Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematological disorder associated with an acquired deficiency in glycophosphatidylinositol-anchor biosynthesis that renders erythrocytes susceptible to complement attack. Intravascular hemolysis via the membrane attack complex is a clinical hallmark of the disease, and C5 blockade is currently the only approved treatment for PNH. However, residual anemia is an emerging observation for many PNH patients receiving anti-C5 treatment. A range of complement-targeted therapeutic approaches, encompassing surface-directed inhibition of C3 convertases, blockade of membrane attack complex assembly or C3 interception using peptidic inhibitors, has yielded promising results and offers leverage for even more effective treatment of PNH. This article discusses recent advances in this rapidly evolving field, integrating critical perspectives from preclinical PNH models and diverse complement modulation strategies with genetic insights and therapy response profiles. It also evaluates the relative efficacy, limitations and benefits afforded by C3 or C5 inhibition in the context of PNH therapeutics.
Keywords: C3 inhibitors, C5 blockade, complement therapeutics, Cp40, AMY-101, eculizumab, extravascular hemolysis, MAC, PNH
Background & overview
Biological origin, pathophysiology & clinical management profile of PNH
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare hematological disorder associated with an acquired deficiency in glycophosphatidylinositol (GPI)-anchor biosynthesis that is manifested within a subset of hematopoietic stem cells (HSCs) bearing a somatic mutation in the X-linked phosphatidylinositol glycan class A (PIGA) gene [1–3]. Recently, other genes of the GPI-biosynthetic pathway (e.g., PIGT) have also been implicated as genetic determinants of PNH [4], yet more research is needed to determine their prevalence and pathological implications. It has been postulated that these GPI-deficient hematopoietic cells expand under selective pressure in the bone marrow [5,6] and gradually produce progeny (mature red blood cells) that become susceptible to autologous complement attack as a result of the combined absence of the GPI-anchored complement regulators CD55 (decay accelerating factor) and CD59 from their surfaces [7,8]. Indeed, chronic, complement-mediated intravascular hemolysis of PNH erythrocytes remains one of the main clinical hallmarks of the disease, along with a pronounced propensity for thromboembolic complications and an underlying bone marrow failure [7,9–12].
Etiology
Although mutations in genes coding for enzymes of the GPI-biosynthetic pathway (usually somatic [1–3], even if germline mutations have also been recently reported [4]) define the genetic basis of the disease [13], this genetic signature is still regarded as insufficient to elicit the full clinical phenotype of the disease. Indeed, scarce PNH-like cell populations harboring PIGA-inactivating mutations can even be detected in normal individuals, with no evidence of the disease [14]. In this context, it is interesting that conditional silencing of the murine PIGA gene fails to recapitulate the human pathophysiology of PNH in mice [15]. Furthermore, there is no evidence that the PIGA-mutated HSCs gain any proliferative advantage over normal HSCs that would favor their clonal expansion in the bone marrow [16]. For a few cases, it has been reported that the aberrant expression of HMGA2, a transcription factor deregulated in several benign tumors, may contribute to the overgrowth of PIGA-mutated cells [17]. The hypothesis that concomitant genetic alterations, other than PIGA mutations, might confer a proliferative advantage to PNH cells has been recently re-evaluated by dissecting the stepwise acquisition of mutations in PNH using whole exome sequencing [18].
These combined genetic and hematologic observations provided the underpinnings for hypothesizing a ‘dual pathophysiology’ of PNH. This hypothesis, also known as ‘escape’ [19] or ‘relative advantage’ theory [3], is based on the essential contribution of a second independent event that would tilt the equilibrium toward the selective expansion of GPI-deficient PNH clones in the bone marrow, leading to their release into the circulation and evolution of the full clinical spectrum of the disease [10,19]. Observations from both human studies and animal disease models support the hypothesis that an (auto)immune-mediated attack on normal HSCs tilts the balance toward relative expansion of PIGA-mutated PNH HSCs that essentially escape a CD8+/CD57+ T-cell-dependent immune attack that targets the GPI moiety on HSC surface proteins [5,20]. Because of this bone marrow-targeted immune derangement, the ‘disguised’ immune-tolerated GPI-deficient HSCs will survive the immune attack, eventually producing committed hematopoietic progenitors and subsequently mature blood cells (i.e., erythrocytes, granulocytes and platelets). Indeed, the presence of autoreactive CD1d-restricted T-cell clones expressing invariant T-cell receptor α-chains that respond to stimulation by GPI-loaded antigen-presenting cells in PNH patients is suggestive of an autoimmune attack that selectively spares the GPI-deficient HCSs and allows the expansion of PNH HSCs and generation of mature progeny blood cells in the circulation [5,21,20].
Pathophysiology
Despite its rather heterogeneous and complex pathophysiological landscape, PNH is consistently characterized by three major clinical manifestations: hemolytic anemia, bone marrow failure and thrombophilia [22,23,7].
Intravascular hemolysis, the most typical manifestation of PNH, is evident in all patients presenting with the disease. However, the extent of hemolysis varies among patients, depending on the size of the PNH clone(s), the phenotype of PNH erythrocytes (type II vs type III, i.e., partial vs complete deficiency of GPI-linked complement regulators) and possibly the level of complement activation (which may vary according to specific medical conditions or patient-specific features) [6]. Typically, hemolysis is chronic, secondary to low-level spontaneous complement activation and subsequent impairment of complement regulation on affected erythrocytes caused by a lack of CD55 and CD59 (Figure 1). In addition to this continuous hemolysis, patients may undergo exacerbations (the so-called ‘paroxysms’) as a result of massive complement activation, often in association with infections or other triggering events [7]. Intravascular hemolysis is associated with hemoglobinuria, laboratory signs of hemolysis (increased lactate dehydrogenase, decreased haptoglobin, increased unconjugated bilirubin and an elevated erythrocyte count resulting from compensatory erythropoiesis) and chronic anemia, with possible fatigue and muscle dystonia [11,22].
Figure 1. Schematic illustration of the fine interplay between complement activation, regulation and PNH pathophysiology.
(A) Complement activation is effectively contained on the surface of normal erythrocytes that express the GPI-linked regulators CD55 (DAF) and CD59. Opsonic C3b may be generated by spontaneous (tick-over) or bystander activation (e.g., due to an infection) but is readily degraded by the plasma enzyme factor I (FI) in concert with the complement regulators factor H (FH), CD55 and CR1 (CD35). Furthermore, CD59 prevents the assembly of the MAC and protects the erythrocyte from autologous complement-mediated lysis (intravascular hemolysis) (B) Lack of GPI-linked CD55 and CD59 leads to the functional ‘dismantling’ of normal complement regulatory activity on the PNH erythrocyte surface, rendering the cell susceptible to autologous complement attack. Complement activation triggered by: spontaneous C3 hydrolysis (via tick-over mechanism); bystander activation; or specific (yet unknown) triggering mechanisms on PNH erythrocyte surfaces leads to C3b generation and surface opsonization that, in the absence of CD55, culminates in uncontrollable C3 fragment opsonization via amplification of the complement response through the alternative pathway and, eventually, in MAC-mediated intravascular hemolysis (i.e., due to the absence of CD59).
The second key clinical feature of PNH is cytopenia, mainly secondary to the underlying bone marrow disorder, which is embedded in the dual pathophysiology of PNH [10,23,24]. Bone marrow failure is usually indicated by clinical signs of neutropenia and thrombocytopenia; however, marrow failure can also contribute to anemia in PNH patients (as documented by a reticulocyte count that is inadequate to sustain normal hemoglobin levels).
The third typical manifestation of PNH is thrombophilia, with thrombosis developing in about 40% of all patients [25]; thus, PNH is one of the medical conditions with the highest risk of thrombosis. Thromboembolic complications may be life-threatening and rank as the main cause of mortality in PNH patients [26–28]. In contrast to marrow failure and hemolysis, the pathogenic mechanisms underlying thrombosis in PNH remain ill-defined. Several thrombogenic pathways have been implicated in PNH (extensively reviewed in [25]), including: direct complement activation on PNH platelets; hemolysis-driven thrombosis, via the generation of procoagulant microvesicles or scavenging of nitric oxide (NO) by hemoglobin released by lysed erythrocytes and dysregulation of fibrinolysis as a result of the missing GPI-linked urokinase-type plasminogen activator receptor. Thrombotic events are largely unpredictable in PNH patients. However, two follow-up studies seeking to identify PNH prognostic factors have indicated that larger PNH granulocyte clones and extensive hemolysis are predictive of venous thrombosis [29,30]. The thrombotic risk is unique to each patient and is possibly a result of additional independent (environmental or genetic) risk factors that may shape the individual’s predisposition to thrombosis.
Clinical management
For decades, the treatment of PNH has been both challenging and disappointing, with the limited options for therapeutic intervention aiming to control the dominant clinical manifestation(s) of the disease rather than tackling the underlying causes. Indeed, blood cell transfusion has remained the most widely used treatment strategy for decades, since no etiological treatment for complement-mediated hemolytic anemia was available until recently. However, the treatment of PNH changed dramatically with the introduction of the anti-complement agent eculizumab (Soliris®, Alexion Pharmaceuticals, Connecticut, USA), a humanized anti-C5 monoclonal antibody (mAb) that selectively prevents activation of C5 and, consequently, the assembly of the lytic membrane attack complex (MAC). Eculizumab was approved by the US FDA and EMA in 2007, based on two large multinational trials that documented its efficacy in controlling intravascular hemolysis, as well as reducing the need for transfusions, stabilizing hemoglobin and resolving all the hemolysis-related symptoms [31,32]. The sustained control of intravascular hemolysis resulted in transfusion-independence in about half of the treated patients and was associated with a reduced risk of thromboembolic events [33] (possibly resulting in improved long-term survival) [34]. However, the anti-C5 treatment proved inadequate in some patients who exhibited residual severe anemia even after receiving eculizumab, revealing noteworthy unmet clinical needs. Recent studies have elegantly demonstrated a genetic basis for this variable response to eculizumab, demonstrating that certain polymorphic variations in complement genes (C5 and CR1) render the carriers refractory or mildly responsive to anti-C5 therapy [35,36]. These genetic studies indicate a need for improved therapeutic schemes in PNH that can circumvent these genetic hurdles by targeting upstream components of the complement cascade, such as C3 (a schematic overview of the complement cascade is provided in Figure 2A).
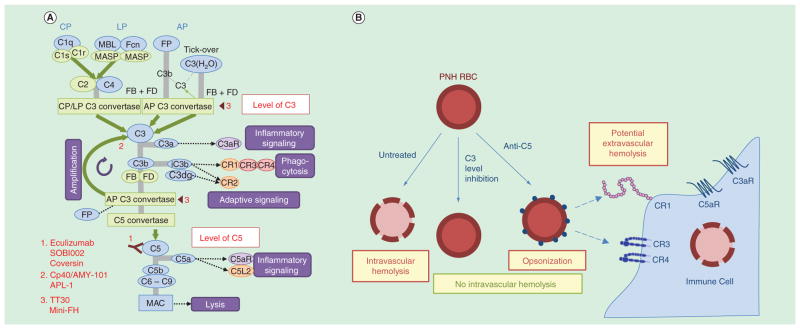
Figure 2. Overview of the complement cascade with an emphasis on therapeutic targets, drug leads and intervention strategies for the treatment of PNH.
(A) Simplified schematic overview of the complement cascade illustrating points of therapeutic intervention in paroxysmal nocturnal hemoglobinuria. i) At the level of C3: Cp40, the Cp40-based therapeutic AMY-101 and APL-1/2 block activation of C3 by any convertases, thereby abrogating generation of C3b (opsonization), amplification via formation of C3 convertases and downstream effector pathways. TT30 and mini-FH target C3 convertases and C3b, accelerating convertase decay and C3b degradation, thereby also inhibiting AP amplification. ii) At the C5 level: Eculizumab, Coversin and SOBI002 all bind to and inhibit cleavage of C5, thereby blocking terminal pathway activation and preventing formation of the MAC. (B) Benefits and limitations of complement-targeted therapeutic strategies for treating PNH. i) Complement modulation at the level of C3 prevents C3 deposition (opsonization) and all downstream effector functions, including lytic pathway activation and MAC-mediated intravascular hemolysis. On the other hand, complement inhibition at the level of C5, prevents MAC-mediated hemolysis (intravascular hemolysis) but allows C3 fragment opsonization to persist, possibly leading to C3-mediated extravascular hemolysis (via engulfment and immune clearance of C3-opsonized PNH erythrocytes by complement receptor bearing macrophages in the hepatosplenic system). AP: Alternative pathway; CP: Classical pathway; FB: Factor B; Fcn: Ficollin; FD: Factor D; FP: Properdin; LP: Lectin pathway; MAC: Membrane attack complex; MASP: Mannose-binding protein-associated serine protease; MBL: Mannose-binding lectin.
Beyond bone marrow failure and breakthrough, which can contribute to anemia in some patients, C3-mediated extravascular hemolysis has emerged as the most likely and relevant cause of suboptimal responses to eculizumab [37,24,38]. This novel route of erythrocyte clearance is mechanistically explicable, since complement regulation remains impaired on PNH erythrocytes because of their lack of CD55, which eventually leads to C3 fragment opsonization and entrapment of C3-bound PNH erythrocytes in hepatosplenic macrophages (Figure 2B). Indeed, recent studies have confirmed that patient-derived PNH erythrocytes are recognized and phagocytozed by primary human macrophages in vitro [39]. Thus, it appears highly feasible that extravascular hemolysis mediated by the activation of the early steps of the complement cascade is a common mechanism that accounts not only for a considerable fraction of PNH patients remaining transfusion-dependent, but also for the mild-to-moderate anemia observed in the majority of eculizumab-treated PNH patients.
Despite recent advances in diagnostics and therapy, PNH remains a hematological disorder with looming clinical complications that impose a devastating socioeconomic burden in terms of patient management and quality of life. While significant improvement in clinical care has been achieved by C5-targeted therapy, bone marrow transplantation remains the only curative treatment option for PNH patients [40]. Both non-myeloablative syngeneic bone marrow transplantation and stem cell transplantation from HLA-matched or -identical siblings have been successfully performed in PNH patients [41,42]. However, allogeneic bone marrow transplantation is associated with significant morbidity and mortality due to complications from acute or chronic graft-versus-host disease [42]. Stem cell transplantation probably remains the best treatment option for PNH patients with underlying bone marrow failure that does not respond to immunosuppressants, or for patients that present with refractory thromboembolic disease [11]. Notably, the additional burden placed on PNH patients by the chronic administration of corticosteroids should not be overlooked, particularly since this treatment is not generally recommended owing to its undesirable complications and side effects and the absence of clear proof of efficacy [43].
Importantly, the annual cost of current complement-targeted therapy exceeds US$400,000 per patient [44], which may limit broader access to this treatment option, for example, in developing countries. Furthermore, as stated above, the therapeutic outcome of anti-C5 therapy is not satisfactory in all PNH patients. These limitations and unmet clinical needs have fueled efforts to search for alternative anti-complement treatment strategies. Important progress has already been achieved; in particular, pre-clinical studies suggest that intervention at the level of C3 offers therapeutic merit in treating PNH when compared with blockage of C5. Recent studies (employing both biologics and small-molecule inhibitors) have suggested that targeted C3 inhibition can efficiently block complement opsonization of erythrocytes in addition to preventing intravascular hemolysis, thereby providing a clear therapeutic benefit. The advent of potent C3 inhibitors, including small peptidic drugs (e.g., Cp40 [45] and the Cp40-based therapeutic AMY-101) and surface-targeted fusion proteins that can modulate activation of the alternative pathway (AP) of complement (e.g., TT30 and mini-FH) [46,47] has shown promise in ameliorating both intravascular and C3-mediated extravascular hemolysis and opens new avenues of opportunity for sustainable and cost–effective PNH therapeutics (especially in the case of Cp40 [48] and the Cp40-based therapeutic AMY-101).
Furthermore, the emerging crosstalk of complement effectors with procoagulant pathways possibly underlying the thrombotic nature of PNH [49,50] highlights the necessity for further exploiting complement as a key target for therapeutic intervention in multiple PNH indications.
Complement dysregulation in the spotlight of PNH pathology
The molecular hallmark of PNH pathology is the clonal expansion of non-malignant HSCs carrying mutations in genes responsible for the synthesis or attachment of preassembled GPI-anchors to proteins destined to be tethered to the cell surface [13,6,4]. PNH clones harboring mutations that interfere with GPI synthesis give rise to progeny erythrocytes that fail to display GPI-anchored proteins on their surface. Among the missing GPI-linked proteins, two membrane-bound complement regulators, CD55 (decay accelerating factor) and CD59, take center stage in mediating the pathogenic role of complement in this hematological disorder [51,52]. CD55 effectively contains C3 activation on host surfaces through destabilization of C3 convertase complexes [53], while CD59 interferes with the assembly of the lytic MAC on the opsonized cell surface, blocking the insertion of C9 into the nascent C5b-8 complex [54]. As a result, PNH erythrocytes enter the circulation unshielded from autologous complement attack that can either be initiated by spontaneous C3 hydrolysis (‘tick-over’) under steady-state conditions [55] or exacerbated by pathogen-driven activation in response to infectious insults or other pathologic states (bystander effect) [6]. PNH erythrocytes are, therefore, subject to persistent surface complement activation that culminates in MAC-mediated intravascular hemolysis (Figure 1), leading to severe anemia and long-term transfusion dependence in PNH patients [24,7].
Although the mechanism by which PNH erythrocytes succumb to complement-mediated lysis has been well established in vitro, the precise triggering cues that lead to initial complement activation and C3 deposition on the PNH cell surface and to MAC-mediated hemolysis in vivo still remain debatable. In addition to spontaneous AP activation and bystander opsonization during infection, classical and lectin pathway activation may contribute as triggering mechanisms involving natural antibodies, activated platelet surfaces and other unidentified surface recognition units and molecular patterns. From a hierarchical perspective, AP-mediated complement activation appears to play a dominant role in exacerbating the clinical course of PNH [56,7].
Chronic, low-level complement activation via spontaneous C3 hydrolysis and formation of the C3(H2O)Bb convertase (tick-over) has been postulated to lead to initial C3 fragment deposition on PNH erythrocytes, fueling persistent AP activation through an amplification loop that operates unhindered because of a lack of surface CD55 expression (Figure 1B) [7]. The so-called ‘paroxysms’ (climactic hemolytic episodes) may manifest themselves in PNH patients as a consequence of massive complement activation during the course of an opportunistic inflammatory or infectious insult [11]. The relative contribution of each of the three canonical pathways (lectin, alternative, classical) to complement activation that can affect PNH pathology remains ill-defined; however, it is reasonable to speculate that more than one pathway can become activated in PNH patients. All initiation pathways converge at the level of C3 cleavage, which in turn relays the signal for downstream activation of the lytic terminal pathway, eventually culminating in MAC assembly and hemolysis. Regardless of the triggering signal, AP activation eventually takes over on PNH erythrocyte surfaces, amplifying C3 activation and causing uncontrolled deposition of C3 fragments (C3b, iC3b, C3dg) because of the absence of the pivotal C3 convertase regulator, CD55 [6]. Even though the causality of complement in inducing severe hemolysis in PNH patients is well established and constitutes a cardinal pathophysiological mechanism underlying this hematological disorder, the contribution of complement effectors to the thrombophilic nature of the disease is also emerging as a distinct pathogenic mechanism that probably operates in parallel with MAC-mediated intravascular hemolysis. It is conceivable that uncontrolled platelet activation in PNH, occurring because of the absence of CD55 from cell surfaces, may lead to platelet aggregation via C3b or iC3b interactions with complement receptors on leukocytes [57–60]. PNH platelet aggregation via complement receptor engagement can activate multiple thrombogenic pathways. Alternatively, the direct activation of C3 and/or C5 by platelets and neutrophil-derived proteases [60,61] could lead to the generation of downstream proinflammatory effectors such as C5a which, in turn, might trigger the extrinsic tissue factor (TF)-dependent procoagulant response and lead to thrombotic complications [62,49]. In this respect, the significant benefit afforded by anti-C5 (eculizumab) treatment in PNH patients in terms of ameliorating thromboembolic complications provides important insight into the close interplay of the complement and coagulation cascades in PNH pathology [24,25].
Complement-targeted therapeutic strategies for attenuating PNH pathology
PNH defines a complex pathology that is tightly intertwined with dysregulated complement activation [63,6,9]. In this respect, it constitutes an ideal paradigm of how a chronic debilitating disease might be effectively contained, in terms of attenuating its clinical manifestations via complement-targeted intervention [46,48]. The approval of eculizumab (anti-C5 mAb) for clinical use in PNH and the significant hematologic benefit afforded to patients by this anti-C5 treatment further underscore the validity of this therapeutic approach [64]. However, complement-targeted therapy for PNH is a field wide open to improvement, especially given the genetic variation studies that have provided insights into the genetic basis by which a significant percentage of patients remain refractory to anti-C5 therapy and consequently, transfusion-dependent [35]. Nonetheless, it should be stressed that careful selection of the target protein, delivery route and dosage regimes for applying complement inhibition to a chronic disorder poses a significant challenge that needs to be addressed before preclinical findings can be translated into effective PNH patient management.
Target selection
In the clinical phenotype of PNH, the combined absence of the GPI-linked complement regulators CD55 and CD59, which act upon different steps of the cascade (i.e., C3 activation and terminal lytic pathway, respectively), defines two ‘hot spots’ for complement interception, that is, inhibition at the level of C3 or C5 (see also Figure 2A). While C5 interception via antibody-mediated blockade (eculizumab) has already proved clinically effective, largely abrogating MAC-dependent intravascular hemolysis and its ensuing thrombotic complications [65], inhibition at the level of C3 offers advantages that might provide new leverage for more effective therapeutic intervention.
C3 is a large plasma protein (183 kDa) that engages in various protein–protein interactions, undergoing structural transitions during the activation process (Figure 2A) [66,67]. Although not considered a ‘druggable target’ in the traditional sense, its large surface area offers potential binding sites for small molecules that may act as protein–protein interaction inhibitors and offer advantages concerning production cost and administration [68]. In addition, there are several regulators of complement activation proteins that control C3 activation under physiological conditions and may be directly used or engineered for therapeutic processes [46,47]. As C3 marks the point of convergence for all initiation pathways, interception of C3 activation is largely independent of underlying triggering mechanisms, which are still ill-defined in PNH. Given the upstream position in the cascade, and its distinct nature from C5, inhibition at the level of C3 should not be influenced by known polymorphisms in C5 and CR1 that have been associated with a phenotype refractory to therapy in some PNH patients. Most importantly in the context of PNH, and as mentioned above, blockage of C3 activation is expected to address both intra- and extravascular hemolysis by preventing opsonization with C3 fragments. Selecting C3 as therapeutic target is, therefore, expected to lead to a more comprehensive treatment option for more patients by reducing transfusion dependence and residual anemia.
The two advantages that are often mentioned in favor of C5-directed therapy are residual complement activity and target concentration. Inhibition of C5 activation selectively blocks the effector arms of the terminal pathway, that is, MAC formation and release of the anaphylatoxin C5a, while leaving opsonization and phagocytic signaling intact. Although likely responsible for extravascular hemolysis in PNH patients, this maintained opsonic activity may also participate in the clearance of pathogens and immune complexes. However, it has been argued that complement-dependent clearance of microbes becomes less important once adulthood is reached. Indeed, individuals with primary C3 deficiencies show an increased risk for infection to a small set of pathogens, primarily in the early stages of their life [69]. Among the most severe risks are infections with Neisseria meningitidis, which is highly susceptible to MAC-mediated lysis; such infections are, therefore, observed in both C3 and C5 deficiency, yet can be prevented by vaccination. Even residual low amounts of C3 appear to be effective for host defense; interruption of inhibitor treatment during an active infection is, therefore, expected to restore complement activity in a feasible timeframe. Finally, inhibition at the level of C3 still allows for some opsonization via the classical and lectin pathway (the deposition of C4b can contribute to phagocytosis but is not sufficient to drive amplification or MAC formation). As for the target concentration, the higher abundance of C3 when compared with C5 (~1.0 and 0.08 mg/ml in plasma, respectively) renders administration of saturating inhibitor concentrations more demanding when targeting C3 itself. Recent studies with small-molecule C3 inhibitors (Cp40 and the Cp40-based therapeutic AMY-101) showed that target saturation is indeed feasible, and that it can be tailored by chemical modifications and administration strategies [48].
Overall, the rational selection of the optimal target for chronic complement modulation in PNH should aim to reconcile the patient’s individual genetic profile (complement haplotype) with the clinical benefit afforded by each therapeutic strategy, as well as consider the socioeconomic burden imposed on the patient and healthcare system.
We present below a comprehensive overview of the currently available biological therapy for PNH and of novel therapeutic strategies that exploit C3 or C5 interception, currently under preclinical or clinical development (Table 1) and showing clear promise for improving treatment.
Table 1.
Overview of complement-targeted therapeutics that have received approval for clinical use or are currently under clinical development for paroxysmal nocturnal hemoglobinuria.
| Complement-targeted PNH therapeutic agent | Target protein | Mode of complement interception | Point of interference in PNH pathology | Route of application | Stage of clinical development | Ref. |
|---|---|---|---|---|---|---|
| Eculizumab (Soliris®, Alexion Pharmaceuticals) | C5 | Inhibition of C5 activation by C5 convertases | MAC formation (intravascular hemolysis) | iv. | US FDA/EMA approved, in clinical use | [64] |
| Coversin (OmCI) (Volution Immuno Pharmaceuticals) | C5 | Inhibition of C5 activation by C5 convertases | MAC formation (intravascular hemolysis) | sc. | Human volunteer Phase I study (completed) | [76] |
| SOBI002, affibody-ABD fusion protein (Swedish Orphan Biovitrum) | C5 | Inhibition of C5 activation by C5 convertases | MAC formation (intravascular hemolysis) | sc. | Phase I study in humans (to be commenced) | [78] |
| TT30 (Alexion Pharmaceuticals) | C3 convertase, C3b | AP C3 convertase decay, cofactor activity for C3b cleavage, inhibition of AP amplification | Opsonization, MAC formation (intra- and extravascular hemolysis) | iv. and sc. | Phase I trial for treatment of PNH (terminated in May 2014) | [46] |
| Cp40/AMY-101 (Amyndas Pharmaceuticals) | C3/C3b/C3c | Inhibition of C3 binding to C3 convertases; inhibition of AP amplification | Opsonization, MAC formation (intra- and extravascular hemolysis) | sc. | Phase I trial for ABO-incompatible renal transplantation (to be commenced); orphan status for PNH | [48] |
| APL-2 (Apellis Pharmaceuticals) | C3/C3b/C3c | Inhibition of C3 binding to C3 convertases; inhibition of AP amplification | Opsonization, MAC formation (intra- and extravascular hemolysis) | sc. | preclinical evaluation completed | [89] |
Therapeutic agents have been classified according to target protein, mode of complement interception, preclinical models of PNH or other preclinical studies and the clinical indications that these agents can potentially treat in human PNH.
ABD: Albumin-binding domain; AP: Alternative pathway; FH: Factor H; iv.: Intravenous; MAC: Membrane attack complex; PNH: Paroxysmal nocturnal hemoglobinuria; sc.: Subcutaneous.
Complement interception at the level of C5
C5 serves as the initial component of the terminal pathway of complement activation that culminates in the assembly of the cytolytic MAC on complement-opsonized surfaces (Figure 2A) [70,63]. Complement activation via all pathways may lead to the convertase-mediated cleavage of C5, which in turn results in the generation of its bioactive fragments, C5b and C5a [68,64]. These C5-derived fragments display potent cytolytic (via the MAC) and a plethora of immunomodulatory and proinflammatory properties (via C5a receptor signaling) that are pertinent to the major clinical manifestations of PNH. Being a debilitating hemolytic disease that renders erythrocytes prone to autologous complement attack, it was reasoned that an inhibitor specifically blocking C5 activation would be highly effective as a rational therapeutic intervention in PNH to treat MAC-mediated intravascular hemolysis and its ensuing complications. Indeed, in 2007, the humanized h5G1.1-mAb eculizumab (Soliris) received the FDA and EMA approval for use in PNH patients [64,65]. This anti-C5 therapy drastically improved quality of life, reducing to a significant extent the transfusion dependence of patients over long periods of follow-up [24]. Two multi-center Phase III clinical studies established eculizumab’s efficacy and safety [31–33]. Eculizumab impairs terminal complement activation by binding native C5 and blocking its proteolytic cleavage into C5a and C5b by C5 convertases, thereby preventing MAC assembly on the cell surface and also ameliorating C5a-triggered inflammatory and procoagulant complications [64,71]. The drug’s profound hematologic benefit is premised on its efficacy in abrogating MAC-mediated intravascular hemolysis in PNH patients [24]. Chronic treatment with eculizumab results in sustained control of intravascular hemolysis, leading to hemoglobin stabilization and transfusion independence in more than half of the treated patients [6].
However, in the eculizumab era, an illuminating medical paradigm also revealed the limitations of this new complement-targeted therapy shedding light on underappreciated pathophysiological aspects of the disease. Despite the fact that patients’ responses in large cohort studies were tremendously improved with anti-C5 therapy, a noteworthy percentage (20–30%) of patients on eculizumab still fail to respond, requiring regular blood transfusions to stabilize hemoglobin levels. These patients present with residual anemia, possibly as a result of the extravascular hemolysis that afflicts surviving PNH erythrocytes and/or an underlying bone marrow hypoplasia. While eculizumab treatment completely prevents intravascular (MAC-mediated) hemolysis, it cannot abolish the activation of C3 via tick-over or bystander effects that can lead to progressive C3 deposition on the surviving erythrocytes through uncontrolled AP amplification [43]. This accumulation of C3-derived opsonic fragments on PNH erythrocytes is expected to provide an ‘eat-me’ signal for professional phagocytes bearing complement receptors (e.g., CR3 on macrophages); such erythrocytes may be engulfed and cleared in extravascular sites such as the reticuloendothelial system in the liver and spleen [6].
In view of the clinical success of eculizumab, and despite the potential limitations mentioned earlier, C5 and the MAC remain important targets for PNH therapy. As a consequence, several groups and companies are exploiting alternative options for inhibiting the terminal complement pathway. Previous studies have shown that a fusion protein consisting of the C3-binding region of CR2, linked to a soluble form of CD59, displays potent MAC inhibitory activity, abrogating MAC-mediated lysis of C3-opsonized cells [72]. Such fusion proteins could offer an alternative for treating MAC-mediated intravascular hemolysis in PNH patients that fail to respond to eculizumab, by targeting CD59 to PNH erythrocyte surfaces opsonized by C3 fragments. Another possible alternative to anti-C5 mAb therapy, currently under development as a PNH therapeutic, is coversin (also termed OmCI, Volution Immuno-Pharmaceuticals), a complement inhibitor of the lipocalin family originally isolated from the soft tick Ornithodoros moubata [73]. Similar to eculizumab, coversin binds C5 and blocks its proteolytic activation by C5 convertases, yet also inhibits the function of leukotriene B4 [74,75]. This relatively small protein (16 kDa) has shown promising results in several preclinical models of disease [75]. Coversin has recently been evaluated as a potential PNH therapeutic in a Phase I clinical trial and showed potent systemic C5 inhibitory effects in a subcutaneous (sc.) administration protocol that could be amenable to clinical use [76]. Notably, its relatively small size should mean lower production costs than the therapeutic antibodies currently on the market. However, besides the need to ascertain their potential efficacy and hematologic benefit in PNH, the use of proteins (such as coversin) derived from evolutionary distant species may raise immunogenicity issues in chronic administration protocols that must be sufficiently addressed before advancement to the clinical stage.
SOBI002 (Swedish Orphan Biovitrum), a recently described C5-targeting affibody fused to an albumin-binding domain, has also been evaluated for its C5 inhibitory activity. Affibodies are small non-immunoglobulin ligands displaying high affinity binding to a wide range of protein targets. These molecules share no sequence/structural homology to antibodies and are isolated from scaffold-constrained combinatorial libraries [77]. SOBI002 binds human C5 with low-nanomolar affinity (KD ~1 nM) and effectively blocks its activation. Addition of the albumin-binding moiety led to an increase in its half-life and stability in both rodent and non-human primate (cynomolgus monkey) plasma, offering favorable pharmacokinetic properties for sustained inhibition [78]. Its high subcutaneous bioavailability as well as its efficacy in preclinical models of C5-driven pathology have earned this novel anti-C5 agent a place in the growing arsenal of potential PNH therapeutics.
From a different perspective, the high expenses typically associated with the chronic treatment involving biological agents, and in particular therapeutic antibodies [79], call for the consideration of novel complement-based therapeutics based on small-sized inhibitors that have significantly lower production costs, such as synthetic peptides [80]. Furthermore, the demanding clinical protocol for PNH therapy, entailing antibody infusions over long periods of time, frequent hospitalization rounds and a high burden on the healthcare system strongly argues for alternative therapeutic strategies that will facilitate patient management and alleviate the clinical symptoms of PNH without imposing a high socioeconomic burden on the patients.
Complement interception at the level of C3
Whereas the clinical use of eculizumab has drastically impacted the natural history of PNH and contributed to the improvement of patient management, this complement-targeted strategy has also propelled our understanding of the pathophysiological basis of this disease. In particular, it has helped unmask pathogenetic mechanisms that are driven by complement activation at earlier stages of the cascade, converging at C3 convertase assembly, and C3-mediated opsonization of PNH erythrocytes through dysregulated AP amplification [6]. Although eculizumab effectively compensates for the lack of CD59 on PNH erythrocytes, it cannot counteract the absence of a complement AP regulator that is crucial for controlling C3 deposition (i.e., CD55). Indeed, C3-mediated extravascular hemolysis is an emerging clinical manifestation in patients receiving anti-C5 that limits its therapeutic efficacy. This medical problem has fueled several investigations aimed at developing alternative complement therapeutics that preferentially target the activation of C3 on the erythrocyte surface [68,67,7]. Such an approach would also intercept C5 activation and MAC assembly, irrespective of the upstream triggering mechanism or pathway involved (Figure 2A).
Antibody-based anti-C3 approaches
Whereas targeting native C3 using monoclonal antibodies is challenging due to its high plasma concentration, a recent study adopted the elegant approach of targeting activated C3 fragments (iC3b/C3b) on the erythrocyte surface with an anti-C3b/iC3b mAb (clone 3E7) [81]. This antibody acts specifically on the AP C3/C5 convertase, because classical pathway-hemolytic activity remains intact in its presence. Both this antibody and its humanized derivative H17 were able to block hemolysis and C3 deposition on PNH erythrocytes in vitro [81]. Albeit promising, these results still need to be translated to the clinic. The presence of Fc-moieties of the IgG1 isotype in these antibodies might have adverse effects in terms of sustaining, rather than abrogating, C3-mediated extravascular hemolysis via recruitment of phagocytes through Fc receptor- and CR3-mediated interactions. Engineering a modified anti-C3b/iC3b mAb lacking the Fc portion could offer greater promise for therapeutic application, but this approach remains to be tested. Indeed, a Fab fragment of H17 has recently been described and tested in a renal disease model [82], but not yet evaluated in PNH.
Surface-targeting of AP C3 inhibitors in PNH
Since PNH pathology mainly reflects the uncontrollable activation of AP and the accumulation of C3 fragments on surviving PNH erythrocytes, devising a therapeutic strategy that would exploit natural AP inhibitors to target both the C3 convertase formation step and the AP amplification loop on PNH cells would be an attractive therapeutic approach. Factor H (FH) is the main fluid-phase regulator of AP activation, preventing formation of the AP C3 convertase, accelerating the decay of existing convertases and also acting as a cofactor for the factor I-mediated degradation of C3b into iC3b [63,83]. Indeed, the fact that FH can effectively block AP amplification regardless of the initial triggering pathway is of paramount importance for controlling PNH hemolysis in vivo [84]. Several strategies have been developed to specifically target its inhibitory activity to the complement-opsonized surface (reviewed in [68]). In this respect, TT30 (Alexion Pharmaceuticals), a recombinant fusion protein consisting of the C3dg/iC3b binding domains of CR2 (i.e., complement control protein [CCP] domains 1–4) and the complement inhibitory domains of FH (CCP1-5), was recently shown to be effective in blocking the MAC-dependent hemolysis of PNH erythrocytes and also abrogating C3 fragment deposition on surviving PNH erythrocytes [46]. This inhibitory effect is dependent on targeting FH to the erythrocyte surface, since only partial inhibition was achieved when CR2-mediated binding of TT30 to PNH cells was blocked [46]. The FH-elicited cofactor activity of TT30 provides an exquisite mechanistic ‘handle’ for augmenting the targeting of this inhibitor to PNH erythrocyte surfaces through the generation of additional docking sites (iC3b/C3dg). These studies provided a proof-of-concept and a well-founded rationale for further development of this surface-targeted AP inhibitor as a PNH therapeutic that might effectively attenuate both intravascular and extravascular hemolysis. A Phase I clinical trial evaluating TT30 as a potential therapeutic option for treating PNH in humans had been initiated, but has recently been reported as terminated [85].
Supporting the emerging therapeutic potential of surface-targeted FH in PNH, a recent study has elaborated on the therapeutic efficacy of a rationally engineered miniaturized version of human FH, employing AP-driven in vitro models of PNH [47]. This so-called mini-FH (43 kDa) combines the regulatory CCP1–4 domains of FH with the C-terminal CCP19–20 domains that are involved in the recognition of self-cell pattern. Despite condensing the size of the parental FH by 70%, mini-FH shows markedly increased inhibitory potency (almost 10-fold) over FH in blocking AP activation. Mini-FH retains the regulatory function of FH (C3 convertase decay and cofactor activities) and displays a unique triple-targeting profile for diseased surfaces by binding oxidative damage markers, polyanionic surfaces and the full spectrum of C3-derived opsonins [47]. Strikingly, the rational design of mini-FH led to the unmasking of a cryptic binding site for iC3b/C3dg, located in FH CCP19-20. This structure-guided minimization increased the targeting capacity of this AP inhibitor for surfaces subjected to high opsonic turnover, as exemplified by PNH erythrocytes. Indeed, mini-FH effectively blocked hemolysis of patient-derived PNH erythrocytes, largely outperforming full-length FH [47]. Mini-FH also prevented surface deposition of C3 fragments on PNH erythrocytes exposed to AP-activated serum. Interestingly, mini-FH appeared to be more potent than TT30 in the same assay format (although not evaluated side-by-side), with full inhibition achieved at concentrations about 10-fold lower than TT30. Although the pharmacokinetic profile and other aspects of this inhibitor remain to be investigated, these findings suggest that mini-FH might be a promising lead compound for novel PNH therapeutics in humans. Notably, its enhanced recognition capacity for self-surfaces heavily decorated with complement opsonic fragments coupled with its AP inhibitory activity should prove valuable leads for preventing both intravascular hemolysis and residual anemia due to C3-mediated extravascular hemolysis in PNH patients.
Toward peptide-based PNH therapeutics
Departing from therapeutic strategies that rely on larger proteins, such as anti-C3 mAbs or fusion proteins with AP regulatory activity (e.g., TT30 and mini-FH), recent studies have produced promising preclinical results by intercepting C3 activation in PNH models with small peptidic inhibitors of the compstatin family [45,48]. Following rigorous structure-guided optimization and activity refinement, a second generation of compstatin derivatives has culminated in the development of AMY-101 (Amyndas Pharmaceuticals, Glyfada, Greece), a novel C3-targeted therapeutic based on the compstatin analog Cp40, that shows subnanomolar binding affinity for C3, increased plasma stability, safety in systemic administration protocols in non-human primates and a favorable pharmacokinetic profile for therapeutic modulation in primate models of inflammatory disease [45,48,86].
To address the challenge of effective systemic delivery and to maintain a sustainable pharmacologic profile of therapeutic modulation in PNH, a series of long-acting polyethylene glycol (PEG) derivatives of Cp40 were designed and evaluated in an in vitro model of PNH [48]. Both unmodified Cp40 and its N-terminal PEGylated derivative (PEG-Cp40) exerted strong inhibitory effects by abrogating PNH erythrocyte hemolysis and attenuating C3 fragment deposition on surviving PNH cells [48]. These findings strongly suggest that compstatin effectively intercepts both MAC-mediated intravascular and C3-mediated extravascular hemolysis in PNH, thus indicating a broader therapeutic benefit over eculizumab treatment. Furthermore, PEGylated Cp40 displayed a favorable pharmacokinetic profile in non-human primates that was highly comparable to that of therapeutic antibodies, with increased plasma stability and a remarkably prolonged elimination half-life of more than 5 days [48]. However, the increased (>10-fold) plasma residence of this PEGylated Cp40 appears to also affect plasma C3 levels, indicating that additional studies are warranted to address its potential effect on the metabolic turnover and clearance of C3. In addition, it remains to be seen whether the comparatively high doses of PEGylated peptide necessary to saturate an abundant plasma protein such as C3 may lead to adverse effects. Indeed, although generally considered safe, PEGylation may change the immunogenicity and safety profiles of peptide/protein drugs at high concentrations, and cases of anti-PEG antibodies or cellular vacuolation have been described [87,88].
Interestingly, unmodified Cp40 and the Cp40-based therapeutic AMY-101 were shown to provide sustained plasma inhibitor levels when injected sc. into cynomolgus monkeys in a multi-dose regimen (12-h intervals), suggesting that AMY-101 may prove a valuable option for long-term systemic treatment of PNH patients (e.g., potentially facilitating self-administration of the drug by the patient). The use of non-PEGylated AMY-101 may also allow for quickly regaining complement activity in case of infection or other complications that require C3 capacity. In August 2014, AMY-101 received orphan status designation from EMA for the treatment of PNH, and clinical development for this indication is currently pursued by Amyndas Pharmaceuticals (Glyfada, Greece). In addition to Cp40, an earlier-generation analog of compstatin (APL-1, Apellis Pharmaceuticals) and its PEGylated derivative (APL-2) have shown in vitro efficacy in protecting PNH erythrocytes from complement-mediated lysis and opsonization [89].
As well as pointing to alternative therapeutic options for treating PNH, these studies on peptidic C3 inhibitors mark important progress for introducing more affordable therapeutic options. As a result, such peptide-based therapies are expected to reduce the burden on patients’ clinical management and the healthcare system at large. It is well appreciated that the use of large proteins as therapeutics, particularly for treating chronic pathologies such as PNH, entails potential drawbacks concerning production costs, stability, administration options and/or immunogenicity. As small peptidic inhibitors, compstatin analogs (e.g., Cp40) can offer a promising alternative for therapeutic intervention in PNH that might afford multiple benefits for PNH treatment: peptide-based C3 inhibitors have already shown promise as safe and potent therapeutics in primate models, production costs for synthetic peptides have decreased significantly in recent years [80], translating into less expensive therapies and lower state subsidies for patient healthcare and C3 inhibition in PNH appears to exert multiple beneficial effects, preventing intravascular hemolysis while also effectively abolishing the pathological sequelae of chronic C3 opsonization of erythrocyte surfaces (i.e., immune-mediated clearance of surviving PNH cells).
Implications of complement-targeted therapy for other PNH manifestations
Therapeutic implications for PNH-associated thrombophilia
Thrombophilia is a cardinal clinical feature of PNH, with thrombotic complications developing in about 40% of all patients [25]. Arterial thrombosis and venous thromboembolism are potentially life-threatening complications, and venous thromboembolism is regarded as the major cause of morbidity and mortality in PNH patients [28,90]. Despite our appreciable knowledge of the pathogenic mechanisms underlying hemolysis and bone marrow failure in PNH, the etiology of thrombophilia in PNH patients remains quite obscure and multifactorial. However, in the era of eculizumab, well-controlled multicenter patient surveys have offered new insights that extend our understanding of the pathogenic landscape of PNH-related thrombosis [90,33]. The tight interconnection of the complement and coagulation cascades at the vascular endothelial interface emerges as a potential pathogenic driver of thrombotic complications in PNH patients [25]. Indeed, complement has been implicated as a key contributor to vascular inflammation and modulates several procoagulant pathways that are relevant to PNH. These include: direct platelet activation due to the absence of GPI-linked complement regulators, hemolysis-driven thrombosis involving procoagulant TF-microparticles, NO depletion caused by the release of free hemoglobin and dysregulated fibrinolysis because of the absence of GPI-anchored anti-coagulant proteins (such as urokinase-type plasminogen activator receptor, heparin sulfate and TF pathway inhibitor) from monocytes and platelets (reviewed in [25]). Recently, two clinical studies enrolling patients on eculizumab therapy have provided valuable insight into the impact of C5 blockade on critical hemostatic parameters and endothelial activation markers that collectively shape the thrombotic state in PNH patients [90,91]. Eculizumab therapy was shown to ameliorate procoagulant responses by controlling intravascular hemolysis and reversing NO depletion by free hemoglobin. Furthermore, eculizumab treatment resulted in a significant reduction in plasma TF-microparticles and also attenuated thrombin generation by modulating thrombin-antithrombin complexes [92]. Interestingly, the beneficial impact of anti-C5 therapy extended to the vascular endothelium, through the downregulation of key endothelial activation markers (e.g., tissue plasminogen activator, vascular cell adhesion molecule, von Willebrand factor). Given that the complement and coagulation cascades forge multiple interconnections that converge at common effectors, it would be worthwhile to investigate whether complement modulation at the level of C3 (e.g., using peptidic C3 inhibitors or AP regulators) could afford greater control of the thrombotic state in PNH patients by blocking procoagulant pathways that are regulated not only by downstream effectors (e.g., crosstalk of the C5a/C5aR axis with the TF-dependent procoagulant pathway), but also by C3 and its bioactive fragments (e.g., C3b-CR1 or iC3b/CR3-dependent, leukocyte-mediated platelet aggregation) [60]. Given the emergence of inflammation of the vascular endothelium as a potentially distinct mechanism of PNH thrombosis, C3 interception may afford greater therapeutic benefit by blunting procoagulant responses that are triggered by the direct interaction of C3 with activated endothelial cells and components of the fibrinolytic pathway. Notably, C3 inhibition by peptidic inhibitors of the compstatin family has already shown promise as a means of intercepting procoagulant responses that exacerbate pathology and has led to the restoration of the anticoagulant nature of the vascular endothelium in various non-human primate models of inflammatory diseases [50,92,93]. Collectively, these studies have paved the way for novel C3-targeted therapeutics that may prove even more effective than eculizumab in treating PNH, by intercepting procoagulant pathways that act independently of intravascular hemolysis and NO depletion.
Insights from gene profiling studies & classification of PNH therapy responders
Recent evidence suggests that patients’ responses to complement-targeted therapies may be more complex and unpredictable than expected, particularly in light of the emerging genetic variation and discrete complement haplotype landscape revealed across different populations. PNH exemplifies such a complement-driven chronic disease whose treatment landscape appears to be affected by genetic factors underlying key pathogenetic mechanisms that lead to intravascular hemolysis and thromboembolic complications. Undeniably, anti-C5 therapy has drastically improved the treatment landscape of PNH; however, several patient cases have emerged worldwide that are associated with suboptimal or poor responses to eculizumab treatment that keep these patients blood transfusion-dependent. Recent studies have elegantly revealed the genetic basis for this variable response to eculizumab, demonstrating that certain polymorphic variations in complement genes (i.e., C5 and CR1) render the carriers refractory or only mildly responsive to anti-C5 therapy [35,36]. The identification of a missense C5 heterozygous mutation (c.2654G-A) within a Japanese cohort of patients receiving eculizumab predicted the polymorphism Arg885His in the native C5 protein, leading researchers to unravel the molecular basis for the poor response to anti-C5 therapy. Despite its normal in vitro hemolytic activity, this mutant C5 did not bind eculizumab, explaining the non-responders’ clinical phenotype. Similarly, a recent study identified a genetic variation in the complement receptor 1 (CR1) locus that also skews therapeutic responses to eculizumab [36]. Two co-dominant alleles have been identified within the CR1 locus, with the H allele associated with high expression of CR1 on erythrocytes and the L allele with low expression. Based on CR1 genotyping among PNH patients, it was concluded that carriers of the (L/L) CR1 genotype are seven-times more likely to be suboptimal responders to eculizumab [36].
These studies have shed light on previously elusive aspects of complement-targeted therapeutics and helped researchers/clinicians reconcile poor clinical responses with genetic variation profiles. In the case of eculizumab, these studies have also prompted the consideration of upstream intervention strategies (e.g., C3 inhibition) for circumventing these genetic hurdles and improving the clinical management of PNH patients. Given that most of the complement genes are polymorphic, harboring variants of unknown clinical significance within the population, it is reasonable to speculate that, in order to elicit full therapeutic efficacy, complement-targeted therapeutics will have to be developed in close conjunction with each patient’s individual genetic repertoire and tailored to the patient’s complement haplotype.
Concluding remarks & outlook
It has long been recognized that the detrimental sequelae of complement dysregulation on the PNH erythrocyte membrane are tightly intertwined with the main clinical manifestations of PNH. Complement-triggered pathways extend key interactions with both humoral and cellular effectors of the activated vascular endothelium and thus contribute to the pathogenesis of venous thrombosis and intravascular hemolysis, leading to chronic anemia and deteriorated quality of life in PNH patients. However, only recently has the treatment landscape of PNH changed, primarily owing to the introduction of the first complement-targeted therapeutic agent, the anti-C5 mAb eculizumab (Soliris). PNH treatment and longitudinal patient management in the era of eculizumab have been drastically improved; however, emerging genetic and epigenetic risk factors have raised some concern regarding the variable efficacy of this therapy and perhaps pointing to alternative complement therapeutics that might improve the treatment landscape, circumventing caveats associated with poor therapeutic responses to eculizumab. Indeed, C3-mediated extravascular hemolysis is emerging as an important pathogenetic mechanism of PNH that is clinically unmasked in patients receiving anti-C5 therapy. This unmasking occurs largely because PNH erythrocytes, escaping MAC-mediated lysis by eculizumab, survive long enough to become heavily decorated with C3-derived opsonic fragments that promote their extravascular immune clearance by phagocytic cells in the liver and spleen. In order to address this problem, research efforts have been directed toward developing a new generation of C3-targeted therapeutics that can effectively abrogate both intravascular and extravascular hemolysis in PNH patients. Significant progress has been made in the preclinical stage by studies focusing on the therapeutic potential of AP inhibitory fusion proteins and a new generation of peptidic C3 inhibitors (e.g., based on the compstatin analog Cp40) that show great promise as alternative PNH therapeutics. Indeed, Cp40 and the Cp40-based therapeutic AMY-101 have been evaluated in non-human primate models of disease and have shown evidence of safety, favorable pharmacokinetic properties and sustained inhibition profiles with sc. administration routes that are highly relevant to affordable, manageable PNH treatment. The strong potential of such small-sized C3 inhibitors to abrogate both intravascular and extravascular hemolysis has been successfully tested in models of PNH pathology, with clear promise for clinical development and translation into the clinic. The comparatively low production costs currently incurred with therapeutic peptide synthesis [80] also point to the introduction of less expensive therapies for PNH patients. Along with these novel C3-based peptidic inhibitors, a fascinating lineup of new C3-and C5-targeted complement therapeutics is being built up, encompassing elegant strategies to block AP activation on erythrocyte surfaces by targeting AP regulators and fusion proteins to the C3b-opsonized surface. It remains to be seen whether these rapidly developing, new anti-complement agents will be added to the arsenal of PNH therapeutics, with more effective results projected for patients. The consolidation of these preclinical complement interception strategies with appropriate pharmacogenomic studies and insights from the expanding genetic landscape of complement-related genes is anticipated to pave the way for the design of more effective PNH therapies tailored to the patient’s own genetic haplotype and inherent response profile.
Expert commentary
Therapy for PNH has been dramatically improved by the introduction of the anti-C5 antibody eculizumab (Soliris), which has demonstrated strong efficacy in treating the majority of PNH patients and possibly changing the natural history of the disease. However, unmet clinical needs are emerging even in the era of anti-C5 treatment, especially concerns about possible residual anemia that can occur through C3-mediated extra-vascular hemolysis. Thus, investigators are currently focusing on developing alternative strategies for complement inhibition that may improve the current results of anti-C5 treatment.
Five-year view
Complement inhibition remains the most effective treatment strategy in PNH, even if recent studies have demonstrated that novel concepts are needed to circumvent the pitfalls of current anti-C5 treatment. Several strategies, which employ distinct candidate agents acting at different levels of the complement cascade, are currently in preclinical development. For some of them, preclinical data have suggested superiority over anti-C5 treatment in the absence of increased toxicity, even if data establishing their possible clinical benefit remain to be collected. However, translational efforts have already begun, and the authors anticipate that in the near future, several novel strategies encompassing complement inhibitors will be tested in clinical trials, potentially improving the treatment of PNH as well as other complement-mediated diseases.
Key issues.
Complement dysregulation on paroxysmal nocturnal hemoglobinuria (PNH) erythrocytes is driven by deficiency in glycophosphatidylinositol-linked CD55 and CD59 and is tightly intertwined with intravascular hemolysis, the main clinical manifestation of PNH.
Anti-C5 antibody therapy (eculizumab, Soliris®, Alexion Pharmaceuticals) has drastically changed the treatment landscape, offering PNH patients an effective therapeutic ‘handle’ for controlling intravascular hemolysis and its ensuing thrombotic complications.
Genetic variations in complement genes (e.g., CR1, C5) have emerged as therapy-modifying factors and partly explain poor clinical responses of a subset of PNH patients to eculizumab therapy.
Residual anemia, presumably due to C3-mediated extravascular hemolysis of surviving PNH erythrocytes, has emerged as a common clinical manifestation during eculizumab treatment, eventually limiting the hematological benefit of this treatment.
A broad spectrum of new C5- and C3-targeted therapeutic strategies aimed at improving response rate, administration options and/or treatment costs has been developed, several of which are currently being evaluated in preclinical models or in Phase I clinical trials.
Targeted modulation of complement activity at the C3 level emerges as an attractive alternative for effective therapeutic intervention in PNH that offers advantages over inhibition of the lytic terminal pathway (C5 level) alone.
Engineered regulators of the C3 convertase and small synthetic C3 inhibitors have been shown to abrogate opsonization of PNH erythrocytes and downstream formation of lytic membrane attack complex, thereby preventing both intravascular and extravascular hemolysis.
Peptidic C3 inhibitors of the compstatin family (e.g., Cp40 and the Cp40-based therapeutic AMY-101) display favorable pharmacokinetic properties, safety and inhibition profiles, thus showing great promise as potent and potentially cost-effective therapeutics for PNH.
Acknowledgments
The authors would like to thank D McClellan for excellent editorial assistance, paid for by the authors. This work was supported by National Institutes of Health grants AI068730, AI030040, EY020633 and AI097805, a pilot grant from the Penn-CHOP Blood Center for Patient Care and Discovery, and funding from the European Union’s Seventh Framework Programme under grant agreement no. 602699 (DIREKT).
Footnotes
Financial & competing interests disclosure
JD Lambris and D Ricklin are inventors of patent and/or patent applications that describe the use of complement inhibitors for therapeutic purposes. JD Lambris is also the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. AM Risitano acknowledges past research funding from Alexion Pharmaceuticals and consultancy with Alnylam and RA Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI. anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73(4):703–11. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 2.Miyata T, Takeda J, Iida Y, et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259(5099):1318–20. doi: 10.1126/science.7680492. [DOI] [PubMed] [Google Scholar]
- 3.Luzzatto L, Bessler M, Rotoli B. Somatic mutations in paroxysmal nocturnal hemoglobinuria: a blessing in disguise? Cell. 1997;88(1):1–4. doi: 10.1016/s0092-8674(00)81850-4. [DOI] [PubMed] [Google Scholar]
- 4.Krawitz PM, Hochsmann B, Murakami Y, et al. A case of paroxysmal nocturnal hemoglobinuria caused by a germline mutation and a somatic mutation in PIGT. Blood. 2013;122(7):1312–15. doi: 10.1182/blood-2013-01-481499. [DOI] [PubMed] [Google Scholar]
- 5.Karadimitris A, Manavalan JS, Thaler HT, et al. Abnormal T-cell repertoire is consistent with immune process underlying the pathogenesis of paroxysmal nocturnal hemoglobinuria. Blood. 2000;96(7):2613–20. [PubMed] [Google Scholar]
- 6.Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Adv Exp Med Biol. 2013;735:155–72. doi: 10.1007/978-1-4614-4118-2_10. [DOI] [PubMed] [Google Scholar]
- 7.Risitano AM. Paroxysmal nocturnal hemoglobinuria and other complement-mediated hematological disorders. Immunobiology. 2012;217(11):1080–7. doi: 10.1016/j.imbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Medof ME, Gottlieb A, Kinoshita T, et al. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987;80(1):165–74. doi: 10.1172/JCI113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risitano AM. Anti-Complement Treatment in Paroxysmal Nocturnal Hemoglobinuria: where we Stand and Where we are Going. Transl Med UniSa. 2014;8:43–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Rotoli B, Luzzatto L. Paroxysmal nocturnal haemoglobinuria. Baillieres Clin Haematol. 1989;2(1):113–38. doi: 10.1016/s0950-3536(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 11.Risitano AM, Rotoli B. Paroxysmal nocturnal hemoglobinuria: pathophysiology, natural history and treatment options in the era of biological agents. Biologics. 2008;2(2):205–22. doi: 10.2147/btt.s1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill A, Richards SJ, Hillmen P. Recent developments in the understanding and management of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2007;137(3):181–92. doi: 10.1111/j.1365-2141.2007.06554.x. [DOI] [PubMed] [Google Scholar]
- 13.Inoue N, Murakami Y, Kinoshita T. Molecular genetics of paroxysmal nocturnal hemoglobinuria. Int J Hematol. 2003;77(2):107–12. doi: 10.1007/BF02983208. [DOI] [PubMed] [Google Scholar]
- 14.Araten DJ, Nafa K, Pakdeesuwan K, Luzzatto L. Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria genotype and phenotype are present in normal individuals. Proc Natl Acad Sci USA. 1999;96(9):5209–14. doi: 10.1073/pnas.96.9.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasinski M, Keller P, Fujiwara Y, et al. GATA1-Cre mediates Piga gene inactivation in the erythroid/megakaryocytic lineage and leads to circulating red cells with a partial deficiency in glycosyl phosphatidylinositol-linked proteins (paroxysmal nocturnal hemoglobinuria type II cells) Blood. 2001;98(7):2248–55. doi: 10.1182/blood.v98.7.2248. [DOI] [PubMed] [Google Scholar]
- 16.Araten DJ, Bessler M, McKenzie S, et al. Dynamics of hematopoiesis in paroxysmal nocturnal hemoglobinuria (PNH): no evidence for intrinsic growth advantage of PNH clones. Leukemia. 2002;16(11):2243–8. doi: 10.1038/sj.leu.2402694. [DOI] [PubMed] [Google Scholar]
- 17.Inoue N, Izui-Sarumar T, Murakami Y, et al. Molecular basis of clonal expansion of hematopoiesis in 2 patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2006;108(13):4232–6. doi: 10.1182/blood-2006-05-025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen W, Makishima H, Clemente M, et al. Hierarchical clonal architecture. in paroxysmal nocturnal hemoglobinuria: stepwise acquisition of mutations. J Clin Invest. 2014 doi: 10.1172/JCI74747. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young NS, Maciejewski JP. Genetic and environmental effects in paroxysmal nocturnal hemoglobinuria: this little PIG-A goes “Why? Why? Why?”. J Clin Invest. 2000;106(5):637–41. doi: 10.1172/JCI11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gargiulo L, Lastraioli S, Cerruti G, et al. Highly homologous T-cell receptor beta sequences support a common target for autoreactive T cells in most patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;109(11):5036–42. doi: 10.1182/blood-2006-10-052381. [DOI] [PubMed] [Google Scholar]
- 21•.Gargiulo L, Papaioannou M, Sica M, et al. Glycosylphosphatidylinositol-specific, CD1d-restricted T cells in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(14):2753–61. doi: 10.1182/blood-2012-11-469353. This study supports the (auto)immune-mediated expansion of glycophosphatidylinositol-deficient hematopoietic stem cells, providing evidence for the presence of an autoreactive T-cell compartment that specifically targets the glycophosphatidylinositol anchor in bone marrow hematopoietic stem cells of paroxysmal nocturnal hemoglobinuria (PNH) patients. [DOI] [PubMed] [Google Scholar]
- 22.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luzzatto L, Gianfaldoni G, Notaro R. Management of paroxysmal nocturnal haemoglobinuria: a personal view. Br J Haematol. 2011;153(6):709–20. doi: 10.1111/j.1365-2141.2011.08690.x. [DOI] [PubMed] [Google Scholar]
- 24.Luzzatto L, Risitano AM, Notaro R. Paroxysmal nocturnal hemoglobinuria and eculizumab. Haematologica. 2010;95(4):523–6. doi: 10.3324/haematol.2009.017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–96. doi: 10.1182/blood-2012-09-311381. [DOI] [PubMed] [Google Scholar]
- 26.Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–8. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 27.Socie G, Mary JY, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology Lancet. 1996;348(9027):573–7. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 28•.de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112(8):3099–106. doi: 10.1182/blood-2008-01-133918. This study serves as the most updated description of the natural history of PNH. [DOI] [PubMed] [Google Scholar]
- 29.Moyo VM, Mukhina GL, Garrett ES, Brodsky RA. Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126(1):133–8. doi: 10.1111/j.1365-2141.2004.04992.x. [DOI] [PubMed] [Google Scholar]
- 30.Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2003;102(10):3587–91. doi: 10.1182/blood-2003-01-0009. [DOI] [PubMed] [Google Scholar]
- 31.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–43. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 32.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–7. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 33.Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–8. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 34.Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–92. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 35•.Nishimura J, Yamamoto M, Hayashi S, et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370(7):632–9. doi: 10.1056/NEJMoa1311084. This study provides evidence for a genetic basis underlying the refractory phenotype of a subset of PNH patients on eculizumab treatment. [DOI] [PubMed] [Google Scholar]
- 36.Rondelli T, Risitano AM, Peffault de Latour R, et al. Polymorphism of the complement receptor 1 gene correlates with the hematologic response to eculizumab in patients with paroxysmal nocturnal hemoglobinuria. Haematologica. 2014;99(2):262–6. doi: 10.3324/haematol.2013.090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113(17):4094–100. doi: 10.1182/blood-2008-11-189944. This study is the first report to demonstrate C3 opsonization of surviving PNH erythrocytes, implicating C3-mediated extravascular hemolysis as an emerging pathological mechanism in patients receiving eculizumab. [DOI] [PubMed] [Google Scholar]
- 38.Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95(4):567–73. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricklin D, Lin Z, Schmidt CQ, et al. Towards a model of complement-mediated extravascular hemolysis of red blood cells in PNH patients under standard treatment: implications for novel therapeutic options. Presented at 11th International Conference on Innate Immunity, Aegean Conferences; Aldemar Olympian Village; Olympia, Greece. 2014. [Google Scholar]
- 40.Peffault de Latour R, Schrezenmeier H, Bacigalupo A, et al. Allogeneic stem cell transplantation in paroxysmal nocturnal hemoglobinuria. Haematologica. 2012;97(11):1666–73. doi: 10.3324/haematol.2012.062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brodsky RA, Luznik L, Bolanos-Meade J, et al. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42(8):523–7. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113(26):6522–7. doi: 10.1182/blood-2009-03-195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risitano AM, Notaro R, Luzzatto L, et al. Paroxysmal nocturnal hemoglobinuria –hemolysis before and after eculizumab. N Engl J Med. 2010;363(23):2270–2. doi: 10.1056/NEJMc1010351. [DOI] [PubMed] [Google Scholar]
- 44.Zuber J, Fakhouri F, Roumenina LT, et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8(11):643–57. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 45.Qu H, Ricklin D, Bai H, et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218(4):496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Risitano AM, Notaro R, Pascariello C, et al. The complement receptor 2/factor H fusion protein TT30 protects paroxysmal nocturnal hemoglobinuria erythrocytes from complement-mediated hemolysis and C3 fragment. Blood. 2012;119(26):6307–16. doi: 10.1182/blood-2011-12-398792. This study presents for the first time the therapeutic potential of a surface-targeted complement AP regulator for abrogating both intravascular and extravascular hemolysis in a preclinical model of PNH. [DOI] [PubMed] [Google Scholar]
- 47••.Schmidt CQ, Bai H, Lin Z, et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J Immunol. 2013;190(11):5712–21. doi: 10.4049/jimmunol.1203548. This study presents a potentially more effective AP inhibition strategy for treating PNH, based on a miniaturized version of a complement AP regulator that unmasks broader binding specificity over a large spectrum of complement opsonins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Risitano AM, Ricklin D, Huang Y, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123(13):2094–101. doi: 10.1182/blood-2013-11-536573. This study presents important preclinical findings on the promising therapeutic profile of a novel C3 peptidic inhibitor that abrogates PNH hemolysis, displays favorable pharmacokinetic properties in a NHP model and a sustained inhibitory profile suitable for PNH treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourtzelis I, Markiewski MM, Doumas M, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116(4):631–9. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silasi-Mansat R, Zhu H, Popescu NI, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116(6):1002–10. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholson-Weller A, March JP, Rosenfeld SI, Austen KF. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci USA. 1983;80(16):5066–70. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holguin MH, Fredrick LR, Bernshaw NJ, et al. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meri S, Morgan BP, Davies A, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 55.Pangburn MK, Muller-Eberhard HJ. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann N Y Acad Sci. 1983;421:291–8. doi: 10.1111/j.1749-6632.1983.tb18116.x. [DOI] [PubMed] [Google Scholar]
- 56.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci USA. 1983;80(17):5430–4. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamad OA, Nilsson PH, Wouters D, et al. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J Immunol. 2010;184(5):2686–92. doi: 10.4049/jimmunol.0902810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamad OA, Ekdahl KN, Nilsson PH, et al. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J Thromb Haemost. 2008;6(8):1413–21. doi: 10.1111/j.1538-7836.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markiewski MM, Nilsson B, Ekdahl KN, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28(4):184–92. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Hamad OA, Back J, Nilsson PH, et al. Platelets, complement, and contact activation: partners in inflammation and thrombosis. Adv Exp Med Biol. 2012;946:185–205. doi: 10.1007/978-1-4614-0106-3_11. [DOI] [PubMed] [Google Scholar]
- 61.Amara U, Flierl MA, Rittirsch D, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185(9):5628–36. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177(7):4794–802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 63.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190(8):3831–8. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256–64. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 65•.Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet. 2009;373(9665):759–67. doi: 10.1016/S0140-6736(09)60001-5. This study presents a comprehensive overview of the clinical development of eculizumab as a PNH therapeutic, discussing the major clinical benefits and limitations of anti-C5 therapy in PNH. [DOI] [PubMed] [Google Scholar]
- 66.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190(8):3839–47. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Adv Exp Med Biol. 2013;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. This study provides a comprehensive and up-to-date overview of the various complement-targeted therapeutic approaches being developed for treating complement-mediated diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reis S, Falcao DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol. 2006;63(3):155–68. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 70.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25(11):1265–75. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matis LA, Rollins SA. Complement-specific antibodies: designing novel anti-inflammatories. Nat Med. 1995;1(8):839–42. doi: 10.1038/nm0895-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song H, He C, Knaak C, et al. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J Clin Invest. 2003;111(12):1875–85. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nunn MA, Sharma A, Paesen GC, et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174(4):2084–91. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- 74.Roversi P, Lissina O, Johnson S, et al. The structure of OMCI, a novel lipocalin inhibitor of the complement system. J Mol Biol. 2007;369(3):784–93. doi: 10.1016/j.jmb.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barratt-Due A, Thorgersen EB, Lindstad JK, et al. Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans. J Immunol. 2011;187(9):4913–19. doi: 10.4049/jimmunol.1101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weston-Davies WH, Westwood JP, Nunn M, et al. Phase I Clinical Trial of Coversin, a Novel Complement C5 and LTB4 Inhibitor. Presented at 7th International Conference on Complement Therapeutics, Aegean Conferences; Aldemar Olympian Village; Olympia, Greece. 2014. [Google Scholar]
- 77.Feldwisch J, Tolmachev V. Engineering of affibody molecules for therapy and diagnostics. Methods Mol Biol. 2012;899:103–26. doi: 10.1007/978-1-61779-921-1_7. [DOI] [PubMed] [Google Scholar]
- 78.Strömberg P. Introducing SOBI002, a small Affibody-ABD fusion protein targeting complement component C5. Presented at 7th International Conference of Complement Therapeutics, Aegean Conferences; Aldemar Olympian Village; Olympia, Greece. 2014. [Google Scholar]
- 79.Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. BMJ. 2012;12:345. doi: 10.1136/bmj.e8346. [DOI] [PubMed] [Google Scholar]
- 80.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010;15(1–2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 81••.Lindorfer MA, Pawluczkowycz AW, Peek EM, et al. A novel approach to preventing the hemolysis of paroxysmal nocturnal hemoglobinuria: both complement-mediated cytolysis and C3 deposition are blocked by a monoclonal antibody specific for the alternative pathway of complement. Blood. 2010;115(11):2283–91. doi: 10.1182/blood-2009-09-244285. This study presents important findings supporting the dual therapeutic merit of an anti-C3 mAb approach targeting both MAC-mediated hemolysis and C3 opsonization on PNH erythrocytes. [DOI] [PubMed] [Google Scholar]
- 82.Paixao-Cavalcante D, Torreira E, Lindorfer MA, et al. A humanized antibody that regulates the alternative pathway convertase: potential for therapy of renal disease associated with nephritic factors. J Immunol. 2014;192(10):4844–51. doi: 10.4049/jimmunol.1303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whaley K, Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976;144(5):1147–63. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferreira VP, Pangburn MK. Factor H mediated cell surface protection from complement is critical for the survival of PNH erythrocytes. Blood. 2007;110(6):2190–2. doi: 10.1182/blood-2007-04-083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Safety and Pharmacokinetics of TT30 in Subjects With Paroxysmal Nocturnal Hemoglobinuria (PNH) Available from: http://clinicaltrials.gov/ct2/show/NCT01335165.
- 86.Maekawa T, Abe T, Hajishengallis E, et al. Genetic and intervention studies implicating complement c3 as a major target for the treatment of periodontitis. J Immunol. 2014;192(12):6020–7. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5(1):113–28. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 88.McDonnell T, Ioannou Y, Rahman A. PEGylated drugs in rheumatology–why develop them and do they work? Rheumatology (Oxford) 2014;53(3):391–6. doi: 10.1093/rheumatology/ket278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaudlay P, Hua H, He G, et al. Red cell complement loading in PNH patients on eculizumab is associated with a C3 polymorphism which influences C3 function, predicts for increased extravascular hemolysis and provides a rationale for C3 inhibition. Presented at Annual Meeting of the American Society of Hematology; New Orleans, LA, USA. 2013. [Google Scholar]
- 90.Helley D, de Latour RP, Porcher R, et al. Evaluation of hemostasis and endothelial function in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Haematologica. 2010;95(4):574–81. doi: 10.3324/haematol.2009.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weitz IC, Razavi P, Rochanda L, et al. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb Res. 2012;130(3):361–8. doi: 10.1016/j.thromres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 92.Lupu F, Keshari RS, Lambris JD, et al. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133(Suppl 1):S28–31. doi: 10.1016/j.thromres.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keshari RS, Silasi-Mansat R, Zhu H, et al. Acute Lung Injury and Fibrosis in a Baboon Model of Escherichia coli Sepsis. Am J Respir Cell Mol Biol. 2014;50(2):439–50. doi: 10.1165/rcmb.2013-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]