Abstract
Exercise (physical activity) has been proposed as a treatment for drug addiction. In rodents, voluntary wheel running reduces cocaine and nicotine seeking during extinction, and reinstatement of cocaine seeking triggered by drug cues. The purpose of this study was to examine the effects of chronic wheel running during withdrawal and protracted abstinence on extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats, and to determine a potential neurobiological correlate underlying the effects. Rats were given extended access to methamphetamine (0.05 mg/kg, 6h/day) for 22 sessions. Rats were withdrawn and were given access to running wheels (wheel runners) or no wheels (sedentary) for three weeks after which they experienced extinction and reinstatement of methamphetamine seeking. Extended access to methamphetamine self-administration produced escalation in methamphetamine intake. Methamphetamine experience reduced running output, and conversely, access to wheel running during withdrawal reduced responding during extinction and, context- and cue-induced reinstatement of methamphetamine seeking. Immunohistochemical analysis of brain tissue demonstrated that wheel running during withdrawal did not regulate markers of methamphetamine neurotoxicity (neurogenesis, neuronal nitric oxide synthase, vesicular monoamine transporter-2) and cellular activation (c-Fos) in brain regions involved in relapse to drug seeking. However, reduced methamphetamine seeking was associated with running-induced reduction (and normalization) of the number of tyrosine hydroxylase (TH) immunoreactive neurons in the periaqueductal gray (PAG). The present study provides evidence that dopamine neurons of the PAG region show adaptive biochemical changes during methamphetamine seeking in methamphetamine dependent rats and wheel running abolishes these effects. Given that the PAG dopamine neurons project onto the structures of the extended amygdala, the present findings also suggest that wheel running may be preventing certain allostatic changes in the brain reward and stress systems contributing to the negative reinforcement and perpetuation of the addiction cycle.
Keywords: Self-administration, BrdU, c-Fos, nNOS, VMAT2, tyrosine hydroxylase, PAG
Introduction
The burden of methamphetamine addiction is increasing in the United States; available SAMHSA reports show 8% of all drug/alcohol treatment admissions involve methamphetamine and treatment studies report frequent relapses to methamphetamine seeking among those that are trying to quit (SAMHSA, 2008). Furthermore, methamphetamine addiction takes emotional and financial tolls on society, cutting across ages, races, ethnicities, and genders. It increases mortality, morbidity, and economic costs. Therefore, successfully reducing risk behaviors, such as methamphetamine abuse, can potentially result in large public health gains ((WHO), 1980; USPSTF, 2008)).
There are no effective medications (FDA approved) to treat methamphetamine addiction (NIDA, 2006; Gonzales et al., 2010; NIDA, 2010, 2011). Currently, the best treatment option is the behavioral treatment approach (Matrix model). However, behavioral therapies are associated with lower beneficial effects compared with pharmacological interventions on maintaining an individual’s abstinence as well as on reducing context- and cue-induced drug craving, a major and persistent obstacle to long-term recovery of methamphetamine addiction (Carroll et al., 1994). Therefore, research on creating a therapeutic intervention that may treat methamphetamine addiction, particularly the relapse (preoccupation/anticipation or craving) stage of addiction has enormous potential to reduce and treat methamphetamine addiction.
In this context, clinical reports have demonstrated that engaging in physical exercise prior to and concurrent with alcohol and drug experience predicts lower levels of alcohol and illicit drug use (Kujala et al., 2007; Korhonen et al., 2009; Terry-McElrath and O'Malley, 2011; Terry-McElrath et al., 2011). Furthermore, physical exercise during drug withdrawal reduces self-rated measures of anxiety, depression and craving (negative affect; (Bock et al., 1999)). These effects may be related to exercise-induced enhanced measures of self-esteem and self-efficacy (Manger and Motta, 2005; Fillipas et al., 2006), which are established behavioral measures that predict treatment success. However, clinical studies do not establish, conclusively, whether physical exercise during drug withdrawal facilitates recovery, and whether physical exercise during drug withdrawal can be an effective intervention in substance abuse treatment programs (Weinstock et al., 2008); preclinical models are employed in prospective experimental designs to address these questions (Smith and Lynch, 2011).
The reinstatement of drug-seeking behavior in rats is widely used as a model of craving to mimic the relapse stage of addiction in human addicts (Shaham et al., 2003) and the predictive validity of this procedure has been demonstrated for several treatment interventions (Epstein et al., 2006). Voluntary wheel running (WR; a form of physical exercise and environmental enrichment) in laboratory animals reduces drug self-administration, attenuates physical signs of withdrawal and lowers relapse to drug seeking (Smith and Lynch, 2011; Lynch et al., 2013). The positive effects of WR are dependent on the intensity of activity, the duration of activity and whether WR was experienced before, during or after drug experience (Lynch et al., 2013). Notably, beneficial effects of WR or forced running performed only during drug withdrawal have been demonstrated in animal models of addiction. For example, running activity during forced withdrawal reduces cocaine seeking during extinction, and decreases cocaine seeking triggered by drug-cues (Lynch et al., 2010; Thanos et al., 2013) and cocaine itself in cocaine experienced rats (Thanos et al., 2013). However, WR during forced withdrawal reduced nicotine seeking during extinction and did not alter nicotine seeking triggered by drug-cues (Sanchez et al., 2013) in nicotine experienced rats, suggesting that WR’s efficacy may vary by the drug self-administered. The purpose of the present study was to characterize the effects of WR during forced withdrawal on methamphetamine seeking during extinction and methamphetamine seeking triggered by drug-context and drug-cues in methamphetamine dependent rats. Brain tissue from sedentary and WR methamphetamine rats was analyzed via immunohistochemistry to determine a potential neurobiological correlate for WR-induced reduction in relapse to methamphetamine seeking.
Materials and Methods
Animals
Surgical and experimental procedures were carried out in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Thirty-eight adult, male Wistar rats (Charles River), 8 weeks old and weighing 200–250 g at the start of the experiment, were housed two per cage in a temperature-controlled vivarium under a reverse light/dark cycle (lights off 8:00 AM–8:00 PM) for at least one week.
Surgery
All rats (methamphetamine and drug naïve controls) underwent surgery for catheter implantation for intravenous methamphetamine self-administration (Mandyam et al., 2007). The drug naïve controls did not experience operant behavior and methamphetamine. The catheters were not disturbed (or removed) until the end of the experimental study (until euthanasia). Animals with indwelling catheters were maintained on antibiotics every day until the end of the study, and did not show any signs of ill health or infections. For surgery, rats were anesthetized with 2–3% of isoflurane mixed in oxygen. They were implanted with a silastic catheter (0.3×0.64mm OD; Dow Corning Co.) into the right external jugular vein under aseptic conditions. The distal end of the catheter was s.c. threaded over the shoulder of the rat where it exited the rat via a metal guide cannule (22G, Plastics One Inc.) that was anchored onto the back of the rat. After surgery, rats were given an analgesic (Flunixin, 2.5 mg/kg, s.c.). Antibiotic (Timentins, 20 mg, i.v.; SmithKline Beecham) was administered daily to the rats for at least 5 days. To extend catheter patency, the catheters were flushed once daily with 0.1 ml of an antibiotic solution of cefazolin (10.0 mg/mL; SavMart Pharmaceuticals) dissolved in heparinized saline (70 U/mL; Baxter Health Care Corp) before each self-administration session and with 0.1 ml of heparinized saline (70 U/mL) after each session. The patency of catheters in the rats was tested using the ultra short-acting barbiturate Brevital (methohexital sodium, 10 mg/ml, 2 mg/rat) whenever a catheter failure was suspected during the study.
Baseline training sessions and maintenance on an extended access schedule
Four to five days after surgery rats (n = 18) were trained to press a lever according to an FR 1 schedule of methamphetamine reinforcement (0.05 mg/kg/injection of methamphetamine hydrochloride, generously provided by the National Institute on Drug Abuse) in operant boxes (Med Associates) under baseline (acquisition) conditions (1h access per day for ten days). The dose of methamphetamine and the schedule of reinforcement were based on seminal studies and our previous publications (Kitamura et al., 2006; Rogers et al., 2008; Orio et al., 2010; Recinto et al., 2012). During daily sessions, a response on the active lever resulted in a 4 second infusion (90–100 µl volume), followed by a 20 second time-out period to prevent overdose. Each infusion was paired for 4 seconds with white stimulus light over the active lever (conditioned stimulus [CS]). Response during the time-out or on the inactive lever was recorded but resulted in no programmed consequences. All animals were housed on a reverse cycle (lights off at 8 am) and were transferred from their home cages to their operant chambers between 9 and 10 am. Training on the first and second day was initiated with two-three priming (noncontingent) infusions of methamphetamine during the first ten minutes. Rats were allowed to respond for the remaining fifty minutes. Acquiring methamphetamine self-administration was defined as maintenance of similar number of infusions over three consecutive days during baseline training sessions. All animals acquired methamphetamine self-administration (Figure 2a). After baseline (acquisition) training, the rats were subjected to long access schedule of methamphetamine reinforcement (LgA, 6h per day under an FR1 schedule). Methamphetamine self-administration was performed 5 days per week and all rats experienced 22 LgA sessions. The sessions for the 6h schedule occurred between 9 am – 4 pm. All eighteen rats completed the self-administration sessions. The remaining six rats served as methamphetamine naïve (and WR naïve) controls.
Figure 2.
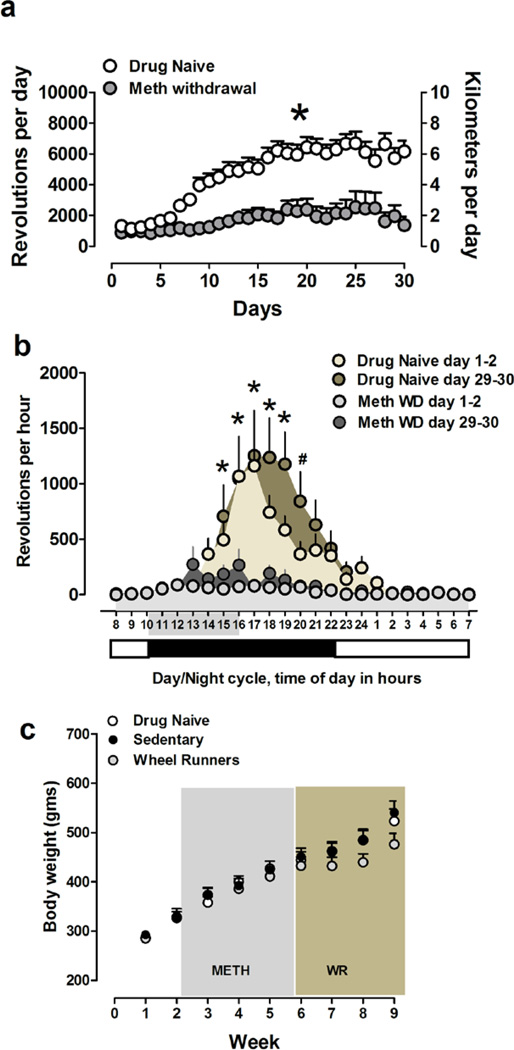
Methamphetamine experience reduces running output. (a–b) Total number of revolutions per day (left y-axis); distance run in kilometers (right y-axis) (a); *p<0.05 compared with drug naïve rats in (a). Total number of revolutions per hour (b) during the first two days of withdrawal and last week of withdrawal in methamphetamine withdrawn rats and at similar time points in drug naïve animals. The numbers on the x-axis shaded in gray in (b) show the time of day when methamphetamine self-administration was conducted prior to withdrawal days; *p<0.05 compared with running activity in the light cycle in drug naïve rats. (c) Body weight in grams over the entire experimental period.
5-bromo-2’-deoxyuridine (BrdU) injections
On the day following the last methamphetamine self-administration, all methamphetamine experienced rats were withdrawn from access to methamphetamine. Methamphetamine experienced and drug naïve rats received one intraperitoneal injection of BrdU (dissolved in 0.9% saline and 0.007N NaOH at 20 mg/ml; 150 mg/kg, i.p.) 16 hours after the end of their last self-administration session to label progenitors in the S phase of the cell cycle and were killed 5 weeks later (Figure 1a). This time point was chosen to prevent labeling cells during peak withdrawal manifestation and high brain and plasma methamphetamine levels after methamphetamine self-administration (Cryan et al., 2003; Segal and Kuczenski, 2006; McFadden et al., 2012b).
Figure 1.
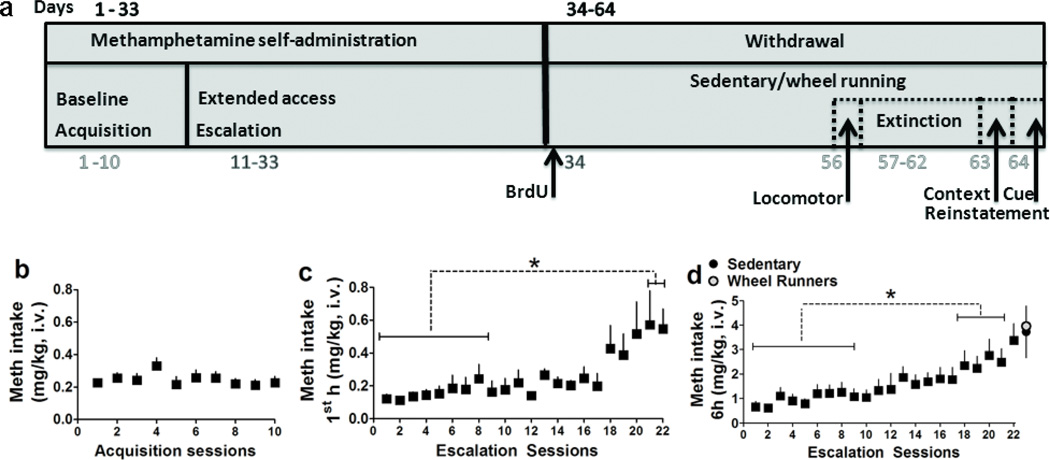
(a) Schematic of behavioral protocol and experimental procedures. Self-administration began with a 10-day acquisition period in which rats were limited to 1h sessions under a fixed ratio 1 (FR1) schedule. Subsequently, rats experienced extended access (6h) sessions under a FR1 schedule. Next, rats were withdrawn from methamphetamine and were given one injection of BrdU on the following day to label progenitors in the hippocampus. After injections rats were placed in home cages equipped with (WR) or without (sedentary) running wheels for 30 days during which methamphetamine was withdrawan. During withdrawal rats extinguished self-administration behavior in a new context and the following day began a within-session context/cue-induced reinstatement paradigm. All animals were killed 60–90 minutes after the last reinstatement session. (b–d) Methamphetamine intake (mg/kg) during acquisition (b) and escalation (c–d) sessions.(c) Amount of methamphetamine consumed during the first hour of the six hour access; (d) amount of methamphetamine consumed over the entire six hour access. At the end of the six hour sessions rats (n = 18) were separated into sedentary or WR groups (n = 9 sedentary group; n = 9 WR group). Data is expressed as mean ± S.E.M. *p<0.05 vs. initial sessions in (c–d).
Wheel running
After BrdU injections, methamphetamine experienced rats were separated into wheel running (WR; n = 9) or sedentary groups (n = 9) and were single housed for the remaining period until euthanasia. A separate set of drug naïve rats (n = 9) were housed with running wheels in their home cage during the same timeframe to determine running output in methamphetamine naïve rats. Wheel running rats were housed in cages equipped with running wheels (Nalgene activity wheels 34.5 cm diameter × 9.7 cm wide with magnetic switches connected to a PC). Daily running activity was monitored and wheel revolutions were collected in one hour bins with VitalView Software (Minimitter Inc.).
Locomotor activity
Locomotor activity was assessed on the day prior to extinction sessions to determine whether WR rats showed signs of hyper activity that may interfere with the drug-seeking behavior during extinction and reinstatement sessions. Sedentary and WR rats were transferred to the locomotor activity room at the start of the dark cycle and were allowed to rest (single housed in rat cages without running wheels) for 1 h prior to placement in the locomotor cages, to reduce the impact of transport stress on activity. Locomotor activity was tested in wire mesh cages (l × w × h: 36 cm × 25 cm × 20 cm) equipped with two photobeams placed 16 cm apart along the long wall (Valdez et al., 2003). Rats were acclimated to the locomotor cages for 20 min to reduce the novelty of the environment. Rats were injected with methamphetamine (1.0 mg/kg; i.p.) at the end of the 20 min acclimation period, then placed back immediately in the same locomotor chambers. Methamphetamine effects on locomotor activity were assessed for 30 minutes post-injection. Computer-recorded photobeam breaks were analyzed as a measure of locomotor activity within the chamber during the testing period.
Extinction sessions
After 30 days of abstinence from self-administration, the rats underwent daily 1 h extinction sessions for six days in an operant chamber (context B) different from the self-administration chamber (context A) (Shaham et al., 2003). During the extinction sessions, the rats were not connected to the infusion apparatus. Responses on either the active or inactive lever were recorded and did not result in programmed consequences (i.e., no infusions, no auditory cues (infusion pump noise) and no CS presentations (cue light)). Extinction training consisted of six sessions and all the rats reached the extinction criterion (< 10 active lever presses per session for two consecutive sessions).
Reinstatement testing
Twenty-four hours after the final extinction session, the rats were placed into the methamphetamine-paired context (i.e., the same operant box used for self-administration sessions; context A) for 1 h, during which they were connected to the infusion apparatus to allow for a similar interaction with the spatial elements of the context similar to the methamphetamine self-administration training. Lever presses were used as a measure of drug seeking, and responses on both the active and inactive lever were recorded. Active lever responses activated the pump (auditory cues) but did not result in infusions of fluids through the catheter or other programmed consequences (i.e., CS presentations; visual cue (cue light)). The following day rats were assessed for methamphetamine seeking triggered by visual cues. During the conditioned-cued reinstatement test, rats were placed into the operant chambers for 1 h (context A) without connections to the infusion apparatus. Each active lever press resulted in a 1-s CS presentation in the absence of drug reinforcement and auditory cues. All of the rats were euthanized 1–2 h post-reinstatement testing.
Perfusions and brain tissue collection
After the last experimental session, rats were fully anesthetized using chloral hydrate (240 mg/kg, i.p.). Rats were then transcardially perfused with phosphate-buffered saline (over 2 minutes at 15ml/min and 4% paraformaldehyde (over 20 minutes at 15ml/min). The brains were dissected out and postfixed in 4% paraformaldehyde at 4°C for 16–20h and sectioned in the coronal plane at a thickness of 40µm on a freezing microtome. The sections through the brain were collected in nine vials (containing 0.1% NaN3 in 1X phosphate-buffered saline (PBS)) and stored at 4°C.
Antibodies, immunohistochemistry, microscopic analysis, and quantification
The following primary antibodies were used for immunohistochemistry (IHC): Ki-67 (1:1000, catalog # RM-9106-S, Thermo Scientific (Mandyam et al., 2008)), BrdU (1:400, catalog # MCA2060, Serotec (Mandyam et al., 2008)), c-Fos (1:1000, catalog # sc-52, Santa Cruz Biotechnology (Recinto et al., 2012)), neuronal nitric oxide synthase (nNOS; 1:2000, catalog # sc-55521, Santa Cruz Biotechnology (Wang and Angulo, 2011)), vesicular monoamine transporter-2 (VMAT2; 1:2000, catalog # AB1598P, EMD Millipore Corporation) and tyrosine hydroxylase (TH; 1:2000, catalog # AB152, EMD Millipore Corporation). The left and right hemispheres of every ninth section through the rat hippocampus (−1.4 to −6.7 mm from bregma) were used for BrdU and Ki-67 quantification. The left and right hemispheres containing the medial prefrontal cortex (mPFC; 2.7 mm from bregma), nucleus accumbens shell (NAc shell; 2.7 mm from bregma), bed nucleus of the stria terminalis (BNST; −0.26 mm from bregma), caudate putamen (−0.26 mm from bregma), medial septal nucleus (−0.26 mm from bregma), amygdala (−2.3 mm from bregma), ventro-lateral periaqueductal gray (PAG; −6.04 mm from bregma; corresponding to A10 dopaminergic cell group (Messanvi et al., 2013)), granule cell layer (GCL; −6.04 mm from bregma), substantia nigra (SN; medial, central, lateral compacta and reticulate; −6.04 mm from bregma), and parabrachial and paranigral subregions of the ventral tegmental area (VTA; −6.04 mm from bregma; (Paxinos and Watson, 1997)) were slide-mounted, coded, and dried overnight prior to IHC. The sections were pretreated (Mandyam et al., 2004), blocked, and incubated with the primary antibodies (BrdU, Ki-67, Fos, nNOS, VMAT2 and TH) followed by biotin-tagged secondary antibodies.
Ki-67 immunoreactive cells in the subgranular zone (SGZ; i.e., cells that touched and were within three cell widths inside and outside the hippocampal granule cell-hilus border) and BrdU cells in the GCL were visually quantified with a Zeiss primostar photomicroscope (600× magnification) and absolute cell counting (counting every immunoreactive cell in the area for analysis as this would give an unbiased estimate of cell counts) in sections through the dentate gyrus (−1.4 to −6.7 mm from bregma; (Paxinos and Watson, 1997)) were quantified (Noori and Fornal, 2011). Cells in the SGZ and GCL were summed and multiplied by 9 to give the total number of cells (Eisch et al., 2000).
nNOS, Fos and TH immunoreactive cells were examined and quantified with a Zeiss Axiophot Microscope equipped with MicroBrightField Stereo Investigator software, a three-axis Mac 5000 motorized stage, a Zeiss digital video camera, PCI color frame grabber, and computer workstation. Live video images were used to draw contours delineating the brain regions indicated in Table 1. The fields of the brain regions for quantification were traced separately at 25x magnification. A 150 × 150 µm frame was placed over the regions of interest using the Stereo Investigator stereology platform. The frame was systematically moved over the tissue to cover the entire contoured area and the labeled cells in each region falling entirely within the borders of the contour were marked and analyzed. Immunoreactive cells were quantified bilaterally (absolute cell counting in the area contoured for analysis) and were summed up for each brain region. A separate group of rats self-administered methamphetamine in a 6h schedule for 22 days and were euthanized 16–20h after their last self-administration session. Behavior data and brain immunohistochemical data from these animals have been previously published in Recinto et al 2012 (Recinto et al., 2012) and is not presented in the current report. Brain tissue enriched in PAG from these animals was used in the present study to evaluate the number of TH neurons in the PAG, to determine the number of TH neurons in animals that only self-administered methamphetamine and did not experience withdrawal and reinstatement sessions. Data from these additional animals are presented in Figure 5.
Table 1.
Quantitative (cell counts in mm2) and densitometric (density) analysis of various markers of methamphetamine toxicity. Data are expressed as mean ± S.E.M; n = 9 sedentary group; n = 9 WR; n = 6 drug naive.
| Quantitative analysis [cell numbers per mm2] of o-FOG expressing cells | ||||
|---|---|---|---|---|
| Brain region | Control | Sed Math | WR Math | p |
| Medial Prefrontal Cortex | 23 ± 5 | 27± 4 | 39 ± 8 | n.s. |
| Nucleus Accumbens Shell | 18 ± 7 | 22 ± 4 | 33 ± 10 | n.s. |
| Granule Cell Layer | 49 ± 7 | 56 ± 8 | 76 ± 15* | 0.07 |
| Granule Cell Layer c-Fos+/BrdU+colabeling | None | None | None | |
| Periaqueductal Gray | 8 ± 1 | 13 ± 3 | 15 ± 5 | n.s. |
| Ventral Tegmental Area | 2 ± 1 | 4 ± 1 | 4.6 ± 1 | n.s. |
| Quantitative analysis (cell numbers per mm2) of NnoS expressing cells | ||||
| Brain region | Control | Sed Meth | WR Meth | |
| Orbital Cortex | 21 ± 4 | 17 ± 2 | 16 ± 2 | n.s |
| Medial Prefrontal Cortex | 48 ± 1 | 44 ± 2 | 44 ± 3 | n.s |
| Nucleus Accumbens Shell | 24 ± 2 | 23 ± 1 | 25 ± 2 | n.s |
| Medial Septal Nucleus | 28 ± 4 | 22 ± 3 | 19 ± 4 | n.s |
| Bed Nucleus of the Stria Terminals | 14 ± 3 | 11 ± 1 | 11 ± 1 | n.s |
| Caudate Putamen (striatum) | 80 ± 7 | 83 ± 5 | 82 ± 6 | n.s |
| Amygdala | 25 ± 7 | 24 ± 3 | 21 ± 2 | n.s |
| Hippocampal Dentate Gyrus | 8 ± 4 | 5 ± 1 | 7 ± 2 | n.s |
| Periaqueductal Gray | 77 ± 9 | 70 ± 5 | 60 ± 9 | n.s |
| Ventral Tegmental Area | 8 ± 4 | 1 ± 0 | 1 ± 1 | n.s |
| Quantitative analysis of VMAT2 Immunoreactivity | ||||
| Brain region | Control | Sed Meth | WR Meth | |
| Nucleus Accumbens Shell (density) | 17 ± 2 | 13 ± 2 | 14 ± 2 | n.s |
| Quantitative analysis of TH Immunoreactivity | ||||
| Brain region | Control | Sed Meth | WR Meth | |
| Nucleus Accumbens Shell (density) | 39 ± 4 | 43 ± 4 | 38 ± 3 | n.s |
| Substantia Nigra (cell numbers per mm2) | 55 ± 4 | 68 ± 2* | 73 ± 6* | < 0.05 |
| Ventral Tegmental Area (cell numbers per mm2) | 75 ± 10 | 99 ± 5* | 101 ± 13 | 0.04 |
p<0.05 vs. drug naïve.
Figure 5.
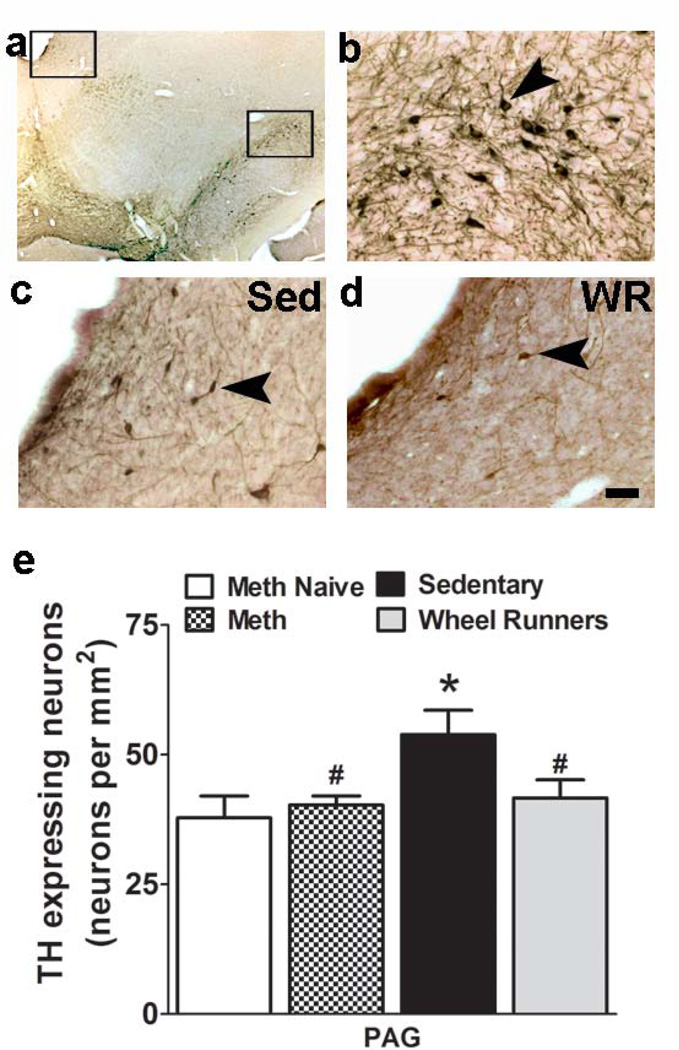
Reinstatement of methamphetamine seeking increases the number of TH immunoreactive dopamine neurons in the PAG. (a–d) Photomicrographs of DAB stained section containing the PAG (top open rectangle) and SN (bottom open rectangle) at low magnification (2.5x; a) from one sedentary animal. (b) TH cells in the SN under higher magnification. (c–d) TH cells in the PAG in sedentary rat (c) and WR rat (d). The top left box in (a) represents the area in (c–d). Scale bar in d applies (a–d); in b-d = 20 um; in a = 200 um. (e) Quantitative analysis of immunoreactive cells stained for TH. Data are expressed as mean ± S.E.M; n = 9 sedentary group; n = 9 WR; n = 5 methamphetamine; n = 6 drug naive. * p<0.05 vs. drug naïve and #p<0.05 vs. sedentary animals.
For c-Fos and BrdU colabeling, approximately 25 BrdU cells from each rat were individually scanned in a confocal microscope. Staining was performed identical to the DAB method, except fluorophores were used for secondary antibodies. BrdU was labeled with CY3 donkey anti-rat and c-Fos was labeled with CY2-donkey anti-rabbit. The combinatorial labeling allowed us to determine whether BrdU cells (CY3-labeled) were co-labeled with c-Fos (Cy2-labeled). None of the BrdU cells were co-labeled with c-Fos in any experimental groups (drug naïve, sedentary or WR).
Density of TH neuron terminals and VMAT2 immunoreactivity were measured from two bilateral sections containing the NAc shell (2.7 mm from bregma; as indicated in the Paxinos and Watson atlas (Paxinos and Watson, 1997)) and the analysis was made by taking four fields corresponding to the area of the shell region. The density of the neuron terminals and immunoreactivity, was measured using an image analysis system (ImageJ). The neuron terminals and immunoreactivity in at least four areas was determined by measuring the percentage of the area occupied by TH neuron terminals or VMAT2 immunoreactivity with respect to a standardized area, using a 10x objective lens and a Zeiss MRm video camera. Gray values, measured in TH-negative or VMAT2-negative areas of the sections, were subtracted as background from the resulting binary picture (Bjorklund and Stromberg, 1997; Casu et al., 2002). All the analysis was performed by an observer blind to the study.
Data analysis
The methamphetamine self-administration data is expressed as the mean mg/kg per session of methamphetamine self-administration. The effect of session duration on methamphetamine self-administration during the 6h session and during the first hour of the 6h session was examined over the 22 escalation sessions using a two-way repeated-measures analysis of variance (ANOVA; session duration × daily session) followed by the Student-Newman-Keuls post hoc test. The pattern of responding for methamphetamine is expressed as the mean mg/kg per hour over 6 h sessions in LgA rats and were compared between the first and > 10th escalation sessions. Differences in the rate of responding between the first and other escalation sessions were evaluated using the paired t-test. Running data is expressed as revolutions per day or per hour. The effect of methamphetamine withdrawal on hours during the day and weeks of running activity was examined over the 24-h period or 30-day period using a two-way repeated-measures (ANOVA; duration × day) followed by the Student-Newman-Keuls post hoc test. For the effect of WR on locomotor activity, extinction and reinstatement of methamphetamine seeking, the two groups (sedentary and WR) were used as between-subjects factors, and differences were assessed by ANOVA followed by paired t-test. For the Ki-67, BrdU, Fos, nNOS, TH and VMAT2 analyses, one-way ANOVA or unpaired Students t-test was used. The data are expressed as mean ± SEM in all graphs. Pearson’s correlations were used to examine the relationship between running output and the extent of reinstatement triggered by drug-context and drug-cues.
Results
Extended access methamphetamine self-administration increases responding for methamphetamine and produces an escalation in methamphetamine intake
Rats underwent surgery for intravenous catheters and were tested for operant responding for intravenous methamphetamine self-administration (Figure 1a). All rats responded on the first day of self-administration. Stable response rates were observed by the third session and were maintained until the last baseline session. Repeated measures ANOVA did not detect a change in the number of active lever presses over acquisition days (Figure 1b). After 10 days of 1h baseline sessions, rats were subjected to 6h extended access (long access; LgA) sessions for 22 days. Methamphetamine intake during the 1st h of the 6h sessions increased over self-administration days, and escalation in methamphetamine intake was evident after 20 sessions of self-administration (Figure 1c; F21,395 = 8.39, p < 0.001). Post hoc analysis revealed an escalation in methamphetamine intake in LgA rats during sessions 21–22 compared with earlier 1–9 sessions (all p < 0.05). Similarly, daily 6h methamphetamine intake significantly increased over self-administration days, and escalation in daily methamphetamine intake was evident after 17 sessions of self-administration (Figure 1d; effect of days of self-administration: F21,395 = 21.85, p < 0.001). Post hoc analysis revealed an escalation in methamphetamine intake in LgA rats during sessions 18–22 compared with earlier 1–9 sessions (all p < 0.05).
Extended access methamphetamine experience reduces running output when wheel running is performed during withdrawal and protracted abstinence
Drug naïve rats increased their running activity over thirty days (Figure 2a; repeated measures ANOVA; effect of days, F20,639 = 48.8, p < 0.001), and post hoc analysis indicated an increase in running output during 6–14 days compared with initial 5 days, after which the output was maintained until the end of the study (p < 0.05).
Methamphetamine withdrawn rats also increased their running activity during the four weeks (Figure 2a; repeated measures ANOVA; effect of days, F8,269 = 24.14, p < 0.05), however, post-hoc analysis did not indicate an increase in running output between days of running.
Running output was significantly lower in methamphetamine withdrawn rats, and this effect was maintained until the end of the study (Figure 2a; repeated measures two-way ANOVA; effect of withdrawal, F1,812 = 17.29, p = 0.0003; and a significant withdrawal × days of running interaction, F29,812 = 6.3, p < 0.0001). Post-hoc analysis demonstrated higher running output in drug naïve rats compared with methamphetamine withdrawn rats from days 10 to 30 (p’s < 0.01).
Microanalysis of running activity in drug naïve rats did not show significant differences in running activity during days 1–2 compared with days 29–30. Repeated measures two-way ANOVA showed a significant effect of time of day on running output (F23,506 = 17.3, p = 0.0001). Post-hoc analysis demonstrated a higher running output during the dark cycle compared with the light cycle in drug naive rats (p < 0.03; Figure 2b).
Microanalysis of running activity in methamphetamine withdrawn rats did not demonstrate an overall reduction in running activity during days 1–2 compared with days 29–30. Repeated measures two-way ANOVA showed a significant days after withdrawal × running output over 24h period interaction (F23,391 = 1.6, p = 0.03), and a significant effect of time of day on running output (F23,391 = 4.6, p = 0.0001). Post-hoc analysis demonstrated a higher running output during the dark cycle compared with the light cycle in methamphetamine withdrawn rats during days 29–30 (p < 0.03; Figure 2b).
Methamphetamine self-administration does not reduce body weight
Drug naïve, sedentary and WR rats gained weight throughout the study. Two-way repeated measures ANOVA indicated a significant increase in body weight over weeks in all groups (effect of weeks, F8,168 = 297.7, p < 0.001; Figure 2c; effect of weeks × experimental group interaction, F16,168 = 3.46, p < 0.001; Figure 2c). Post hoc analysis did not indicate significant differences between groups.
Running activity during withdrawal does not alter locomotor activity
Locomotor activity assessed weeks into withdrawal was not different between sedentary and WR animals (two-way repeated measures ANOVA, no effect of WR, F1,90 = 0.61, n.s.; Figure 3a). Methamphetamine priming (1mg/kg, i.p.) increased locomotor activity and this effect was seen in sedentary and WR rats (effect of methamphetamine, F9,90 = 22.05, p < 0.001; Figure 3a).
Figure 3.
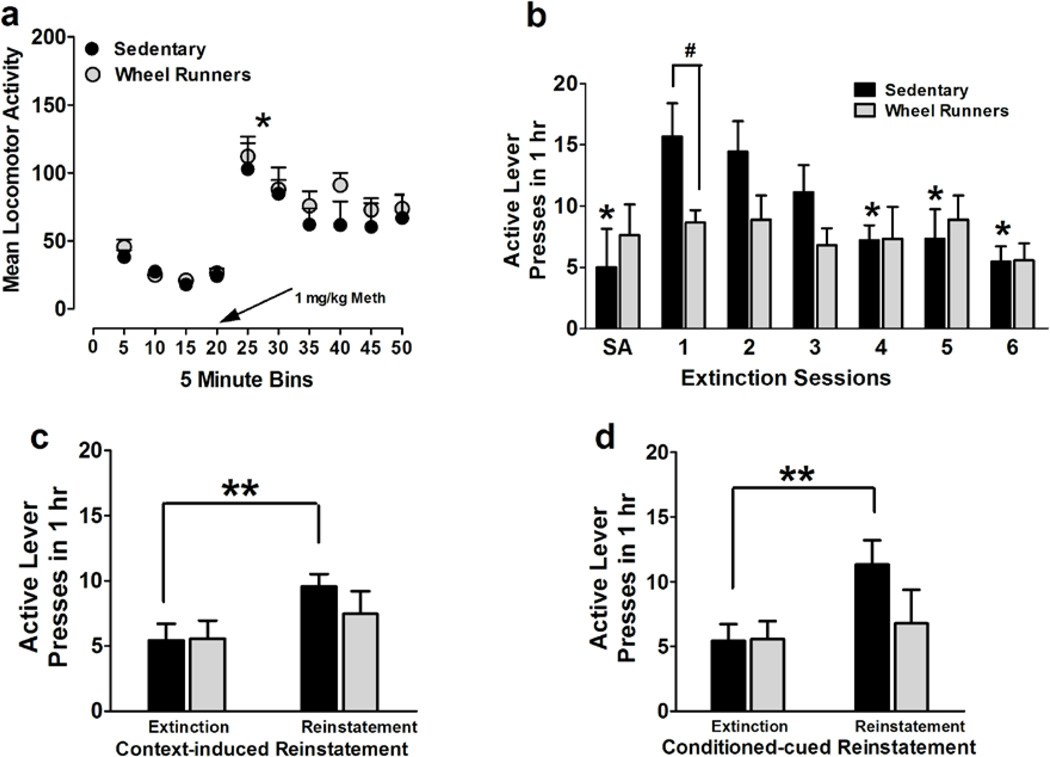
(a) Locomotor activity measured as crossovers before and after an intraperitoneal injection of methamphetamine. *p<0.05 compared with activity before methamphetamine injection in sedentary and WR animals. (b–d) Extinction and reinstatement of methamphetamine seeking. (b) Increased active lever responding during first extinction session compared with the first hour responding during the last self-administration (SA) session; Decreased lever responding on the previously methamphetamine paired lever in daily 1h extinction trials in a operant box different from the one used for self-administration sessions. The extinction criterion was reached after day 4 in both groups. WR significantly reduced extinction responding compared with sedentary animals (*p≤0.05, compared with day 1 of extinction
Responding in sedentary group; # p<0.05 vs. sedentary rats). (c–d) Methamphetamine seeking triggered by context (c) and cues (d); reinstatement expressed as active lever presses. n = 9 in each group; **p<0.01, compared with extinction responding.
Running activity during withdrawal reduces responding during extinction and reinstatement of methamphetamine seeking triggered by methamphetamine context and cues
After 30 days of withdrawal from methamphetamine, sedentary and WR rats were tested on extinction sessions. The number of active lever presses on the first day of extinction was significantly higher than the number of active lever presses during the first hour of self-administration session on the last day of methamphetamine session in sedentary rats; this was not evident in WR rats (Figure 3b; p = 0.05 by paired t test). During extinction sessions, methamphetamine was not available and lever responding readily declined across daily 1-hour extinction sessions, as seen in Figure 3b (repeated measures two-way ANOVA; effect of day, F5,80 = 6.2, p = 0.0001; and a significant wheel running × days of extinction interaction, F5,80 = 2.9, p = 0.01). Post-hoc analysis demonstrated higher responding in sedentary rats compared with WR rats on the first day of extinction (Figure 3b; p < 0.05). Sedentary and WR groups extinguished equally prior to reinstatement testing. Following extinction, sedentary and WR rats were tested on contexual reinstatement and conditioned-cued reinstatement (Figure 3c, d). It should be noted that the extent of reinstatement in sedentary rats is not robust and could be attributed to the extended period of withdrawal after the last methamphetamine session, the change in time spent in the operant box during extinction session (1h) vs. self-administration session (6h) and the cues during methamphetamine sessions and extinction sessions. However, sedentary rats showed higher responding on the previously drug-paired lever during context-induced reinstatement when compared with the last extinction session, and this effect was not seen in WR rats (repeated measures two-way ANOVA; effect of reinstatement, F1,16 = 8.0, p = 0.01). Post-hoc analysis demonstrated higher responding during reinstatement compared with extinction in sedentary rats (Figure 3c; p < 0.01). Sedentary rats showed higher responding on the previously drug-paired lever during conditioned-cued reinstatement when compared with the last extinction session, and this effect was not seen in WR rats (repeated measures two-way ANOVA; effect of reinstatement, F1,16 = 4.3, p = 0.05). Post-hoc analysis demonstrated higher responding during reinstatement compared with extinction in sedentary rats (Figure 3d; p < 0.01).
Running output does not correlate with the extent of reinstatement triggered by drug-context
Pearson’s correlations did not demonstrate a significant relationship between running output (measured as revolutions per 24-hour period prior to reinstatement testing) and reinstatement to methamphetamine seeking triggered by drug-context (measured as percent change in lever responses compared with the last extinction session; R2 = 0.157, n.s.) or drug-cues (R2 = 0.152, n.s.).
Wheel running during withdrawal does not alter neurogenesis in the dentate gyrus of the hippocampus
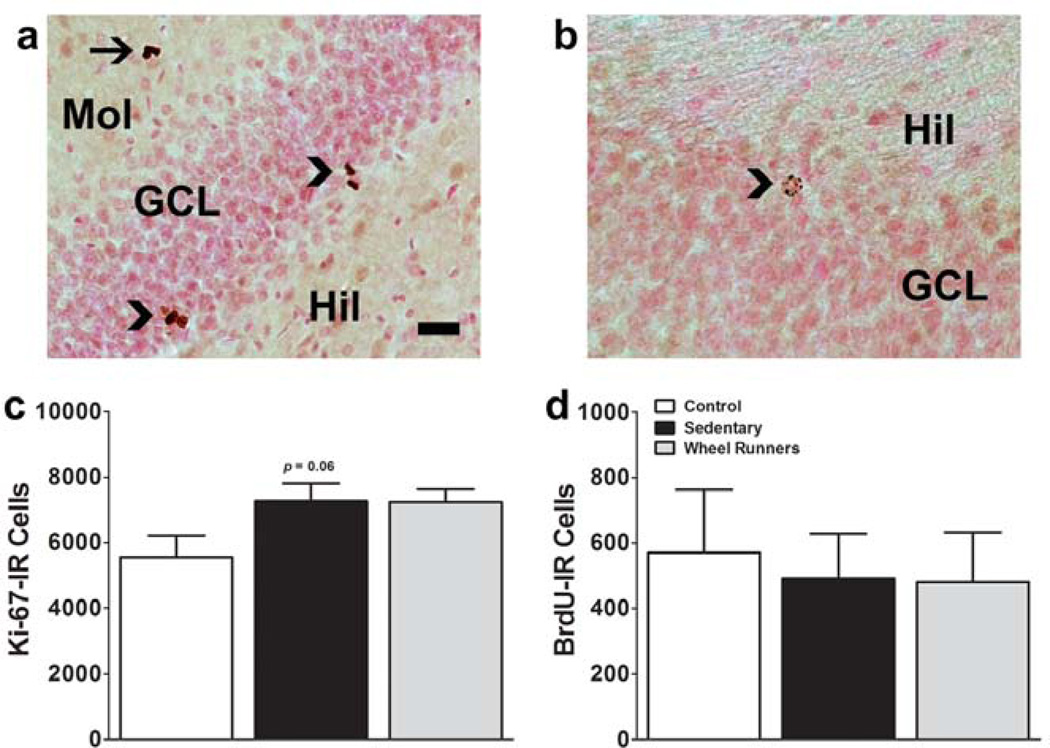
The effect of withdrawal with or without running on the developmental stages of progenitors in the hippocampal dentate gyrus was examined in the SGZ in control (methamphetamine and WR naïve), sedentary and WR rats (Figure 4a–d). Withdrawal from methamphetamine self-administration in sedentary rats showed a strong trend towards increases in the number of proliferating progenitors in the SGZ compared with drug naïve controls (Ki-67; Figure 4c; p = 0.06; (Recinto et al., 2012)). Running experience during withdrawal and protracted abstinence did not alter the number of proliferating progenitors (Ki-67; Figure 4c). WR during withdrawal did not alter the number of surviving progenitors in the granule cell layer (BrdU; Figure 4d).
Figure 4.
Reinstatement of methamphetamine seeking does not alter the number of proliferating cells or surviving cells in the dentate gyrus of the hippocampus. (a–b) Photomicrographs of DAB stained Ki-67 cells (a) and 30-day-old BrdU cells (b) from one control drug naïve rat. Scale bar in (a) applies (a–b) = 30 um in (a) and 20 um in (b). (c–d) Quantitative analysis of immunoreactive cells stained for Ki-67 (c) and BrdU (d). Data are expressed as mean ± S.E.M; n = 9 sedentary group; n = 9 WR; n = 6 drug naive.
Running activity during withdrawal and protracted abstinence prevents withdrawal-induced upregulation of tyrosine hydroxylase positive dopamine neurons in the PAG
Markers of neuronal activation (c-Fos) and toxicity (nNOS, VMAT2, TH) were examined in various regions of the brain implicated in the binge/intoxication stage (NAc shell, SN and VTA), withdrawal/negative affect stage (BNST, caudate putamen, PAG) and preoccupation/anticipation or craving stage of addiction (mPFC, amygdala, hippocampal granule cell layer) in control (methamphetamine and WR naïve), sedentary and WR rats. Mean total c-Fos cell counts did not differ in any brain region between any experimental groups (Table 1; c-Fos). Mean total nNOS cell counts did not differ in any region between any experimental groups (Table 1; nNOS). Mean total density of VMAT2 expressing cells in the NAc shell did not differ between the experimental groups. In the VTA and SN, mean total TH cell counts differed between the experimental groups (Table 1, TH; two-way ANOVA, significant effect of treatment F2,42 = 10.78, p = 0.03 and significant effect of brain region F1,42 = 22.4, p = 0.004). Post-hoc analysis showed higher number of TH cells in sedentary and WR rats compared with controls in the SN (Table 1, TH; p < 0.05) and in the VTA (Table 1, TH; p < 0.05), and higher number of TH cells bodies in the VTA compared with SN (p < 0.05). In the NAc shell there was no difference in the density of TH terminals between control, sedentary and WR rats (one-way ANOVA, n.s.). In the PAG withdrawal and protracted abstinence increased the number of TH neurons in sedentary animals compared with controls and methamphetamine animals and this effect was reduced in WR rats (one-way ANOVA, F3,29 = 3.3, p = 0.03). Post-hoc analysis showed higher number of TH cells in sedentary rats compared with controls, methamphetamine and WR rats in the PAG (Figure 5d; p < 0.05).
Discussion
The goal of this study was to determine if WR during withdrawal and protracted abstinence would reduce subsequent methamphetamine seeking triggered by drug-context and drug-cues in a model of methamphetamine dependence. Consistent with our hypothesis, WR during withdrawal significantly attenuated methamphetamine seeking. The effects of WR were apparent under extinction conditions. This finding is consistent with previous studies in cocaine and nicotine experienced animals, in which WR performed during withdrawal significantly lessened subsequent cocaine and nicotine seeking during extinction (Lynch et al., 2010; Zlebnik et al., 2010; Sanchez et al., 2013). Furthermore, as reported in Lynch et al (2010), our study demonstrates that WR during withdrawal reduces conditioned-cued reinstatement, but significantly extend the findings with methamphetamine, a mechanistically distinct, more potent and toxic stimulant drug of abuse. Importantly, the generality of the effect of WR during withdrawal was confirmed on drug context-induced reinstatement in addition to conditioned-cued reinstatement. Running output during withdrawal was significantly lower in methamphetamine experienced rats when compared with drug naïve controls which demonstrate that even modest levels of WR is sufficient to produce a beneficial effect on methamphetamine-seeking. Most importantly, the present findings provide concurrent evidence that modest WR during withdrawal exerts protection against methamphetamine relapse by ameliorating withdrawal and protracted abstinence- induced increases in the levels of TH expressing dopamine neurons with significant effects in the PAG. Our findings provide novel evidence that running activity during withdrawal protects against methamphetamine toxicity in the midbrain. These results add to a growing body of preclinical literature supporting a promising role for physical activity during withdrawal in reducing relapse and drug-induced neuroadaptations in the extended reward regions (Smith and Lynch, 2011; Lynch et al., 2013).
Running output during withdrawal was significantly lower in methamphetamine experienced rats compared with drug-naïve controls; in fact, running output in methamphetamine experienced rats did not escalate over weeks of running activity as seen in controls. Because methamphetamine was not available during running sessions, the present findings suggest that rather than the behaviors (self-administration and WR) competing, the reinforcing value of the wheel and methamphetamine reward competed (Kanarek et al., 1995; Serwatkiewicz et al., 2000; Miller et al., 2011; Engelmann et al., 2013). Furthermore, it is possible that chronic methamphetamine exposure via extended access self-administration altered the circadian rhythms of running behavior by modulating the circadian oscillators in the suprachiasmatic nucleus, the food entrainable oscillator and a separate methamphetamine-sensitive circadian oscillator (Tataroglu et al., 2006; Mohawk and Menaker, 2009; Pendergast and Yamazaki, 2014). Microanalysis of running activity over the 24h light:dark cycle during acute withdrawal and protracted abstinence did not demonstrate significant shifts in activity suggesting that methamphetamine reduced overall circadian activity during withdrawal. The interaction between methamphetamine and wheel running behavior is also evident on the first day of extinction, where sedentary rats had significantly higher methamphetamine seeking (active lever responding) compared with WR rats. The effect of WR on extinction cannot be explained as a consequence of motoric incapacity, because WR did not reduce either spontaneous or methamphetamine-induced locomotor activity. Thus, WR can be considered a nondrug alternative and extending access to WR during withdrawal is a preventive strategy that could be employed to reduce relapse to methamphetamine seeking in methamphetamine dependent subjects.
The reinstatement of drug-seeking behavior is a valid animal model of relapse and relapse can be triggered by the drug, environmental cues (drug-context, drug-cues) and stress (Shaham et al., 2003). The stimuli triggering drug seeking can activate neural circuits in the brain reward regions and these reflect distinct functional interactions between distinct brain regions of the neural circuitry of drug-seeking behavior triggered by drug-context and drug-cues (See et al., 2001; Kalivas and McFarland, 2003; Fuchs et al., 2005; Hiranita et al., 2006; Fuchs et al., 2007; Mantsch et al., 2010; Smith and Aston-Jones, 2011). Here, we examined the effects of voluntary WR during withdrawal and protracted abstinence on drug-context and conditioned-cued reinstatement. WR generally reduced active lever responses during drug-context and conditioned-cued reinstatement. Taken together, these findings suggest that WR during withdrawal and abstinence equi-effectively reduces methamphetamine seeking triggered by drug-context and drug-cues and these effects could be associated with WR-induced regulation of the neurobiological substrates that underlie drug-context and conditioned-cued reinstatement (Johnson and Mitchell, 2003; Farmer et al., 2004; Berchtold et al., 2005; Lin et al., 2012; Peterson et al., 2013).
Detailed immunohistochemical analysis followed by stereological quantification was performed to determine the possible neurobiological correlate underling WR-induced reduction of reinstatement of methamphetamine seeking. Analysis of c-Fos expression was conducted to measure neuronal activation, as the activation of the immediate early gene c-fos is regulated by drugs of abuse (Hyman et al., 1993) and WR (Clark et al., 2011). Cell quantification demonstrated that WR-induced reduction of reinstatement was not associated with significant changes in the number of c-Fos expressing cells, suggesting that WR during withdrawal did not alter the activation of neurons in the reward circuitry in response to methamphetamine reinstatement triggered by drug-context and drug-cues. While previous reports have indicated that neuronal activation occurs following methamphetamine seeking and WR in the brain regions associated with relapse to drug seeking (Clark et al., 2011; Clark et al., 2012; Recinto et al., 2012), a lack of effect in the current study may be attributable to differences in the amount of animal handling, schedule of reinstatement, the cues that triggered drug seeking, exposure to enriched environment, length of withdrawal period before testing, and anatomical subregions analyzed.
Analysis of Ki-67 and BrdU cell numbers were conducted to measure developmental stages of hippocampal neurogenesis, as the generation of and survival of progenitors in the hippocampus is regulated by reinforcing doses of drugs of abuse (Eisch and Harburg, 2006; Mandyam and Koob, 2012) and WR (van Praag et al., 1999b; van Praag et al., 1999a). It has been demonstrated that acute-to-early withdrawal from methamphetamine self-administration (16–24h after the last self-administration session) reduces proliferation, differentiation and survival of hippocampal progenitors and these changes are relative to the amount of methamphetamine consumed (Mandyam et al., 2008; Yuan et al., 2011). However, protracted abstinence from methamphetamine self-administration produces compensatory changes in proliferation and survival of hippocampal progenitors (visualized as increases in proliferation and survival) compared with acute-to-early withdrawal time point (Recinto et al., 2012). The affects noted during protracted abstinence in animals that were not exposed to any reinstatement sessions were abolished in animals that underwent drug-primed reinstatement of methamphetamine seeking, suggesting that re-exposure to the drug environment triggered neurobiological alterations in the hippocampal neurogenic niche such that these alterations affected the enhanced proliferation observed in animals that did not reinstate (Recinto et al., 2012). The current findings are similar to the findings reported in the Recinto et al study and show that proliferation and survival is not affected by reinstatement of drug seeking, but significantly extend the findings with cue-induced reinstatement. Importantly, the current study demonstrates that WR-induced reduction in reinstatement of methamphetamine seeking was not associated with significant changes in the number of proliferating or surviving cells in the hippocampus. However, one minor limitation of the present results is the lack of phenotype identification of surviving cells. Inevitably, the fact that methamphetamine and WR independently regulate multiple phenotypes of newly born cells (neurons, microglia, oligodendroglia and astroglia) suggests that some caution is warranted when interpreting these findings. Nevertheless, this data leads us to conclude that running activity during withdrawal prevented other measures of toxicity produced by methamphetamine to inhibit reinstatement of methamphetamine seeking.
In the context of the above hypothesis, most notable with methamphetamine neurotoxicity is the increases in markers of oxidative stress (nNOS), apoptosis, astrogliosis and neuroinflammatory responses, and decreases in levels of dopamine and TH, levels and activity of dopamine transporter and VMAT2 in the striatum as demonstrated in self-administration studies (Schwendt et al., 2009; Krasnova et al., 2010; McFadden et al., 2012b; McFadden et al., 2012a; Engelmann et al., 2013). Analysis of the number of nNOS cells demonstrated that protracted abstinence and reinstatement of methamphetamine seeking did not produce significant changes in the striatum and other regions; the data suggest that the number of nNOS cells previously reported to be enhanced during acute withdrawal in the ventral striatum were not enhanced by drug seeking (Engelmann et al., 2013). WR during withdrawal and protracted abstinence did not alter the number of nNOS cells. Regulation of VMAT2 by methamphetamine via enhanced nNOS activity has been linked to cytosolic dopamine-derived oxidative stress by methamphetamine (Larsen et al., 2002; Eyerman and Yamamoto, 2007; McFadden et al., 2012a). Analysis of the density of VMAT2 in the striatum demonstrated that protracted abstinence and reinstatement of methamphetamine seeking did not affect VMAT2 levels. These findings suggest that decreases in VMAT2 function in the striatum seen during methamphetamine self-administration (McFadden et al., 2012a) were transient and were not visualized as reduced VMAT2 immunoreactivity after protracted abstinence and reinstatement of methamphetamine seeking in extended access animals (present results). Furthermore, a lack of effect on nNOS and VMAT2 expression by reinstatement of methamphetamine seeking suggests that WR-induced reduction of reinstatement of methamphetamine seeking was due to amelioration of other markers of methamphetamine toxicity (Hofford et al., 2014).
The effect of withdrawal and protracted abstinence on mesolimbic and nigrostriatal dopamine neurons was evaluated by TH immunoreactivity, a marker for methamphetamine neurotoxicity. Protracted abstinence and reinstatement of methamphetamine seeking in the sedentary animals increased the number of TH positive neurons in the VTA (the region containing the cell bodies of the mesolimbic dopamine neurons) and the SN (the region containing the cell bodies of the nigrostriatal dopamine neurons) and WR during protracted abstinence did not alter the increases. These results add to the growing literature on methamphetamine self-administration and TH toxicity, where extended access methamphetamine self-administration enhanced TH mRNA and protein expression in the VTA and SN during acute withdrawal (Stefanski et al., 2002; Shepard et al., 2006). The current findings are significant and extend the previous reports to demonstrate that the number of TH expressing dopamine neurons is enhanced in the VTA and SN during protracted abstinence and reinstatement of methamphetamine seeking triggered by drug-context and drug-cues. Protracted abstinence and reinstatement of methamphetamine seeking did not produce any changes in the density of TH fibers in the ventral striatum (nucleus accumbens shell region) and was not affected by WR during withdrawal. Taken together our results demonstrate that during extended access methamphetamine self-administration, which produces maladaptive patterns of methamphetamine intake, methamphetamine seeking-induced TH depletion was not evident (Ricaurte et al., 1982; Larsen et al., 2002; Boger et al., 2009; Keller et al., 2011). Enhanced TH levels in the VTA and SN suggest transient elevation of dopamine in these regions, which could contribute to escalation of methamphetamine intake and enhanced reinstatement in animals experiencing extended access sessions (Shepard et al., 2006; Rogers et al., 2008). However, WR-induced reduction in reinstatement of methamphetamine seeking was not attributable to reduction in TH levels in the VTA and SN.
The number of TH neurons in the regions adjacent to the VTA was examined. For example, the dopamine neurons of the PAG are considered independent of the VTA and greater than 50% of these neurons project onto brain structures such as the central nucleus of the amygdala and bed nucleus of the stria terminalis and contribute to the dopaminergic innervations in these regions (Ottersen, 1981; Grove, 1988; Li et al., 1993; Hasue and Shammah-Lagnado, 2002). The functional significance of the dopaminergic projections from the PAG to the extended amygdala (composed of the central nucleus of the amygdala, bed nucleus of the stria terminalis, and a transition zone in the medial (shell) subregion of the nucleus accumbens) is unknown and the projections are hypothesized to influence γ-aminobutyric acid (GABA)ergic neuronal activity in the central nucleus of the amygdala (Freedman and Cassell, 1994). The neural areas of the extended amygdala are of relevance to the withdrawal/negative affect stage of methamphetamine addiction as they harbor brain neurochemical systems (e.g., corticotrophin-releasing factor, CRF) involved in arousal-stress modulation that could contribute to maladaptive patterns of drug intake and enhanced relapse associated with extended access methamphetamine self-administration (Koob, 2013). Particularly interesting is the evidence for increased CRF expression in the extended amygdala in withdrawn methamphetamine animals and blockade of reinstatement of methamphetamine seeking with CRF antagonists (Nawata et al., 2012). Given that the PAG is rich in opioid receptors, endogenous opioid peptides, and mediates physiological functions, many of which are consistent with withdrawal/negative affect state behaviors (Bandler and Shipley, 1994), and harbors reciprocal connections to the extended amygdala (Ottersen, 1981; Grove, 1988; Stinus et al., 1990; Li et al., 1993; Harris and Aston-Jones, 1994; Hasue and Shammah-Lagnado, 2002), it is tempting to speculate that the PAG dopamine neurons play a role in the expression of withdrawal/negative affect symptoms associated with methamphetamine dependence via dopaminergic innervation in the extended amygdala. Protracted abstinence and reinstatement of methamphetamine seeking in methamphetamine dependent animals increased the number of TH neurons in the PAG. Running activity during withdrawal and protracted abstinence prevented methamphetamine seeking-associated increases in TH neurons in the PAG. These novel findings indicate that WR during withdrawal and protracted abstinence ameliorated the dopamine-associated neuroadaptations in the PAG which contributed to the reduced reinstatement of methamphetamine seeking in methamphetamine dependent animals.
In sum, the analysis of multiple neurotoxicity markers in various brain regions associated with the distinct stages of addiction provides information as to potential neurobiological correlates associated with WR-induced reduction in methamphetamine seeking. A limitation of the present study is that multiple neurotoxicity markers assessed are concurrent to the behavioral correlates and causality of each of these markers in relapse to methamphetamine seeking is unknown. Conversely, this data lays the groundwork for future studies aimed at integrating the observed neuronal changes in the PAG in methamphetamine dependence. The study may provide a novel therapeutic approach to reduce methamphetamine relapse.
Highlights.
Extended access Meth produces maladaptive patterns of Meth intake and Meth dependence
Access to running wheels during Meth withdrawal reduces Meth seeking in Meth dependent rats
Dopamine neurons of the PAG region show adaptive biochemical changes during Meth seeking
Wheel running during withdrawal rescues and abolishes adaptive changes in the PAG
Acknowledgements
The study was supported by funds from the National Institute on Drug Abuse (DA022473 and DA034140 to CDM), National institute on Alcoholism and Alcohol Abuse (AA020098 and AA06420 to CDM) and Alcohol Beverage Medical Research Foundation to CDM. We appreciate the technical support of Elena Crawford for StereoInvestigator, and Robert Lintz, Ilham Polis and Yanabel Grant for assistance with intravenous self-administration studies. We thank Derrick Babb from the LSSI TSRI program and Mathew Soleiman from the independent study program at UCSD for assistance with immunohistochemistry. We thank Drs. Miranda Staples and Sucharita Somkuwar for critical reading of the manuscript. This is publication number 26041 from The Scripps Research Institute.
References
- (WHO) WHO; 1980. [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bjorklund L, Stromberg I. Dopaminergic innervation of striatal grafts placed into different sites of normal striatum: differences in the tyrosine hydroxylase immunoreactive growth pattern. Exp Brain Res. 1997;113:13–23. doi: 10.1007/BF02454138. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Granholm AC, McGinty JF. Minocycline restores striatal tyrosine hydroxylase in GDNF heterozygous mice but not in methamphetamine-treated mice. Neurobiol Dis. 2009;33:459–466. doi: 10.1016/j.nbd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Casu MA, Colombo G, Gessa GL, Pani L. Reduced TH-immunoreactive fibers in the limbic system of Sardinian alcohol-preferring rats. Brain Res. 2002;924:242–251. doi: 10.1016/s0006-8993(01)03296-6. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Kohman RA, Deyoung EK, Rhodes JS. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012 doi: 10.1002/hipo.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fillipas S, Oldmeadow LB, Bailey MJ, Cherry CL. A six-month, supervised, aerobic and resistance exercise program improves self-efficacy in people with human immunodeficiency virus: a randomised controlled trial. Aust J Physiother. 2006;52:185–190. doi: 10.1016/s0004-9514(06)70027-7. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature. 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Darna M, Wilmouth CE, Dwoskin LP, Bardo MT. Environmental enrichment reduces methamphetamine cue-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behav Brain Res. 2014;270:151–158. doi: 10.1016/j.bbr.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Kosofsky BE, Nguyen TV, Cohen BM, Comb MJ. Everything activates c-fos--how can it matter? NIDA Res Monogr. 1993;125:25–38. [PubMed] [Google Scholar]
- Johnson RA, Mitchell GS. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain Res. 2003;983:108–114. doi: 10.1016/s0006-8993(03)03039-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Marks-Kaufman R, D'Anci KE, Przypek J. Exercise attenuates oral intake of amphetamine in rats. Pharmacol Biochem Behav. 1995;51:725–729. doi: 10.1016/0091-3057(95)00022-o. [DOI] [PubMed] [Google Scholar]
- Keller CM, Salvatore MF, Pruett BS, Guerin GF, Goeders NE. Biphasic dopamine regulation in mesoaccumbens pathway in response to non-contingent binge and escalating methamphetamine regimens in the Wistar rat. Psychopharmacology (Berl) 2011;215:513–526. doi: 10.1007/s00213-011-2301-9. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Frontiers in psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Kujala UM, Rose RJ, Kaprio J. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: a longitudinal population-based twin study. Twin Res Hum Genet. 2009;12:261–268. doi: 10.1375/twin.12.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Rose RJ. Physical activity in adolescence and smoking in young adulthood: a prospective twin cohort study. Addiction. 2007;102:1151–1157. doi: 10.1111/j.1360-0443.2007.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Takada M, Shinonaga Y, Mizuno N. The sites of origin of dopaminergic afferent fibers to the lateral habenular nucleus in the rat. J Comp Neurol. 1993;333:118–133. doi: 10.1002/cne.903330110. [DOI] [PubMed] [Google Scholar]
- Lin TW, Chen SJ, Huang TY, Chang CY, Chuang JI, Wu FS, Kuo YM, Jen CJ. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem. 2012;97:140–147. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Piehl KB, Acosta G, Peterson AB, Hemby SE. Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry. 2010;68:774–777. doi: 10.1016/j.biopsych.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. Journal of Neuroscience Research. 2004;76:783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int J Emerg Ment Health. 2005;7:49–57. [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Stout KA, Vieira-Brock PL, Allen SC, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration acutely decreases monoaminergic transporter function. Synapse. 2012a;66:240–245. doi: 10.1002/syn.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012b;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messanvi F, Eggens-Meijer E, Roozendaal B, van der Want JJ. A discrete dopaminergic projection from the incertohypothalamic A13 cell group to the dorsolateral periaqueductal gray in rat. Front Neuroanat. 2013;7:41. doi: 10.3389/fnana.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Vaillancourt BD, Wright MJ, Jr, Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Menaker M. A new (and different) circadian pacemaker. Cell Cycle. 2009;8:2861–2862. doi: 10.4161/cc.8.18.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawata Y, Kitaichi K, Yamamoto T. Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by footshock. Pharmacol Biochem Behav. 2012;101:297–302. doi: 10.1016/j.pbb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- NIDA. Research reports: Methamphetamine abuse and addiction. The Science of Drug Abuse and Addiction. 2006 [Google Scholar]
- NIDA. Drug Facts: Methamphetamine. The Science of Drug Abuse and Addiction. 2010 [Google Scholar]
- NIDA. Topics in brief: methamphetamine addiction: progress, but need to remain vigilant. The Science of Drug Abuse and Addiction. 2011 [Google Scholar]
- Noori HR, Fornal CA. The appropriateness of unbiased optical fractionators to assess cell proliferation in the adult hippocampus. Front Neurosci. 2011;5:140. doi: 10.3389/fnins.2011.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addict Biol. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 3°. edition Edition. San Diego: Academic Press; 1997. [Google Scholar]
- Pendergast JS, Yamazaki S. Effects of light, food, and methamphetamine on the circadian activity rhythm in mice. Physiol Behav. 2014;128:92–98. doi: 10.1016/j.physbeh.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–1287. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2007 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. 2008 [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology (Berl) 2013;227:403–411. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Human methamphetamine pharmacokinetics simulated in the rat: single daily intravenous administration reveals elements of sensitization and tolerance. Neuropsychopharmacology. 2006;31:941–955. doi: 10.1038/sj.npp.1300865. [DOI] [PubMed] [Google Scholar]
- Serwatkiewicz C, Limebeer C, Eikelboom R. Sensitization of amphetamine-induced wheel running suppression in rats: dose and context factors. Psychopharmacology (Berl) 2000;151:219–225. doi: 10.1007/s002130000446. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Frontiers in psychiatry. 2011;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Lee SH, Yasar S, Cadet JL, Goldberg SR. Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol. 2002;439:59–68. doi: 10.1016/s0014-2999(02)01301-8. [DOI] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J Biol Rhythms. 2006;21:185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction. 2011;106:1855–1865. doi: 10.1111/j.1360-0443.2011.03489.x. discussion 1866–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM, Johnston LD. Exercise and substance use among American youth, 1991–2009. Am J Prev Med. 2011;40:530–540. doi: 10.1016/j.amepre.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Stamos J, Robison LS, Heyman G, Tucci A, Wang GJ, Robinson JK, Anderson BJ, Volkow ND. Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behav Brain Res. 2013;239:8–14. doi: 10.1016/j.bbr.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USPSTF. United States Preventive Services Task Force (USPSTF) Screening for illicit drug use. 2008 [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Angulo JA. Synergism between methamphetamine and the neuropeptide substance P on the production of nitric oxide in the striatum of mice. Brain Res. 2011;1369:131–139. doi: 10.1016/j.brainres.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Barry D, Petry NM. Exercise-related activities are associated with positive outcome in contingency management treatment for substance use disorders. Addict Behav. 2008;33:1072–1075. doi: 10.1016/j.addbeh.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan CJ, Quiocho JM, Kim A, Wee S, Mandyam CD. Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacol Biochem Behav. 2011;100:98–108. doi: 10.1016/j.pbb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology (Berl) 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]