Abstract
Recombinant outer membrane protein H (rOmpH) is a potential fowl cholera vaccine candidate. The present study was aimed at developing rOmpH formulations for intranasal administration. The rOmpH was purified and formulated with either Escherichia coli enterotoxin B (LTB) or CpG oligodeoxynucleotides (ODN) as an adjuvant. Antibody responses in chickens intranasally immunized with rOmpH in combination with 2 different adjuvants were significantly increased (P<0.05) post immunization. Chicken survival rates showed that rOmpH formulated with ODN and LTB elicited 90% and 70% protection, respectively. Our findings indicated that rOmpH formulated with ODN elicited protection better than that formulated with LTB. Therefore, the vaccines formulations in the present study can be considered new intranasal vaccine formulations for fowl cholera in chickens.
Keywords: intranasal vaccine, Pasteurella multocida, recombinant outer membrane protein H
Pasteurella multocida, a Gram-negative facultative bacterium, is the causative agent of fowl cholera (FC) in avian species. The disease affects the poultry industry very severely as the high morbidity and the high mortality result in large economic losses. P. multocida A: 1, A: 3 and A: 4 have been found to be the major causes of fowl cholera throughout the world [14,15,16]. Commercially available vaccines, at present, are live attenuated vaccines and bacterin vaccines [5]. Live attenuated vaccines provide protective immunity, but the residual of virulence can affect the laying rate, and it is possible that an outbreak can occur. Outer membrane protein H (OmpH) is a major outer membrane protein found in the P. multocida envelope. It is a porin protein that is highly conserved among the Gram-negative bacterial species. Early studies on OmpH have found that the protein has potential as a FC vaccine candidate [6, 19]. Our previous study formulated a recombinant OmpH-based fowl cholera vaccine for chickens by intramuscular administration [19]. The route of vaccine administration plays an essential and significant role in practical usage. Administration by a parenteral route, particularly in the case of the intramuscular route, is generally practiced; however, this carries the risk of needle stick injury or pain. Mucosal vaccination is a noninvasive method and has several advantages over traditional systemic vaccines, such as less risk of needle stick injury, pain or cross-contamination [8, 13, 21]. Moreover, mucosal vaccination is widely considered to be more acceptable and simpler to administer orally or nasally than vaccination via injection. Additionally, the primary reason for using mucosal vaccines is that the mucosal surface is the first-line host defense mechanism. Enormous bacterial or viral infections inspire challenge of developing mucosal vaccines targeted at inducing local immunity against adhesion and colonization at the mucosal surface [8]. As a consequence, it is very challenging to develop new types of vaccine formulations with good efficacy, good safety, lower cost of production and practicality with regard to herd health production. Although the route of avian P. multocida infection is mainly the respiratory tract, an intranasal fowl cholera vaccine in chickens has not been formulated yet. Thus, the present study was aimed at developing an intranasal OmpH-based fowl cholera vaccine formulations by investigating the antibody responses against the vaccines and the protective efficacy in meeting the related challenges.
MATERIALS AND METHODS
Bacterial strains, plasmids, media and growth conditions: P. multocida strain X-73 (serovar A:1, ATCC15742) was grown in tryptose broth (TB; Difco Laboratories, Sparks, MD, U.S.A.) at 37°C for 6 hr and then subcultured on dextrose starch agar (DSA; Difco) at 37°C for 18 hr. E. coli strain PQE-ompH [19] was grown at 37°C in Luria-Bertani (LB) broth or on LB agar supplemented with 100 µg/ml ampicillin and 25 µg/ml kanamycin (Sigma-Aldrich, St. Louis, MO, U.S.A.).
Preparation of recombinant OmpH expressed from an E. coli host: The recombinant OmpH (rOmpH) was expressed according to our previous study [19]. Briefly, E. coli strain PQE-ompH glycerol stock was streaked on LB agar containing 100 µg/ml ampicillin and 25 µg/ml kanamycin, and incubated at 37°C for 18 hr. After incubation, a single colony was picked and inoculated into 20 ml LB broth containing 100 µg/ml ampicillin and 25 µg/ml kanamycin. The culture was grown at 37°C for 18 hr, with horizontal shaking at 210 rpm. A one-liter culture (LB broth, 100 µg/ml ampicillin and 25 µg/ml kanamycin) was inoculated at the ratio 1:50 with the overnight culture and allowed to continue growing under the same growth conditions until an OD600 of 0.5–0.7 (mid-log phase) was reached. Recombinant protein expression was subsequently induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG; Amresco, Solon, OH, U.S.A.) to a final concentration of 1 mM, and the culture was incubated for an additional 4–5 hr. Finally, the cells were harvested by centrifugation at 4,000 × g for 20 min at 4°C and kept at −20°C for further application.
Purification of rOmpH: The purification process for the recombinant protein in this study was a modified form of the electroelution method. Briefly, the cell pellets were lysed in native lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole; pH 8.0), with gentle shaking at 4°C for 1 hr. Then, the suspension was centrifuged at 10,000 rpm at 4°C for 30 min. The supernatant was saved and placed into the chamber of an electroelutor (Nativen, ATTO, Tokyo, Japan). Approximately 1,500 µg of the total protein was run on a preparative 12.5% sodium dodecyl sulfate (SDS) polyacrylamide gel column (10 mm stacking gel and 30 mm separating gel) in sample buffer (4% SDS, 50 mM Tris, 20% glycerol, 0.005% bromophenol blue). The conditions for the protein collection were calculated according to the manufacturer’s instruction (delay time, 200 min; EP time, 2 min; filling time, 100 sec; collecting time, 120 sec; 15 mA). The rOmpH fractions were collected in the collection buffer (371 mM Tris, 5% sucrose; pH 8.8) and kept at −20°C for further analysis.
Electrophoresis and immunoblotting: Samples were subsequently analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the Laemmli method [10] in order to detect the expressed target recombinant protein. The samples were prepared in sample buffer (50 mM Tris, 5% β-mercaptoethanol, 20% glycerol, 0.005% bromophenol blue, 4% SDS) and boiled at 95°C for 5 min. Thereafter, they were analyzed on a 12.5% SDS-PAGE slab gel in a mini-slab apparatus (Bio-Rad Laboratories, Hercules, CA, U.S.A.). The SDS-PAGE slab gels were then subjected to staining with Coomassie Brilliant Blue R-250 (Sigma-Aldrich) for protein band detection. For the immunoblotting procedure, the proteins were transferred from the SDS-PAGE slab gel to a nitrocellulose membrane (Bio-Rad). Then, the membranes were incubated with a dilution of 1:5,000 Anti-6-His-tag horseradish peroxidase conjugated antibody (Anti-HisG-HRP Antibody, Invitrogen, Carlsbad, CA, U.S.A.) in blocking buffer (1% BSA, 0.05% Tween20 in PBS) for 1 hr at room temperature to detect the 6×His-tag rOmpH or incubated with a dilution of 1:1,000 chicken serum against rOmpH from a previous study [19] in blocking buffer for 1 hr at room temperature and subsequently incubated with a 1:1,500 dilution of HRP-conjugated rabbit anti-chicken IgY (IgG; Alpha Diagnostic International, San Antonio, TX, U.S.A.) for 1 hr at room temperature. The proteins were visualized via incubation with 3,3′diaminobenzidine (DAB; Invitrogen).
Chickens: One hundred and thirteen Hi-sex brown chickens at the age of 21-week were used in this study (RPM Farm & Feed Co., Ltd., Chiang Mai, Thailand). Briefly, there were 19 chickens per group in groups 1–3; there were 9 chickens for tracheal lavage and 10 chickens were left for challenge exposure. There were 14 chickens in groups 4–7; there were 9 chickens for tracheal lavage and 5 chickens for challenge exposure. The animal welfare committee of the Faculty of Veterinary Medicine, Chiang Mai University, was in control of the use of the laboratory animals, in accordance with the laboratory animal ethics. The experiments followed the Guide for the Care and Use of Agricultural Animals in Research and Teaching (the Ag Guide, FASS 2010).
Immunization: The rOmpH concentration was measured using a BCA Protein Assay Kit (Pierce®, Rockford, IL, U.S.A.), according to the manufacturer’s instructions. The immunogen was formulated at a desired concentration with the following mucosal adjuvants: 10 µg ODN 2007 (5′- tcg tcg ttg tcg ttt tgt cgt t −3′; ODN; InvivoGen, San Diego, CA, U.S.A.) or 3 µg E. coli Heat-Labile Enterotoxin, B subunit (LTB, Sigma-Aldrich), in 60 µl per dosage. Additionally, rOmpH was also emulsified with an equal volume of Freund’s incomplete adjuvant (Sigma-Aldrich) for intramuscular immunization. The chickens were divided into 6 groups based on the vaccine formulation and route of vaccine administration (Table 1). All the chickens were also observed for clinical signs and behavioral changes before and after immunization.
Table 1. Protections conferred in chickens vaccinated with the vaccine formulations upon challenge exposure with 100 LD50 of live P. multocida strain X-73.
| Group | Vaccine formulation (per dose) | Route | No. of immunizationsa) | No. of survivors / challenged (% protection) |
|---|---|---|---|---|
| 1 | 50 µg rOmpH + 10 µg ODN | IN b) | 19 | 9/10 (90)d) |
| 2 | 50 µg rOmpH + 3 µg LTB | IN | 19 | 7/10 (70)d) |
| 3 | 100 µg rOmpH + Freund’s incomplete | IM c) | 19 | 10/10 (100)d) |
| 4 | DLD bacterin e) | IM | 14 | 5/5 (100)d) |
| 5 | 10 µg ODN | IN | 14 | 0/5 (0) |
| 6 | 3 µg LTB | IN | 14 | 0/5 (0) |
| 7 | 50 µg rOmpH | IN | 14 | 0/5 (0) |
a) Three chickens were collected from each group for the tracheal lavage on days 0, 14 and 28. b) Intranasal administration at 60 µl per dose. c) Intramuscular administration at 1 ml per dose. d) Statistically significant as compared with the control groups, P<0.05. e) The DLD bacterin vaccine is a bacterin vaccine which manufactured by the Bureau of Veterinary Biologics, Department of Livestock Development, Ministry of Agriculture and Cooperatives, Thailand.
Determination of antibody responses: Antibody responses, serum IgY and secretory IgA in chickens were determined by ELISA. Blood samples were collected on days 0, 7, 14, 21, 28 and 35 of the experiments. In addition, three chickens in each group were sampled and euthanized on days 0, 14 and 28 of the experiments, and the tracheal lavage was performed. Then, the sera or tracheal lavage solutions were subjected to immunoblotting and ELISA procedures. Antibody responses in the chicken sera were determined by measuring of the IgY titers using a commercial indirect ELISA test kit (ProFLOK®, Synbiotics, Kansas City, MO, U.S.A.). In additions, the secretory IgA was determined with an IgA Chicken ELISA Kit (ab157691, Abcam®, Cambridge, U.K.). The average log titers and the standard error of the mean of each group were calculated according to the manual’s recommendation.
Challenge exposure: All the chickens were challenge-exposed with a 100 LD50 dose via intranasal administration. The non-immunized control chickens were also exposed to the bacteria in the same manner. The birds were observed for their mortality rates and clinical signs for 7 days. Necropsies and bacterial isolation were undertaken for dead chickens. The gross lesions were recorded, and lungs, livers, spleens, kidneys and hock joints were collected for bacterial isolation by direct culture using blood agar plates.
Statistical analyses: Fisher’s exact test was used to evaluate the efficacy of the different formulations. The level of significance was P<0.05. The differences in ELISA antibody titers between the vaccinated groups and the non-vaccinated control group were analyzed using the Student’s t-test.
RESULTS
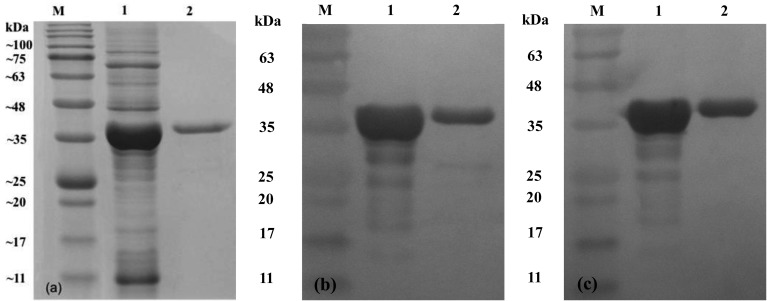
Expression and purification of recombinant proteins: The E. coli strain PQE-ompH whole cell lysates showed an overexpressed band at approximately 39 kDa (6×histidine tag included) on SDS-PAGE (Fig. 1). The rOmpH fractions from the electroelutor showed nice clear bands with the same target molecular mass as shown in Fig. 1. The E. coli strain PQE-ompH whole cell lysate and the purified rOmpH fractions were probed with chicken serum against rOmpH from a previous study [19] or the anti-HisG-HRP antibody 6×histidine tag-rOmpH in order to confirm the overexpressed band, as illustrated in Fig. 1. The results showed the immunoreactivity of the immunized chicken serum against rOpmH in both the E. coli strain PQE-ompH cell lysates (prior to purification) and the purified rOmpH fractions.
Fig. 1.
Immunoblotting of the rOmpH used in this study. The samples were run on a 12.5% SDS-polyacrylamide gel (a) and transferred to a nitrocellulose membrane. Immunoblotting of the rOmpH was done by probing with chicken sera against the rOmpH (b) or the anti-HisG-HRP antibody (c). Lane M, protein ladder; lane 1, cell lysates of the E. coli host (prior to purification); and lane 2, rOmpH fraction purified from the electroelutor. The numbers on the left indicate the positions of the molecular mass standards (in kilodaltons).
Protectivity: A total volume of 60 µl of bacterial suspension containing 2.8 × 108 CFU of strain X-73 was intranasally challenge-exposed. The protectivity in the chickens immunized with the LTB-based vaccine was 70%, while that in those immunized with the ODN-based vaccine was 90%. However, Fisher’s exact test analysis indicated no significant difference in the protection conferred by these two formulations (P<0.05). Complete protection of the chickens from fowl cholera was obtained by vaccination with the Department of Livestock Development (DLD; Ministry of Agriculture and Cooperatives, Thailand) vaccine or by intramuscular rOmpH vaccine formulations. Also, there was no significant difference between the proportions of protection conferred by these vaccine formulations (P<0.05). Moreover, there was no significant difference between the proportions of protection conferred by these 4 vaccine formulations (P<0.05). No survivor was observed in LTB- or ODN-immunized chickens exposed to the bacterial strain.
Clinical signs, gross lesions and bacterial isolation: There was no behavior change observed in any of the chickens after immunization in the OmpH group. In the DLD group, mild depression was observed at 24 hr after immunization. Additionally, it needs to be mentioned that egg laying continued at the same rate (data not shown). However, mild inflammation was observed at the injection site in all the chickens, but this did not interfere with the behavior of the chickens and it was healed within 3–5 days after injection. Chickens in the non-immunized control groups started to show clinical signs, such as depression, anorexia, severe diarrhea, and loss of appetite, at 6–8 hr after challenge exposure. At 12 hr, some chickens were found dead, and all of the chickens in this group died within 24 hr.
The necropsy results demonstrated lesions of fowl cholera, for example, multiple necrotic foci in the liver and/or spleen, lung congestion and edema, multiple petechiae in the liver, hemorrhage in the small intestines and splenomegaly, in all the chicken carcasses. Moreover, there were 2 cases that showed fibrinopurulent peritonitis and salpingitis. In addition, P. multocida was recovered in pure cultures from the specimens of all the dead chickens.
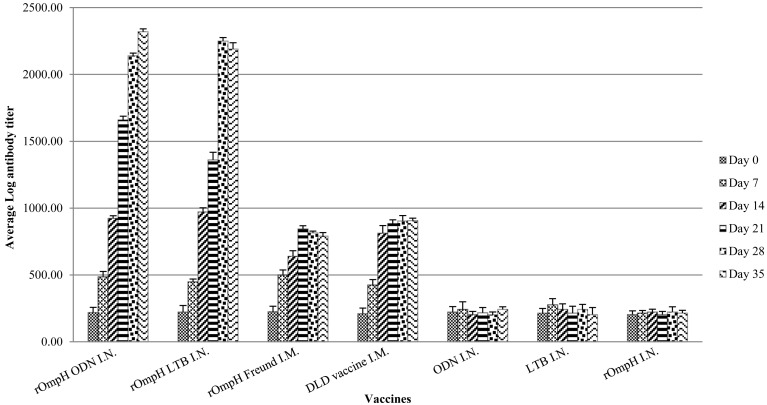
Serum IgY profile: Serum IgY was detected in chickens immunized with the intranasal vaccine (Fig. 2). The levels of chicken serum IgY titers were found to be empirically increased after immunization with the DLD bacterin or rOmpH plus Freund’s incomplete adjuvant vaccine. Moreover, the serum IgY titers derived from both intranasal vaccine formulations were observed to have slightly increased after immunization. However, there was a significant difference in serum IgY titers after day 28 between parenteral vaccines and intranasal vaccines. On the other hand, there was no change in antibody titer in any chicken immunized with LTB or ODN. Therefore, the results indicated that the intranasal vaccine formulations are able to induce serum IgY against P. multocida strain.
Fig. 2.
The antibody responses in the sera of chickens immunized with vaccines. (1) rOmpH + ODN via intranasal route, (2) rOmpH + LTB via intranasal route, (3) rOmpH + Freund’s incomplete adjuvant via intramuscular route, (4) DLD bacterin vaccine via intramuscular route, (5) ODN via intranasal route, (6) LTB via intranasal route and (7) rOmpH without adjuvant via intranasal route. The data were calculated as described in the test kit’s manual and presented as the log mean end-point titers, and the bars indicate the standard error of the means.
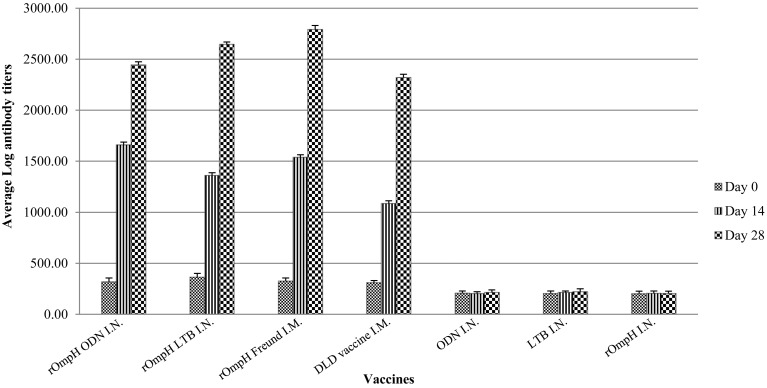
Secretory IgA profile: Secretory IgA was also detected in chickens immunized with the intranasal vaccine as illustrated in Fig. 3. The chicken secretory IgA titers were found to have slightly increased after immunization with the DLD bacterin or rOmpH plus Freund’s incomplete adjuvant vaccine. Interestingly, secretory IgA titers derived from both intranasal vaccine formulations were observed to have empirically increased in the first 2 weeks of the experiments, and there was no difference in secretory IgA titers after day 14 between parenteral vaccines and intranasal vaccines. However, this phenomenon remained significant difference along the experiments. The results revealed that the level of secretory IgA among the vaccine groups showed no significant difference during the experiments. On the other hand, there was no change in the titer of secretory IgA antibodies against the P. multocida strain in any chicken immunized with LTB or ODN. Therefore, the results indicated that the intranasal vaccine formulations are able to induce secretory IgA against P. multocida strain.
Fig. 3.
The antibody responses in the tracheal larvage of chickens immunized with vaccines. (1) rOmpH + ODN via intranasal route, (2) rOmpH + LTB via intranasal route, (3) rOmpH + Freund’s incomplete adjuvant via intramuscular route, (4) DLD bacterin vaccine via intramuscular route, (5) ODN via intranasal route, (6) LTB via intranasal route and (7) rOmpH without adjuvant via intranasal route. The data were calculated as described in the test kit’s manual and presented as the log mean end-point titers, and the bars indicate the standard error of the means.
DISCUSSION
Outer membrane proteins (Omps) of P. multocida are some of the virulent factors that play an important role in pathogenesis [7]. Among its Omps, OmpH is one of the major outer membrane proteins [11]. It is highly immunogenic, exposing epitopes and is found in the envelope of P. multocida. Early studies on OmpH have shown that besides its structural and functional relation to the porin superfamily among Gram-negative bacteria, it has also been identified as an adhesive protein that mediates bacteria adhering to host cells during the initial stage of bacterial infection [11, 20]. The outer membrane protein and its recombinant protein have been recognized as vaccine candidates including for fowl cholera [17, 19]. Vaccine formulations have been created and tested against experimental infections in animal models. The application was also done for the protectivity in pig and cattle. The iron-regulated OMP-based vaccine provided efficient protection in rabbits and calves [1]. The recombinant OmpH-based vaccine provided cross-protection for mice and chickens against challenges with avian P. multocida strains [19]. Administration via various routes of administration, including the intranasal route, was performed. This previous study employed the outer membrane (OM) and outer membrane vesicles (OMVs) as an intranasal vaccine candidate to a murine model against P. multocida infection. Protection was obtained, and the results showed that the OM and OMVs had the potential to act as a vaccine candidate against bovine pasteurellosis [17].
Currently, there is a need for mucosal vaccines against pathogens that invade via the mucosal surfaces. This route of vaccine delivery would also eliminate needle injections. One of the challenges in the development of mucosal vaccines is the lack of safe and effective mucosal adjuvants. The present investigation tried to clarify and elucidate the protection conferred by immunization with rOmpH vaccine formulations in natural host chickens. Protection against challenge exposure to the parental strain X-73 was obtained by using two intranasal fowl cholera vaccine formulations and the intramuscular vaccine formulation of rOmpH in the chickens. Likewise, increased ELISA titers were induced by immunization (Fig. 2). These results support the supposition that rOmpH vaccines prepared from the E. coli expression vector system and purified by electroelution were able to induce sufficient protection against the parental strain. The bacterial OmpH has previously been recognized as a vaccine candidate against pasteurellosis [6, 9]. The protection induced in the present study using intranasal immunization with the rOmpH vaccine formulations is of significant interest. Our results include a comparison of chicken immunization with rOmpH with or without adjuvants. The results demonstrated that there were low antibody responses and protectivity conferred by immunization with rOmpH without an adjuvant in chickens. Therefore, we concluded that the protectivity conferred by the intranasal vaccine formulations came from the collaboration between rOmpH and the adjuvants, which were added the vaccine formulations. However, the host defense mechanism and modification of the OMPs during infection have an effect on protection in vivo [2]. The expression of the OMPs of P. multocida was found to change during the infection in the hosts [2]. The OMPs from the host samples were classified into 35 proteins, and it was concluded that the OMPs would modify themselves during infection in the host in order to function. Therefore, the protein modification in the host and the host response mechanism to the bacterial protein need to be determined.
Adjuvants have been tested and approved for enhancing the immunogenicity of mucosal immunity; examples include toxin-based adjuvants, such as cholera toxin (CT) and E. coli heat-labile enterotoxin (LT); immunostimulatory adjuvants, such as synthetic oligonucleotides containing an unmethylated CpG motif (CpG ODN); and particulate adjuvants, such as immunostimulating complexes (ISCOM) [13, 18, 21, 22]. The most common uses of mucosal adjuvants are as CTs and LTs, but their native forms are too toxic for use. For this reason, mutant or subunit forms of these adjuvants have been developed. E. coli heat-labile enterotoxin subunit B (LTB) has been recognized as an effective adjuvant for mucosal vaccine formulations [3, 4, 18]. LTB is a potential adjuvant that binds to the GM1 ganglioside receptor, a glycosphingolipid present on the mucosal surface of mammalian cells. LTB stimulates strong systemic and mucosal immune responses [4]. Early studies have examined the activity of LTB as a mucosal adjuvant in vaccines against a variety of bacterial, fungal and viral pathogens. However, a high dose of LTB is still a potent enterotoxin [4, 13]. As the results of our investigation regarding chickens immunized with a LTB adjuvant showed no adverse effects, such as signs of nervousness or abnormal behaviors, during the experiment, it can be safely assumed that the dosage of LTB used in this study is safe for chickens.
In recent times, the oligonucleotide synthetic ligands for Toll-like receptor 9 (TLR-9), CpG ODN, have found preliminary use in clinical trials as modifiers of potential adjuvants for mucosal vaccines including animal vaccine models [4, 12, 13, 18, 21]. The CpG motif stimulates cells that express TLR-9, thereby initiating an immunomodulating cascade. It has been found to, after nasal administration, markedly enhance both innate and adaptive mucosal immunity in domestic animals, such as cattle, horses, pigs and pet animals’ models [8, 11, 14]. A previous study investigated the development of a P. multocida vaccine with CpG ODN in pigs [11]. The study reported that the immunostimulatory CpG ODN could modulate the immune response toward a Th1-like response when coadministered to piglets during swine P. multocida living vaccine (SPML) vaccination and suggested that CpG ODN may be applicable to husbandry animals as vaccine adjuvants and immunoprotective agents.
In conclusions, our vaccine formulations administered intranasally successfully induced homologous serum IgY and secretory IgA against avian P. multocida strain infection in vivo and elicited good protection in chickens. However, it is suggested that further studies need to be conducted on protection against various heterologous P. multocida strains or field strains and protectivity in different hosts.
Acknowledgments
The authors would like to express their appreciation to the staff of the Central Laboratory, Faculty of Veterinary Medicine, Chiang Mai University, for the assistance provided for the experiment. This research was financially supported by the National Science and Technology Development Agency (NSTDA), Ministry of Science and Technology, Thailand, grant No. P-10-11170, and partially supported by the Faculty of Veterinary Medicine, Chiang Mai University.
REFERENCES
- 1.Ahmad T. A., Rammah S. S., Sheweitab S. A., Haroun M., El-Sayed L. H.2014. Development of immunization trials against Pasteurella multocida. Vaccine 32: 909–917. doi: 10.1016/j.vaccine.2013.11.068 [DOI] [PubMed] [Google Scholar]
- 2.Boyce J. D., Cullen P. A., Nguyen V., Wilkie I., Adler B.2006. Analysis of the Pasteurella multocida outer membrane sub-proteome and its response to the in vivo environment of the natural host. Proteomics 6: 870–880. doi: 10.1002/pmic.200401342 [DOI] [PubMed] [Google Scholar]
- 3.Conceição F. R., Moreira A. N., Dellagostin O. A.2006. A recombinant chimera composed of R1 repeat region of Mycoplasma hyopneumoniae P97 adhesin with Escherichia coli heat-labile enterotoxin B subunit elicits immune response in mice. Vaccine 24: 5734–5743. doi: 10.1016/j.vaccine.2006.04.036 [DOI] [PubMed] [Google Scholar]
- 4.Freytag L. C., Clements J. D.2005. Mucosal adjuvants. Vaccine 23: 1804–1813. doi: 10.1016/j.vaccine.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 5.Glisson J. R., Hofacre C. L., Christensen J. P.2003. Fowl cholera. pp. 657–690. In: Diseases of Poultry (Saif, Y. M., Barnes, H. J., Glisson, J. R., Fadly, A. M., McDougald, L. R. and Swayne, D. E. eds.), Wiley-Blackwell Press, New Jersey. [Google Scholar]
- 6.Gong Q., Qu N., Niu M., Qin C., Cheng M., Sun X., Zhang A.2013. Immune responses and protective efficacy of a novel DNA vaccine encoding outer membrane protein of avian Pasteurella multocida. Vet. Immunol. Immunopathol. 152: 317–324. doi: 10.1016/j.vetimm.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Harper M., Boyce J. D., Adler B.2006. Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol. Lett. 265: 1–10. doi: 10.1111/j.1574-6968.2006.00442.x [DOI] [PubMed] [Google Scholar]
- 8.Holmgren J., Czerkinsky C.2005. Mucosal immunity and vaccines. Nat. Med. 11: S45–S53. doi: 10.1038/nm1213 [DOI] [PubMed] [Google Scholar]
- 9.Kharb S., Charan S.2011. Mucosal immunization provides better protection than subcutaneous immunization against Pasteurella multocida (B:2) in mice preimmunized with outer membrane proteins. Vet. Res. Commun. 35: 457–461. doi: 10.1007/s11259-011-9484-8 [DOI] [PubMed] [Google Scholar]
- 10.Laemmli U. K.1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. doi: 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 11.Luo Y., Glisson J. R., Jackwood M. W., Hancock R. E., Bains M., Cheng I. H., Wang C.1997. Cloning and characterization of the major outer membrane protein gene (ompH) of Pasteurella multocida X-73. J. Bacteriol. 179: 7856–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marandi M., Dubreuil J. D., Mittal K. R.1996. The 32 kDa major outer-membrane protein of Pasteurella multocida capsular serotype D. Microbiology 142: 199–206. doi: 10.1099/13500872-142-1-199 [DOI] [PubMed] [Google Scholar]
- 13.Ogra P. L., Faden H., Welliver R. C.2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14: 430–445. doi: 10.1128/CMR.14.2.430-445.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhoades K. R., Rimler R. B.1987. Capsular groups of Pasteurella multocida isolates from avian host. Avian Dis. 31: 895–898. doi: 10.2307/1591048 [DOI] [PubMed] [Google Scholar]
- 15.Rimler R. B., Glisson J. R.1997. Fowl cholera. pp. 143–159. In: Diseases of Poultry (Calnek, B. W. and Barnes, H. J. eds.), Iowa, State University Press, Ames. [Google Scholar]
- 16.Rimler R. B., Rhoades K. R.1989. Pasteurella multocida and fowl cholera. pp. 37–74, 95–114. In: Pasteurella and Pasteurellosis (Adlam, C. and Rutter, J. M. eds.), Academic Press Limited, London. [Google Scholar]
- 17.Roier S., Fenninger J. C., Leitner D. R., Rechberger G. N., Reidl J., Schild S.2013. Immunogenicity of Pasteurella multocida and Mannheimia haemolytica outer membrane vesicle. Int. J. Med. Microbiol. 303: 247–256. doi: 10.1016/j.ijmm.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan E. J., Daly L. M., Mills K. H. G.2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19: 293–304. doi: 10.1016/S0167-7799(01)01670-5 [DOI] [PubMed] [Google Scholar]
- 19.Sthitmatee N., Numee S., Yamashita K., Takahashi N., Kataoka Y., Sawada T.2008. Protection of chickens from fowl cholera by vaccination with recombinant adhesive protein. Vaccine 26: 2398–2407. doi: 10.1016/j.vaccine.2008.02.051 [DOI] [PubMed] [Google Scholar]
- 20.Mutwiri G., Pontarollo R., Babiuk S., Griebel P., van Drunen Littel-van den Hurk S., Mena A., Tsang C., Alcon V., Nichani A., Ioannou X., Gomis S., Townsend H., Hecker R., Potter A., Babiuk L. A.2003. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet. Immunol. Immunopathol. 91: 89–103. doi: 10.1016/S0165-2427(02)00246-5 [DOI] [PubMed] [Google Scholar]
- 21.Woodrow K. A., Bennett K. M., Lo D. D.2012. Mucosal vaccine design and delivery. Annu. Rev. Biomed. Eng. 14: 17–46. doi: 10.1146/annurev-bioeng-071811-150054 [DOI] [PubMed] [Google Scholar]
- 22.Linghua L., Xingshan T., Fengzhen Z.2007. CpG oligodeoxynucleotides augment the immune responses of piglets to swine Pasteurella multocida living vaccine in vivo. Res. Vet. Sci. 83: 171–181. doi: 10.1016/j.rvsc.2006.11.012 [DOI] [PubMed] [Google Scholar]