Abstract
Pharmacokinetic (PK) parameters of marbofloxacin (MRFX) in Korean cattle, Hanwoo, were determined following its intravenous (i.v.) or intramuscular (i.m.) administration at a dose of 2 mg/kg. Area under the curve (AUC0–24 hr), half-life (t1/2) and total body clearance (CLB) of i.v. MRFX were 6.87 hr∙µg/ml, 2.44 hr and 0.29 l/kg∙hr, respectively, and the corresponding values for i.m. administration of MRFX were 5.07 hr∙µg/ml, 2.44 hr and 0.39 l/kg∙hr. The suggested optimal doses of MRFX in Hanwoo cattle, calculated by integration of PK data obtained in the present study and previously reported minimum inhibitory concentration (MIC) for MRFX against susceptible (MIC ≤1 µg/ml) and intermediate (MIC ≤2 µg/ml) pathogenic bacteria, were 2.1 and 4.2 mg/kg/day by i.v. route and 3.9 and 7.8 mg/kg/day by i.m. route.
Keywords: Hanwoo, Korean cattle, marbofloxacin, pharmacokinetics
Marbofloxacin (MRFX) is one of the fluoroquinolones that exhibits concentration-dependent bactericidal activity against gram-positive and gram-negative bacteria [1, 3]. Owing to this broad spectrum of antibacterial activity, MRFX is used in the treatment of bacterial infections in animals [8,9,10]. The pharmacokinetics (PK) of MRFX has been investigated in different animal species, including cow, in order to overcome interspecies differences in PK and consequently minimize dosage errors (therapeutic failures, toxic effects or development of bacterial resistance) [2, 5, 6, 11, 14]. Hanwoo is a type of Korean native cattle that is typically raised on a restricted-feeding system that results in high fat, low muscle and minimal connective tissues in comparison with those in other breeds [7]. These differences in physical traits could influence the disposition of drugs and therefore influence drug dosage in Hanwoo cattle.
Understanding the relationship between dosage regimens and the concentration-time profiles is very important to optimize the drug dosage. This can be achieved by integrating the PK parameters of the drug with its pharmacodynamic (PD) profile. In the context of the reported study, PD was defined as interaction of MRFX with a group of pathogens represented by Enterococcus faecium, Escherichia coli, Mycoplasma bovis, Mannheimia haemolytica and Pasteurella multocida, all of which are known to cause diarrhea and respiratory disease in cattle [8,9,10]. Since the successful treatment outcome of antibiotics, including fluoroquinolones, can be facilitated by integrating PK/PD parameters, the optimal dosage should be determined in terms of PK/PD relationships between factors, such as peak concentration Cmax, minimum inhibitory concentration (MIC) and area under the time-concentration curve (AUC) that corresponds to MIC (AUIC) [5, 14, 15].
The aim of the present study therefore was to evaluate the PK profile of MRFX in Hanwoo cattle when administered through intravenous (i.v.) and intramuscular (i.m.) routes at a dose of 2 mg/kg. The rationale behind this approach was to utilize the data obtained for PK/PD modeling and to estimate the appropriate dose of MRFX in Hanwoo cattle.
Six male Hanwoo cattle, weighing 300 ± 10 kg (between 11 and 13 months of age), were randomly divided into 2 groups of 3 animals each and scheduled to receive MRFX in a two-period crossover manner. During the first part of the study, three animals from a group received i.v. MRFX administered over 40 sec at a dose of 2 mg/kg, and the animals in the other group received the same dose of MRFX via the i.m. route. After an interval of 21 days, the treatments were reversed. The animals were housed indoors and fed with a drug-free commercial pellet diet and water ad libitum. Applicable animal welfare requirements as prescribed by Gyeongsangbuk-do Livestock Research Institute (GDLR 2009-01, Andong, Korea) were followed during the course of study.
Blood samples were collected before and at 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 12 and 24 hr after MRFX administration. The samples were centrifuged at 2,000 × g for 15 min, and the supernatant serum was stored at 20°C until analysis using high-performance liquid chromatography (HPLC). Serum concentration of MRFX was assayed using Agilent1100 series HPLC system comprising HP ODS Hypersil column (4.6 × 250 mm, 5 µm). An isocratic mobile phase composed of HPLC-grade acetonitrile: potassium phosphate monobasic (0.05 M, ACS reagent, Sigma® ≥99.0% purity, pH=2.9) (80:20% v/v) at a flow rate of 1 ml/min was used. The UV detection wavelength and column temperature were set at 295 nm and 30°C, respectively. Validation of analytical methods was performed according to a previously described method [5], and it revealed linearity of standard curve (r2= 0.99). Recovery was found to be 97.05 ± 3.62%, and coefficient of variation (the inter- and intra-day) was <10%. Limit of detection (LOD) and limit of quantitation (LOQ) were 0.012 and 0.062 µg/ml, respectively. Pharmacokinetic analysis of MRFX was performed using Phoenix WinNonlin 6.0 (Pharsight Corp., St. Louis, MO, U.S.A.) software program. The individual serum concentration data were analyzed by performing nonlinear least-squares regression analysis. The best fit was achieved with a one-compartment model for both i.v. and i.m. administration. The absolute bioavailability (F) following i.m. administration was calculated using the following equation:
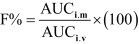 ……………………………… (1) ……………………………… (1)
|
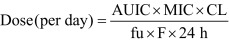 ……………………………… (2) ……………………………… (2)
|
Plasma protein binding of MRFX was evaluated using pooled plasma, harvested from study cattle prior to MRFX administration. The free fraction of MRFX in plasma was calculated by a previously reported method [6].
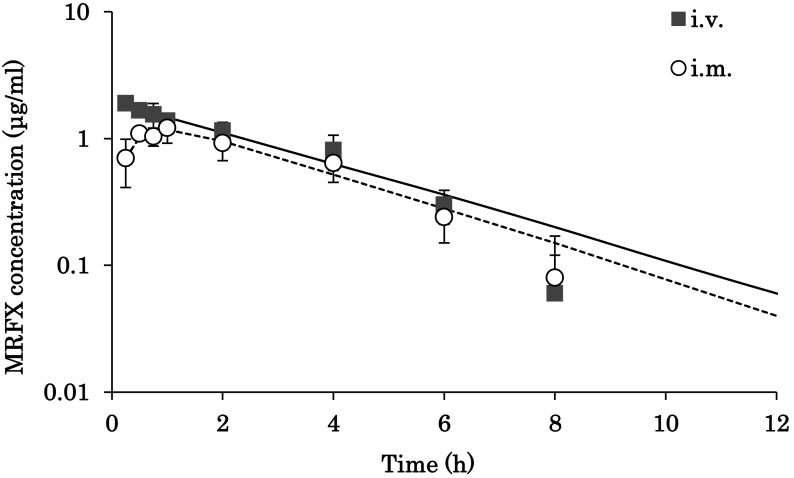
The serum concentration versus time profiles of MRFX following a single dose (2 mg/kg) administration by i.v. and i.m. routes are presented in Fig. 1, and the pharmacokinetic parameters are summarized in Table 1. MRFX, administered by i.m. in Hanwoo cattle, achieved a peak serum concentration (Cmax) of 1.16 µg/ml with relative rapidity at 0.95 hr and demonstrated moderate bioavailability (73%). The Cmax of MRFX observed in the present study was in accordance with previously reported values in lactating cows (1.66 µg/ml) [13] and in calves (1.4 µg/ml) [6]. The elimination half-lives (t1/2) of MRFX after i.v. and i.m. administrations (2.44 and 2.24 hr, respectively) were almost similar, indicating that rate of absorption does not affect the elimination rate of MRFX in Hanwoo cattle. These observations were similar to those reported in lactating cows (2.53 hr) [13]. In contrast, longer t1/2 were reported in cross-bred Simmental calves (4.60 hr) [6], buffalo calves (4.60 hr) [2], sheep (3.96 hr) [15] and goats (t1/2, 7.18 hr for i.v. and 6.70 hr for i.m.) [17]. The AUC0–24 hr values of MRFX achieved after 6.8 µg·hr/ml (i.v.) and 5.07 µg·hr/ml (i.m.) administration in the present study were comparable with corresponding results in lactating cows (7.65 µg·hr/ml) [13]. Likewise, the volume of distribution (Vss, 1.02 l/kg) observed in the current study was in line with previously reported values (1.5 l/kg) in lactating cows [13].
Fig. 1.
Semi-logarithmic plot of serum concentration (mean ± SD) versus time after single intravenous (i.v.) and intramuscular (i.m.) administration of marbofloxacin (2 mg/kg) in Hanwoo cow (n=6). The markers (full squares and empty circles) represent the observed points, and the lines (solid and dashed) represent the predicted values.
Table 1. Pharmacokinetics parameters (mean ± SD) of marbofloxacin after single dose (2 mg/kg body weight) i.v. and i.m. administration in Hanwoo cattle (n= 6).
| PK parameters | Units | i.v. | i.m. |
|---|---|---|---|
| AUC0–24 hr | hr·µg/ml | 6.87 ± 0.52 | 5.07 ± 0.42 |
| K01_HL | hr | - | 0.27 ± 0.05 |
| K10_HL | hr | 2.44 ± 0.23 | 2.24 ± 0.31 |
| CLB/F | l/kg·hr | 0.29 ± 0.02 | 0.39 ± 0.03 |
| Tmax | hr | - | 0.95 ± 0.09 |
| Cmax | µg/ml | - | 1.16 ± 0.04 |
| AUMC0–24hr | hr·µg/ml | 24.22 ± 4.10 | - |
| MRT0–24hr | hr | 3.52 ± 0.33 | - |
| Vss | l/kg | 1.02 ± 0.03 | - |
| F (%) | - | - | 73.00 ± 6.07 |
SD: Standard deviation, i.v.: Intravenous, i.m.: Intramuscular, AUC0–24hr: Area under the curve from point of administration to 24 hr after administration, K01_HL: Half-life of absorption, K10_HL: Elimination half-life, CLB/F: Total body clearance, Tmax: Time taken to achieve maximum drug concentration, Cmax: Maximum serum concentration, AUMC: Area under the first moment curve, MRT: Mean residence time, Vss: Volume of distribution at steady state, F (%): Percent of absolute bioavailability.
An optimal dosage of drugs, derived on the basis of PK and PD parameters, can be determined through the use of an equation reported previously [5]. In the reported study, we sought to ascertain whether the calculated MRFX dose of 2 mg/kg, administered either i.v. or i.m., could achieve the desired PK-PD endpoints, such as Cmax/MIC ratio of 10 or more or AUC0–24 hr/MIC (AUIC) of 125.Moreover, a Cmax/MIC ratio of ≥10 for fluoroquinolones is associated with efficacy and low incidence of resistance development [5], and the peak concentration of MRFX observed in our study corresponded to this favorably. Schentag et al. [12] concluded from their study that the AUIC ratio for quinolones should be more than 125 in order to prevent selective pressure that leads to increased development of drug-resistant bacterial sub-populations. The optimum MRFX dose, 2 mg/kg, required to achieve the target AUC0–24 hr/MIC of 125 is reported to be effective against a homogenous population of P. multocida, E. coli and M. haemolytica isolates (MIC, ≤0.03 µg/ml) as well as Staphylococcus aureus and coagulase-negative staphylococci with MIC centered around 0.25 µg/ml [9]. Cattle with bacterial infections usually show a better PK profile - higher Cmax, faster Tmax and longer t1/2 - than healthy cattle [6]. Despite this, we recommend optimal dosage prediction with guidelines for the interpretation of MIC depending on the complexity of the clinical situation.
A broad spectrum of activity against a range of pathogens is a desirable feature in an antibacterial agent, and an appropriate PD parameter that could be used to evaluate this is the MIC cutoff limit. In this study, we considered the MIC breakpoint for the aerobic pathogenic bacteria isolated from cattle, including E. faecium and M. haemolytica, as prescribed by CLSI guidelines (CLSI 2008) [4] –susceptible (MIC ≤1 µg/ml) and intermediate(MIC ≤2 µg/ml). Using these benchmarks, we concluded that the administered dose (2 mg/kg/day) was inadequate for achieving the target end point associated with efficacy of fluoroquinolones, and to arrive at an optimal dose for desired effect, specific equation described was used.
The protein binding of MRFX in this reported study was 21%, indicating that the free/unbound fraction of MRFX (fu) was 0.79. In addition, we found the bioavailability (F) of MRFX in Hanwoo cattle to be 1.00 (i.v.) and 0.73 (i.m.). Further, taking into consideration the required AUC0–24 hr/MIC ratio of 125 for effective antibacterial activity and the CLSI-defined MIC breakpoints against susceptible and intermediate pathogens, the calculated doses of MRFX predicted for achieving the target PK-PD indices were found to be 2.1 (susceptible) and 4.2 (intermediate) mg/kg/day by the i.v. route and 3.9 (susceptible) and 7.8 (intermediate) mg/kg/day by the i.m. route. Therefore, a higher dose of MRFX should be considered for treatment of unclear bacterial infections in Hanwoo cattle. However, additional studies may be necessary to confirm the PK profile of MRFX in diseased animals and also compare that in different age-related to total body water to facilitate the drug’s optimal use in the treatment of bovine disease.
Acknowledgments
This research was supported in part by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2011-0021670) and in part by a grant from the Next-Generation BioGreen 21 Program (No. PJ009007), Rural Development Administration, Republic of Korea.
REFERENCES
- 1.Aliabadi F. S., Lees P.2002. Pharmacokinetics and pharmacokinetic/pharmacodynamic integration of marbofloxacin in calf serum, exudate and transudate. J. Vet. Pharmacol. Ther. 25: 161–174. doi: 10.1046/j.1365-2885.2002.00399.x [DOI] [PubMed] [Google Scholar]
- 2.Baronil E. E., Rodríguez C., Crudeli G., Perone C., Rubio S., de Lucas J. J., San Andrés M. I.2007. Pharmacokinetics of marbofloxacin after single intravenous administrations in buffalo calves. Ital. J. Anim. Sci. 6: 838–841. [Google Scholar]
- 3.Brown S. A.1996. Fluoroquinolones in animal health. J. Vet. Pharmacol. Ther. 19: 1–14. doi: 10.1111/j.1365-2885.1996.tb00001.x [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute (CLSI)2008. Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters for Veterinary Antimicrobial Agents; Approved Guideline. 3rd ed. CLSI DocumentM37-A3. Clinical and Laboratory Standards Institute. [Google Scholar]
- 5.Elias G., Lee J. S., Hwang M. H., Park Y. S., Cho K. H., Kim Y. H., Park S. C.2009. Pharmacokinetics and pharmacokinetic/pharmacodynamic integration of orbifloxacin in Korean Hanwoo cattle. J. Vet. Pharmacol. Ther. 32: 219–228. doi: 10.1111/j.1365-2885.2008.01027.x [DOI] [PubMed] [Google Scholar]
- 6.Ismail M., El-Kattan Y. A.2007. Comparative pharmacokinetics of marbofloxacin in healthy and Mannheimia haemolyticainfected calves. Res. Vet. Sci. 82: 398–404. doi: 10.1016/j.rvsc.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 7.Jo C., Cho S. M., Cho S. H., Chang J., Nam K. C.2012. Keys to production and processing of Hanwoo beef: a perspective of tradition and science. Anim. Front. 2: 32–38. doi: 10.2527/af.2012-0060 [DOI] [Google Scholar]
- 8.Kim J. S., Heo J. H., Jung M. H., Cho M. H., Kim N. C., Lee K. C., Seo J. L., Son S. G.2001. Isolation and antimicrobial drug susceptibility of Pasteurella spp. from pneumonic calves and cows. J. Vet. Cli. Med. 18: 98–104. [Google Scholar]
- 9.Kroemer S., Galland D., Guerin-Faublee V., Giboin H., Woehrle-Fontaine F.2012. Survey of marbofloxacin susceptibility of bacteria isolated from cattle with respiratory disease and mastitis in Europe. Vet. Rec. 170: 53–57. doi: 10.1136/vr.100246 [DOI] [PubMed] [Google Scholar]
- 10.Lee W. W., Lee S. M., Park M. S., Lee G. S., Kim G. H.2011. Isolation and Antimicrobial susceptibility of Enterococci from cattle and pigs. Annual Rep. Busan Metropolitan city Inst. Health Envirom. 20: 247–252. [Google Scholar]
- 11.Regnier A., Concordet D., Schneider M., Boisrame B., Toutain P. L.2003. Population pharmacokinetics of marbofloxacin in aqueous humor after intravenous administration in dogs. Am. J. Vet. Res. 64: 889–893. doi: 10.2460/ajvr.2003.64.889 [DOI] [PubMed] [Google Scholar]
- 12.Schentag J. J., Gilliland K. K., Paladino J. A.2001. What have we learned from pharmacokinetic and pharmacodynamic theories? Clin. Infect. Dis. 32Suppl 1: S39–46. doi: 10.1086/319375 [DOI] [PubMed] [Google Scholar]
- 13.Schneider M., Vallé M., Woehrlé F., Boisramé B.2004. Pharmacokinetics of marbofloxacin in lactating cows after repeated intramuscular administrations and pharmacodynamics against mastitis-isolated strains. J. Dairy Sci. 87: 202–211. doi: 10.3168/jds.S0022-0302(04)73159-8 [DOI] [PubMed] [Google Scholar]
- 14.Shem-Tov M., Ziv G., Glickman A., Saran A.1997. Pharmacokinetics and penetration of marbofloxacin from blood into the milk of cows and ewes. Zentralbl. Veterinarmed. A 44: 511–519. doi: 10.1111/j.1439-0442.1997.tb01137.x [DOI] [PubMed] [Google Scholar]
- 15.Sidhu P. K., Landoni M. F., Aliabadi F. S., Lees P.2010. PK–PD integration and modeling of marbofloxacin in sheep. Res. Vet. Sci. 88: 134–141. doi: 10.1016/j.rvsc.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 16.Toutain P. L., DelCastillo J. R., Bousquet-Melou A.2002. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 73: 105–114. doi: 10.1016/S0034-5288(02)00039-5 [DOI] [PubMed] [Google Scholar]
- 17.Waxman S., Rodríguez C., González F., De Vicente M. L., San Andrés M. I., San Andrés M. D.2001. Pharmacokinetic behavior of marbofloxacin after intravenous and intramuscular administrations in adult goats. J. Vet. Pharmacol. Ther. 24: 375–378. doi: 10.1046/j.1365-2885.2001.00357.x [DOI] [PubMed] [Google Scholar]