argued to make the protein more aggregate-prone than WT αSyn (Conway et al., 1998; El-Agnaf et al., 1998), providing a mechanistic link between protein aggregation and disease. Moreover, duplication or triplication of the wild-type (WT) αSyn gene is sufficient to cause disease(Singleton et al., 2003; Chartier-Harlin et al., 2004), demonstrating that even modest increases in the WT protein are pathogenic. The accumulation of αSyn correlates with a near selective loss of dopaminergic neurons and an increase in activated glial cells in the substantia nigra in the PD brain (Croisier et al., 2005). To model αSyn-dependent processes in PD, a wide array of αSyn overexpression models in rodents have been developed. The sub-chronic adeno-associated virus-mediated overexpression of αSyn in the substantia nigra of mice leads to MHCII-dependent microglial activation, inflammatory cytokine release, lymphocyte infiltration and the subsequent degeneration of dopaminergic neurons (St Martin et al., 2007; Theodore et al., 2008; Cao et al., 2012; Harms et al., 2013; Rockenstein et al., 2014). Importantly, numerous transgenic αSyn animal models have corroborated these more acute results, further linking αSyn exposure to the promotion of neuroinflammation, in vivo (reviewed in (Sekiyama et al., 2012). Early studies examined αSyn immunization as a potential therapeutic treatment for PD-like synucleinopathy and found that human αSyn-expressing transgenic mice vaccinated against the human protein displayed reduced αSyn aggregates and decreased neurodegeneration (Masliah et al., 2005). This study was one of the first to suggest that immune cells can play a role in mitigating αSyn-dependent neuropathology.
There is little debate that changes in the metabolism or aggregation properties of aSyn can cause PD, yet the precise species and/or conformation of the offending protein is not clear. Both a soluble-oligomeric form (El-Agnaf et al., 2006; Paleologou et al., 2009; Winner et al., 2011; Rockenstein et al., 2014) and the fibrillar specie (Lashuel et al., 2002; Karpinar et al., 2009; Volpicelli-Daley et al., 2011) have been implicated in pathology. Likewise, the true physiological form of the protein remains unknown (Croke et al., 2008; Bartels et al., 2011; Wang et al., 2011; Fauvet et al., 2012; Lashuel et al., 2013). Regardless, the accumulation of toxic levels of αSyn can lead to the activation of both microglia (Ahn et al., 2012; Béraud et al., 2013; Harms et al., 2013) and astroglia (Fellner and Stefanova, 2013), and foster neuroinflammation that likely contributes to neurodegeneration. Thus, understanding the interplay between the αSyn protein and microglia, as well as the role of microglia in promoting or attenuating the progression of PD pathology, is critical. Such future work will likely identify under-appreciated aspects of disease pathogenesis, and may ultimately afford novel opportunities to design effective therapeutic strategies.
Is the Neuronal Secretion of αSyn Linked to Pathogenesis?
The discovery that neurons naturally secrete αSyn, a protein once considered to be strictly intracellular, was the first indication that extracellular αSyn might play a role in disease. αSyn was first identified as a neuronal protein enriched within presynaptic terminals and involved in learning and memory (originally named synelfin) in songbirds (George et al., 1995). Over the past few years, however, several studies have now demonstrated that αSyn is not only secreted from neurons (Luk et al., 2012a; Ejlerskov et al., 2013; Paillusson et al., 2013) but also subsequently taken up by surrounding neurons (Kordower et al., 2008; Desplats et al., 2009; Ahn et al., 2012) and glia (Lee et al., 2008b; Su et al., 2009; Lee et al., 2010b; Fellner et al., 2013; Kim et al., 2013). The observation that cerebral spinal fluid (CSF) (Mollenhauer and Schlossmacher, 2010; Mollenhauer et al., 2012; 2013) and interstitial fluid of PD patients contain detectable levels of αSyn (Emmanouilidou et al., 2011; Shi et al., 2014) provide further support that the neuronal release of αSyn could contribute to its pathogenicity. Braak et al. have proposed a model correlating the progression of PD pathology with the subsequent clinical phenotypes. It postulates that Lewy body pathology originates in the dorsal motor nucleus of the vagus nerve (as well as in olfactory structures) and proceeds in a neuron-to-neuron manner rostrally through the medulla, midbrain, forebrain, before eventually reaching the cerebral cortex (Braak et al., 2004). In this particular model, the later stages of the disease are associated with subsequent (or concomitant) neuronal loss and the resultant clinical symptoms. While the Braak model fits well with more recent demonstrations of the spreading of αSyn pathology in animal models (described below), it is has also been the subject of some controversy (Burke et al., 2008). Similarly, three independent investigations of the postmortem evaluation of patients who had received fetal ventral midbrain cell transplantation revealed mixed results with respect to emergent Lewy body pathology and signs of neurodegeneration in the grafted cells (Kordower et al., 2008; Mendez et al., 2008; Desplats et al., 2009). Nevertheless, their findings were largely consistent with the hypothesis that αSyn spread from the host brain into implanted tissues, and suggested a pathological mechanism for secreted αSyn.
Currently, numerous studies have now described the transfer of neuronally-secreted αSyn species to other neurons in culture and in vivo (Lee et al., 2005; 2008b; Hansen et al., 2011; Kordower et al., 2011; Lee et al., 2013; Braidy et al., 2014). While the mechanism of αSyn release is still not known, some have proposed that this process involves exosomes (Emmanouilidou et al., 2010; Alvarez-Erviti et al., 2011; Danzer et al., 2012), but this has not been broadly observed (Paillusson et al., 2013; Chutna et al., 2014). Recent in vivo studies have shown that that αSyn injected into rodent olfactory bulb (Rey et al., 2013), vagus nerve (AAV-αSyn) (Ulusoy et al., 2013), or striatum (Luk et al., 2012b) displays a rostral spreading of αSyn into regions of the brain characteristic of the pathology described in the Braak model. The informative study by Rey and colleagues injected different preparations of human αSyn into the olfactory bulb of mice, and detected human αSyn within interconnected neural structures that was consistent with both retrograde and anterograde transport of the injected αSyn (Rey et al., 2013). Independent studies have modeled αSyn-induced disease progression through stereotaxic injection of protofibrillar αSyn material into the brains of mice, showing age-dependent seeding of endogenous αSyn into Lewy Body-like aggregates (Luk et al., 2012a) followed by substantial neuronal loss and glial activation weeks later (Luk et al., 2012b). Recently, one group purified aggregated αSyn from Lewy body deposits of PD patients, injected the material into the brains of mice and non-human primates, and likewise observed αSyn pathology and nigrostriatal neurodegeneration (Recasens et al., 2014). However, one caveat regarding the current generation of studies may be that the concentrations of αSyn used are orders of magnitude higher than the human CSF αSyn levels recently reported: the direct quantification of CSF αSyn has been variable (reviewed in (Mollenhauer, 2014)), but levels have been reported to be as low as 1.31 ng/mL (90.5 pM) (Mondello et al., 2014) and as high as 39.67 ng/mL (2.7 nM) (Parnetti et al., 2014). Nevertheless, these in vivo data support the hypothesis that αSyn is secreted from healthy neurons, that this pool of the protein can play a role in PD pathogenesis, and these models provide excellent opportunities to explore the regulatory mechanisms involved in the inter-neuronal transfer of αSyn in PD.
Does α-Synuclein represent a pathologically relevant stimulator of microglial activation?
The literature supports multiple ways in which αSyn can influence microglia function and contribute to the etiology of PD. One model that has been widely investigated is the ability of pathologic conformations or toxic levels of αSyn to activate microgliosis and stimulate neuroinflammation. The loss of dopaminergic neurons in the substantia nigra and Lewy-body pathology characteristic of PD brains is often accompanied by signs of neuroinflammation, as an accumulation of CD68+ amoeboid microglia have been observed at the sites of neuronal loss (McGeer et al., 1988; Imamura et al., 2003; Hirsch and Hunot, 2009; Marinova-Mutafchieva et al., 2009). It is debatable whether microglial recruitment and activation occurs before or after the appearance of cytoplasmic αSyn inclusions and/or the first signs of neuronal dysfunction or loss. One could predict that the aberrant accumulation of αSyn in neurons would result in the increased release of a pathogenic αSyn specie, and the subsequent recruitment of microglia to affected brain regions prior to their activation by extracellular αSyn. Some groups have suggested specific cell-surface receptors such as TLR4 (Stefanova et al., 2011; Fellner et al., 2013), TLR2 (Kim et al., 2013), FCγR (Cao et al., 2012) or PAR-1 (Lee et al., 2010a) are required for αSyn-induced microglial activation. However, each of these studies has identified a different αSyn conformation as the primary ligand for microglial activation, as well as a distinct receptor with which it binds. This lack of consensus may indicate a non-specific nature of αSyn-induced microgliosis, or perhaps the capacity for multiple receptors to sense changes in the levels of extracellular αSyn, regardless of conformation. Investigations into the oligomeric secreted forms of αSyn are gaining momentum (Angot et al., 2012; Chai et al., 2013; Prots et al., 2013), but questions remain as to which αSyn specie is the most pathologically relevant. The recent use of neuronally secreted αSyn in this context may be particularly informative (Kim et al., 2013).
While it seems likely that extracellular αSyn can affect microglial cells, the efficacy of different secreted species in activating microgliosis is still undetermined (for an in-depth review see (Sanchez-Guajardo et al., 2013)). The monomeric and fibrillar αSyn species have both been shown to elicit an inflammatory response in microglia, either evidenced through analysis of cytokine secretion (Klegeris et al., 2008; Lee et al., 2009; Béraud et al., 2013; Austin et al., 2006; Su et al., 2008; Couch et al., 2011)or reactive oxygen species (ROS) production (Thomas et al., 2007; Fellner et al., 2013). However, it is difficult to accurately identify the pathogenic αSyn specie when both the function and the physiologically relevant form of αSyn are under debate (Cookson, 2009; Dettmer et al., 2013; Gurry et al., 2013). αSyn aggregates (soluble oligomers and fibrils) have displayed more consistent results with respect to activating microglia than monomeric αSyn (Zhang et al., 2005; Sanchez-Guajardo et al., 2010; Béraud et al., 2013; Kim et al., 2013). A recent study showed that soluble monomeric αSyn did not elicit an immune response in the microglia-like BV2 cell line, but relatively low doses of aggregated material dramatically altered TNF-α secretion from these cells (Béraud et al., 2013). These data suggest that aggregated αSyn is more likely to induce microgliosis than the monomeric conformation. One important point is that αSyn preparations utilized in the aforementioned microglial activation studies have been either recombinant, bacterially-expressed unfolded monomer or fibrillar aggregate at concentrations 50–1000 fold higher than the highest recently reported CSF αSyn values (Parnetti et al., 2014) from healthy human control subjects, which must be taken into account. Given the robust literature recently accumulated on neuronal secretion and models for in vitro (and in vivo) seeding of synucleinopathy, it will be valuable to examine more physiologically relevant preparations and concentrations of neuronally secreted αSyn with respect to microglial activation in the near future.
The clearance of extracellular αSyn by microglia
The removal and degradation of neuronally-secreted αSyn could be a key measure taken by microglia to prevent the spread of αSyn and its deposition into Lewy body inclusions. αSyn released from neuronal cells is not only taken up by other neurons, but also the non-neuronal cells of the brain. Astrocytes express relatively low levels of αSyn (Solano et al., 2000; Mori et al., 2002; Miller et al., 2005), but display large αSyn deposits in the brains of PD (Braak et al., 2007; Lee et al., 2010b) and Dementia with Lewy-Body (DLB) (Wakabayashi et al., 2000; Terada et al., 2003) patients. Similarly, in Multiple Systems Atrophy, oligodendrocytes primarily accumulate αSyn protein into glial cytoplasmic inclusions despite not expressing aSyn, further implicating the glial accumulation of αSyn in this disease (Spillantini et al., 1998; Tu et al., 1998; Dickson et al., 1999). In contrast, microglia appear to be the most efficient scavengers of extracellular αSyn (Lee et al., 2008a), and it is plausible that they actively buffer neurons from the aberrant accumulation and/or spread of the αSyn protein in PD. In the aforementioned study by Rey et al., injected αSyn was rapidly transferred to interconnected brain regions, but after 72 hours the exogenously added αSyn was absent from those neurons, and appeared in microglia of those same nuclei. These data provide experimental evidence that microglia rapidly internalize αSyn and may play a role in clearing secreted αSyn, not just in vitro but also in vivo (Rey et al., 2013).
The data describing microglial internalization of αSyn varies greatly, and can largely depend on the cellular system employed (immortalized vs. primary cell cultures) and the αSyn species being analyzed. Some in vitro studies have suggested that monomeric αSyn enters microglia in a clathrin-, caveolae-, dynamin-independent manner, as chemical inhibitors of each pathway and over-expression of dynamin K44A, a dominant negative form of dynamin, had no effect on the microglial uptake of monomeric αSyn (Park et al., 2009). Some groups have even proposed that αSyn diffuses freely in and out of cells, avoiding lysosomal degradation (Park et al., 2008; Lee et al., 2008a), while the fibrillar form of αSyn has been reported to be readily taken up by microglia and degraded by the lysosome (Liu et al., 2007; Lee et al., 2008a). Most investigations into the degradation of αSyn have been performed in neurons or neuronal-like cultures, and there is no consensus on the main cellular pathway of αSyn degradation. Both the ubiquitin-proteosome system (UPS) and the ALP have been implicated (Webb et al., 2003; Cuervo et al., 2004), and again outcomes appear to depend largely on the αSyn conformation being analyzed (for an in depth review see (Xilouri et al., 2013)). Briefly, soluble short-lived proteins are typically ubiquitinated and delivered for processing at the proteosome, while organelles, long-lived proteins, and aggregates of mis-folded proteins are degraded through microautophagy, macroautophagy, or chaperone-mediated autophagy via ALP. Consequently, in neurons, where αSyn is readily produced, an equilibrium likely exists between UPS degradation of soluble αSyn and ALP degradation of insoluble αSyn aggregates. Conversely, microglia do not express αSyn, and the internalization of aggregated αSyn likely results in the lumenal accumulation of the protein and clearance via ALP. As loss-of-function mutations in lysosomal proteins, such as the Gaucher’s disease-linked protein glucocerebrocidase, result in synuclein accumulation, deficits in microglial ALP are a probable contributor in the progression of PD (for an in depth review see (Bourdenx et al., 2014)).
Do Pathogenic Mutations in LRRK2 Alter The Interactions Between Microglia and αSyn?
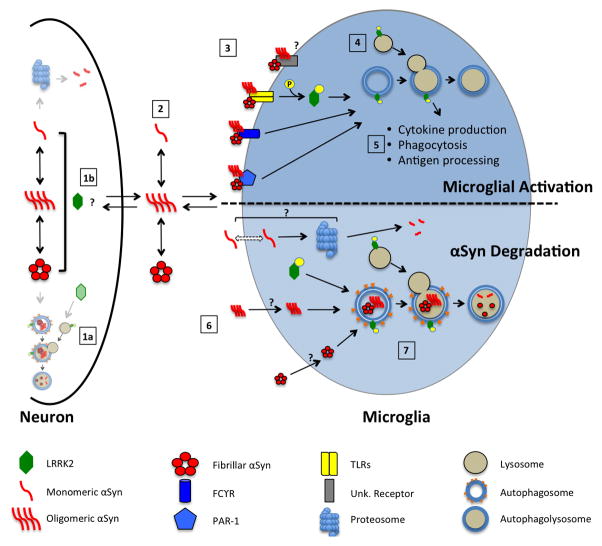
The robust responsiveness of microglia to extracellular αSyn and the relatively high LRRK2 expression in these cells compared to neurons suggest that LRRK2 plays a role in αSyn-induced microglial activation. The diversity of the changes in microglia due to αSyn are clear, and largely dependent on the αSyn species being analyzed, but taken together with the proposed role of LRRK2 in regulating microglia responsiveness, there is an enticing opportunity to investigate the relationship between these two PD-linked proteins across neurons and non-neuronal cells. We propose a model that may delineate how mutations in LRRK2 could exacerbate αSyn-induced microglial pathology and neuroinflammation, either by modulating the activation of microglia or regulating the clearance of internalized synuclein (Figure 2).
Figure 2. Opportunities for pathogenic mutations in LRRK2 to dysregulate microglial function.
In the neuron, αSyn may exist in an equilibrium between soluble (monomer, oligomer) and insoluble species (fibrillar). It could then be degraded by LRRK2-directed autophagy (1a) or be secreted into the extracellular milieu (scale of neuronal αSyn degradation pathways reduced for clarity). Neuronal αSyn is released via an unknown mechanism, and given the numerous ties between LRRK2 function and membrane dynamics, this process could be affected by LRRK2 (1b). As in the neuron, αSyn in the extracellular milieu may form higher order structures or remain as a monomer, where it can then interact with surrounding microglia (2). Extracellular αSyn could re-enter neurons, or interact with surrounding microglia. At the cell surface level, αSyn aggregates may activate different microglia receptors to initiate cell activation, where as monomeric αSyn may be inert. An activation of TLRs, or other as yet unidentified cell-surface receptors, would result in phosphorylation and membrane translocation of LRRK2 protein (3). LRRK2 recruited to ALP vesicles assist in the microglial activation response (4), modulating processes including the production/secretion of cytokines, phagocytosis of αSyn, or antigen processing to promote immune tolerance (5). Independently, it is believed that αSyn species are internalized by microglia (by a yet unknown mechanism) (6), potentially by passive diffusion (monomer), or by more active or receptor-mediated means (oligomer/fibrillar). LRRK2 may then regulate the degradation of these species through a lysosomal pathway (7). Dysregulation of these LRRK2-implicated pathways by pathogenic PD-associated missense mutations could then affect these pathways to result in neuroinflammation and accumulation of αSyn, respectively.
LRRK2 could mediate αSyn-induced microglial activation, based on either a commonality of receptor pathways responding to αSyn, or a potential involvement in multiple microglial signaling cascades. Initially, different α-synuclein conformations in the neuron may exist in an equilibrium, forming or dissociating as necessary. This synuclein could be degraded by some cellular mechanism, potentially by LRRK2-directed autophagic degradation (Point 1a, Figure 2), or could exit the cell by an unknown process. One possible method could be through exosome secretion (Emmanouilidou et al., 2010; Alvarez-Erviti et al., 2011; Danzer et al., 2012), a process potentially mediated by LRRK2 (Fraser et al., 2013) (Point 1b, Figure 2). This extracellular synuclein will also likely exist in some dynamic equilibrium of species and may then either be taken up by surrounding neurons, or interact in some capacity with microglia (Point 2, Figure 2). Studies in microglia and other monocytes reported that LRRK2 expression and phosphorylation were increased upon TLR2 or TLR4 stimulation (Gillardon et al., 2012; Moehle et al., 2012; Schapansky et al., 2014), and both of these cell surface receptors have been implicated in the αSyn-induced activation of microglia (in vitro, in vivo or both) (Fellner et al., 2013; Kim et al., 2013) (Points 3–5, Figure 2). In addition, a recent report demonstrates that a LRRK2 KO rat showed reduced microglial activation in response to both LPS (a TLR4 agonist) and αSyn (Daher et al., 2014), suggesting a requirement for intact LRRK2 signaling to initiate microglial activation. However, this finding awaits confirmation, as deletion of LRRK2 in mice was associated not with reduced monocyte activation but rather exaggerated inflammation in a model of colitis (Liu et al., 2011). However, the Daher study did show an increase in LRRK2 protein levels in the midbrain after treatment with LPS or αSyn that suggests that microglial inflammation is linked either directly or indirectly to LRRK2 function. These data provided early support for a role of LRRK2 in microglial activation, and potentially the response to αSyn. The role of LRRK2 in microglial responses to αSyn may also involve other signaling pathways with which LRRK2 has been associated. For example, more recent studies in microglia have proposed that LRRK2 is downstream of an ERK5-dependent response to IFNγ (Kuss et al., 2014). LRRK2 has also been implicated in ERK and JNK pathways (Bravo-San Pedro et al., 2012; Chen et al., 2012; Zhu et al., 2013), Wnt signaling (Sancho et al., 2009; Berwick and Harvey, 2012), NFAT regulation (Liu et al., 2011; Greggio et al., 2012) and others (Yuan et al., 2011; Dzamko et al., 2012). Alternatively, LRRK2 may modulate αSyn-induced microgliosis in a yet unidentified pathway (Figure 2, Point 3, “unidentified receptor”). In addition to the ligand-receptor model for αSyn-induced activation of microglia, one must also consider the possibility that the internalization and glial accumulation of pathologic αSyn, regardless of its conformation or aggregation state, may itself represent a challenge or stress to microglia resulting in their activation in a non-receptor mediated fashion. This responsiveness, as well, is yet another opportunity for mutations in LRRK2 to dysregulate microglial behavior in PD-relevant ways. While we are at the early stages of dissecting its non-autonomous contribution to the neurodegeneration that occurs in PD, the potential for LRRK2-dependent regulation of microgliosis and neuroinflammation is a critically important area of current investigation.
Independent of the ability of αSyn to simulate microglia, these cells have a well-established potential for the degradation of secreted, aggregation-prone proteins in the brain (reviewed in (Fu et al., 2014). Given the numerous links between LRRK2 and the ALP degradation pathway summarized in the present review, it is entirely possible that pathogenic mutations in LRRK2 impact the ability of microglia to internalize and/or degrade neuronally secreted αSyn (Points 6 & 7, Figure 2). The links between αSyn and autophagy are in fact many-fold, as conditional knockout of the autophagy protein ATG7 in the murine substantia nigra resulted in an age-dependent loss of dopaminergic neurons and an accumulation of αSyn comparable to that seen in the later stages of PD (Hara et al., 2006; Komatsu et al., 2006). An independent study showed that inhibition of autophagy leads to the increased exocytosis of αSyn (Lee et al., 2013), which would likely increase the rate of disease progression. Directly related to this theme, our group recently showed that inhibition of endogenous LRRK2 kinase activity impaired the autophagic clearance of a disease-linked aggregate-prone protein in microglial cells (Schapansky et al., 2014). One could imagine that a deficiency in the ALP machinery, as seen in LRRK2 animal models (Herzig et al., 2012; Hinkle:2012bx Baptista et al., 2013; Miklavc et al., 2014)), could hinder microglial clearance of αSyn. The elevation of αSyn protein and markers of autophagy in the kidneys of LRRK2 KO mice (Tong et al., 2010) certainly suggests that dysregulation of the LRRK2/ALP axis can lead to αSyn accumulation. The alteration of such processes and their role in PD pathogenesis is not limited to inherited mutations in LRRK2, but is likely impacted in normal aging (Terman and Brunk, 2005; Combaret et al., 2009; Barnett and Brewer, 2011; Cuervo and Macian, 2014), which is the single greatest risk factor in idiopathic PD (Hindle, 2010; Collier et al., 2011; Chinta et al., 2013).
Outlook
Here we describe multiple opportunities for the αSyn protein to impinge upon microglial function and how these interactions may contribute to PD pathogenesis and disease progression. From a classic ligand-receptor model, pathologic αSyn species may be produced and secreted by neurons where they stimulate microgliosis, neuroinflammation, and hasten neuronal loss. In a more passive manner, microglia may serve as vigilant protectors, internalizing and degrading pathologic αSyn and attenuating propagation of synucleinopathy, and this homeostatic process may be altered through inherited LRRK2 mutations affecting microglial function, or simply through aging. It is noteworthy that distinct literatures support the potential involvement of LRRK2 in both capacities, and that these two models are not mutually exclusive for their relevance to PD pathogenesis nor the potential dysregulation by disease-linked mutations in LRRK2. It has been suggested that in the rodent brain that the density of microglia is highest in the substantia nigra (Lawson et al., 1990). Given that this brain region is most vulnerable to αSyn deposition and neuron loss in PD, it is interesting to speculate that perhaps these cells have a greater intrinsic vulnerability that requires an unusually high density of microglial to buffer them from challenges, such as pathogenic αSyn. Clearly, there is much work to do, but present and emerging data continue to make the compelling case that non-cell autonomous pathways likely contribute to the etiology and progression of PD, even in the context of a neuronal protein such as αSyn.
Highlights.
Review of relationships between the PD-linked protein α-synuclein and microglia
Synthesis of available literature regarding the functions of LRRK2 in microglia
Proposed models reconciling LRRK2 function with α-synuclein/microglia interactions
Acknowledgments
We would like to thank T. Young-Pearse and R. Charan for critical reading of the manuscript. Funding for authors was supplied by the Michael J. Fox Foundation [M.J.L] and the National Institutes of Health (NS072604 to M.J.L.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn T-B, Langston JW, Aachi VR, Dickson DW. Relationship of neighboring tissue and gliosis to α-synuclein pathology in a fetal transplant for Parkinson’s disease. Am J Neurodegener Dis. 2012;1:49–59. [PMC free article] [PubMed] [Google Scholar]
- Alegre-Abarrategui J, Christian H, Lufino MMP, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJA, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot E, Steiner JA, Lema Tomé CM, Ekström P, Mattsson B, Björklund A, Brundin P. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS ONE. 2012;7:e39465. doi: 10.1371/journal.pone.0039465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MAS, Dave KD, Frasier MA, Sherer TB, Greeley M, Beck MJ, Varsho JS, Parker GA, Moore C, Churchill MJ, Meshul CK, Fiske BK. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS ONE. 2013;8:e80705. doi: 10.1371/journal.pone.0080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett A, Brewer GJ. Autophagy in aging and Alzheimer’s disease: pathologic or protective? J Alzheimers Dis. 2011;25:385–394. doi: 10.3233/JAD-2011-101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011 doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Smith KA, LaVoie MJ. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry. 2010;49:5511–5523. doi: 10.1021/bi100157u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Harvey K. LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D, Hathaway HA, Trecki J, Chasovskikh S, Johnson DA, Johnson JA, Federoff HJ, Shimoji M, Mhyre TR, Maguire-Zeiss KA. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein α-synuclein. J Neuroimmune Pharmacol. 2013;8:94–117. doi: 10.1007/s11481-012-9401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu S-W, Savitt JM, Waldvogel HJ, Faull RLM, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- Bonilla DL, Bhattacharya A, Sha Y, Xu Y, Xiang Q, Kan A, Jagannath C, Komatsu M, Eissa NT. Autophagy regulates phagocytosis by modulating the expression of scavenger receptors. Immunity. 2013;39:537–547. doi: 10.1016/j.immuni.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, LaVoie MJ, Petsko GA, Ringe D. Proteostasis and movement disorders: Parkinson’s disease and amyotrophic lateral sclerosis. Cold Spring Harb Perspect Biol. 2011;3:a007500. doi: 10.1101/cshperspect.a007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M, Bezard E, dehay B. Lysosomes and alpha-synuclein form a dangerous duet leading to neuronal cell death Blesa J, ed. Frontiers in neuroanatomy. 2014;8:1–7. doi: 10.3389/fnana.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Braidy N, Gai WP, Xu YH, Sachdev P, Guillemin GJ, Jiang X-M, Ballard JWO, Horan MP, Fang ZM, Chong BH, Chan DKY. Alpha-synuclein transmission and mitochondrial toxicity in primary human foetal enteric neurons in vitro. Neurotox Res. 2014;25:170–182. doi: 10.1007/s12640-013-9420-5. [DOI] [PubMed] [Google Scholar]
- Bravo-San Pedro JM, Niso-Santano M, Gómez-Sánchez R, Pizarro-Estrella E, Aiastui-Pujana A, Gorostidi A, Climent V, López de Maturana R, Sanchez-Pernaute R, López de Munain A, Fuentes JM, González-Polo RA. The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Dauer WT, Vonsattel JPG. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar M, Zach S, Carlson CB, Brockmann K, Gasser T, Gillardon F. Leucine-rich repeat kinase 2 functionally interacts with microtubules and kinase-dependently modulates cell migration. Neurobiol Dis. 2013;54:280–288. doi: 10.1016/j.nbd.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Cao S, Standaert DG, Harms AS. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J Neuroinflammation. 2012;9:259. doi: 10.1186/1742-2094-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CC, Pereira AC, de Sales Marques C, Moraes MO. Leprosy susceptibility: genetic variations regulate innate and adaptive immunity, and disease outcome. Future Microbiol. 2011;6:533–549. doi: 10.2217/fmb.11.39. [DOI] [PubMed] [Google Scholar]
- Chai Y-J, Kim D, Park J, Zhao H, Lee S-J, Chang S. The secreted oligomeric form of α-synuclein affects multiple steps of membrane trafficking. FEBS Lett. 2013;587:452–459. doi: 10.1016/j.febslet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Chan D, Citro A, Cordy JM, Shen GC, Wolozin B. Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2 (LRRK2) J Biol Chem. 2011;286:16140–16149. doi: 10.1074/jbc.M111.234005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin M-C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Weng Y-H, Chien K-Y, Lin K-J, Yeh T-H, Cheng Y-P, Lu C-S, Wang H-L. Cell death and Differentiation. Cell Death Differ. 2012;19:1623–1633. doi: 10.1038/cdd.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Lieu CA, Demaria M, Laberge R-M, Campisi J, Andersen JK. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson’s disease? J Intern Med. 2013;273:429–436. doi: 10.1111/joim.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BP, Song DY, Sugama S, Shin DH, Shimizu Y, Kim SS, Kim YS, Joh TH. Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia. 2006;53:92–102. doi: 10.1002/glia.20265. [DOI] [PubMed] [Google Scholar]
- Chutna O, Gonçalves S, Villar-Piqué A, Guerreiro P, Marijanovic Z, Mendes T, Ramalho J, Emmanouilidou E, Ventura S, Klucken J, Barral DC, Giorgini F, Vekrellis K, Outeiro TF. The small GTPase Rab11 co-localizes with α-synuclein in intracellular inclusions and modulates its aggregation, secretion and toxicity. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu391. [DOI] [PubMed] [Google Scholar]
- Cirnaru MD, Marte A, Belluzzi E, Russo I, Gabrielli M, Longo F, Arcuri L, Murru L, Bubacco L, Matteoli M, Fedele E, Sala C, Passafaro M, Morari M, Greggio E, Onofri F, Piccoli G. LRRK2 kinase activity regulates synaptic vesicle trafficking and neurotransmitter release through modulation of LRRK2 macro-molecular complex. Front Mol Neurosci. 2014;7:49. doi: 10.3389/fnmol.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12:359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Béchet D, Taillandier D, Mosoni L, Attaix D. Skeletal muscle proteolysis in aging. Curr Opin Clin Nutr Metab Care. 2009;12:37–41. doi: 10.1097/MCO.0b013e32831b9c31. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y, Alvarez-Erviti L, Sibson NR, Wood MJA, Anthony DC. The acute inflammatory response to intranigral α-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation. 2011;8:166. doi: 10.1186/1742-2094-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RKB, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croke RL, Sallum CO, Watson E, Watt ED, Alexandrescu AT. Hydrogen exchange of monomeric alpha-synuclein shows unfolded structure persists at physiological temperature and is independent of molecular crowding in Escherichia coli. Protein Sci. 2008;17:1434–1445. doi: 10.1110/ps.033803.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Macian F. Autophagy and the immune function in aging. Current Opinion in Immunology. 2014;29C:97–104. doi: 10.1016/j.coi.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Daher JPL, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of -synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, Lee J-D, Patricelli MP, Nomanbhoy TK, Alessi DR, Gray NS. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat Chem Biol. 2011;7:203–205. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee H-J, Bae E-J, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee S-J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. Journal of Biological Chemistry. 2013;288:6371–6385. doi: 10.1074/jbc.M112.403311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J, Sima A, D’Amato C, Albin R, Gilman S, Yen SH. Widespread alterations of alpha-synuclein in multiple system atrophy. AJPA. 1999;155:1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2012;21:1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett EA, Zhao J, Mork CN, Hu D, Nichols RJ. Phosphorylation of LRRK2 serines 955 and 973 is disrupted by Parkinson’s disease mutations and LRRK2 pharmacological inhibition. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07537.x. [DOI] [PubMed] [Google Scholar]
- Dzamko N, Deak M, Hentati F, Reith AD, Prescott AR, Alessi DR, Nichols RJ. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J. 2010;430:405–413. doi: 10.1042/BJ20100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Inesta-Vaquera F, Zhang J, Xie C, Cai H, Arthur S, Tan L, Choi H, Gray N, Cohen P, Pedrioli P, Clark K, Alessi DR. The IkappaB Kinase Family Phosphorylates the Parkinson’s Disease Kinase LRRK2 at Ser935 and Ser910 during Toll-Like Receptor Signaling. PLoS ONE. 2012;7:e39132. doi: 10.1371/journal.pone.0039132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejlerskov P, Rasmussen I, Nielsen TT, Bergström A-L, Tohyama Y, Jensen PH, Vilhardt F. Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion. Journal of Biological Chemistry. 2013;288:17313–17335. doi: 10.1074/jbc.M112.401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Bodles AM, Guthrie DJ, Harriott P, Irvine GB. The N-terminal region of non-A beta component of Alzheimer’s disease amyloid is responsible for its tendency to assume beta-sheet and aggregate to form fibrils. Eur J Biochem. 1998;258:157–163. doi: 10.1046/j.1432-1327.1998.2580157.x. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OMA, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. The FASEB Journal. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, Vekrellis K. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS ONE. 2011;6:e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvet B, Mbefo MK, Fares M-B, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. Journal of Biological Chemistry. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Stefanova N. The role of glia in α-synucleinopathies. Mol Neurobiol. 2013;47:575–586. doi: 10.1007/s12035-012-8340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Boulanger LM. Role of immune molecules in the establishment and plasticity of glutamatergic synapses. Eur J Neurosci. 2010;32:207–217. doi: 10.1111/j.1460-9568.2010.07342.x. [DOI] [PubMed] [Google Scholar]
- Fraser KB, Moehle MS, Daher JPL, Webber PJ, Williams JY, Stewart CA, Yacoubian TA, Cowell RM, Dokland T, Ye T, Chen D, Siegal GP, Galemmo RA, Tsika E, Moore DJ, Standaert DG, Kojima K, Mobley JA, West AB. LRRK2 Secretion in Exosomes is Regulated by 14-3-3. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49:1422–1434. doi: 10.1007/s12035-013-8620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- Gardet A, Benita Y, Li C, Sands BE, Ballester I, Stevens C, Korzenik JR, Rioux JD, Daly MJ, Xavier RJ, Podolsky DK. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J Immunol. 2010;185:5577–5585. doi: 10.4049/jimmunol.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Giesert F, Hofmann A, Bürger A, Zerle J, Kloos K, Hafen U, Ernst L, Zhang J, Vogt-Weisenhorn DM, Wurst W. Expression analysis of Lrrk1, Lrrk2 and Lrrk2 splice variants in mice. PLoS One. 2013 doi: 10.1371/journal.pone.0063778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P, Luzón-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P, Rivero-Ríos P, Fdez E, Ramírez MB, Ferrer I, Aiastui A, López de Munain A, Hilfiker S. LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu395. [DOI] [PubMed] [Google Scholar]
- Greggio E, Civiero L, Bisaglia M, Bubacco L. Parkinson’s disease and immune system: is the culprit LRRKing in the periphery? J Neuroinflammation. 2012;9:94. doi: 10.1186/1742-2094-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gurry T, Ullman O, Fisher CK, Perovic I, Pochapsky T, Stultz CM. The dynamic structure of α-synuclein multimers. J Am Chem Soc. 2013;135:3865–3872. doi: 10.1021/ja310518p. [DOI] [PubMed] [Google Scholar]
- Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, Kabbach G, Venderova K, Girardin SE, Bulman DE, Scherzer CR, LaVoie MJ, Gris D, Park DS, Angel JB, Shen J, Philpott DJ, Schlossmacher MG. Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm. 2011;118:795–808. doi: 10.1007/s00702-011-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergström A-L, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li J-Y, Brundin P. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. MHCII Is Required for -Synuclein-Induced Activation of Microglia, CD4 T Cell Proliferation, and Dopaminergic Neurodegeneration. J Neurosci. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, Kornfeld H, Fitzgerald KA, Lavelle EC. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. Journal of Biological Chemistry. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DG, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. The Lancet Neurology. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckaerts L, Cleynen I, Brinar M, John JM, Van Steen K, Rutgeerts P, Vermeire S. Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm Bowel Dis. 2011;17:1392–1397. doi: 10.1002/ibd.21486. [DOI] [PubMed] [Google Scholar]
- Herzig MC, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig MC, Bidinosti M, Schweizer T, Hafner T, Stemmelen C, Weiss A, Danner S, Vidotto N, Stauffer D, Barske C, Mayer F, Schmid P, Rovelli G, van der Putten PH, Shimshek DR. High LRRK2 Levels Fail to Induce or Exacerbate Neuronal Alpha-Synucleinopathy in Mouse Brain Lewis P, ed. PLoS ONE. 2012;7:e36581. doi: 10.1371/journal.pone.0036581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi S, Moore DJ, Yamamoto R, Minegishi M, Sato K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Hino H, Kosaka K, Emson PC, Wada K, Dawson VL, Dawson TM, Arai H, Iseki E. Abnormal localization of leucine-rich repeat kinase 2 to the endosomal-lysosomal compartment in lewy body disease. J Neuropathol Exp Neurol. 2009;68:994–1005. doi: 10.1097/NEN.0b013e3181b44ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle JV. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- Hinkle KM, Yue M, Behrouz B, Dächsel JC, Lincoln SJ, Bowles EE, Beevers JE, Dugger B, Winner B, Prots I, Kent CB, Nishioka K, Lin W-L, Dickson DW, Janus CJ, Farrer MJ, Melrose HL. LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor co-ordination behaviors. Mol Neurodegener. 2012;7:25. doi: 10.1186/1750-1326-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot SP. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? The Lancet Neurology. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, Alessi DR. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson’s disease. J Parkinsons Dis. 2013;3:493–514. doi: 10.3233/JPD-130250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinar DP, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho D-H, Suk J-E, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee H-J, Lee S-J. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis J, Oosting M, Plantinga TS, van der Meer JWM, Joosten LAB, Crevel RV, Netea MG. Autophagy modulates the Mycobacterium tuberculosis-induced cytokine response. Immunology. 2011;134:341–348. doi: 10.1111/j.1365-2567.2011.03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-I, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, Sortwell C, Steece-Collier K, Collier TJ. Transfer of host-derived α synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari U, Tan EK. LRRK2 in Parkinson’s disease: genetic and clinical studies from patients. FEBS J. 2009;276:6455–6463. doi: 10.1111/j.1742-4658.2009.07344.x. [DOI] [PubMed] [Google Scholar]
- Kuss M, Adamopoulou E, Kahle PJ. Interferon-γ induces leucine-rich repeat kinase LRRK2 via extracellular signal-regulated kinase ERK5 in macrophages. J Neurochem. 2014 doi: 10.1111/jnc.12668. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee E-J, Woo M-S, Moon P-G, Baek M-C, Choi I-Y, Kim W-K, Junn E, Kim H-S. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. The Journal of Immunology. 2010a;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Cho E-D, Lee KW, Kim J-H, Cho S-G, Lee S-J. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp Mol Med. 2013;45:e22. doi: 10.1038/emm.2013.45. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23661100&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Patel S, Lee S-J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Suk J-E, Bae E-J, Lee J-H, Paik SR, Lee S-J. Assembly-dependent endocytosis and clearance of extracellular a-synuclein. The International Journal of Biochemistry & Cell Biology. 2008a;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Suk J-E, Bae E-J, Lee S-J. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008b;372:423–428. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct Transfer of -Synuclein from Neuron to Astroglia Causes Inflammatory Responses in Synucleinopathies. Journal of Biological Chemistry. 2010b;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-B, Park SM, Ahn KJ, Chung KC, Paik SR, Kim J. Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem Biophys Res Commun. 2009;381:39–43. doi: 10.1016/j.bbrc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Lee S-J, Lim H-S, Masliah E, Lee H-J. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res. 2011;70:339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, et al. Parkinson’s disease-related LRRK2 G2019S mutation results from independent mutational events in humans. Hum Mol Genet. 2010;19:1998–2004. doi: 10.1093/hmg/ddq081. [DOI] [PubMed] [Google Scholar]
- Li X, Wang QJ, Pan N, Lee S, Zhao Y, Chait BT, Yue Z. Phosphorylation-dependent 14-3-3 binding to LRRK2 is impaired by common mutations of familial Parkinson’s disease. In: Li X, Wang QJ, Pan N, Lee S, Zhao Y, Chait BT, Yue Z, editors. PLoS ONE. Vol. 6. 2011. p. e17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Tsai P-I, Wu R-M, Chien C-T. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3β. J Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhou Y, Wang Y, Fong H, Murray TM, Zhang J. Identification of proteins involved in microglial endocytosis of alpha-synuclein. J Proteome Res. 2007;6:3614–3627. doi: 10.1021/pr0701512. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol. 2011;12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael E, Zhao J, Rudenko IN, Beylina A, Gao F, Wetter J, Beullens M, Bollen M, Cookson MR, Baekelandt V, Nichols RJ, Taymans J-M. Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. Biochem J. 2013 doi: 10.1042/BJ20121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luerman GC, Nguyen C, Samaroo H, Loos P, Xi H, Hurtado-Lorenzo A, Needle E, Noell GS, Galatsis P, Dunlop J, Geoghegan KF, Hirst WD. Phosphoproteomic Evaluation Of Pharmacological Inhibition Of Leucine-Rich Repeat Kinase 2 Reveals Significant Off-Target Effects Of Lrrk-2-In-1. J Neurochem. 2013 doi: 10.1111/jnc.12483. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Pathological - Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological -synuclein initiates a rapidly progressive neurodegenerative -synucleinopathy in mice. Journal of Experimental Medicine. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D, Dowman J, Hammond R, Leete T, Inoue K, Abeliovich A. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, MacCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A. RAB7L1 Interacts with LRRK2 to Modify Intraneuronal Protein Sorting and Parkinson’s Disease Risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamais A, Chia R, Beilina A, Hauser DN, Hall C, Lewis PA, Cookson MR, Bandopadhyay R. Arsenite Stress Downregulates Phosphorylation and 14-3-3 Binding of Leucine-rich Repeat Kinase 2 (LRRK2) Promoting Self-Association and Cellular Redistribution. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M113.528463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandemakers W, Snellinx A, O’Neill MJ, De Strooper B. LRRK2 expression is enriched in the striosomal compartment of mouse striatum. Neurobiol Dis. 2012;48:582–593. doi: 10.1016/j.nbd.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem. 2009;110:966–975. doi: 10.1111/j.1471-4159.2009.06189.x. [DOI] [PubMed] [Google Scholar]
- Marker DF, Puccini JM, Mockus TE, Barbieri J, Lu S-M, Gelbard HA. LRRK2 kinase inhibition prevents pathological microglial phagocytosis in response to HIV-1 Tat protein. J Neuroinflammation. 2012;9:261. doi: 10.1186/1742-2094-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci USA. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Matta S, et al. LRRK2 Controls an Endo A Phosphorylation Cycle in Synaptic Endocytosis. Neuron. 2012;75:1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson“s and Alzheimer”s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklavc P, Ehinger K, Thompson KE, Hobi N, Shimshek DR, Frick M. Surfactant Secretion in LRRK2 Knock-Out Rats: Changes in Lamellar Body Morphology and Rate of Exocytosis. PLoS ONE. 2014;9:e84926. doi: 10.1371/journal.pone.0084926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in Mammalian Autophagy Research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, Desilva TM, Cowell RM, West AB. LRRK2 Inhibition Attenuates Microglial Inflammatory Responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B. Quantification of α-synuclein in cerebrospinal fluid: how ideal is this biomarker for Parkinson’s disease? Parkinsonism & Related Disorders. 2014;20(Suppl 1):S76–S79. doi: 10.1016/S1353-8020(13)70020-8. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Schlossmacher MG. CSF synuclein: adding to the biomarker footprint of dementia with Lewy bodies. J Neurol Neurosurg Psychiatr. 2010;81:590–591. doi: 10.1136/jnnp.2010.206391. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Otte B, Ng J, Spreer A, Lange P, Sixel-Döring F, Hakimi M, Vonsattel J-P, Nussbaum R, Trenkwalder C, Schlossmacher MG. α-Synuclein in human cerebrospinal fluid is principally derived from neurons of the central nervous system. J Neural Transm. 2012;119:739–746. doi: 10.1007/s00702-012-0784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Döring F, Ebentheuer J, Trenkwalder C, Schlossmacher MG. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci Lett. 2013;532:44–48. doi: 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Mondello S, Constantinescu R, Zetterberg H, Andreasson U, Holmberg B, Jeromin A. CSF α-synuclein and UCH-L1 levels in Parkinson’s disease and atypical parkinsonian disorders. Parkinsonism & Related Disorders. 2014;20:382–387. doi: 10.1016/j.parkreldis.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- Muda K, Bertinetti D, Gesellchen F, Hermann JS, Zweydorf von F, Geerlof A, Jacob A, Ueffing M, Gloeckner CJ, Herberg FW. Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14-3-3. Proc Natl Acad Sci USA. 2014;111:E34–E43. doi: 10.1073/pnas.1312701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- Nalls MA, et al. Genetic comorbidities in Parkinson’s disease. Hum Mol Genet. 2014;23:831–841. doi: 10.1093/hmg/ddt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RJ, Dzamko N, Hutti JE, Cantley LC, Deak M, Moran J, Bamborough P, Reith AD, Alessi DR. Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson’s disease. Biochem J. 2009;424:47–60. doi: 10.1042/BJ20091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RJ, Dzamko N, Morrice NA, Campbell DG, Deak M, Ordureau A, Macartney T, Tong Y, Shen J, Prescott AR, Alessi DR. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem J. 2010;430:393–404. doi: 10.1042/BJ20100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein SJ, Kuo S-H, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo AM. Interplay of LRRK2 with chaperone-mediated autophagy. Nature Publishing Group; 2013. pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillusson S, Clairembault T, Biraud M, Neunlist M, Derkinderen P. Activity-dependent secretion of alpha-synuclein by enteric neurons. J Neurochem. 2013;125:512–517. doi: 10.1111/jnc.12131. [DOI] [PubMed] [Google Scholar]
- Paisán-Ruíz C, Nath P, Washecka N, Gibbs JR, Singleton AB. Comprehensive analysis of LRRK2 in publicly available Parkinson’s disease cases and neurologically normal controls. Hum Mutat. 2008;29:485–490. doi: 10.1002/humu.20668. [DOI] [PubMed] [Google Scholar]
- Paleologou KE, Kragh CL, Mann DMA, Salem SA, Al-Shami R, Allsop D, Hassan AH, Jensen PH, El-Agnaf OMA. Detection of elevated levels of soluble alpha-synuclein oligomers in postmortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132:1093–1101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- Park JY, Kim K-S, Lee S-B, Ryu J-S, Chung KC, Choo Y-K, Jou I, Kim J, Park SM. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem. 2009;110:400–411. doi: 10.1111/j.1471-4159.2009.06150.x. [DOI] [PubMed] [Google Scholar]
- Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric α-synuclein, not aggregated α-synuclein: Implications for Parkinson’s disease. Glia. 2008;56:1215–1223. doi: 10.1002/glia.20691. [DOI] [PubMed] [Google Scholar]
- Parnetti L, et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov Disord. 2014;29:1019–1027. doi: 10.1002/mds.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peral de Castro C, Jones SA, Ní Cheallaigh C, Hearnden CA, Williams L, Winter J, Lavelle EC, Mills KHG, Harris J. Autophagy regulates IL-23 secretion and innate T cell responses through effects on IL-1 secretion. The Journal of Immunology. 2012;189:4144–4153. doi: 10.4049/jimmunol.1201946. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Cherra SJ, Liu Y-J, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prots I, Veber V, Brey S, Campioni S, Buder K, Riek R, Böhm KJ, Winner B. α-Synuclein oligomers impair neuronal microtubule-kinesin interplay. Journal of Biological Chemistry. 2013;288:21742–21754. doi: 10.1074/jbc.M113.451815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Recasens A, dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut P-O, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Rey NL, Petit GH, Bousset L, Melki R, Brundin P. Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013;126:555–573. doi: 10.1007/s00401-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Nuber S, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Trejo-Morales M, Gerez J, Picotti P, Jensen PH, Campioni S, Riek R, Winkler J, Gage FH, Winner B, Masliah E. Accumulation of oligomer-prone α-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain. 2014;137:1496–1513. doi: 10.1093/brain/awu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M. Neuroimmunological processes in Parkinson’s disease and their relation to α-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro. 2013;5:113–139. doi: 10.1042/AN20120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho RM, Law BMH, Harvey K. Mutations in the LRRK2 Roc-COR tandem domain link Parkinson’s disease to Wnt signalling pathways. Hum Mol Genet. 2009;18:3955–3968. doi: 10.1093/hmg/ddp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, Jiménez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals JM, Memo M, Alberch J, López-Barneo J, Vila M, Cuervo AM, Tolosa E, Consiglio A, Raya A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013;23:1034–1040. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapansky J, Nardozzi JD, Felizia F, LaVoie MJ. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiyama K, Sugama S, Fujita M, Sekigawa A, Takamatsu Y, Waragai M, Takenouchi T, Hashimoto M. Neuroinflammation in Parkinson’s Disease and Related Disorders: A Lesson from Genetically Manipulated Mouse Models of α-Synucleinopathies. Parkinsons Dis. 2012;2012:271732. doi: 10.1155/2012/271732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3:130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu S-C, Quinn JF, Galasko DR, Banks WA, Zhang J. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano SM, Miller DW, Augood SJ, Young AB, Penney JB. Expression of alpha-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: genes associated with familial Parkinson’s disease. Ann Neurol. 2000;47:201–210. [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J Neurochem. 2007;100:1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-Like Receptor 4 Promotes α-Synuclein Clearance and Survival of Nigral Dopaminergic Neurons. AJPA. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr Protein Pept Sci. 2013;14:16–20. doi: 10.2174/1389203711314010004. [DOI] [PubMed] [Google Scholar]
- Terada S, Ishizu H, Yokota O, Tsuchiya K, Nakashima H, Ishihara T, Fujita D, Uéda K, Ikeda K, Kuroda S. Glial involvement in diffuse Lewy body disease. Acta Neuropathol. 2003;105:163–169. doi: 10.1007/s00401-002-0622-9. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. The aging myocardium: roles of mitochondrial damage and lysosomal degradation. Heart Lung Circ. 2005;14:107–114. doi: 10.1016/j.hlc.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenet J, Pescini Gobert R, Hooft van Huijsduijnen R, Wiessner C, Sagot YJ. Regulation of LRRK2 Expression Points to a Functional Role in Human Monocyte Maturation. PLoS ONE. 2011;6:e21519. doi: 10.1371/journal.pone.0021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MP, Chartrand K, Reynolds A, Vitvitsky V, Banerjee R, Gendelman HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson’s disease. J Neurochem. 2007;100:503–519. doi: 10.1111/j.1471-4159.2006.04315.x. [DOI] [PubMed] [Google Scholar]
- Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci USA. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Rusconi R, Pérez-Revuelta BI, Musgrove RE, Helwig M, Winzen-Reichert B, Monte DAD. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med. 2013;5:1119–1127. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno J, Asano K, Matsushita T, Matsumoto T, Kiyohara Y, Iida M, Nakamura Y, Kamatani N, Kubo M. Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2011;17:2407–2415. doi: 10.1002/ibd.21651. [DOI] [PubMed] [Google Scholar]
- Vancraenenbroeck R, De Raeymaecker J, Lobbestael E, Gao F, De Maeyer M, Voet A, Baekelandt V, Taymans J-M. In silico, in vitro and cellular analysis with a kinome-wide inhibitor panel correlates cellular LRRK2 dephosphorylation to inhibitor activity on LRRK2. Front Mol Neurosci. 2014;7:51. doi: 10.3389/fnmol.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon PJ, Tang D. Eat-me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid Redox Signal. 2013;18:677–691. doi: 10.1089/ars.2012.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM-Y. Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- Wang W, et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. LRRK2 Regulates Mitochondrial Dynamics and Function through Direct Interaction with DLP1. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Cowell RM, Daher JPL, Moehle MS, Hinkle KM, Melrose HL, Standaert DG, Volpicelli-Daley LA. Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J Comp Neurol. 2014;522:Spc1. doi: 10.1002/cne.23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Melrose HL, Zhao C, Hinkle KM, Yue M, Kent C, Braithwaite AT, Ogholikhan S, Aigner R, Winkler J, Farrer MJ, Gage FH. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41:706–716. doi: 10.1016/j.nbd.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Brekk OR, Stefanis L. α-Synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol. 2013;47:537–551. doi: 10.1007/s12035-012-8341-2. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen J, Shi C, Jing Z, Song N. Autophagy gene polymorphism is associated with susceptibility to leprosy by affecting inflammatory cytokines. Inflammation. 2014;37:593–598. doi: 10.1007/s10753-013-9773-1. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Cao P, Smith MA, Kramp K, Huang Y, Hisamoto N, Matsumoto K, Hatzoglou M, Jin H, Feng Z. Dysregulated LRRK2 Signaling in Response to Endoplasmic Reticulum Stress Leads to Dopaminergic Neuron Degeneration in C. elegans. PLoS ONE. 2011;6:e22354. doi: 10.1371/journal.pone.0022354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L, Xu Q, Ye Y, Li X, Liu Y, Tashiro S-I, Onodera S, Ikejima T. Autophagy enhanced phagocytosis of apoptotic cells by oridonin-treated human histocytic lymphoma U937 cells. Arch Biochem Biophys. 2012;518:31–41. doi: 10.1016/j.abb.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong J-S, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. The FASEB Journal. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang C, Yu M, Cui J, Liu L, Xu Z. ULK1 and JNK are involved in mitophagy incurred by LRRK2 G2019S expression. Protein & Cell. 2013:4. doi: 10.1007/s13238-013-3910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]