Abstract
Xenotransplantation ( xeno-Tx) is considered as an alternative solution to overcome the shortage of human donor organs. However, the success of xeno-Tx is hindered by immune reactions against xenogeneic cells (e. g. of porcine origin). More specifically, activation of innate immune mechanisms such as complement and triggering of the coagulation cascade occur shortly after xeno-Tx, and adhesion of human leukocytes to porcine endothelium is another early critical step mediating the immune attack. To investigate the therapeutic potential of complement inhibition in the context of xenogeneic interactions, we have employed a whole-blood model in the present study. Incubation of human blood with porcine endothelial cells (PAECs) led to activation of complement and coagulation as well as to increased leukocyte adhesion. The observed responses can be attributed to the pig-to-human xenogeneicity, since the presence of human endothelium induced a minor cellular and plasmatic inflammatory response. Importantly, complement inhibition using a potent complement C3 inhibitor, compstatin analogue Cp40, abrogated the adhesion of leukocytes and, more specifically, the attachment of neutrophils to porcine endothelium. Moreover, Cp40 inhibited the activation of PAECs and leukocytes, since the levels of the adhesion molecules E-selectin, ICAM-1, ICAM-2, and VCAM-1 on PAECs and the surface expression of integrin CD11b on neutrophils were significantly decreased. Along the same line, inhibition of CD11b resulted in decreased leukocyte adhesion. Taken together, our findings provide a better understanding of the mechanisms regulating the acute innate immune complications in the context of xeno-Tx and could pave the way for complement-targeting therapeutic interventions.
Keywords: complement C3, xenotransplantation, leukocyte adhesion, compstatin
Introduction
The transplantation of non-human organs to human recipients (xeno-transplantation; xeno- Tx) is regarded as an alternative approach for bridging the gap between the increasing number of patients with end-stage organ failure waiting for transplantation and the limited availability of grafts [1]. Pig-derived xenografts, such as isolated islets of Langerhans, can be suitable because of the anatomical and physiological similarities they share with human organs [2, 3].
Nevertheless, several complications of porcine organ transplantation have been described, principally the hyperacute rejection (HAR), which occurs shortly after xeno-Tx and induces vigorous innate immune responses that can result in rapid graft loss. HAR is largely mediated by the binding of naturally occurring antibodies (nAbs) of human recipients against the carbohydrate galactose-α(l-3)-galactose [Galα(l-3)Gal] epitope on the porcine endothelium, with subsequent complement activation [4, 5].
Xeno-Tx of islets is associated with rapid innate immune reactions taking place intravascularly as a result of the exposure of porcine islets to human blood. These reactions include a series of thrombo-inflammatory processes termed Immediate Blood-Mediated Inflammatory Reaction (IBMIR). More specifically, activation of complement and coagulation cascades together with platelet activation and binding thereof to the islet surface, as well as adhesion of leukocytes to the graft orchestrate IBMIR resulting in disruption of islet integrity and massive islet loss [6–8].
The complement system can be activated through 3 principal routes: The classical pathway (CP), induced by the binding of complement-fixing antibodies, and the lectin pathway (LP), initiated by surface interactions of mannose-binding lectin or ficolins [9], converge at the CP/LP C3 convertase. The alternative pathway (AP) is constantly activated, albeit at a low rate. Nascent C3b is deposited on cell surfaces and induces the generation of AP C3 convertases. In the absence of sufficient regulation, this generates more C3b and amplifies the complement response, eventually leading to the formation of C5 convertases. Cleavage of C5 releases the pro-inflammatory anaphylatoxin C5a and initiates assembly of the membrane attack complex (C5b-9) on target cell surfaces. Interestingly, thrombin or other proteases can also directly activate C5 independent of C3 [10]. Membrane-bound and soluble complement regulatory proteins (CRPs) such as Factor H, CD46, CD55, and CD59 tightly control complement activation on healthy host cells [11].
Although the CP is the dominant initiator of complement activation in HAR, amplification of C3b generation by the AP-driven positive feedback loop is also critical [12]. The degree of involvement of the LP in xenograft rejection is less clear [13]. Moreover, the failure of complement regulation in the foreign organ also affects the overall complement activation. Of interest, CRPs have been reported to control the activation and the subsequent damage of the xenogeneic endothelium as a result of complement activation [14–17]. However, the crosstalk between complement and the endothelium is broad with complement components affecting endothelial cell functions related to inflammation or angiogenesis [18–21].
It is also evident that thrombotic complications are a major issue in xeno-Tx [22, 23]; especially they are considered a key element of IBMIR that is elicited upon islet xeno-Tx. Tissue factor is considered an initiator of xenograft thrombosis [22]. It is expressed on activated endothelial cells (ECs), monocytes, platelets, and microparticles; its binding to activated factor VII (VIIa) triggers thrombin generation and clot formation [24]. In addition, tissue factor is expressed by islets and its levels were found elevated after islet exposure to xenogeneic blood, thereby contributing to the effects of IBMIR [25].
The vascular endothelium of transplanted organs including islets makes up the interface between donor and recipient, and therefore it is the primary target of the immune response. Activation of the endothelium results in increased surface expression of cellular adhesion molecules (CAMs), thereby triggering the adhesion of recipients’ leukocytes to the donor endothelium [26–28]. Leukocyte adhesion to the porcine endothelium is key for the establishment and propagation of the host’s immune attack on the graft.
Whereas the role of complement has been investigated in the xeno-Tx setting, and a role for complement inhibition in the activation of xenogeneic endothelial cells has been described [29], its involvement in the early steps of leukocyte adhesion to the donor endothelium is less well studied. In this report, we utilized a relevant whole-blood model to demonstrate that complement inhibition regulates the xenogeneic adhesive interactions between human blood leukocytes and the porcine endothelium.
Materials and Methods
Endothelial cell cultures
Porcine aortic endothelial cells (PAECs) were obtained from PELOBiotech (PB-P304K-05) and were maintained in 0.2 % gelatin- treated culture plates in medium (PELOBiotech, P211–500) at 37 °C, in 5 % CO2. Human umbilical vein endothelial cells (HUVECs, Lonza; C2519A) were cultured with supplemented medium (PromoCell; C220–10, C39245). In all experiments, endothelial cell cultures were used between passages 2–5.
RNA extraction and quantitative real-time PCR analysis
Total RNA was isolated using the TRIzol reagent (Molecular Research Center Inc.) according to the manufacturer’s instructions. cDNA was synthesized from 2 μg of isolated RNA with the iScript Advanced cDNA synthesis kit (Biorad, CA, USA). Quantitative real-time PCR was performed in a Biorad CFX384 Real time system (Biorad) using the SsoFast EvaGreen Supermix (Biorad). The amplification conditions were: one cycle of denaturation at 95 °C (30 s), followed by 45 cycles of 5 s at 95 °C, 5 s at 60 °C, and 5 s at 70 °C. Melting curve analyses were performed to ensure the specificity of the qPCR products. The 2−ΔΔCT method was used to quantify the target gene expression. For each sample, the following primer sequences were used: porcine E-selectin (FWD: 5-TGCTCTCCCTTTGGTGCTTC-3, REV: 5-GGTTTCTGTAGAGGCGCTGT- 3); vascular cell adhesion protein 1 porcine (VCAM-1) (FWD: 5-TGCGGGAAATTGAAAGGGGA-3; REV: 5-ACTGGCTTCCCAACTTCTGG-3), intercellular adhesion molecule 1 porcine (ICAM-1) (FWD: 5-ACTTATGTCCTGCCAGCCAC-3, REV: 5-GTTCACAGAAACGGGTGTGC-3); intercellular adhesion molecule 2 porcine (ICAM-2) (FWD: 5-CCTGGGGACTGTTCATGGC- 3, REV: 5-GGTTTCTCGAACGCCTCCTT-3); and glyceraldehyde 3-phosphate dehydrogenase porcine (GAPDH) (FWD: 5-ACATGGCCTCCAAGGAGTAAGA-3, REV: 5-GATCGAGTTGGGGCTGTGACT- 3).
Treatment of blood with inhibitors
The potent peptidic C3 inhibitor Cp40 (dTyr-Ile-[Cys-Val- Trp(Me)-Gln-Asp-Trp-Sar-His-Arg-Cys]-mIle-NH2) [30, 31], a compstatin analogue, was used to block complement activation. For the inhibition of C5a receptor (C5aR), the cyclic hexapeptide PMX53 (AcF-[OPdChaWR]) [32], or its inactive control, AcdChaP-WFRO- NH2 (both synthesized in-house), were used. Blocking of integrin Mac-1 was performed with an anti-human antibody against CD11b (Biolegend, 301312, clone ICRF44) or the appropriate mouse IgG1 isotype control (Biolegend, 400124). Blood samples were pretreated for 10 min with either 20 μM Cp40, 10 μM PMX53, or the inactive control for PMX53, 10 μg/ml anti- CD11b, or its isotype control prior to incubation with PAECs.
In vitro whole-blood model
Blood samples were obtained from healthy donors (n = 6) in heparin- washed syringes. Samples pretreated with either inhibitors or their respective controls (1 part blood/1 part culture medium) were then added to confluent EC monolayers (in 12- or 24-well plates) and incubated for 2 h at 37 °C with shaking. After the incubation, blood was collected for the assessment of neutrophil activation and for plasma isolation. Wells were washed once with PBS, and adherent cells were trypsinized. Afterwards, lysis of red blood cells was performed, and the cell suspensions were either stained with a mouse anti-human CD45 antibody (BD Pharmigen, 555483) or used for RNA isolation. The relative adhesion of blood leukocytes to the PAEC monolayer was measured by FACS and is presented as the ratio of CD45+ leukocytes to CD45− PAECs. Adherent neutrophil and monocyte subpopulations were identified according to their FSC/SSC characteristics and CD14+ staining, respectively. Activation of neutrophils was determined by assessing the presence of CD11b using flow cytometry. In brief, lysis of red blood cells was performed, and leukocytes were stained with a mouse anti-human antibody against CD11b (BD Pharmigen, 558123). Results are given as mean fluorescence intensity (MFI).
Measurement of activation of complement and coagulation pathways
Levels of complement activation were examined by measuring the soluble terminal complement complex (TCC) in EDTA-treated samples (Hycult, HK328). Formation of thrombin-antithrombin (TAT) complexes was used to assess the activation of the coagulation cascade (American Diagnostica, ADG833).
Statistical analysis
Data are presented as means ± SE of the means. Paired t-tests or Wilcoxon matched-pairs tests were used to assess statistical significance between the analyzed groups. All statistical analyses were performed with GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA), and values of p ≤ 0.05 were considered significant.
Results
Porcine endothelium induces activation of complement and coagulation cascades in a whole-blood model
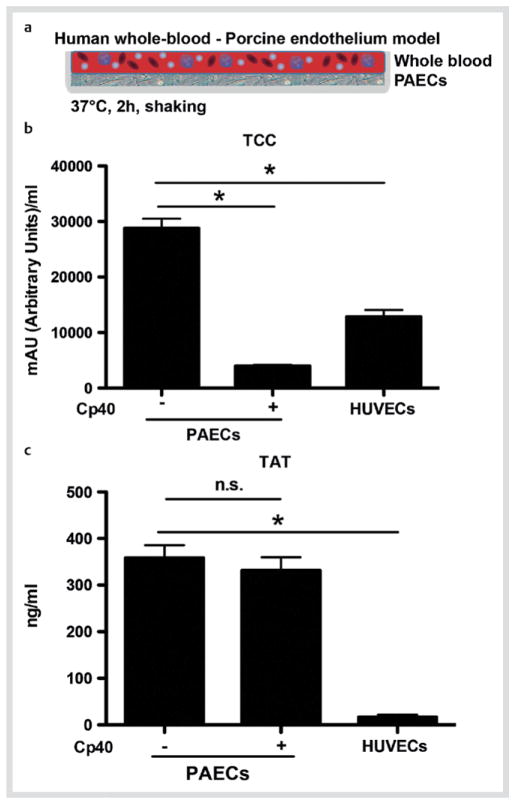
The recognition of porcine xenografts and their immune attack by the host via complement are critical initial steps that shape the subsequent host immune responses [12]. We therefore developed a whole-blood model in order to study the possible effect of complement on the interactions taking place between the leukocytes of the recipient and the porcine endothelium of the donor (Fig. 1a). Incubation of human blood with a confluent monolayer of PAECs induced the formation of both TCC and TAT complexes (as a measure of activation of complement and coagulation pathways in isolated plasma, respectively) (Fig. 1b, c). The observed induction of TCC and TAT formation in blood that was co-cultured with PAECs was much higher than that in blood incubated with human endothelial cells (HUVECs). These data indicate that the elicited responses were specific to the xenogeneic human-to-pig response. In agreement with previous reports [33], the PAEC-induced activation of complement was suppressed when the blood was pretreated with the compstatin analogue Cp40 (Fig. 1b). Conversely, complement inhibition did not affect the activation of coagulation (Fig. 1c).
Fig. 1.
Activation of complement and coagulation pathways in response to the xenogeneic pig-to-human interaction. a Depiction of the xenogeneic model, where human whole blood is incubated with a confluent PAEC monolayer for 2 h at 37 °C under shaking. b Levels of soluble TCC and c amounts of TAT complexes in plasma isolated from blood incubated with confluent monolayers of either HUVECs or PAECs. The incubation on PAECs was performed in the presence or absence of the complement inhibitor Cp40 (20 μM). The soluble TCC levels or the TAT complexes were measured by ELISA (n = 6). * p < 0.05; paired t-test. (Color figure available online only).
Xenogeneic induction of human leukocyte adhesion to PAECs can be efficiently reduced by inhibiting C3 cleavage
Given the observed complement activation upon incubation of human blood with PAECs and based on the fact that leukocyte activation and adhesion to the donor organ is a key component of IBMIR that subsequently leads to graft rejection, we assessed the effect of complement inhibition on cell adhesive events. In our model, exposure of blood to the xenogeneic porcine endothelial cell layer resulted in profound adhesion of leukocytes, as shown by the increased ratio of CD45+ to CD45− endothelial cells; in contrast, the presence of human ECs had a minimal effect on leukocyte adhesion (Fig. 2a). Notably, blocking of complement activation at the C3 level with Cp40 effectively decreased the adhesion of leukocytes to the PAEC monolayer (Fig. 2b).
Fig. 2.
Human leukocytes adhere to PAECs in a complement-dependent manner. a, b Relative blood leukocyte adhesion to a HUVECs or PAECs (n = 3) and b to PAECs in the presence or absence of Cp40 (20 μM) (n = 6). In a, data are shown as % of control; leukocyte adhesion to HUVEC was set as the 100 % control. In b, data are shown as % of control; leukocyte adhesion to PAEC in the absence of inhibitor (−) was set as the 100 % control. c, d Adhesion of c neutrophils and d monocytes to the PAEC monolayer in the presence or absence of Cp40 (n = 6). The results are presented as % of control. The relative adhesion to PAEC of c neutrophils or d monocytes in the absence of inhibitor (−) was set as the 100 % control. * p < 0.05; paired t-test.
Neutrophil and monocyte leukocyte subpopulations are major players in xeno-Tx-associated immune responses including IBMIR, and both are affected by complement activation [12, 34, 35]. For this reason, we further analyzed the effect of complement inhibition specifically on the adhesion of neutrophils and monocytes to the porcine ECs. Flow cytometric analysis revealed that the percentages of neutrophils and monocytes that adhered to PAEC monolayer were clearly increased. However, Cp40 treatment exerted a preferential inhibitory effect that was restricted to neutrophil adhesion (Fig. 2c, d).
Complement activation mediates the upregulation of adhesion molecules in PAECs after exposure to human blood
Having shown that complement inhibition reduces the adhesion of human leukocytes to porcine EC, we next performed a gene expression analysis of adhesion molecules that are implicated in leukocyte-endothelial cell interactions. To this end, mRNA expression levels of the adhesion molecules ICAM-1, ICAM-2, VCAM-1, and E-selectin were measured in PAECs after incubation with blood. Interestingly, the expression of all the tested adhesion molecules was significantly elevated after the exposure of blood to PAECs, thus confirming the involvement of these molecules in the shaping of immune responses following xeno- Tx (Fig. 3a–d). Of particular note was the fact that the preincubation of blood with Cp40 showed that complement significantly contributes to the activation of PAECs, as the levels of ICAM-1, ICAM-2, VCAM-1, and E-selectin were all significantly decreased (Fig. 3a–d).
Fig. 3.
Complement-dependent expression of leukocyte adhesion molecules in PAECs. a–d The relative expression of a ICAM-1, b VCAM-1, c ICAM-2, and d E-selectin mRNA in PAECs obtained after incubation with human blood in the presence or absence of Cp40 (20 μM) was determined with qPCR (n = 6). The 2 −ΔΔCT method was used to quantify the target gene expression. Relative mRNA expression is shown as % of control. The mRNA expression of cells incubated in the absence of Cp40 (Cp40 –) was set as the 100 % control. * p < 0.05; Wilcoxon matched-pairs test.
Blockade of Mac-1 integrin attenuates the leukocyte adhesion to PAECs
C5a anaphylatoxin is generated during the complement activation process and plays a key role in immune cell recruitment and function in several inflammatory conditions [9, 36, 37]. Therefore, we hypothesized that complement activation and subsequent generation of this effector can have an impact on the leukocyte adhesion to porcine ECs. To test this hypothesis, we pretreated blood samples with a specific antagonist that blocks C5aR signaling. In our model, the inhibition of anaphylatoxin signaling did not interfere with the capacity of leukocytes to adhere to PAECs (Fig. 4a). We therefore continued to address the potential role of iC3b, which is also generated upon C3 cleavage. The C3 cleavage product iC3b interacts with the leukocyte integrin Mac-1 (also known as complement receptor 3, CR3), and this interaction participates in many inflammatory processes involving the activation of complement [9, 38–41]. For this reason, we evaluated the effect of inhibiting Mac-1 on leukocyte adhesion in our whole-blood assay. Interestingly, pretreatment of the blood with a blocking anti-CD11b Ab resulted in a partial inhibition of leukocyte adhesion to PAECs, as compared to samples incubated with an isotype control (Fig. 4b). The involvement of Mac-1 signaling in the complement-associated xenogeneic interactions was further supported by the finding that complement inhibition using the compstatin analogue Cp40 led to decreased surface expression of CD11b (the alpha integrin subunit in Mac-1) on neutrophils (Fig. 4c); indeed, CD11b surface expression is an established marker of neutrophil activation.
Fig. 4.
The effect of C5aR or Mac-1 antagonism on blood leukocyte adhesion to PAECs. a–c Relative leukocyte adhesion to the PAEC monolayer after pretreatment of blood with a the C5aR antagonist PMX-53 (10 μM) or its inactive control peptide (10 μM) (n = 6), b the anti-CD11b antibody or isotype control (10 μg/ml, n = 3). The adhesion of leukocytes is presented as % of control. Relative adhesion to PAECs in the absence of inhibitors or their respective controls (−) was set as the 100 % control. c The effect of Cp40 on neutrophil activation after incubation of blood with PAECs. Cell activation was measured by the presence of the subunit CD11b of integrin Mac-1 on the surface of neutrophils obtained from blood incubated with PAECs, in the presence or absence of Cp40 (20 μM), using flow cytometry. The results are presented as ratios of the mean fluorescence intensity (MFI) of cells stained with an anti-CD11b antibody (n = 3). * p < 0.05; paired t-test.
Discussion and Conclusions
In order for the transplantation of pig organs or tissues, such as pancreatic islets, to humans to reach clinical practice, several immune-mediated limitations have to be overcome. In particular, adverse effects related to the activation of intravascular inflammation and IBMIR upon contact between human blood and the transplanted islets still remain the major problem [3, 12]. Complement has moved into the spotlight in biocompatibility research in a variety of clinical applications, ranging from extracorporeal circulation of blood [42–44] and implant surgery [45] to islet xeno-Tx [12]. Although this system presents a suitable target for therapeutic intervention, its role in leukocyte adhesion to the donor endothelium, as revealed by relevant whole-blood assays, has not been thoroughly investigated thus far.
In this study, we utilized a whole-blood model to explore the importance of complement inhibition in the xenogeneic interactions between leukocytes and pig endothelium occurring after Tx. Our model allows the exploration of the xenogeneic interactions in the presence of all the partners involved (leukocyte subsets and plasma), which we think offers substantial advantages over isolated systems that are based on purified neutrophils or monocytes stimulated with either isolated porcine serum or single inflammatory mediators [46–49]. Another whole-blood system has been used in the past, in which beads were coated with PAECs and then were exposed to human blood [50]. However, in this previous work, the authors focused on other aspects within the xenogeneic response rather than the cell adhesive interactions that were investigated in the present experimental setting. We have demonstrated that complement activation resulting from exposure of human blood to porcine endothelium regulates the adhesive events of leukocytes to the porcine endothelium. Of note, the inability of blood leukocytes to adhere to PAECs in the presence of the potent compstatin analogue Cp40 underscores the critical contribution of complement to the process of leukocyte adhesion. We also found that complement inhibition regulates the adhesion process not only quantitatively but also qualitatively, since treatment with Cp40 preferentially decreased neutrophil attachment. Neutrophils are the initiators of the acute inflammatory response, and they are capable of releasing inflammatory mediators and producing reactive oxygen species, thus fostering the invasion of additional inflammatory cells and propagating the immune response [51–53]. The deleterious role of neutrophils is also established in IBMIR following islet xeno-Tx [54]. Moreover, we showed that exposure of human blood to PAECs caused a profound increase in the expression levels of the adhesion molecules E-selectin, ICAM-1 and 2, and VCAM-1, which was dependent on the activation of complement. Leukocyte recruitment from the vasculature to the islet xenografts dramatically affects the outcome of the Tx [4]. The rolling and adhesion of recipients’ leukocytes to porcine endothelium are crucial in this multi-step process and are mediated by cross-species interactions. More specifically, pig E-selectin interacts with the neutrophil PSGL-1, porcine ICAM-1 and 2 bind to β2 integrins [4, 49], and porcine VCAM-1 interacts with the monocyte β1-integrin VLA-4 [49]. Our present findings with regard to the complement-dependent expression of porcine E-selectin and VCAM-1 are in line with a recent report utilizing different xeno-Tx models [55].
Our study also shows that the specific inhibition of the β2-integrin Mac-1 resulted in attenuated leukocyte adhesion and that complement inhibition at the C3 level decreased the presence of Mac-1 on the surface of neutrophils. These findings, together with the inability of C5aR antagonist to inhibit leukocyte adhesion, suggest a primary role for the interaction between Mac-1 and the C3 activation product iC3b in leukocyte adhesive events after exposure to PAECs.
Importantly, complement inhibition using compstatin analogues has shown promising effects in many disease models, and compstatin derivative Cp40 is in clinical development for transplantation and other indications [56]. Together, the present model and current findings contribute to a better understanding of the inflammatory mechanisms involved in the rejection of cells and organs of porcine origin and confirm that administration of soluble complement inhibitors such as Cp40 could prove beneficial in the clinical setting of islet xeno-Tx.
Acknowledgments
The research leading to these results was supported in part by the European Community’s Seventh Framework Program under grant agreement No. 602699 (DIREKT), by an European Commission International Reintegration Grant (2010-268108), by the Deutsche Forschungsgemeinschaft (SFB-TRR 127 Project A3), and by the U.S. National Institutes of Health (grant AI068730).
Footnotes
Conflict of Interest
J.D.L. and D.R. are the inventors of patents and/or patent applications that describe the use of complement inhibitors for therapeutic purposes. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. The remaining authors declare no competing financial interests.
References
- 1.Cozzi E, Bosio E, Seveso M, Vadori M, Ancona E. Xenotransplantation-current status and future perspectives. Br Med Bull. 2005;75–76:99–114. doi: 10.1093/bmb/ldh061. [DOI] [PubMed] [Google Scholar]
- 2.Pierson RN, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, Hering BJ, Cooper DK. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y-G, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 4.Schneider MKJ, Seebach JD. Current cellular innate immune hurdles in pig-to-primate xenotransplantation. Curr Opin Organ Transplant. 2008;13:171–177. doi: 10.1097/MOT.0b013e3282f88a30. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DK. Depletion of natural antibodies in non-human primates– a step towards successful discordant xenografting in humans. Clin Transplant. 1992;6:178–183. [PubMed] [Google Scholar]
- 6.Goto M, Tjernberg J, Dufrane D, Elgue G, Brandhorst D, Ekdahl KN, Brandhorst H, Wennberg L, Kurokawa Y, Satomi S, Lambris JD, Gianello P, Korsgren O, Nilsson B. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15:225–234. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1014. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16:620–626. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 11.Morgan BP, Berg CW, Harris CL. Homologous restriction in complement lysis: roles of membrane complement regulators. Xenotransplantation. 2005;12:258–265. doi: 10.1111/j.1399-3089.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowan PJ, d’Apice AJ. Complement activation and coagulation in xenotransplantation. Immunol Cell Biol. 2009;87:203–208. doi: 10.1038/icb.2008.107. [DOI] [PubMed] [Google Scholar]
- 13.Bongoni AK, Kiermeir D, Jenni H, Wünsch A, Bähr A, Ayares D, Seebach JD, Wolf E, Klymiuk N, Constantinescu MA, Vögelin E, Rieben R. Activation of the lectin pathway of complement in pig-to-human xenotransplantation models. Transplantation. 2013;96:791–799. doi: 10.1097/TP.0b013e3182a3a52b. [DOI] [PubMed] [Google Scholar]
- 14.Dalmasso AP, Platt JL, Bach FH. Reaction of complement with endothelial cells in a model of xenotransplantation. Clin Exp Immunol. 1991;86 (Suppl 1):31–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmasso AP, Vercellotti GM, Platt JL, Bach FH. Inhibition of complement- mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991;52:530–533. doi: 10.1097/00007890-199109000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Gou D, Zhen C, Jiang D, Mao X, Li W, Chen S, Cai C. Protection of xenogeneic cells from human complement-mediated lysis by the expression of human DAF, CD59 and MCP. FEMS Immunol Med Microbiol. 2001;31:203–209. doi: 10.1111/j.1574-695X.2001.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 17.Miyagawa S, Mikata S, Shirakura R, Matsuda H, Nagasawa S, Terados A, Hatanaka M, Matsumoto M, Seya T. C5b-8 step lysis of swine endothelial cells by human complement and functional feature of transfected CD59. Scand J Immunol. 1996;43:361–366. doi: 10.1046/j.1365-3083.1996.d01-50.x. [DOI] [PubMed] [Google Scholar]
- 18.Sweigard JH, Yanai R, Gaissert P, Saint-Geniez M, Kataoka K, Thanos A, Stahl GL, Lambris JD, Connor KM. The alternative complement pathway regulates pathological angiogenesis in the retina. FASEB J. 2014;28:3171–3182. doi: 10.1096/fj.14-251041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer HF, Chung K-J, Orlova VV, Choi EY, Kaul S, Kruhlak MJ, Alatsatianos M, DeAngelis RA, Roche PA, Magotti P, Li X, Economopoulou M, Rafail S, Lambris JD, Chavakis T. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116:4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silasi-Mansat R, Zhu H, Popescu NI, Peer G, Sfyroera G, Magotti P, Ivanciu L, Lupu C, Mollnes TE, Taylor FB, Kinasewitz G, Lambris JD, Lupu F. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laudes IJ, Chu JC, Huber-Lang M, Guo R-F, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- 22.Cowan PJ, Robson SC, d’Apice AJF. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase H, Ezzelarab MB, Ekser B, Cooper DKC. The role of platelets in coagulation dysfunction in xenotransplantation, and therapeutic options. Xenotransplantation. 2014;21:201–220. doi: 10.1111/xen.12085. [DOI] [PubMed] [Google Scholar]
- 24.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, Picha KM, Soderman AR, Jordan RE, Bugelski PJ, Horninger D, Lark M, Davis JE, Alejandro R, Berggren PO, Zimmerman M, O’Neil JJ, Ricordi C, Kenyon NS. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation. 2007;84:308–315. doi: 10.1097/01.tp.0000275401.80187.1e. [DOI] [PubMed] [Google Scholar]
- 26.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 27.Chavakis T. Leucocyte recruitment in inflammation and novel endogenous negative regulators thereof. Eur J Clin Invest. 2012;42:686–691. doi: 10.1111/j.1365-2362.2012.02677.x. [DOI] [PubMed] [Google Scholar]
- 28.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13:1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalmasso AP, Platt JL. Prevention of complement-mediated activation of xenogeneic endothelial cells in an in vitro model of xenograft hyperacute rejection by C1 inhibitor. Transplantation. 1993;56:1171–1176. doi: 10.1097/00007890-199311000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del Vecchio L, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu H, Ricklin D, Bai H, Chen H, Reis ES, Maciejewski M, Tzekou A, DeAngelis RA, Resuello RR, Lupu F, Barlow PN, Lambris JD. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1674. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 33.Saethre M, Schneider MKJ, Lambris JD, Magotti P, Haraldsen G, Seebach JD, Mollnes TE. Cytokine secretion depends on Galalpha(1,3)Gal expression in a pig-to-human whole blood model. J Immunol. 2008;180:6346–6353. doi: 10.4049/jimmunol.180.9.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spahn JH, Li W, Kreisel D. Innate immune cells in transplantation. Curr Opin Organ Transplant. 2014;19:14–19. doi: 10.1097/MOT.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satyananda V, Hara H, Ezzelarab MB, Phelps C, Ayares D, Cooper DKC. New concepts of immune modulation in xenotransplantation. Transplantation. 2013;96:937–945. doi: 10.1097/TP.0b013e31829bbcb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Zhou W. Anaphylatoxins in organ transplantation. Semin Immunol. 2013;25:20–28. doi: 10.1016/j.smim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W. The new face of anaphylatoxins in immune regulation. Immunobiology. 2012;217:225–234. doi: 10.1016/j.imbio.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Mitroulis I, Kang Y-Y, Gahmberg CG, Siegert G, Hajishengallis G, Chavakis T, Choi EY. Developmental endothelial locus-1 attenuates complement- dependent phagocytosis through inhibition of Mac-1-integrin. Thromb Haemost. 2014;111:1004–1006. doi: 10.1160/TH13-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb Haemost. 2009;102:191–197. doi: 10.1160/TH08-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 2013;34:1–6. doi: 10.1016/j.it.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo B-H, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kourtzelis I, Markiewski MM, Doumas M, Rafail S, Kambas K, Mitroulis I, Panagoutsos S, Passadakis P, Vargemezis V, Magotti P, Qu H, Mollnes TE, Ritis K, Lambris JD. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson B, Larsson R, Hong J, Elgue G, Ekdahl KN, Sahu A, Lambris JD. Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation. Blood. 1998;92:1661–1667. [PubMed] [Google Scholar]
- 44.Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Kourtzelis I, Rafail S, DeAngelis RA, Foukas PG, Ricklin D, Lambris JD. Inhibition of biomaterial-induced complement activation attenuates the inflammatory host response to implantation. FASEB J. 2013;27:2768–2776. doi: 10.1096/fj.12-225888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang XF, Feng MF. Adherence of human monocytes and NK cells to human TNF-alpha-stimulated porcine endothelial cells. Immunol Cell Biol. 2000;78:633–640. doi: 10.1046/j.1440-1711.2000.00970.x. [DOI] [PubMed] [Google Scholar]
- 47.Morigi M, Zoja C, Colleoni S, Angioletti S, Imberti B, Donadelli R, Remuzzi A, Remuzzi G. Xenogeneic serum promotes leukocyte-endothelium interaction under flow through two temporally distinct pathways: role of complement and nuclear factor-kappaB. J Am Soc Nephrol. 1999;10:2197–2207. doi: 10.1681/ASN.V10102197. [DOI] [PubMed] [Google Scholar]
- 48.Rollins SA, Johnson KK, Li L, Birks C, Matis LA, Rother RP. Role of porcine P-selectin in complement-dependent adhesion of human leukocytes to porcine endothelial cells. Transplantation. 2000;69:1659–1667. doi: 10.1097/00007890-200004270-00023. [DOI] [PubMed] [Google Scholar]
- 49.Robinson LA, Tu L, Steeber DA, Preis O, Platt JL, Tedder TF. The role of adhesion molecules in human leukocyte attachment to porcine vascular endothelium: implications for xenotransplantation. J Immunol. 1998;161:6931–6938. [PubMed] [Google Scholar]
- 50.Banz Y, Cung T, Korchagina EY, Bovin NV, Haeberli A, Rieben R. Endothelial cell protection and complement inhibition in xenotransplantation: a novel in vitro model using whole blood. Xenotransplantation. 2005;12:434–443. doi: 10.1111/j.1399-3089.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 51.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 52.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, Garlanda C, Danese S, Salvatori G, Sassano M, Evangelista V, Rossi B, Zenaro E, Constantin G, Laudanna C, Bottazzi B, Mantovani A. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 53.Woodfin A, Voisin M-B, Beyrau M, Colom B, Caille D, Diapouli F-M, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moberg L, Korsgren O, Nilsson B. Neutrophilic granulocytes are the predominant cell type infiltrating pancreatic islets in contact with ABO-compatible blood. Clin Exp Immunol. 2005;142:125–131. doi: 10.1111/j.1365-2249.2005.02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bongoni AK, Kiermeir D, Jenni H, Bähr A, Ayares D, Klymiuk N, Wolf E, Voegelin E, Constantinescu MA, Seebach JD, Rieben R. Complement dependent early immunological responses during ex vivo xenoperfusion of hCD46/HLA-E double transgenic pig forelimbs with human blood. Xenotransplantation. 2014;21:230–243. doi: 10.1111/xen.12090. [DOI] [PubMed] [Google Scholar]
- 56.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]