Abstract
Background: Bipolar I disorder is a highly heritable psychiatric illness with undetermined predisposing genetic and environmental risk factors. We examined familial contributions to hippocampal morphology in bipolar disorder, using a population‐based twin cohort design. Methods: We acquired high‐resolution brain MRI scans from 18 adult patients with bipolar I disorder [BPI; mean age 45.6 ± 8.69 (SD); 10 lithium‐treated], 14 non‐bipolar co‐twins, and 32 demographically matched healthy comparison twins. We used three‐dimensional radial distance mapping techniques to visualize hippocampal shape differences between groups. Results: Lithium‐treated BPI patients had significantly larger global hippocampal volume compared to both healthy controls (9%) and non‐bipolar co‐twins (12%), and trend‐level larger volumes relative to non‐lithium‐treated BPI patients (8%). In contrast, hippocampal volumes in non‐lithium‐treated BPI patients did not differ from those of non‐bipolar co‐twins and control twins. 3D surface maps revealed thicker hippocampi in lithium‐treated BPI probands compared with control twins across the entire anterior‐to‐posterior extent of the cornu ammonis (CA1 and 2) regions, and the anterior part of the subiculum. Unexpectedly, co‐twins also showed significantly thicker hippocampi compared with control twins in regions that partially overlapped those showing effects in the lithium treated BPI probands. Conclusions: These findings suggest that regionally thickened hippocampi in bipolar I disorder may be partly due to familial factors and partly due to lithium‐induced neurotrophy, neurogenesis, or neuroprotection. Unlike schizophrenia, hippocampal alterations in co‐twins of bipolar I disorder probands are likely to manifest as subtle volume excess rather than deficit, perhaps indicating protective rather than risk effects. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: bipolar disorder, magnetic resonance imaging, hippocampus, shape, volume, mood disorders, twin, morphology
INTRODUCTION
Bipolar I is a highly heritable psychiatric disorder, with estimates of additive genetic contributions to disease liability in the range of 69–100% [Kieseppa et al.,2004]; see also [Bertelsen et al.,1977; Cardno et al.,1999; Kendler et al.,1993; McGuffin et al.,2003]. Nevertheless, the predisposing genes and their mechanisms of action are yet to be determined. One approach to search for susceptibility genes for complex disorders is to use endophenotypic markers, or measures thought to be closer to a disorder's pathophysiology than clinical diagnoses [Gottesman and Gould,2003]. Declarative memory impairments, that may involve hippocampal pathology, have been observed in patients with bipolar disorder [Frey et al.,2007] and their unaffected relatives [Gourovitch et al.,1999; Kieseppa et al.,2005]. In this study, we therefore examined hippocampal morphology in twin pairs discordant for bipolar I disorder, using surface‐based anatomical modeling methods [Thompson et al.,2004] to determine the extent to which hippocampal morphological abnormalities in bipolar I disorder are associated with predisposing familial or treatment‐related factors.
Hippocampal volumes in adult and pediatric patients with bipolar disorder have either been shown to be enlarged [Beyer et al.,2004; Kemmerer et al.,1994], reduced [Blumberg et al.,2003; Frazier et al.,2005; Rossi et al.,1991] or equivalent [Altshuler et al.,2000; Brambilla et al.,2003; Hauser et al.,2000; Pearlson et al.,1997; Sax et al.,1999; Strakowski et al.,1999,2002; Strasser et al.,2005] to those of a variety of control groups. These discrepancies may be in part due to differences in clinical characteristics such as duration and severity of illness, age, number of prior episodes, and medication use. More recently, consistent with the lithium‐induced neurotrophy, neuroprotection, or neurogenesis [Chen et al.,2000; Manji et al.,2000], several studies reported larger hippocampal volumes in lithium‐treated patients with bipolar disorder [Bearden et al.,2007b; Germana et al.,2010; Yucel et al.,2007,2008] but see some conflicting findings [Javadapour et al.,2010; Rimol et al.,2010]. In addition, unmedicated bipolar patients may show localized hippocampal reductions compared with healthy controls [Bearden et al.,2007b], effects that may not be observed in small samples when confounding medication effects are not to some extent taken into account [Mamah et al.,2010]. These findings suggest that bipolar illness may be associated with subtle hippocampal volume deficits, which may be attenuated or reversed by lithium treatment.
Very few neuroanatomic studies of bipolar disorder have examined those at genetic high risk for the illness. Apart from the small twin study by Noga et al. [2001], in which an absence of typical asymmetry of the hippocampi was observed among six healthy co‐twins of six bipolar probands when compared with 12 control twins, to our knowledge, none of the bipolar disorder studies that included relatives [Ahearn et al.,1998,2002; Gulseren et al.2006; Hajek et al.,2008a,b;2009; Kieseppa et al.,2003; McDonald et al.,2006; McIntosh et al.,2006; Noga et al.,2001; van der Schot et al.,2009] have examined hippocampal morphology (for review see Hajek et al. [2005]). Here we set out to create the first detailed hippocampal surface maps comparing patients with bipolar I disorder and non‐bipolar co‐twins with control twins, using a highly sensitive method that creates group average maps of three‐dimensional (3D) hippocampal surface models, thus providing detailed maps of local hippocampal thickness measurements [Thompson et al.,2004]. Hippocampal surface anatomy was carefully matched across individuals to provide accurate and spatially refined localizations of group differences.
Based on findings to date, we hypothesized that: (1) non‐lithium‐treated bipolar I patients and non‐bipolar co‐twins of BPI patients would show hippocampal volume deficits relative to healthy control twins, and (2) that lithium‐treated bipolar I patients would have larger hippocampi than non‐lithium‐treated patients, non‐bipolar co‐twins, and healthy control twins.
METHODS
Subjects
The National Hospital Discharge Register of Finland was searched for patients with ICD‐8 [WHO,1967] codes of 296.10 or 296.30, or a DSM‐III‐R codes of 296.4, 296.5, or 296.6 during 1969 to 1991. Subsequently, the National Population Register and the Finnish Twin Cohorts [Kaprio et al.,1990] were queried to locate twins born between 1940 and 1969. This comprehensive search identified 59 twins, who were invited to participate in the study with their co‐twin [Kieseppa et al.,2000].
All participants were assessed with the Structured Clinical Interview for DSM‐IV Disorders (SCID [Spitzer et al.,1997]), as detailed elsewhere [Cannon et al.,2000; Kieseppa et al.,2000,2004]. Zygosity was determined based on genetic marker analysis in all control and index pairs [Cannon et al.,2002].
Because our collection method was population‐based, we included all valid cases, including individual twin subjects. The numbers of bipolar twins and co‐twins were relatively small, and we wanted to maximize the utility of the information available to us. Thus, after diagnostic ascertainment, 18 BPI probands (6 without a co‐twin; 10 Lithium‐treated) and 14 co‐twins (6 without a proband) were selected for imaging. Only four of the bipolar twins were from monozygotic pairs. In cases for which the co‐twin did not have a proband that was included in the study, proband diagnosis of BPI was based on all information obtained from medical records, forensic reports, and interviews with health personnel and the co‐twin. Two twin pairs were concordant for BPI. One index pair was opposite sex (male/female). Exclusion criteria for all groups were any other psychotic or mood disorders, neurological disorders affecting the brain, brain injuries, or current substance abuse. Nine out of ten lithium‐treated patients and three out of eight non‐lithium‐treated patients received antidepressant medication. None of the bipolar patients was treated with valproate. Five out of the eight non‐lithium‐treated patients received no medication (see Tables I and II for sample details). Three of the patients in the non‐lithium group in our sample had a prior history of lithium use 20 years before they participated in this study (1 for 2 months, and the others for 2 years). None of the subjects had lithium toxicity events within the 12 months prior to the time of image acquisition.
Table I.
Sample demographics
| Lithium‐treated BPI Probands (N = 10) | BPI probands no lithium (N = 8) | Co‐twins (N = 14) | Control twins (n = 32) | P‐value | |
|---|---|---|---|---|---|
| Age in years (SD) | 45.60 (8.69) | 42.50 (6.76) | 44.64 (7.38) | 47.19 (3.86) | 0.21 |
| Sex (Female)a | 7 (70) | 3 (38) | 9 (64) | 15 (47) | 0.37 |
| Education (SD)b | 4.30 (2.36) | 3.63 (2.97) | 3.00 (2.04) | 3.69 (1.09) | 0.40 |
| Monozygotica | 2 (20) | 2 (25) | 1 (7) | 6 (19) | 0.57 |
| Right handeda | 10 (100.00) | 8 (100) | 14 (100) | 31 (96.88) | 1.00 |
| Life‐time alcohol dependencea | 1 (10) | 4 (50) | 1 (7) | 2 (6) | 0.02 |
| Current alcohol dependencea | 1 (10) | 0 (0) | 0 (0) | 1 (3) | 0.53 |
| Lifetime anxiety disordera | 3 (30.00) | 4 (50) | 1 (7) | 2 (6) | 0.009 |
| Lithium (mg/day)c | 900 (600–1,200) | 0 | 0 | 0 | |
| Any psychotropic medicationa | 9 (90) | 3 (38) | 0 | 0 | |
| Length of use of medication (years)c | 14.3 (4.5–26) | 8.3 (0.3–27) | 0 | 0 |
Data represented as n (%).
Education classified according the Structural Clinical Interview for DSM‐IV.
Data represented as mean (range).
Table II.
Twin sample by group and zygosity
| Diagnostic status of twin pair (number of pairs) | Zygosity | N pairs | N participants |
|---|---|---|---|
| MRI data available for both twins | |||
| BPI Li+/BPI Li− | MZ | 1 | 2 |
| BPI Li+/BPI Li− | DZ | 1 | 2 |
| BPI Li+/Healthy | MZ | 1 | 2 |
| BPI Li+/Healthy | DZ | 4 | 8 |
| BPI Li−/Healthy | DZ | 3 | 6 |
| MRI data only for probands | |||
| BPI Li+/(Healthy) | DZ | 3 | 3 |
| BPI Li−/(Healthy) | MZ | 1 | 1 |
| BPI Li−/(Healthy) | DZ | 2 | 2 |
| MRI data only for co‐twins | |||
| (BPI)/Healthy | DZ | 6 | 6 |
| Healthy/(Healthy) | MZ | 6 | 6 |
| Healthy/(Healthy) | DZ | 26 | 26 |
| Total | 54 | 64 |
BPI Li+, Bipolar I patient on Lithium; BPI Li−, Bipolar I patient not on lithium.
A control group of 32 twins from 32 healthy twin pairs without any psychotic or mood disorder, of which six from MZ pairs, was recruited from the same Finnish twin cohort and matched to the mean age [F(2,61) = 1.72, P = 0.19], sex (χ = 1.24, P = 0.54) and zygosity distributions (χ = 1.37, P = 0.57) of the patient and co‐twin samples. The groups showed a trend‐level difference in the frequency of lifetime alcohol abuse (Fisher's Exact Test; χ = 5.35, P = 0.08) and significant differences in the frequency of lifetime anxiety disorder diagnoses (Fisher's Exact Test; χ = 10.29, P = 0.009), both with higher prevalence in the bipolar disorder patients.
The study was approved by the Ministry of Social Affairs and Health in Finland, the Ethics Committee of the National Public Health Institute of Finland, and the University of California Los Angeles Institutional Review Board and complied with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written, informed consent was obtained from all participants after they had received a complete description of the study.
Image Acquisition
The scanning protocol was identical to that used in our other twin studies [Cannon et al.,2002; Thompson et al.,2001]. Briefly, T1‐weighted MP‐RAGE (magnetization prepared rapid gradient echo) scans of the brain (TR/TE = 11.4/4.4 ms, sagittal orientation, matrix = 256 × 256 × 128, FOV = 250 mm, resolution = 0.98 × 0.98 × 1.2) were acquired on a 1.0‐Tesla scanner (Siemens, Inselin, NJ) at a private medical center (Teslamed, Helsinki, Finland).
Hippocampal Shape Protocol
Prior to tracing, to correct for differences in image intensity and overall brain size, individual brain images were corrected for radio frequency field non‐uniformity using Montreal Neurological Institute's (MNI) N3 [Sled et al.,1998] and aligned to the ICBM‐305 average brain template with a least‐squares 9‐parameter registration model implemented using MNI's “mritotal” [Collins et al.,1994]. Hippocampal measures were taken as outlined in Figure 1. Intra‐rater (AM) and inter‐rater (AM, GDH) reliabilities as measured by intraclass correlations were high for left and right hippocampal volume (> 0.90; > 0.90), surface area (>0.95; >0.93), and length (>0.92; >0.86). Reliability maps for the radial measures using this method have been reported elsewhere [Thompson et al.,2004].
Figure 1.
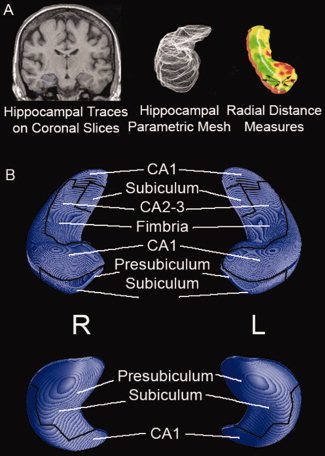
3D Representation of Hippocampal Cytoarchitectonic Subregions. Figure adapted from Frisoni et al., Neuroimage2006, 32, 104‐110. Traces outlining the hippocampus—including the cornu ammonis (CA) regions 1–4, dentate gyrus (DG), and subiculum—were created with MultiTracer (http://air.bmap.ucla.edu/MultiTracer/) on coronal slices using a protocol described previously (Thompson et al.,2004). Subsequently, anatomical mesh modeling methods (Thompson et al.,1996) were used to match equivalent hippocampal surface points across subjects and groups. Briefly, top and bottom hippocampal surfaces were digitized to create parametric meshes of 150 × 100 surface points. This procedure allows for measurements to be made at corresponding 3D surface locations in each subject that may then be compared statistically. A 3D medial space curve through the centroid of the hippocampal cross‐sections was computed, and at each surface point the radial distance between the surface point and the center was computed (a) allowing detailed mapping of hippocampal morphology (b). Part A of the figure was adapted from Figure 7 in Thompson et al. British Journal of Radiology (2007) 80, S78‐S91.
Statistical Analyses
Group differences in hippocampal morphology were examined using mixed model regression analyses [Proc Mixed, Statistical Analysis Software (SAS)] predicting hippocampal volume, length, surface area, and thickness (i.e., radial distances from the medial core of the hippocampus) from variables including group (lithium‐treated BPI patients, BPI not on lithium, co‐twin, control), hemisphere (left, right), and a group × hemisphere interaction (Table III). Hemisphere entered the model as a within‐subject repeated measure. Non‐independence of measures among co‐twins was controlled for by including twin pair as a random variable and adjusting the model error degrees of freedom with the Satterthwaite option. Sex, age, lifetime alcohol dependence, and lifetime anxiety disorder entered the model as covariates. Significant volumetric findings are represented in bar graphs and least square mean differences in radial distance. Where the direction of the effect was predicted p‐values reported are one‐tailed. P‐values describing the significance of group differences were plotted at each hippocampal surface point using a color code to produce a statistical map. Permutation methods [Bullmore et al.,1999; Thompson et al.,2003] were used to assess the significance of the statistical maps, and to correct for multiple comparisons. In each case, the covariate (group membership) was permuted 100,000 times on an SGI Reality Monster supercomputer with 64 internal R10000 processors, and a null distribution was developed for the area of the hippocampal surface with group difference statistics above a fixed threshold (P < 0.05) in the significance maps. An algorithm was then used to determine the significance probability for the overall difference patterns in each map [Thompson et al.,2003], after the appropriate correction for multiple comparisons.
Table III.
Absolute hippocampal measures (SD) by group and hemisphere
| BPI lithium (n = 10) | BPI No lithium (n = 8) | Co‐twin (n = 14) | Control twin (n = 32) | |
|---|---|---|---|---|
| Volume (mL) | ||||
| Left | 3,828 (221) | 3,436 (196) | 3,463 (292) | 3,534 (396) |
| Right | 3,877 (272) | 3,570 (293) | 3,549 (294) | 3,619 (347) |
| Area (mm2) | ||||
| Left | 1,865 (125) | 1,748 (80) | 1,744 (110) | 1,699 (131) |
| Right | 1,861 (110) | 1,771 (116) | 1,766 (115) | 1,698 (115) |
| Length (mm) | ||||
| Left | 36.6 (1.7) | 36.0 (2.8) | 36.4 (2.0) | 36.0 (2.2) |
| Right | 35.3 (2.8) | 35.3 (2.3) | 35.9 (2.2) | 35.7 (1.9) |
RESULTS
Hippocampal Volume
Groupwise comparisons of hippocampal volumes were performed to provide a context for the 3D hippocampal maps. There were significant main effects of group (BPI Li+, BPI Li‐, co‐twin, control) [F(3,56) = 3.15, P = 0.03] and hemisphere [F(1,60) = 5.53, P = 0.02] on hippocampal volume. BPI Li+ patients had significantly larger hippocampal volumes than the non‐bipolar co‐twins (t 56 = 2.96, P < 0.003) and control twins (t 56 = 2.58, P = 0.005), and trend‐level significantly larger hippocampal volumes than the BPI Li‐ patients (t 56 = 1.60, P < 0.06) (Fig. 2). Across groups, the right hippocampus was larger than the left (t 60 = 2.35; P = 0.02).
Figure 2.
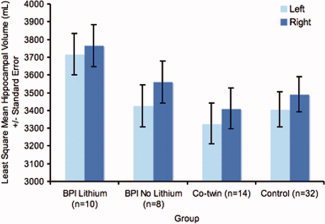
Hippocampal Volumes in Lithium‐Treated Bipolar I Disorder Patients, Non‐Lithium‐Treated Bipolar I Disorder Patients, Non‐Bipolar Co‐twins, and Healthy Twins. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Hippocampal Surface Area
There was a main effect of group [F(3,55) = 6.79, P = 0.0006] and lifetime alcohol dependence [F(1,55) = 4.31, P = 0.04] on hippocampal surface area. BPI Li+ patients (LSM = 1,810 mm2, SE = 38) had significantly larger hippocampal surface areas than control twins (t 55 = 4.43, P < 0.0001; LSM = 1,639 mm2, SE = 35) and non‐bipolar co‐twins (t 55 = 2.79, P = 0.007; LSM = 1,692 mm2; SE = 38), and non‐significantly larger hippocampal areas than the BPI Li‐ patients (t 55 = 1.03, P = 0.31; LSM = 1,756 mm2, SE = 36). The BPI Li‐ group had larger hippocampal surface areas than the control group (t 55 = 2.27, P = 0.03). Across groups, those with lifetime alcohol dependence (LSM = 1,672 mm2, SE = 45) had smaller hippocampal areas than those without (t 55 = 2.07, P = 0.04; LSM = 1,777 mm2, SE = 20).
Hippocampal Length
Across groups, left hippocampi were longer than right hippocampi (t 60 = 2.76, P = 0.008) and those with a lifetime anxiety disorder had shorter hippocampi than those without (t 56 = −2.15, P = 0.04). None of the other predictors showed significant main or interaction effects.
3D Hippocampal Maps
3D hippocampal surface maps showed significantly larger hippocampus thickness in the BPI Li+ patients compared with the control twins (L: P < 0.04, R: P < 0.001, for BPI Li+ > control, permutation test), and significantly larger right hemisphere hippocampus thickness in BPI Li+ patients compared with the unaffected co‐twins (L: P = 0.31, R: P < 0.03, for BPI Li+ > co‐twin, permutation test), across the entire anterior‐to‐posterior extent of the cornu ammonis (CA 1 and 2) regions, and the anterior part of the subiculum, but no significantly larger hippocampus thickness in the BPI Li+ group compared with the BPI Li‐ group (L: P = 0.20, R: P = 0.36, for BPI Li+ > BPI Li‐, permutation test) (Fig. 3).
Figure 3.
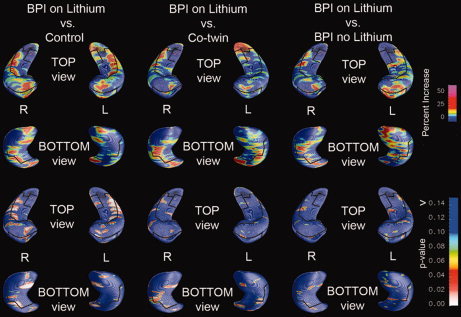
3D Hippocampal Maps: Lithium‐treated bipolar probands vs. control twins (left), vs. non‐bipolar co‐twins (middle), and vs. non‐lithium‐treated bipolar probands (right). The top two rows reflect percent gray matter increase and the bottom two rows corresponding P‐values.
Further map‐wise comparisons revealed that the non‐bipolar co‐twins also had larger hippocampus thickness relative to controls, along the border of the cornu ammonis (CA 1 and 2) region and the anterior subiculum, which were significant on the right side (L: P = 0.34; R: P = 0.01, for Co‐twin > Control, permutation test). To some extent, these areas overlapped with regions showing lithium‐associated effects in BPI probands (Fig. 4).
Figure 4.
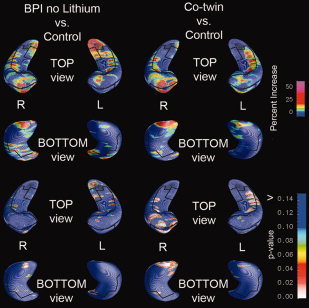
3D Hippocampal Maps: Non‐lithium treated bipolar probands vs. controls (left) and co‐twins vs. controls (right). The top two rows reflect percent gray matter increase and the bottom two rows corresponding p‐values for the contrasts of non‐lithium treated BPI Probands vs. Controls and Co‐twins vs. Controls. The maps show a significant increase in right hippocampal volume for co‐twins compared with controls, in particular in CA1.
DISCUSSION
The principal findings of this study were as follows: (1) global hippocampal volume was larger in bipolar I patients treated with lithium, relative to age‐ and sex‐matched healthy control twins and non‐bipolar co‐twins of bipolar probands, and trend‐level larger relative to patients not treated with lithium, with the excess most pronounced in the cornu ammonis 1 and 2 and the anterior subiculum hippocampal sub‐regions, and (2) despite no differences in global hippocampal volume, significant regional right hippocampal thickening was present in non‐bipolar co‐twins compared with control twins, and the thicker regions partially overlapped those showing lithium‐associated regional thickening in bipolar I probands.
Lithium treatment was associated with significantly larger hippocampal volumes in BPI versus healthy control twins (9%) and non‐bipolar co‐twins (12%), and trend‐level larger hippocampal volumes compared with non‐lithium‐treated BPI probands (8%). These findings corroborate prior observations of larger lithium‐associated hippocampal volumes in bipolar patients, in cross‐sectional [Bearden et al.,2007b; Beyer et al.,2004; Germana et al.,2010; Yucel et al.,2008] and longitudinal designs [Yucel et al.,2007], and the observation that patient‐control group differences are positively associated with the percent of lithium‐treated patients across studies [Kempton et al.,2008]. The hippocampus is known to be a brain region in which neurogenesis occurs even in adulthood [van Praag et al.,2002]. It is therefore plausible that the regional hipopcampal thickening in Li+ compared with Li‐ patients is in part due to lithium‐induced neurogenesis. The extent to which the observed hippocampal thickening is due to lithium‐induced neurogenesis, neurotropy, or neuroprotection, as reported in preclinical studies [Chen et al.,2000; Frey et al.,2006], warrants investigation in future studies. It must be noted that three of the patients in the non‐lithium group in our sample had a prior history of lithium use. However, their exposure was relatively short (1 for 2 months, and the others for 2 years), in each case occurred more than 20 years before they participated in this study, and their mean hippocampal volumes were similar to those not treated with lithium (3,373 mm3 and 3,519 mm3 for left and right hippocampus, respectively). No significant associations between the hippocampal morphological measures and lithium dose were observed.
The identification of abnormal hippocampal structure may help to elucidate the underlying pathophysiological mechanisms associated with bipolar illness, and may also indicate functional systems that may be disturbed in the illness. Hippocampal pathology may underlie the declarative memory impairments observed in patients with bipolar disorder [Frey et al.,2007] and their unaffected relatives [Gourovitch et al.,1999; Kieseppa et al.,2005]. In fact, Yucel et al. [2007,2008] reported an increase in hippocampal volume and verbal declarative memory performance after lithium treatment [Yucel et al.,2007], though practice effects were not controlled for.
As cortical gray matter is larger in lithium‐treated patients versus controls [Moore et al.,2000; Sassi et al.,2002], it is unlikely that lithium effects are limited to the hippocampus. Detailed mapping of lithium effects on cortical gray matter in this sample is currently in progress. One such prior study found regional differences in the magnitude of lithium effects, with greatest effects in the bilateral cingulate and paralimbic cortices [Bearden et al.,2007a]. Possible differences in subcortical versus cortical lithium effects warrant further exploration, particularly with regard to their timing and association with treatment response. For instance, lithium‐associated gray matter effects may be exacerbated in the hippocampus, as lithium acts on several biochemical pathways that may exert relatively large effects in the hippocampus; for review see [Shaltiel et al.,2007]. More specifically, some of its effects appear to include a reduction of glutamate‐induced, NMDA receptor‐mediated excitotoxicity [Nonaka and Chuang,1998] and an increase in the expression of brain‐derived neurotrophic factor (BDNF) [Frey et al.,2006; Hashimoto et al.,2002]. While further research is needed, it has been suggested that lithium may also be of use in the treatment of other neuropsychiatric disorders [Chen et al.,2000] and possibly also in neurodegenerative disorders.
Other studies have found larger gray matter volumes in lithium vs. non‐lithium‐treated bipolar patients, but our finding of regionally larger hippocampal thickness in non‐bipolar co‐twins relative to controls is novel. This finding clearly warrants replication, though it is consistent with a recent study that reported larger gray matter volume in the left parahippocampal gyrus in healthy offspring from parents diagnosed with bipolar disorder [Ladouceur et al.,2008]. The pattern of larger local hippocampal thickness among the non‐lithium treated bipolar I patients was very similar to that of the non‐ill co‐twins, but was not statistically significant. Although co‐twins had never taken lithium or other mood ‐stabilizing medication, they nevertheless showed thickened hippocampi in similar hippocampal regions as bipolar I patients on lithium therapy ‐ specifically, portions of the cornu ammonis (CA 1 and 2) subfields and the anterior subiculum. The observation that the thickening of the hippocampi in lithium treated patients is in similar areas as the hippocampal thickening in the unaffected and unmedicated co‐twins may suggest that lithium acts on similar molecular pathways as familial (genetic or shared environmental) risk or protective factors for mood instability.
Usually mean differences observed in co‐twins of patients with psychiatric disorders are interpreted as risk effects. However, given that lithium, used as a first‐line treatment for bipolar disorder, is associated with greater hippocampal thickness, it is tempting to speculate that the thicker hippocampi in the co‐twins compared with controls protect these co‐twins against the pathological fluctuations in mood observed in bipolar I patients, and that lithium‐induced hippocampal thickening may lead to similar “protective” effects in patients. Clearly, caution is warranted given the cross‐sectional nature of these findings, but the thicker hippocampi in the co‐twins of BPI patients relative to the controls is a striking addition to the current findings in the literature and clearly warrants follow‐up with larger family and twin samples.
Among the weaknesses of this study are the relatively small sample sizes for each of the groups, and the cross‐sectional rather than longitudinal design with regard to the lithium treatment effects. Furthermore, we cannot exclude possible confounding effects of antidepressants. In addition to lithium, 9 of the Li+ and 3 of the Li‐ patients were treated with anti‐depressant medications, which have recently been shown to be associated with larger hippocampal volumes in depressed patients [Malykhin et al.,2010]. Within‐subject longitudinal studies are needed to better disentangle potential effects of both lithium and anti‐depressants on regional hippocampal morphology in bipolar disorder patients. Lifetime diagnoses of anxiety disorder and alcohol dependence were present more often bipolar disorder patients compared with controls. Excessive alcohol intake in general [Wilhelm et al.,2008] and anxiety in bipolar disorder [Simeonova et al.,2009] are correlated with reduced hippocampal volume and are therefore not likely to explain the observed effects. Because of the small number of monozygotic twin pairs, we were unable to compare monozygotic vs. dizygotic twin pairs and due to the small number of twin pairs overall we were unable to fully implement matched‐pair comparisons, which is a key strength of the twin design. Strengths of the study are that this is the first study to examine regional hippocampal alterations in bipolar probands and non‐bipolar co‐twins; we included a homogeneous group of probands with bipolar I disorder diagnoses only; and study participants were ascertained from a population‐based twin sample, such that the findings can be generalized to all twins in the cohort.
The familial liability for bipolar I disorder, in non‐bipolar co‐twins, appears to be associated with larger regional hippocampus thickness—larger thickness that is also observed in bipolar I patients treated with lithium. These findings are promising in that they show regional effects on hippocampal morphology, but the study of lithium effects requires pre and posttreatment follow‐up. Genetic and environmental influences on hippocampal morphology should also be examined with studies that study sufficient numbers of monozygotic and dizygotic co‐twins. Future work should also examine whether lithium treatment modulates limbic‐cortical network activity and connectivity [Mayberg,1997,2003] using physiological measures such as functional magnetic resonance imaging and electroencephalography both pre and postlithium treatment.
Acknowledgements
The authors thank Ulla Mustonen for her role in recruitment and clinical evaluation as well as the twins who participated in the study. The Finnish Twin Cohort study forms part of the Academy of Finland Centre of Excellence in Complex Disease Genetics.
REFERENCES
- Ahearn EP, Steffens DC, Cassidy F, Van Meter SA, Provenzale JM, Seldin MF, Weisler RH, Krishnan KR ( 1998): Familial leukoencephalopathy in bipolar disorder. Am J Psychiatry 155: 1605–1607. [DOI] [PubMed] [Google Scholar]
- Ahearn EP, Speer MC, Chen YT, Steffens DC, Cassidy F, Van Meter S, Provenzale JM, Weisler RH, Krishnan KR ( 2002): Investigation of Notch3 as a candidate gene for bipolar disorder using brain hyperintensities as an endophenotype. Am J Med Genet 114: 652–658. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J ( 2000): An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 48: 147–162. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. ( 2007a) Greater cortical gray matter density in lithium‐treated patients with bipolar disorder. Biol Psychiatry 62: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Soares JC. ( 2007b): Three‐dimensional mapping of hippocampal anatomy in unmedicated and lithium‐treated patients with bipolar disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen A, Harvald B, Hauge M ( 1977): A Danish twin study of manic‐depressive disorders. Br J Psychiatry 130: 330–351. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Kuchibhatla M, Payne ME, Moo‐Young M, Cassidy F, Macfall J, Krishnan KR ( 2004): Hippocampal volume measurement in older adults with bipolar disorder. Am J Geriatr Psychiatry 12: 613–620. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS ( 2003): Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 60: 1201–1208. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2003): MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res 37: 287–295. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio‐Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M ( 2000): The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet 67: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold‐Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J ( 2002): Cortex mapping reveals regionally specific patterns of genetic and disease‐specific gray‐matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA 99: 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM ( 1999): Heritability estimates for psychotic disorders: The Maudsley twin psychosis series. Arch Gen Psychiatry 56: 162–168. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji‐Bozorgzad N, Manji HK ( 2000): Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75: 1729–1734. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J ( 2005): Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 162: 1256–1265. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Rosa AR, Martins MR, Valvassori SS, Reus GZ, Hatch JP, Quevedo J, Kapczinski F ( 2006): Lithium increases nerve growth factor levels in the rat hippocampus in an animal model of mania. Behav Pharmacol 17: 311–318. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F ( 2007): The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol 18: 419–430. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM ( 2006): In vivo neuropathology of the hippocampal formation in AD: A radial mapping MR‐based study. Neuroimage 32: 104–110. [DOI] [PubMed] [Google Scholar]
- Germana C, Kempton MJ, Sarnicola A, Christodoulou T, Haldane M, Hadjulis M, Girardi P, Tatarelli R, Frangou S ( 2010): The effects of lithium and anticonvulsants on brain structure in bipolar disorder. Acta Psychiatr Scand 122: 481–487. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD ( 2003): The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–645. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE ( 1999): Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry 45: 639–646. [DOI] [PubMed] [Google Scholar]
- Gulseren S, Gurcan M, Gulseren L, Gelal F, Erol A ( 2006): T2 hyperintensities in bipolar patients and their healthy siblings. Arch Med Res 37: 79–85. [DOI] [PubMed] [Google Scholar]
- Hajek T, Carrey N, Alda M ( 2005): Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disord 7: 393–403. [DOI] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Bernier D, Slaney C, Propper L, Grof P, Macqueen G, Duffy A, Alda M. ( 2008a): Subgenual cingulate volumes in affected and unaffected offspring of bipolar parents. J Affect Disord 108: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Bernier D, Slaney C, Propper L, Macqueen G, Duffy A, Alda M. ( 2008b) Pituitary volumes in relatives of bipolar patients: High‐risk study. Eur Arch Psychiatry Clin Neurosci 258: 357–362. [DOI] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Slaney C, Propper L, Macqueen G, Duffy A, Alda M. ( 2009): Striatal volumes in affected and unaffected relatives of bipolar patients—High‐risk study. J Psychiatr Res 43: 724–729. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM ( 2002): Lithium induces brain‐derived neurotrophic factor and activates TrkB in rodent cortical neurons: An essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology 43: 1173–1179. [DOI] [PubMed] [Google Scholar]
- Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM ( 2000): MRI‐based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. J Affect Disord 60: 25–32. [DOI] [PubMed] [Google Scholar]
- Javadapour A, Malhi GS, Ivanovski B, Chen X, Wen W, Sachdev P ( 2010): Hippocampal volumes in adults with bipolar disorder. J Neuropsychiatry Clin Neurosci 22: 55–62. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Rose RJ ( 1990): Population‐based twin registries: Illustrative applications in genetic epidemiology and behavioral genetics from the Finnish Twin Cohort Study. Acta Genet Med Gemellol (Roma) 39: 427–439. [DOI] [PubMed] [Google Scholar]
- Kemmerer M, Nasrallah HA, Sharma S, Olson SC, Martin R, Lyn MB ( 1994): Increased hippocampal volume in bipolar disorder. Biol Psychiatry 35: 615–747. [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM ( 2008): Meta‐analysis, database, and meta‐regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65: 1017–1032. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Pedersen N, Johnson L, Neale MC, Mathe AA ( 1993): A pilot Swedish twin study of affective illness, including hospital‐ and population‐ascertained subsamples. Arch Gen Psychiatry 50: 699–700. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Kaprio J, Lonnqvist J. ( 2000): Accuracy of register‐ and record‐based bipolar I diagnoses in Finland—A study of twins. Acta Neuropsychiatrica 12: 106–109. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, Kaprio J, Lonnqvist J ( 2003): Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry 54: 896–905. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J ( 2004): High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry 161: 1814–1821. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Tuulio‐Henriksson A, Haukka J, Van Erp T, Glahn D, Cannon TD, Partonen T, Kaprio J, Lonnqvist J ( 2005): Memory and verbal learning functions in twins with bipolar‐I disorder, and the role of information‐processing speed. Psychol Med 35: 205–215. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C, Monk K, Kupfer DJ, Phillips ML ( 2008): Subcortical gray matter volume abnormalities in healthy bipolar offspring: Potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry 47: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, Coupland NJ ( 2010): Structural changes in the hippocampus in major depressive disorder: Contributions of disease and treatment. J Psychiatry Neurosci 35: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Wang L, Csernansky JG, Rice JP, Smith M, Barch DM ( 2010): Morphometry of the hippocampus and amygdala in bipolar disorder and schizophrenia. Bipolar Disord 12: 341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G ( 2000): Lithium up‐regulates the cytoprotective protein Bcl‐2 in the CNS in vivo: A role for neurotrophic and neuroprotective effects in manic depressive illness. J Clin Psychiatry 61 ( Suppl 9), 82–96. [PubMed] [Google Scholar]
- Mayberg HS ( 1997): Limbic‐cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481. [DOI] [PubMed] [Google Scholar]
- Mayberg HS ( 2003): Modulating dysfunctional limbic‐cortical circuits in depression: Towards development of brain‐based algorithms for diagnosis and optimised treatment. Br Med Bull 65: 193–207. [DOI] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM ( 2006): Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 163: 478–487. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A ( 2003): The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 60: 497–502. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM ( 2006): Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet 141: 76–83. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK ( 2000): Lithium‐induced increase in human brain grey matter. Lancet 356: 1241–1242. [DOI] [PubMed] [Google Scholar]
- Noga JT, Vladar K, Torrey EF ( 2001): A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res 106: 25–34. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Chuang DM ( 1998): Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport 9: 2081–2084. [DOI] [PubMed] [Google Scholar]
- WHO ( 1967): Manual of he International Statistical Classification of Diseases, Injuries and Causes of Death. Geneva: World Health Organization. [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY ( 1997): Ziskind‐Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry 41: 1–14. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema‐Notestine C, Hagler DJ Jr, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I ( 2010): Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 68: 41–50. [DOI] [PubMed] [Google Scholar]
- Rossi A, Stratta P, Di Michele V, Gallucci M, Splendiani A, de Cataldo S, Casacchia M ( 1991): Temporal lobe structure by magnetic resonance in bipolar affective disorders and schizophrenia. J Affect Disord 21: 19–22. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2002): Increased gray matter volume in lithium‐treated bipolar disorder patients. Neurosci Lett 329: 243–245. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE Jr, Hawkins JM ( 1999): Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry 156: 139–141. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Chen G, Manji HK ( 2007): Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol 7: 22–26. [DOI] [PubMed] [Google Scholar]
- Simeonova DI, Jackson V, Attalla A, Karchemskiy A, Howe M, Adleman N, Chang K ( 2009): Subcortical volumetric correlates of anxiety in familial pediatric bipolar disorder: A preliminary investigation. Psychiatry Res 173: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC ( 1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Gibbon M, Williams JB ( 1997): The Structured Clinical Interview for DSM‐IV Axis I and II Disorders (SCID I‐II). Washington, DC: American Psychiatric Press. [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER ( 1999): Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 56( 3), 254–260. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM ( 2002): Ventricular and periventricular structural volumes in first‐ versus multiple‐episode bipolar disorder. Am J Psychiatry 159( 11), 1841–1847. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD ( 2005): Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: A pilot study. Biol Psychiatry 57: 633–639. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW ( 1996): High‐resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage 3: 19–34. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold‐Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW ( 2001): Genetic influences on brain structure. Nat Neurosci 4: 1253–1258. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW ( 2003): Dynamics of gray matter loss in Alzheimer's disease. J Neurosci 23: 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW ( 2004): Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage 22: 1754–1766. [DOI] [PubMed] [Google Scholar]
- van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PC, Nuboer V, Schnack HG, van Baal GC, Boomsma DI, Nolen WA, Hulshoff Pol HE, Kahn RS ( 2009): Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry 66( 2), 142–151. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH ( 2002): Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J, Frieling H, Hillemacher T, Degner D, Kornhuber J, Bleich S ( 2008): Hippocampal volume loss in patients with alcoholism is influenced by the consumed type of alcoholic beverage. Alcohol Alcohol 43: 296–299. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, Macqueen GM. ( 2007) Bilateral hippocampal volume increases after long‐term lithium treatment in patients with bipolar disorder: A longitudinal MRI study. Psychopharmacology 195: 357–367. [DOI] [PubMed] [Google Scholar]
- Yucel K, Taylor VH, McKinnon MC, Macdonald K, Alda M, Young LT, Macqueen GM. ( 2008): Bilateral Hippocampal Volume Increase in Patients with Bipolar Disorder and Short‐term Lithium Treatment. Neuropsychopharmacology 33: 361–367. [DOI] [PubMed] [Google Scholar]


