Abstract
Cardiovascular effects of total intravenous anesthesia using ketamine-medetomidine-propofol drug combination (KMP-TIVA) were determined in 5 Thoroughbred horses undergoing surgery. The horses were anesthetized with intravenous administration (IV) of ketamine (2.5 mg/kg) and midazolam (0.04 mg/kg) following premedication with medetomidne (5 µg/kg, IV) and artificially ventilated. Surgical anesthesia was maintained by controlling propofol infusion rate (initially 0.20 mg/kg/min following an IV loading dose of 0.5 mg/kg) and constant rate infusions of ketamine (1 mg/kg/hr) and medetomidine (1.25 µg/kg/hr). The horses were anesthetized for 175 ± 14 min (range from 160 to 197 min). Propofol infusion rates ranged from 0.13 to 0.17 mg/kg/min, and plasma concentration (Cpl) of propofol ranged from 11.4 to 13.3 µg/ml during surgery. Cardiovascular measurements during surgery remained within clinically acceptable ranges in the horses (heart rate: 33 to 37 beats/min, mean arterial blood pressure: 111 to 119 mmHg, cardiac index: 48 to 53 ml/kg/min, stroke volume: 650 to 800 ml/beat and systemic vascular resistance: 311 to 398 dynes/sec/cm5). The propofol Cpl declined rapidly after the cessation of propofol infusion and was significantly lower at 10 min (4.5 ± 1.5 µg/ml), extubation (4.0 ± 1.2 µg/ml) and standing (2.4 ± 0.9 µg/ml) compared with the Cpl at the end of propofol administration (11.4 ± 2.7 µg/ml). All the horses recovered uneventfully and stood at 74 ± 28 min after the cessation of anesthesia. KMP-TIVA provided satisfactory quality and control of anesthesia with minimum cardiovascular depression in horses undergoing surgery.
Keywords: cardiovascular function, equine, ketamine, medetomidine, propofol
General anesthesia for prolonged duration surgical procedures in horses is generally accomplished by administering inhalant anesthetics. The inspired concentration of inhalant anesthetics required in providing a surgical plane of anesthesia frequently contributes to intraoperative hypotension and hypoventilation [28]. Some studies have suggested that TIVA in horses produces better cardiopulmonary values than inhalation anesthesia [1, 2, 19, 31].
A variety of TIVA techniques have been investigated for short surgical procedures in horses [34]. The use of TIVA for longer surgical procedures has generally included combinations of centrally acting muscle relaxants (guaifenesin and midazolam), α2-adrenoceptor agonists or both in conjunction with ketamine [12, 19, 29, 35] and medetomidine-propofol drug combination with or without ketamine [3, 4, 30].
Propofol’s rapid onset of action and short context-sensitive half-life are conductive to rapid recovery from anesthesia following short duration procedures [23]. Propofol, however, can produce marked cardiorespiratory depression and is considered unsatisfactory when used by itself for induction and maintenance of anesthesia in horses [18, 30]. Combining propofol with various sedative and analgesic drugs could provide an alternative method for improving the quality and safety of anesthesia in horses and potentially decrease the total dose of drug required [4, 10, 17, 20, 30].
Towards this end, several studies have investigated and considered propofol drug combinations suitable for TIVA in horses for periods lasting longer than 2 hr [1, 3, 4]. The combination of ketamine-medetomidine-propofol for TIVA (KMP-TIVA) was demonstrated to provide better maintenance of surgical anesthesia and decreased the requirement of propofol compared to propofol infusion alone in horses [30]. Another study reported that cardiovascular function was preserved within acceptable values during extended KMP-TIVA in horses [31]. However, detailed evaluation of cardiovascular function has not been reported in horses undergoing a surgical procedure. Furthermore, propofol Cpl has not been examined in horses anesthetized or recovering from KMP-TIVA. The purpose of the study reported here was to determine cardiovascular effects of KMP-TIVA during surgery in horses.
MATERIALS AND METHODS
Animals: Five healthy Thoroughbred horses (3 mares and 2 stallions; mean ± SD weight was 482 ± 53 kg [range, 428 to 564 kg]; mean age was 11.2 ± 8.4 years [range, 2 to 18 years old]) were used for the present study. Food but not water was withheld from the horses for 12 hr before anesthesia. Horses were cared for according to principles of the Guide for the Care and Use of Laboratory Animals prepared by Rakuno Gakuen University. The Animal Care and Use Committee of School of Veterinary Medicine, Rakuno Gakuen University approved the study (Approved No. 3-2003).
Anesthesia and surgical procedure: Each horse was premedicated with medetomidine (Domitor; Meiji Seika Co., Tokyo, Japan) (5 µg/kg, IV) via a 14-gauge, 13.3-cm catheter (BD Angiocath; Becton-Dickinson Sys Inc., Sandy, UT, U.S.A.) placed percutaneously into a jugular vein. Five minutes later, anesthesia was induced with midazolam (Midazolam injection 0.5%; Fuji Seiyaku Co., Tokyo, Japan) (0.04 mg/kg, IV) and ketamine (Ketalar 100; Sankyo Co., Tokyo, Japan) (2.5 mg/kg, IV). The horses were orotracheally intubated, positioned in left lateral recumbency on an inflated airbed surgical table and connected to a large animal circle anesthesia system that incorporated a ventilator (Mallard Medical ventilator Rachel Model 2800 L.A.A.V; Mallard Medical Inc., Redding, CA, U.S.A.) and breathed 100% oxygen (5 l/min). A loading dose of propofol (Rapinovet; Schering-Plough Animal Health Co., Tokyo, Japan) (0.5 mg/kg, IV) was administered to all the horses, and a constant rate infusion (CRI) of ketamine (1 mg/kg/hr) and medetomidine (1.25 µg/kg/hr) and propofol was started (KMP-TIVA); the CRI of the ketamine and medetomidine (KM) drug combination was administered by use of a syringe infusion pump (STC-521; Terumo Co., Tokyo, Japan). Propofol was administered with an infusion pump (Subratek 3030; JMS, Hiroshima, Japan): the infusion rate adjusted as needed to prevent response to surgical stimulation. The initial rate of propofol infusion was 0.2 mg/kg/min. The CRI of propofol was increased by 0.025 mg/kg/min when spontaneous movement occurred. A bolus of propofol (200 mg, IV) was administered when the movement remained uncontrolled by increasing the infusion rate of propofol. The rate of propofol infusion was decreased by 0.025 mg/kg/min when purposeful movement was not detected during surgery i.e. decreased in increments until the lowest effective level was reached.
Lactated Ringer’s solution (Solulact; Terumo Co.) was administered IV at a rate of 10 ml/kg/hr to all horses. The urinary bladder was catheterized to maintain an empty bladder during anesthesia. Breathing was controlled using intermittent positive pressure ventilation (IPPV) after induction to anesthesia and adjusted to maintain the partial pressure of arterial carbon dioxide (PaCO2) at 40 to 50 mmHg until the end of anesthesia. The respiratory rate was set at 6 breaths/min, tidal volume at 15 ml/kg and peak intra airway pressure of 20 to 25 cmH2O.
Following aseptic preparation of the operative site and instrumentation (mentioned later), the same person (K.Y.) performed the surgical translocations of the right carotid artery in all the horses. A 30 cm long skin incision was made immediately dorsal to the right jugular furrow in each horse at approximately 60 min after the induction of anesthesia. The right carotid artery was identified and surgically relocated to a subcutaneous position. The KMP-TIVA, monitoring and fluid administration were discontinued shortly after completing the carotid artery translocation surgery. The horses were transported to a 3.5 m × 3.5 m padded recovery stall. The endotracheal tube was removed when horses regained the swallowing reflex. All horses recovered with assistance using head and tail ropes. Flunixin meglumine (Banamine 5%; Dai Nippon Seiyaku Co., Osaka, Japan) (1 mg/kg, IV) and penicillin G procaine (4 × 106 U/horse, IM) combined with dihydrostreptomycin sulfate (5 g/horse, IM) (Mycillinsol Meiji; Meiji Seika Co., Ltd.) were administered every 12 hr for 3 days.
Instrumentations for measuring cardiorespiratory parameters: All horses were instrumented with vascular catheters for sampling arterial blood and measurement of hemodynamic data during the first 30 to 60 min of KMP-TIVA. The area over the right jugular furrow and the right metatarsal were clipped and prepared aseptically. An 18-gauge catheter (Supercath; Medikit Co., Tokyo, Japan) was placed in the right dorsal third of the metatarsal artery. An 8-french introducer (Exacta percutaneous sheath introducer 8Fr; Ohmeda, Swindon, U. K.) was placed in the right jugular vein, and a 9-french introducer (Exacta percutaneous sheath introducer 9Fr; Ohmeda) was placed in the right jugular vein 30 cm proximal to the 8-french introducer. A triple-lumen 7-french 100-cm Swan-Ganz ballooned-tipped thermistor catheter (Criti-Cath SP-5107; Ohmeda) was placed in the pulmonary artery via an 8-french introducer and advanced into the jugular vein and into the pulmonary artery. Catheter position was confirmed by the characteristic pressure wave for each heart’s chamber. An 8-french 100-cm catheter (Intervec super guiding catheter; Fuji Systems Co., Tokyo, Japan) was placed in the right atrium via the 9-french introducer. The distance between the tip of the Swan-Ganz catheter and the 8-french catheter was adjusted to 40–50 cm. The proximal end of the Swan-Ganz, right atrial and arterial catheters were connected to saline (0.9%NaCl) solution-filled extension tubing to pressure transducers (CDX-A90; Cobe Laboratories, Tokyo, Japan) and a hemodynamic monitor (DS-5300; Fukuda Denshi, Tokyo, Japan). The transducers were positioned at the level of the heart before calibration.
Cardiorespiratory measurements: Cardiovascular measurements and arterial blood gas data were recorded every 20 min and during 2.5 to 3.0 hr of anesthesia. Arterial blood pressure (ABP; mmHg), pulmonary artery pressure (PAP; mmHg) and right atrial pressure (RAP; mmHg) were measured by connecting the catheters placed in the dorsal metetarsal artery, pulmonary artery and right atrium, respectively, to pressure transducers and a hemodynamic monitor. Cardiac output (CO; l/min) was measured by the thermodilution technique [22] using 40 ml of 0°C 5% dextrose injected manually for approximately 2 sec through an 8-french catheter placed in the right atrium. The CO was measured at least 3 times, and the mean value was calculated. Heart rate (HR; beats/min), electrocardiogram (Apex-Base lead), ABP, PAP, RAP and CO were recorded at predetermined times. Cardiac index (CI; ml/kg/min), stroke volume (SV; ml/beat) and systemic vascular resistance (SVR; dynes/sec/cm5) were calculated using standard formulas [27]. Arterial blood samples were anaerobically collected from the dorsal metatarsal artery into heparinized syringes for immediate blood gas (PaO2 and PaCO2) and pH analysis (Rapidlab 348; Bayer Medical Co., Tokyo, Japan).
Measurements of plasma propofol concentration: Arterial blood samples (8 ml) were collected from the catheter placed in the dorsal metatarsal artery at selected time intervals for determination of plasma propofol concentration. The selected sampling times were: just before skin incision, 30 and 60 min after beginning surgery, at the end of surgery, at end of propofol infusion, 10 min after the cessation of propofol infusion, after removal of the endotracheal tube and just after the horses regained a standing position.
Plasma concentration (Cpl) of propofol was determined by high performance liquid chromatography (HPLC) using fluorescence detection [25, 32]. The blood samples were mixed with heparine sodium (10 unit per 1 ml of blood) and centrifuged (1,580 × g for 15 min) immediately to separate plasma. Then, the plasma samples were stored at −80°C. Each plasma sample (200 µl) was mixed with 100% methanol (400 µl) and the top clear layer (300 µl) extracted after centrifugation (1,580 × g for 15 min). Another 100% methanol (400 µl) was mixed with the precipitate and the top clear layer (300 µl) again separated by centrifugation. These extracts were combined, and 200 µl of the extract was mixed with purified water (600 µl) and stored at −80°C until HPLC analysis.
The instruments using HPLC analysis consisted of dual pumps (DP-8020; Tosoh Co.), auto-sampler (AS-8020; Tosoh Co.), reversed-phase column (TSK-GEL ODS-80TS; Tosoh Co.), integration software (LC8020; Tosoh Co.), degasser (GASTORR 702; Eyela Co.) and intelligent fluorescence detector (FP-2020 plus; JASCO Co., Tokyo, Japan). Propofol within each extract sample was separated with the reversed-phase column using a linear gradient mobile phase from methanol-water-ammonium acetate (24: 75.94: 0.06) to 100% methanol delivered at 1 ml/min and detected by the fluorescence detector set at 276 nm (excitation) and 310 nm (emission). The limit of detection for propofol was 0.05 µg/ml. The apparent elimination half-life (terminal half-life: t1/2) was calculated from the terminal log-linear portion of the post-infusion propofol plasma decay curve.
Evaluation of quality of anesthesia and recovery: The quality of anesthetic induction, transition to TIVA (the first 20 min of anesthesia), anesthetic maintenance (from the first 20 min to the end of anesthesia i.e drug infusions stopped) and recovery from anesthesia were evaluated using scoring system [30] (Table 1). The times from the cessation of anesthesia to extubation, first movement, sternal position and standing were recorded.
Table 1. Criteria for scoring the quality of anesthetic induction, transition to infusion, maintenance of anesthesia and recovery from anesthesia following TIVA in horses.
| Score | Criteria |
|---|---|
| Anesthetic induction | |
| 0 (Poor) | Ataxia and paddling; danger to horse and handler |
| 1 (Fair) | Purposeful paddling with or without attempts to regain feet |
| 2 (Satisfactory) | Ataxia with or without paddling |
| 3 (Good) | Horse takes 1 or 2 steps before falling to ground; no paddling |
| 4 (Excellent) | Horse sinks smoothly to the ground |
| Transition to infusion | |
| 0 (Poor) | Multiple incremental bolus IV doses (200 mg each) of propofol needed |
| 1 (Fair) | One or 2 additional bolus IV doses of propofol needed during first 20 min |
| 2 (Good) | Appeared to be in light plane of anesthesia; responded to a bolus IV injection of propofol |
| 3 (Excellent) | Smooth transition; additional propofol injection not required |
| Anesthetic maintenance | |
| 0 (Poor) | Multiple incremental IV bolus doses (200 mg each) of propofol required to maintain surgical plane of anesthesia |
| 1 (Fair) | One or 2 additional bolus IV doses of propofol (within a period of 5 min) to control movement after the first 20 min |
| 2 (Good) | Appeared to be in light plane of anesthesia; responded to a bolus IV injection of propofol |
| 3 (Excellent) | Smooth anesthetic period; depth of anesthesia responded to increase or decrease in propofol infusion rate |
| Anesthetic recovery | |
| 0 (Unable to stand) | Horse cannot stand for >2 hr after multiple attempts to stand; excitement is evident; injury or high risk of injury |
| 1 (Poor) | Multiple attempts to stand; excitement is evident; high risk of injury |
| 2 (Fair) | Multiple attempts to stand; substantial ataxia |
| 3 (Satisfactory) | Stands after 1 to 3 attempts; prolonged ataxia but no excitement |
| 4 (Good) | Stands after 1 or 2 attempts; mild, short-term ataxia |
| 5 (Excellent) | Stands after first attempt; no ataxia |
Statistical analysis: Data are expressed as mean ± SD. A repeated measures ANOVA was used to compare changes in cardiovascular and respiratory data. A Student paired t-test was used to determine differences between time points when appropriate. Differences were considered significant when P<0.05.
RESULTS
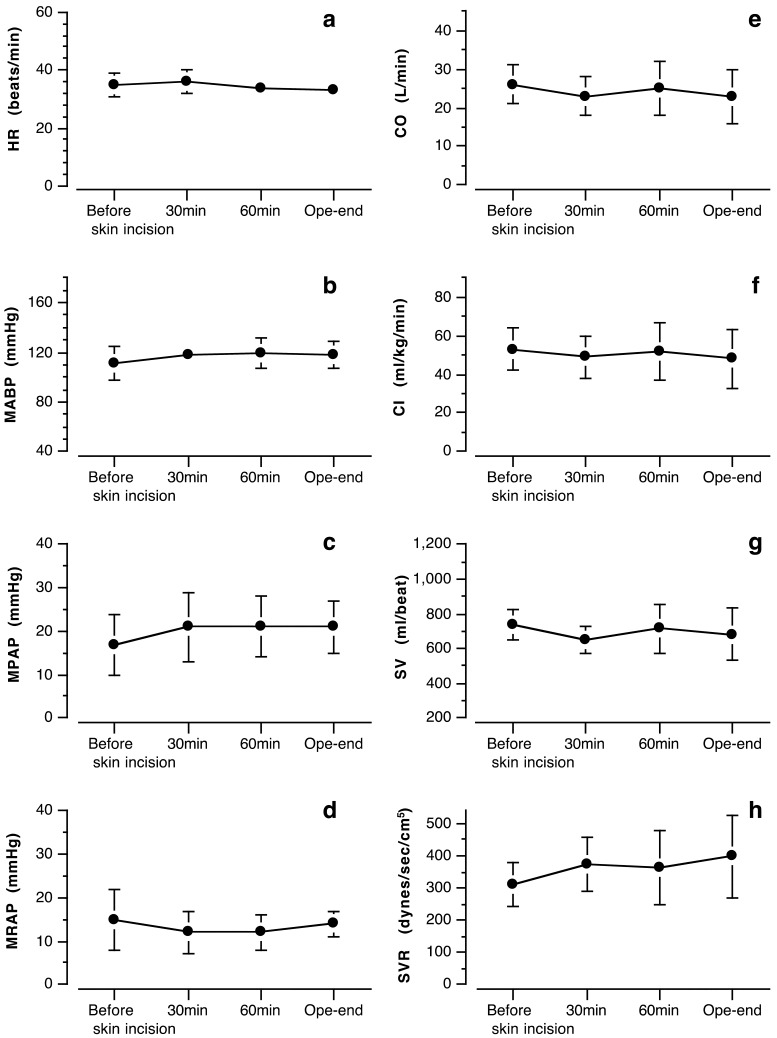
Cardiovascular effects: Cardiovascular parameters were stable and preserved within normal limits for anesthetized horses (Fig. 1). Mean values of HR ranged from 33 to 37 beats/min, and mean ABP (MABP) ranged from 111 to 119 mmHg in all horses. CI and SV values ranged from 48 to 53 ml/kg/min and from 650 to 800 ml/beat during surgery, respectively. SVR ranged from 311 to 398 dynes/sec/cm5. Mean PAP (MPAP) and mean RAP (MRAP) ranged from 17 to 21 mmHg and from 12 to 15 mmHg during surgery, respectively (Fig. 1).
Fig. 1.
Cardiovascular values during surgery in 5 horses undergoing KMP-TIVA. Plots and error bars showed mean value and standard deviation from 5 horses, respectively. HR: heart rate, MABP: mean arterial blood pressure, MPAP: mean pulmonary artery pressure, MRAP: mean right atrial pressure, CO: cardiac output, CI: cardiac index, SV: stroke volume, SVR: systemic vascular resistance, Before skin incision: just before skin incision, 30 min: 30 min into surgery, 60 min: 60 min into surgery, Ope-end: at the end of operation.
Respiration and arterial blood gases: The mean time after induction of anesthesia to the start of IPPV was 15.8 ± 2.9 min. The PaCO2 and PaO2 were maintained at 49 to 54 mmHg and 581 to 617 mmHg, throughout anesthesia, respectively (Table 2).
Table 2. Respiratory rate, blood gas values and arterial pH (mean ± SD) in 5 horses on IPPV undergoing carotid artery translocation via KMP-TIVA.
| Variable | Pre value | Before skin incision | 30 min | 60 min | Ope-end |
|---|---|---|---|---|---|
| RR (breaths/min) | 15 ± 3 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| pHa | ND | 7.45 ± 0.04 | 7.46 ± 0.04 | 7.47 ± 0.04 | 7.48 ± 0.05 |
| PaCO2 (mmHg) | ND | 54 ± 5 | 51 ± 7 | 51 ± 7 | 49 ± 6 |
| PaO2 (mmHg) | ND | 598 ± 127 | 581 ± 115 | 589 ± 109 | 617 ± 111 |
Data showed mean ± standard deviation from 5 horses. Pre value was measured before any medications were administered. ND: Not determined. Before skin incision: Just before skin incision, 30 min: 30 min into surgery, 60 min: 60 min into surgery, Ope-end: At the end of operation. RR: Respiratory rate, pHa: Arterial blood pH, PaCO2: Partial pressure of arterial carbon dioxide. PaO2: Partial pressure of arterial oxygen.
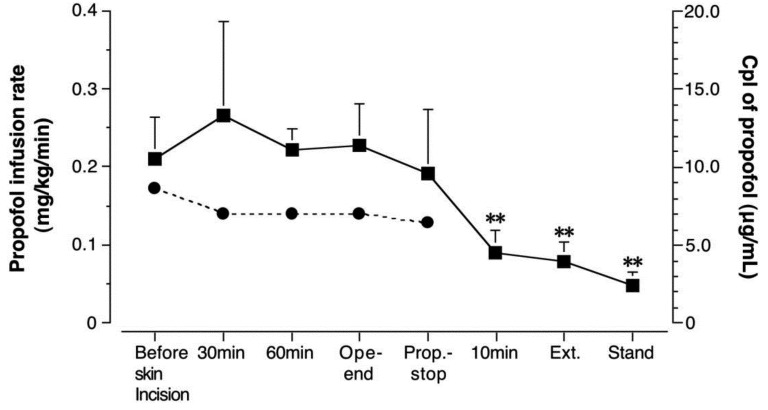
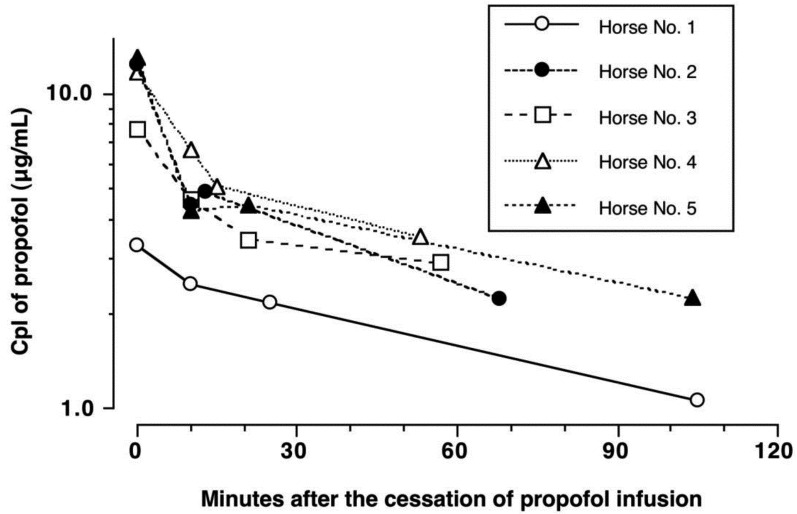
Propofol infusion rate and plasma concentration: The CRI of propofol ranged from 0.17 ± 0.01 mg/kg/min before skin incision to 0.13 ± 0.01 mg/kg/min at the time of cessation of propofol infusion (Fig. 2). Propofol Cpl was 10.6 ± 2.6 µg/ml just before skin incision, 13.3 ± 6.0 µg/ml at 30 min, 11.1 ± 1.4 µg/ml at 60 min and 11.4 ± 2.7 µg/ml at the end of surgery. The propofol Cpl declined rapidly after cessation of propofol infusion. The propofol Cpl was significantly decreased (P<0.01) at 10 min after the cessation of KMP-TIVA (4.5 ± 1.5 µg/ml), extubation (4.0 ± 1.2 µg/ml) and after standing (2.4 ± 0.9 µg/ml) compared to the end of surgery. The t1/2 for propofol was estimated to be 76.6 ± 18.8 min (Fig. 3).
Fig. 2.
Propofol infusion rates and plasma concentration (Cpl) in 5 horses undergoing KMP-TIVA. Closed circles showed mean value of propofol infusion rates from 5 horses. Closed squares and error bars showed mean value of Cpl and its standard deviation, respectively. Values significantly (**P<0.01) lower than the Cpl of propofol at the end of operation. Before skin incision (just before skin incision); 30 min (30 min after beginning surgery); 60 min (60 min after beginning surgery); Ope-end (at the end of operation); Prop.-stop (at the end of propofol infusion); 10 min (10 min after cessation of propofol administration), Ext (just after extubation); Stand (just after the horses stood).
Fig. 3.
Semilogarithmic plots of propofol plasma concentration (Cpl) versus time after the cessation of propofol infusion in individual 5 horses anesthetized with KMP-TIVA for surgery. The terminal half-life of propofol (t1/2) was estimated to be 80 min for Horse No.1, 68 min for Horse No.2, 85 min for Horse No.3, 50 min for Horse No.4 and 100 min for Horse No.5 (mean ± SD: 76.6 ± 18.8 min).
Quality of anesthesia and anesthetic events: Induction to anesthesia was smooth and excitement-free with adequate muscle relaxation (score 4) in all horses. All horses became recumbent within 1–2 min from the time of ketamine-midazolam administration. There were no limb movements and/or head shaking after becoming recumbent. Transition to the KMP-TIVA was uneventful (score 3) in all horses. The horses remained anesthetized for 175 ± 14 min (range from 160 to 197 min). The quality of anesthesia was considered to be excellent (score 3) in all horses. The times to extubation, first movement and sternal recumbency were 19 ± 5 (ranged from 13 to 25) min, 15 ± 8 (ranged from 5 to 24) min and 65 ± 29 (ranged from 38 to 99) min, respectively. The number of attempts to stand and time to standing after the cessation of KMP-TIVA were 1.2 ± 0.4 (ranged from 1 to 2) and 74 ± 28 (ranged from 42 to 105) min, respecively. The quality of recovery from anesthesia was judged to be excellent or good, and recovery was uneventful. One horse had a recovery score of 5 (excellent), whereas 4 horses had a recovery score of 4 (good).
DISCUSSION
The administration of KMP-TIVA produced stable and satisfactory anesthesia for surgery in all our horses. Cardiovascular function in the horses remained within acceptable ranges for healthy resting horses [27], and hypoventilation and hypoxia were effectively prevented by IPPV. The recovery from KMP-TIVA was uneventful, and its quality was excellent to good. However, the longer duration of anesthesia resulted in longer recovery, although the propofol Cpl declined rapidly after the cessation of anesthesia.
Nolan and Hall [23] conjectured that the 95% of effective dose of propofol to prevent a response to surgical stimulus for horses might be greater than 0.28 mg/kg/min when horses are anesthetized with propofol alone. The propofol infusion rates required to maintain surgical anesthesia ranged from 0.18 ± 0.04 mg/kg/min to 0.22 ± 0.03 mg/kg/min in horses premedicated with α2-adrenoceptor agonists and induced to anesthesia with either midazolam (0.04 mg/kg IV) and ketamine (2.5 mg/kg IV) or propofol (2 mg/kg IV) [23, 30]. We were able to achieve, an excellent surgical plane of anesthesia with a lower infusion rate of propofol (0.13 mg/kg/min) in horses anesthetized with KMP-TIVA, and this finding is similar to our previous report in horses anesthetized with KMP-TIVA for the surgical translocation of their carotid artery [30]. The reduction in propofol requirements is most likely due to the anesthetic and analgesic interactive effects of propofol when combined with ketamine and medetomidine. Ketamine, a dissociative anesthetic drug, induces analgesia by antagonizing N-methyl-D-aspartate (NMDA) receptors [14]. Medetomidine is an α2-adrenoceptor agonist that activates α2-receptors located in the spinal cord and brain stem, resulting in sedation and analgesia [8].
To determine pharmacokinetic parameters, plasma concentrations of drugs are usually measured, because plasma is more readily handled and stored than blood. However, blood levels of propofol were measured in previous reports [10, 24], because the measurement of the drug in plasma is reported to yield propofol concentrations lower than in blood, indicating that propofol is more intimately associated with the red blood cells. On the other hand, plasma concentration monitoring is recommended during propofol infusion, but immediate centrifugation is needed [9]. In the present study, we recorded somewhat higher propofol concentrations (11 to 13 µg/ml) compared with previous studies that reported the blood concentration of propofol ranged from 2.26 to 6.45 µg/ml [24] and 3.27 to 9.44 µg/ml [10] during 60 min infusion of propofol in ponies. The plasma propofol concentrations at extubation and standing in the present study were also higher than the values obtained in the previous reports [10, 24]. Those reports [10, 24] were carried out in ponies, weighing 85 to 196 kg and 250 to 280 kg, while the study reported here was investigated in thoroughbred, weighing 428 to 564 kg. This difference may influence the pharmacokinetics and pharmacodynamics of propofol. Furthermore, it was pointed out that the concentration of propofol could be underestimated when whole blood was used during propofol infusion because plasma concentrations of propofol remained 30% higher than whole blood concentrations [9].
The t1/2 for propofol was reported to be 69.0 ± 8.0 min in ponies premedicated with detomidine 20 µg/kg IV, given ketamine 2.2 mg/kg IV for induction of anesthesia, followed by a bolus injection of propofol 0.5 mg/kg IV and maintained with an infusion of propofol at 0.136 mg/kg/min for 60 min and ketamine at 3 mg/kg/min for 45 min [24]. These ponies stood up at 30.0 ± 20.8 min after the cessation of anesthesia [24]. Smooth and satisfactory recovery after castration was also reported in ponies premedicated with detomidine 20 µg/kg IV, given ketamine 2.2 mg/kg IV for induction of anesthesia, followed by a bolus injection of propofol 0.5 mg/kg IV and maintained with an infusion of propofol at 0.124 mg/kg/min and ketamine at 2.4 mg/kg/min for 60 min [10]. These ponies stood up at 14.9 ± 10.1 min after the cessation of anesthesia [10]. Compared to these reports in ponies, the recovery was longer in our horses anesthetized with KMP-TIVA for about 3 hr. On the other hand, the Cpl of propofol declined rapidly after the cessation of KMP-TIVA, and the estimated t1/2 of propofol in the present study (76.6 ± 18.8 min) is similar to that reported in a previous study in ponies [24]. The duration of propofol infusion may not affect the clearance of propofol after the cessation of infusion in horses. The longer duration of KMP-TIVA might induce larger degrees of accumulation of ketamine and medetomidine, thus influencing rate of recovery from anesthesia in the present study.
Systemic hypotension and hypotensive events, MABP <50 to 60 mmHg, are responsible for most drug-related complications in anesthetized horses [7, 11, 15, 21, 36]. Marked reductions in MABP and tissue blood flow (CO) are responsible for the development of myopathy, myositis, rhabdomyolysis, spinal cord ischemia and degeneration, spinal cord malacia, cerebral necrosis, transient or permanent blindness, acute cardiac collapse and prolonged recovery from anesthesia [7, 11, 15, 21, 36]. Alternatively, MABP values in excess of 70 mmHg during anesthesia are considered to be important for preventing post-operative myopathy in horses [7, 11, 15, 21, 36]. In the present study, the MABP ranged from 111 to 118 mmHg during surgery and other cardiovascular parameters, except for the SVR remained within acceptable ranges for healthy resting horses [27]. Mean ± standard deviation of SVR in standing, conscious, unsedated healthy resting horses are 262.4 ± 63.4 and 265 ± 81 dynes/sec/cm5 [27]. The SVR during KMP-TIVA mildly increased and was ranged around its upper limit for the healthy resting horses [27]. This mild increase in SVR and preserved CO within the acceptable range contributed to the good ABP in the horses anesthetized with KMP-TIVA for surgery. KMP-TIVA provided a minimum cardiovascular depression in horses undergoing surgery.
Surgical stimulation causes stress and adrenergic responses resulting increases in HR and ABP when anesthetic and/or analgesic level is insufficient in patients. In the present study, the cardiovascular changes throughout anesthesia were similar to our previous report in horses anesthetized with KMP-TIVA for 4 hr without surgical stimulation [31]. Therefore, the adrenergic response to surgical stimulation seemed to be completely controlled in our horses. It has been demonstrated that TIVA using a combination of detomidine, an α2-adrenoceptor agonist, ketamine and guaifenesin caused less cardiorespiratory depression than halothane anesthesia and reduced stress as suggested by decreases in plasma arginine vasopressin, β-endorphin, ACTH, cortisol and catecholamine concentrations during anesthesia [16, 29]. Medetomidine has a greater selectivity of α2-adrenoceptors and comparative behavioral and neurochemical effects that suggest less endocrine changes during prolonged anesthesia in horses [5, 8, 33]. Ketamine produces an apparent analgesia by antagonizing NMDA receptors [14]. We inferred that KMP-TIVA provided sufficient anesthesia and analgesia and produced reduction of the stress induced by surgical stimulation.
Most spontaneously breathing horses hypoventilate during anesthesia. Drug-induced decreases in respiratory rate or tidal volume produce hypercarbia, which can result in respiratory acidosis and, when severe, can produce hypoxemia and lactic acidosis [21]. The maximum value that the PaCO2 should be allowed to increase before instituting controlled ventilation remains controversial [26]. PaCO2 values greater than 70 mmHg may activate the central nervous system, making it more difficult to maintain a stable plane of anesthesia [21]. Initiating controlled ventilation early during the maintenance phase of anesthesia provides the best opportunity to prevent hypercarbia and maximize PaO2 [6, 21]. However, controlling ventilation may decrease CO and ABP secondary to increases in intrathoracic pressure, which can decrease venous return [13, 21]. Our previous study demonstrated that horses administered KMP-TIVA and spontaneously breathing room air (21% O2) developed hypercarbia (PaCO2 range, 47 to 77 mmHg) and hyoxemia (PaO2 range, 27 to 77 mmHg) shortly after induction of anesthesia [30]. That study and data from the current study suggest that hypoventilation may be a consequence of KMP-TIVA that hypercarbia and hypoxemia can be effectively prevented by IPPV and that cardiovascular function is maintained within acceptable limits for horses. Importantly, KMP-TIVA preserved cardiovascular function in horses even with controlled ventilation.
Our data demonstrated that induction of anesthesia with medetomidine-ketamine-midazolam and maintenance of anesthesia with KMP-TIVA provided satisfactory quality and control of anesthesia with minimum cardiovascular depression in horses undergoing surgery. However, the longer duration of KMP-TIVA may result in longer recovery.
Acknowledgments
Dr. Umar was supported by a study fellowship awarded by Monbukagakusho, Tokyo, Japan.
REFERENCES
- 1.Bettschart-Wolfensberger R., Bowen M. I., Freeman S. L., Feller R., Bettschart R. W., Nolan A., Clarke K. W.2001. Cardiopulmonary effects of prolonged anesthesia via propofol-medetomidine infusion in ponies. Am. J. Vet. Res. 62: 1428–1435. doi: 10.2460/ajvr.2001.62.1428 [DOI] [PubMed] [Google Scholar]
- 2.Bettschart-Wolfensberger R., Bowen M. I., Freeman S. L., Weller R., Clarke K. W.2003. Medetomidine-ketamine anaesthesia induction followed by medetomidine-propofol in ponies: infusion rates and cardiopulmonary side effects. Equine Vet. J. 35: 308–313. doi: 10.2746/042516403776148354 [DOI] [PubMed] [Google Scholar]
- 3.Bettschart-Wolfensberger R., Freeman S. L., Jäggin-Schmucker N., Clarke K. W.2001. Infusion of a combination of propofol and medetomidine for long-term anesthesia in ponies. Am. J. Vet. Res. 62: 500–507. doi: 10.2460/ajvr.2001.62.500 [DOI] [PubMed] [Google Scholar]
- 4.Bettschart-Wolfensberger R., Kalchofner K., Neges K., Kästner S., Fürst A.2005. Total intravenous anesthesia in horses using medetomidine and propofol. Vet. Anaesth. Analg. 32: 348–354. doi: 10.1111/j.1467-2995.2005.00202.x [DOI] [PubMed] [Google Scholar]
- 5.Bryant C. E., England G. C. W., Clarke K. W.1991. Comparisons of the sedative effects of medetomidine and xylazine in horses. Vet. Rec. 129: 421–423. doi: 10.1136/vr.129.19.421 [DOI] [PubMed] [Google Scholar]
- 6.Day T. K., Gaynor J. S., Muir W. W., 3rd, Bednarski R. M., Mason D. E.1995. Blood gas values during intermittent positive pressure ventilation and spontaneous ventilation in 160 anesthetized horses positioned in lateral or dorsal recumbency. Vet. Surg. 24: 266–276. doi: 10.1111/j.1532-950X.1995.tb01330.x [DOI] [PubMed] [Google Scholar]
- 7.Duke T., Filzek U., Read M. R., Read E. K., Ferguson J. G.2006. Clinical observation surrounding an increased incidence of postanesthetic myopathy in halothane-anesthetized horses. Vet. Anaesth. Analg. 33: 122–127. doi: 10.1111/j.1467-2995.2005.00189.x [DOI] [PubMed] [Google Scholar]
- 8.England G. C. W., Clarke K. W.1996. Alpha2 adrenoceptor agonists in the horse − a review. Br. Vet. J. 152: 641–657. doi: 10.1016/S0007-1935(96)80118-7 [DOI] [PubMed] [Google Scholar]
- 9.Fan S. Z., Yu H. Y., Chen Y. L., Liu C. C.1995. Propofol concentration monitoring in plasma or whole blood by gas chromatography and high-perfomance liquid chromatography. Anesth. Analg. 81: 175–178. [DOI] [PubMed] [Google Scholar]
- 10.Flaherty D., Reid J., Welsh E., Monteiro A. W., Lerche P., Nolan A.1997. A pharmacodynamic study of propofol or propofol and ketamine in ponies undergoing surgery. Res. Vet. Sci. 62: 179–184. doi: 10.1016/S0034-5288(97)90143-0 [DOI] [PubMed] [Google Scholar]
- 11.Grandy J. L., Steffey E. P., Hodgson D. S., Woliner M. J.1987. Arterial hypotension and the development of postanesthetic myopathy in halothan-anesthetized horses. Am. J. Vet. Res. 48: 192–197. [PubMed] [Google Scholar]
- 12.Greene S. A., Thurmon J. C., Tranquilli W. J., Benson G. J.1986. Cardiopulmonary effects of continuous intravenous infusion of guaifenesin, ketamine, and xylazine in ponies. Am. J. Vet. Res. 47: 2364–2367. [PubMed] [Google Scholar]
- 13.Hodgson D. S., Steffey E. P., Grandy J. L., Woliner M. J.1986. Effects of spontaneous, assisted, and controlled ventilatory modes in halothane-anesthetized geldings. Am. J. Vet. Res. 47: 992–996. [PubMed] [Google Scholar]
- 14.Klepstad P., Maurset A., Moberg E. R., Oye I.1990. Evidence of a role for NMDA receptors in pain perception. Eur. J. Pharmacol. 187: 513–518. doi: 10.1016/0014-2999(90)90379-K [DOI] [PubMed] [Google Scholar]
- 15.Lindsay W. A., Robinson G. M., Brunson D. B., Brunson D. B., Majors L. J.1989. Induction of equine postanesthetic myositis after halothane-induced hypotension. Am. J. Vet. Res. 50: 404–410. [PubMed] [Google Scholar]
- 16.Luna S. P. L., Taylor P. M., Wheeler M. J.1996. Cardiorespiratory, endocrine and metabolic changes in ponies undergoing intravenous or inhalation anesthesia. J. Vet. Pharmacol. Ther. 19: 251–258. doi: 10.1111/j.1365-2885.1996.tb00046.x [DOI] [PubMed] [Google Scholar]
- 17.Mama K. R., Pascoe P. J., Steffey E. P., Kollias-Baker C.1998. Comparison of two techniques for total intravenous anesthesia in horses. Am. J. Vet. Res. 59: 1292–1298. [PubMed] [Google Scholar]
- 18.Mama K. R., Steffey E. P., Pascoe P. J.1995. Evaluation of propofol as a general anesthetic for horses. Vet. Surg. 24: 188–194. doi: 10.1111/j.1532-950X.1995.tb01317.x [DOI] [PubMed] [Google Scholar]
- 19.Mama K. R., Wagner A. E., Steffey E. P., Kollias-Baker C., Hellyer P. W., Golden A. E., Brevard L. F.2005. Evaluation of xylazine and ketamine for total intravenous anesthesia in horses. Am. J. Vet. Res. 66: 1002–1007. doi: 10.2460/ajvr.2005.66.1002 [DOI] [PubMed] [Google Scholar]
- 20.Matthews N. S., Hartsfield S. M., Hague B., Carroll G. L., Short C. E.1999. Detomidine-propofol anesthesia for abdominal surgery in horses. Vet. Surg. 28: 196–201. doi: 10.1053/jvet.1999.0196 [DOI] [PubMed] [Google Scholar]
- 21.Muir W. W., Hubbell J. A. E.2009. Anesthetic-associated complications. pp. 397–417. In: Equine Anesthesia: Monitoring and Emergency Therapy, 2nd ed. (Muir, W. W. and Hubbell, J. A. E. eds.), Mosby-Year Book, St. Louis. [Google Scholar]
- 22.Muir W. W., Skarda R. T., Milne D. W.1976. Estimation of cardiac output in the horse by thermodilution techniques. Am. J. Vet. Res. 37: 697–700. [PubMed] [Google Scholar]
- 23.Nolan A. M., Hall L. W.1985. Total intravenous anaesthesia in the horse with propofol. Equine Vet. J. 17: 394–398. doi: 10.1111/j.2042-3306.1985.tb02533.x [DOI] [PubMed] [Google Scholar]
- 24.Nolan A., Reid J., Welsh E., Flaherty D., McComack R., Monteiro A. M.1996. Simultaneous infusions of propofol and ketamine in ponies premedicated with detomidine: a pharmacokinetic study. Res. Vet. Sci. 60: 262–266. doi: 10.1016/S0034-5288(96)90051-X [DOI] [PubMed] [Google Scholar]
- 25.Plummer G. F.1987. Improved method for the determination of propofol in blood by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 421: 171–176. doi: 10.1016/0378-4347(87)80394-8 [DOI] [PubMed] [Google Scholar]
- 26.Rogovik A., Goldman R.2008. Permissive hypercapnia. Emerg. Med. Clin. North Am. 26: 941–952. doi: 10.1016/j.emc.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Schwartzward C. C., Bonagura J. D., Muir W. W.2009. The cardiovascular system. pp. 37–100. In: Equine Anesthesia: Monitoring and Emergency Therapy, 2nd ed. (Muir, W. W. and Hubbell, J. A. E. eds.), Mosby-Year Book, St. Louis. [Google Scholar]
- 28.Steffey E. P., Howland D.1980. Comparison of circulatory and respiratory effects of isoflurane and halothane anaesthesia in horses. Am. J. Vet. Res. 41: 821–825. [PubMed] [Google Scholar]
- 29.Taylor P. M., Luna S. P. L., Sear J. W., Wheeler M. J.1995. Total intravenous anesthesia in ponies using detomidine, ketamine and guaifenesin: pharmacokinetics, cardiopulmonary and endocrine effects. Res. Vet. Sci. 59: 17–23. doi: 10.1016/0034-5288(95)90024-1 [DOI] [PubMed] [Google Scholar]
- 30.Umar M. A., Yamashita K., Kushiro T., Muir W. W.2006. Evaluation of total intravenous anesthesia with propofol or ketamine-medetomidine-propofol combination in horses. J. Am. Vet. Med. Assoc. 228: 1221–1227. doi: 10.2460/javma.228.8.1221 [DOI] [PubMed] [Google Scholar]
- 31.Umar M. A., Yamashita K., Kushiro T., Muir W. W.2007. Evaluation of cardiovascular effects of total intravenous anesthesia with propofol or a combination of ketamine-medetomidine-propofol in horses. Am. J. Vet. Res. 68: 121–127. doi: 10.2460/ajvr.68.2.121 [DOI] [PubMed] [Google Scholar]
- 32.Yamashita K., Akashi N., Katayama Y., Uchida Y., Umar M. A., Itami T., Inoue H., Sams R. A., Muir W. W.2009. Evaluation of bispectral index (BIS) as an indicator of central nervous system depression in horses anesthetized with propofol. J. Vet. Med. Sci. 71: 1465–1471. doi: 10.1292/jvms.001465 [DOI] [PubMed] [Google Scholar]
- 33.Yamashita K., Kishihara K., Haramaki S., Tsukiyama K., Tagami M., Izumisawa Y., Kotani T.1999. Comparison of the sedative effects of medetomidine, detomidine and xylazine in horses. J. Jpn. Vet. Med. Assoc. 52: 498–503(in Japanese with English summary). doi: 10.12935/jvma1951.52.498 [DOI] [Google Scholar]
- 34.Yamashita K., Muir W. W.2009. Intravenous anesthetic and analgesic adjuncts to inhalation anesthesia. pp. 260–276. In: Equine Anesthesia: Monitoring and Emergency Therapy, 2nd ed. (Muir, W. W. and Hubbell, J. A. E. eds.), Mosby-Year Book, St. Louis. [Google Scholar]
- 35.Yamashita K., Wijayathilaka T. P., Kushiro T., Umar M. A., Taguchi K., Muir W. W.2007. Anesthetic and cardiopulmonary effects of total intravenous anesthesia using a midazolam, ketamine and medetomidine drug combination in horses. J. Vet. Med. Sci. 69: 7–13. doi: 10.1292/jvms.69.7 [DOI] [PubMed] [Google Scholar]
- 36.Young S. S., Taylor P. M.1993. Factors influencing the outcome of equine anesthesia: a review of 1,314 cases. Equine Vet. J. 25: 147–151. doi: 10.1111/j.2042-3306.1993.tb02926.x [DOI] [PubMed] [Google Scholar]