Key Points
Collagen 4 binds to the VWF A1 domain, and this binding is reduced or abrogated by select VWF A1 domain sequence variations.
Platelet binding to collagen 4 under flow conditions is dependent on the presence of VWF.
Abstract
Von Willebrand factor (VWF) contains binding sites for platelets and for vascular collagens to facilitate clot formation at sites of injury. Although previous work has shown that VWF can bind type IV collagen (collagen 4), little characterization of this interaction has been performed. We examined the binding of VWF to collagen 4 in vitro and extended this characterization to a murine model of defective VWF–collagen 4 interactions. The interactions of VWF and collagen 4 were further studied using plasma samples from a large study of both healthy controls and subjects with different types of von Willebrand disease (VWD). Our results show that collagen 4 appears to bind VWF exclusively via the VWF A1 domain, and that specific sequence variations identified through VWF patient samples and through site-directed mutagenesis in the VWF A1 domain can decrease or abrogate this interaction. In addition, VWF-dependent platelet binding to collagen 4 under flow conditions requires an intact VWF A1 domain. We observed that decreased binding to collagen 4 was associated with select VWF A1 domain sequence variations in type 1 and type 2M VWD. This suggests an additional mechanism through which VWF variants may alter hemostasis.
Introduction
Von Willebrand factor (VWF) plays several key roles in coagulation. It binds factor VIII (FVIII) via the D′ and D3 domains, stabilizing FVIII in circulation,1 and links platelets to exposed vascular collagens via binding sited for platelets in the VWF A1 and C1 domains2 and binding sites for collagen in the A1 and A3 domains.3 Defects in the ability of VWF to bind collagen have been reported to result in von Willebrand disease (VWD). To date, most reported VWF sequence variations affect the ability of VWF to bind type I (collagen 1) or type III collagen (collagen 3),4-6 although defects in binding to type VI collagen (collagen 6) have also been reported7 and may actually occur at increased frequency compared with defects in collagen 3 binding.
The vascular endothelium also contains type IV collagen (abbreviated here as collagen 4 for clarity). Collagen 4 is a key component of the basement membrane.8 Previous work has shown that collagen 4 can support platelet adhesion through a VWF-dependent mechanism.9,10 Defects in collagen 4 have been reported to be associated with intracranial hemorrhage and hemorrhagic stroke in humans.11-13 A murine model with defective collagen 4 demonstrated a high rate of perinatal cerebral hemorrhage.14
Because collagen 4 has been previously shown to bind VWF,9 we hypothesized that defects in VWF binding to collagen 4 could result in bleeding. Current VWD testing examines VWF-platelet interactions routinely, albeit indirectly, through the ristocetin cofactor activity assay.15 In some instances, VWF binding to collagens 1 and 3 is measured through collagen-binding assays,16,17 but no current testing would specifically evaluate the capacity of VWF to bind collagen 4. We report here on the characterization of the binding of VWF to collagen 4 in both healthy controls and in subjects with types 1, 2, and 3 VWD. Additional in vitro studies with recombinant VWF, murine expression studies, and examination of VWF–collagen 4 interactions under flow conditions were performed to further understand VWF binding to collagen 4.
Methods
Patient samples
Plasma samples were analyzed from subjects enrolled in the Zimmerman Program for the Molecular and Clinical Biology of VWD (Zimmerman Program).18 The study was approved by the institutional review board at the site of enrollment and informed consent was obtained from all enrolled subjects. Subjects were classified based on the phenotypic diagnosis assigned after review of VWF laboratory and VWF gene sequencing results. VWF gene sequencing, including intron-exon boundaries, was performed as previously described.19 Bleeding scores were calculated according to the ISTH Bleeding Assessment Tool.20 VWF antigen enzyme-linked immunosorbent assay (ELISA) (VWF:Ag), VWF ristocetin cofactor activity (VWF:RCo), and VWF binding to collagen 3 (VWF:CB3) were performed by the reference laboratory (BloodCenter of Wisconsin Hemostasis Reference Laboratory, Milwaukee, WI) as previously described.21 Blood group was determined by reverse blood typing.18
Collagen 4 binding
VWF binding to collagen 4 (VWF:CB4) was measured by coating maleic anhydride plates (Pierce) with 1 μg/mL human type 4 collagen (Southern Biotech, Birmingham, AL) diluted in phosphate-buffered saline (PBS, pH 7.4) and incubating overnight. Plates were blocked with 1% bovine serum albumin (BSA) in PBS. VWF samples were diluted in blocking buffer (PBS with 1% BSA) at pH 7.4, applied to the collagen 4-coated plate, and incubated at room temperature for 1 hour. A minimum of 2 dilutions were tested for each sample. Detection of bound VWF used a combination of 2 biotinylated anti-VWF monoclonal antibodies (AVW-1 and AVW-15, BloodCenter of Wisconsin), neither of which binds VWF via the A1 domain. Because there is no international standard for collagen 4, values were compared with a plasma standard assigned an arbitrary collagen 4 binding value of 100 U/dL (which is similar to the assigned collagen 3 binding value of 107). Reproducibility studies were performed by 3 technicians over 10 days. Confirmation of collagen binding to ELISA plates was performed using both monoclonal and polyclonal anti–collagen IV antibodies (Sigma-Aldrich/Merck, St. Louis, MO).
In vitro expression studies
Recombinant VWF constructs were generated using the QuikChange XL kit (Agilent, Santa Clara, CA) to introduce specific sequence variants into full-length wild-type (WT) human or murine A/J recombinant VWF as previously described.6 DNA was expressed in HEK293T cells, and supernatants collected at 72 hours. Expression was quantified by VWF:Ag.18 Multimer distribution was characterized by gel electrophoresis.22 VWF:CB3 and VWF binding to collagen 6 (VWF:CB6) was measured as previously described.21 VWF:CB4 was measured as described previously.
Scanning alanine mutagenesis
Amino acids 1387 through 1412 of the VWF A1 domain were individually mutated to alanine using the QuikChange XL kit as described here before to alter the sequence of full-length WT human VWF. For each residue, the WT codon was changed to GCC to replace the original amino acid with alanine in the expressed VWF protein. Each alanine mutant was expressed in HEK293T cells and characterized as described under “in vitro expression studies.”
In vivo model using hydrodynamic tail-vein injection
A plasmid containing the full-length murine WT VWF gene with an enhanced murine transthyretin promoter for hydrodynamic delivery and subsequent liver-based expression was the kind gift of Drs. David Lillicrap and Luigi Naldini. Site-directed mutagenesis was used as described previously to produce the 1399H mutant construct. Plasmid DNA (50 μg) was diluted in sterile PBS to a volume consistent with 10% of the mouse body weight. Bolus injections were performed in <7 seconds via the tail vein of VWF-deficient mice.23 Mice were between 15 and 25 weeks of age at the time of injection. Blood was collected from the inferior vena cava at 24 hours after tail-vein injection. Samples were collected into 3.2% citrate for use in VWF assays. Alternately, heparin and PPACK were used for anticoagulation of whole-blood samples for in vitro flow studies.
In vitro flow studies
Vena8Fluor+ biochips (Cellix, Dublin, UK) were coated overnight with 50 μg/mL of collagen 4 diluted in glacial acetic acid. Whole-blood samples obtained from the inferior vena cava of mice injected with either WT murine VWF DNA, 1399H murine VWF DNA, or from VWF-deficient mice not injected were analyzed and compared with results obtained from WT mice. Platelets were stained with 20 μg/mL quinacrine dihydrochloride (Calbiochem/Millipore, Billerica, MA) for visualization.24 A shear rate of 1111 s−1 was used to flow samples over the collagen-coated chip.25 Images were analyzed at 180 seconds. Platelet adhesion was measured by counting the number of platelet aggregates per image (at ×10 original magnification) by a blinded observer.
Statistics
Statistical analysis was performed using SAS (SAS Institute, Cary, NC). Because the data were not normally distributed, a nonparametric test (Wilcoxon signed-rank test) was used to compare paired samples. Correction for multiple testing yielded an adjusted P value of .017 (.05/3). When laboratory assay values were below the lower limit of detection, a low value (half the limit of detection, based on an assumption of a uniform distribution of the points below the limit of detection) was assigned for calculation of ratios. For the VWF:RCo assay, results <10 IU/dL were assigned a value of 5. For the VWF:CB3 assay, results <1 IU/dL were assigned a value of 0.5. For the VWF:CB4 assay, results <1.6 U/dL were assigned a value of 0.8. We also examined the data omitting these low values, and this did not affect the statistical significance of the results.
Results
VWF binding to collagen: Zimmerman Program healthy controls
A series of plasma samples from healthy human controls and subjects with varying types of VWD were examined to characterize collagen 4 binding in human subjects. Table 1 summarizes statistical values for the healthy controls and VWD subjects. Samples from 246 healthy control subjects were available for analysis. The median VWF:CB4/VWF:Ag ratio for the healthy controls was 0.94, and the median VWF:CB3/VWF:Ag ratio was 1.06 (P < .001). Because this was a novel assay, confirmation of collagen 4 binding to ELISA plates was performed with anti–collagen 4 antibodies and demonstrated the presence of collagen 4. No binding occurred with antibodies against collagens 1 and 3. Reproducibility studies showed a mean value of 28 U/dL for a type 1 control with a standard deviation (SD) of 2.6. The coefficient of variation for the assay was <10% for both a single sample run on multiple occasions and for a control sample run by 2 different users performing the assay over a period of 5 days. Healthy controls had collagen 4 binding comparable with collagen 3 binding, with the exception of 2 subjects (1% of the healthy controls) previously reported to be heterozygous for the p.R1399H sequence variation. This sequence variation has been previously reported in ∼2% of a Caucasian population26 and is associated with decreased collagen 6 binding.7 In the current study, collagen-4 binding was reduced for all subjects with p.R1399H, despite a normal VWF:CB3/VWF:Ag ratio. The median VWF:RCo/VWF:Ag was 0.97, which was not significantly different from VWF:CB4/VWF:Ag ratio.
Table 1.
VWF laboratory values for healthy controls and type 1 VWD subjects
| Healthy controls (n = 246) | Type 1 VWD subjects (n = 299) | |||||
|---|---|---|---|---|---|---|
| Mean | Median | IQR | Mean | Median | IQR | |
| VWF:Ag | 127 | 111 | 88-150 | 33 | 36 | 22-46 |
| VWF:RCo | 122 | 114 | 82-148 | 32 | 35 | 20-44 |
| VWF:RCo/VWF:Ag ratio | 0.98 | 0.97 | 0.86-1.10 | 1.00 | 0.97 | 0.84-1.12 |
| VWF:CB3 | 132 | 120 | 94-161 | 37 | 41 | 22-52 |
| VWF:CB3/VWF:Ag ratio | 1.06 | 1.06 | 0.96-1.17 | 1.12 | 1.13 | 1.02-1.25 |
| VWF:CB4 | 123 | 107 | 75-153 | 29 | 30 | 16-42 |
| VWF:CB4/VWF:Ag ratio | 0.96 | 0.94 | 0.78-1.09 | 0.86 | 0.88 | 0.74-0.98 |
IQR, interquartile range (25th-75th).
Data are given in IU/dL with the exception of VWF:CB4, which was normalized to an international standard and is given in U/dL. For subjects with values below the lower limit of detection for each assay, an arbitrary value was assigned for calculation purposes (5 IU/dL for VWF:RCo <10 IU/dL, 0.5 IU/dL for VWF:CB3 <1 IU/dL, and 0.8 U/dL for VWF:CB4 <1.6 U/dL).
Because blood group is known to affect VWF:Ag levels,27 we also examined VWF:CB4 by blood group. The lowest mean VWF:CB4 was seen in group O subjects (mean 96 U/dL vs 148 U/dL for all other blood groups, P < .001). This difference was attenuated when VWF:CB4/VWF:Ag ratios were examined (mean 0.92 for group O subjects vs 1.00 for all other blood groups, P = .03). Group O subjects comprised 48% of the sample, group A comprised 37%, group B comprised 13%, and group AB comprised 3%.
VWF binding to collagen: type 1 VWD subjects
For the type 1 VWD subjects, 299 had VWF:Ag or VWF:RCo below the defined lower limit of normal for each assay (≤50 IU/dL for VWF:Ag or ≤54 IU/dL for VWF:RCo) and were included in this analysis. Many of these subjects likely represent “low VWF” and do not necessarily have VWD, but for potential consideration of collagen-binding defects, those with VWF:Ag >30 were not excluded. For the type 1 VWD subjects, the median VWF:CB4/VWF:Ag ratio was 0.88, significantly lower than that seen for the healthy controls (P < .001). The median VWF:RCo/VWF:Ag ratio was 0.97 (P < .001 compared with VWF:CB4/VWF:Ag) and the median VWF:CB3/VWF:Ag ratio was 1.13 (P < .001 compared with VWF:CB4/VWF:Ag). The decrease in collagen 4 binding suggests a specific defect in some subjects currently classified as type 1 VWD.
A total of 15 subjects had VWF:CB4/VWF:Ag ratios <0.5. The median bleeding score for subjects with a decreased VWF:CB4/VWF:Ag ratio was 6 compared with a median bleeding score of 5 for the type 1 subjects without a collagen 4 binding defect (P = NS). Fifteen of 301 (5%) type 1 VWD subjects had a defect in collagen 4 binding. There were 4 subjects enrolled in the Zimmerman Program with type 1 VWD who had undetectable or nearly absent binding to collagen 4 and VWF:Ag >10 IU/dL. Three of these subjects demonstrated heterozygosity for p.R1399H (overall prevalence of 1.3% of type 1 subjects) and had a second sequence variation, either p.C2693Y or p.P812Rfs*31. One subject with undetectable collagen 4 binding had an isolated p.R1315C sequence variation; this variation was also seen in an additional subject with a VWF:CB4/VWF:Ag ratio of 0.4. The subject with p.R1315C had a normal multimer distribution, although this sequence variation has been reported to be associated with decreased high-molecular-weight multimers and type 2A VWD.28 Collagen 4 binding defects are summarized in Table 2.
Table 2.
VWF variants with decreased collagen 4 binding
| VWF variant | VWF types | VWF:RCo/VWF:Ag ratio (range) | VWF:CB4/VWF:Ag ratio (range) | Bleeding score (range) |
|---|---|---|---|---|
| p.R1399H | Healthy controls type 1 VWD | 0.93-1.13 | 0-0.31 | 0-8 |
| p.R1315C | Type 1 | 0.82-1.17 | 0-0.59 | 2-12 |
| p.S1358N | Type 2M | 0.54 | 0.4 | 12 |
| p.Q1402P | Type 2M | 0.38 | 0 | 14 |
| p.R1392_Q1402del | Type 2M | 0.41 | 0 | 11 |
| p.I1425F and p.R1399H | Type 2M | 0.34 | 0.48 | 22 |
Data were also available on a cohort of subjects initially enrolled as type 1 VWD but whose enrollment laboratory data were within normal limits. Four of 118 (3%) subjects with a preexisting diagnosis of type 1 VWD but currently normal laboratory results had VWF:CB4/VWF:Ag <0.5. One had a ratio of 0.31 and was heterozygous for the p.R1399H sequence variation. Two subjects (with ratios of 0.36 and 0.47) had no VWF sequence variations that were likely to be causative. The fourth subject did not have sufficient DNA for sequencing. Bleeding scores for 3 of the 4 subjects were ≥8 vs a median bleeding score of 5 for all subjects in this group with a previous diagnosis of type 1 VWD.
VWF binding to collagen: type 2 VWD subjects
For the type 2A and type 2B subjects, VWF:CB4/VWF:Ag ratios were decreased compared with both healthy controls and type 1 VWD subjects, as were VWF:CB3/VWF:Ag ratios (Table 3). This decrease in VWF:CB4/VWF:Ag ratio is consistent with expected decreases in collagen binding because of the loss of high-molecular-weight multimers in this cohort.22,29 Median VWF:CB4/VWF:Ag ratio for the type 2A subjects was 0.50, which was not significantly different from the VWF:CB3/VWF:Ag ratio (P = NS). VWF:RCo/VWF:Ag ratios were even lower for the type 2A subjects, with a median of 0.29 (P < .005 compared with VWF:CB4/VWF:Ag). For type 2B subjects, the median VWF:CB4/VWF:Ag ratio was 0.52, similar to the results seen for the type 2A group. The median VWF:RCo/VWF:Ag ratio was 0.47 (P = NS compared with VWF:CB4/VWF:Ag). Although the VWF:RCo/VWF:Ag ratio was significantly lower in type 2A VWD compared with type 2B VWD subjects (P < .02), no difference in VWF:CB4/VWF:Ag or VWF:CB3/VWF:Ag ratio was observed between these subtypes. A trend toward lower VWF:CB3/VWF:Ag ratios was seen for both type 2A and type 2B subjects compared with VWF:CB4/VWF:Ag.
Table 3.
VWF laboratory values for type 2 VWD subjects
| Type 2A (n = 43) | Type 2B (n = 19) | Type 2M (n = 15) | Type 2N (n = 8) | |||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| VWF:Ag | 27 | 21-35 | 37 | 22-44 | 43 | 25-64 | 75 | 55-94 |
| VWF:RCo | 5 | 5-14 | 14 | 12-22 | 17 | 11-32 | 87 | 52-126 |
| VWF:RCo/VWF:Ag ratio | 0.29 | 0.19-0.47 | 0.47 | 0.32-0.67 | 0.45 | 0.39-0.55 | 1.08 | 1.01-1.29 |
| VWF:CB3 | 8 | 4-24 | 12 | 6-22 | 43 | 27-69 | 95 | 59-108 |
| VWF:CB3/VWF:Ag ratio | 0.31 | 0.17-0.70 | 0.37 | 0.26-0.56 | 1.08 | 0.97-1.11 | 1.12 | 1.09-1.20 |
| VWF:CB4 | 13 | 8-22 | 16 | 8-26 | 23 | 14-45 | 84 | 54-98 |
| VWF:CB4/VWF:Ag ratio | 0.50 | 0.40-0.60 | 0.52 | 0.36-0.60 | 0.67 | 0.48-0.89 | 1.06 | 0.78-1.24 |
| Bleeding score | 8 | 4-11 | 10 | 4-13 | 11 | 4-12 | 8.5 | 2.5-16.5 |
IQR, interquartile range.
Data are given in IU/dL with the exception of VWF:CB4, which was normalized to an international standard and is given in U/dL. For subjects with values below the lower limit of detection for each assay, an arbitrary value was assigned for calculation purposes (5 IU/dL for VWF:RCo <10 IU/dL, 0.5 IU/dL for VWF:CB3 <1 IU/dL, and 0.8 U/dL for VWF:CB4 <1.6 U/dL).
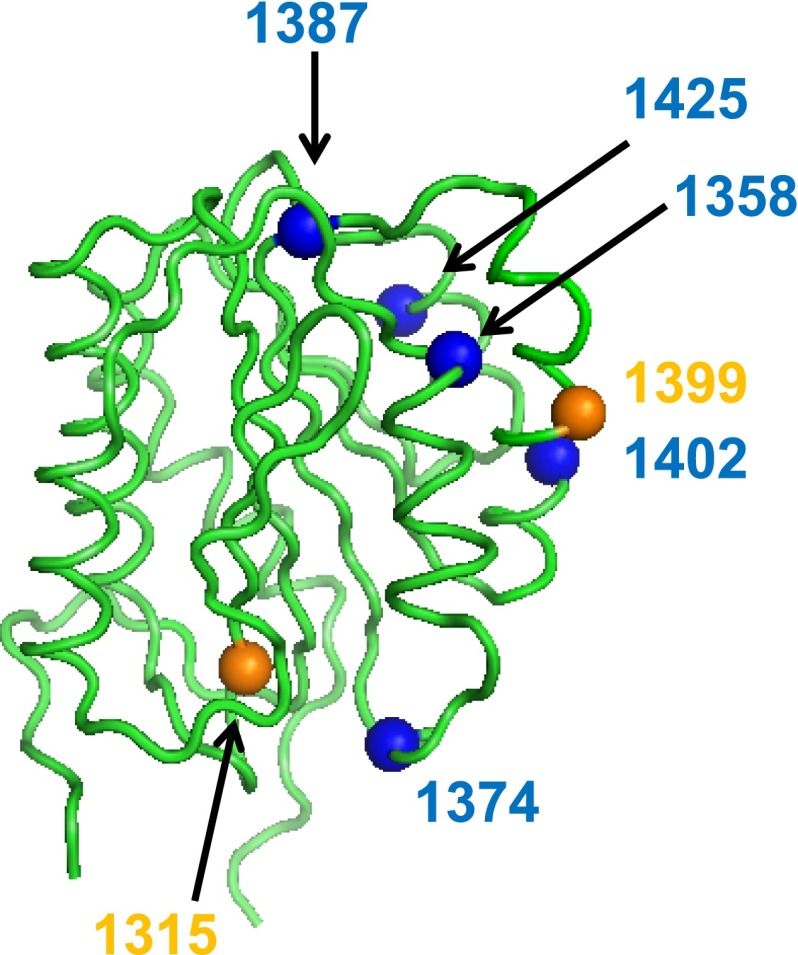
Collagen 4 binding in the type 2M cohort varied from normal to undetectable. All subjects by definition had reduced VWF:RCo/VWF:Ag ratios (median 0.45). The median VWF:CB3/VWF:Ag ratio was 1.08 (P < .001 compared with VWF:RCo/VWF:Ag). The median VWF:CB4/VWF:Ag ratio was 0.67 (P = NS compared with VWF:RCo/VWF:Ag and P < .001 compared with VWF:CB3/VWF:Ag). Abnormal VWF binding to collagen 4 with a VWF:CB4/VWF:Ag ratio <0.5 was observed for VWF A1 domain variants p.S1358N, p.R1392_Q1402del, and p.Q1402P, and also in a subject heterozygous for p.I1425F and p.R1399H. Figure 1 shows the location of those variants that altered collagen 4 binding in the VWF A1 domain crystal structure (1AUQ).30 Table 2 summarizes the VWF variants with defects in collagen 4 binding.
Figure 1.
Location of VWF A1 domain sequence variations and their effect on collagen 4 binding. The VWF A1 domain crystal structure30 is shown here with orange spheres to indicate Zimmerman Program A1 domain sequence variations found in type 1 subjects, and the blue spheres indicate sequence variations found in type 2M subjects that were associated with reduced or absent VWF–collagen 4 binding.
Bleeding scores for the type 2M subjects with a collagen 4 binding defect and a platelet-binding defect were increased compared with the type 2M subjects with only a platelet-binding defect or subjects with only a collagen 4 binding defect. The median bleeding score was 13 for those type 2M subjects with a defect in collagen 4 binding (n = 4) compared with a median bleeding score of 6 for type 2M subjects without a defect in collagen 4 binding (n = 11, P < .05). Subjects with an isolated collagen 4 binding defect had a median bleeding score of 6. Four of 15 (27%) type 2M subjects had a collagen 4 binding defect. An additional 15 subjects with laboratory findings otherwise consistent with type 1 VWD had an isolated collagen binding defect to type 4 collagen, yielding a prevalence of 6% when both type 1 and type 2M subjects were considered.
Type 2N subjects had normal VWF:CB4/VWF:Ag ratios (median 1.06, P = NS compared with healthy controls). Type 3 subjects had VWF:CB4 below the lower limit of detection for the assay, as expected, given the undetectable VWF:Ag in this patient group. Samples from type 3 VWD subjects receiving recent prophylaxis with VWF-containing concentrates were excluded from this analysis.
VWF binding to collagen 4 in vitro
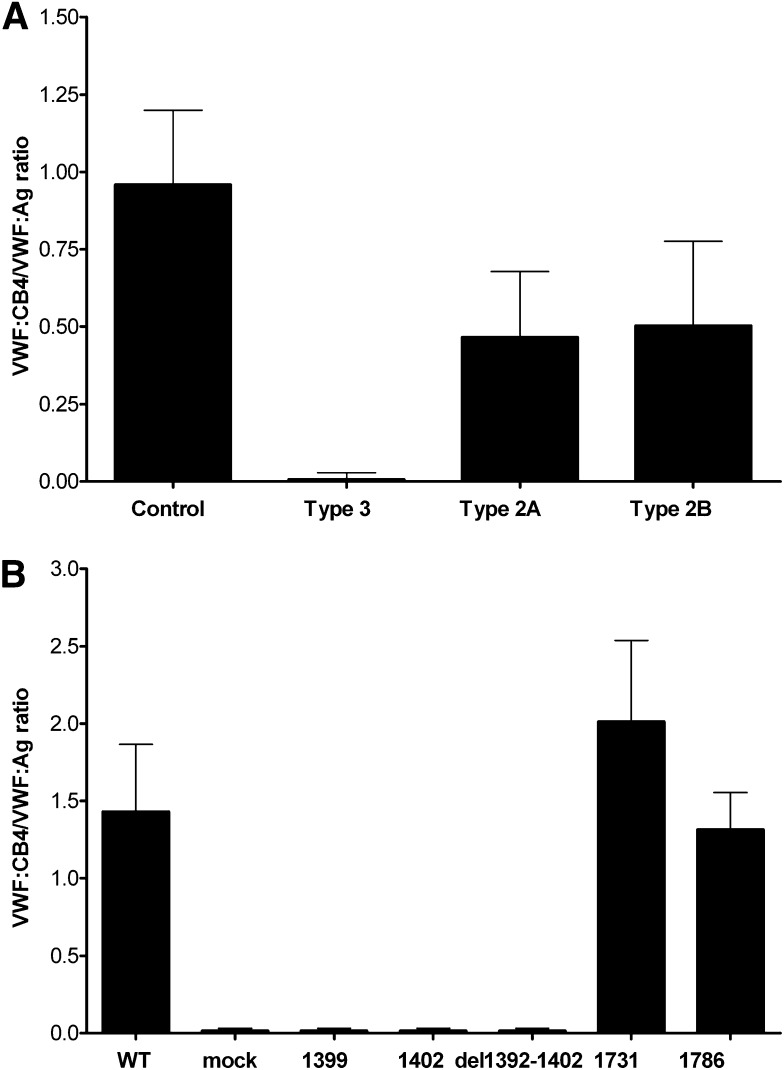
Direct binding of VWF to collagen 4 was observed for both plasma-derived and recombinant VWF when collagen 4 was coated on an amine-binding plate. Binding was reduced for plasma from type 2A and type 2B VWD subjects lacking high-molecular-weight multimers, and was absent for plasma from type 3 VWD subjects with undetectable multimer distribution (Figure 2A). Several recombinant VWF variants also demonstrated markedly decreased binding to collagen 4. An 11-amino-acid deletion in the VWF A1 domain (p.R1392_Q1402del)31 also abrogated binding to collagen 4 (Figure 2B), as did 2 single-nucleotide sequence variations (p.R1399H and p.Q1402P), both of which have been implicated in abnormal interactions with collagen 6.32 Two sequence variations that alter VWF binding to collagen 3, p.S1731T and p.H1786D, did not affect VWF binding to collagen 4 (Figure 2B).
Figure 2.
Collagen 4 binding to VWF depends on multimeric structure and intact VWF A1 domain. Binding of VWF samples to collagen 4 was measured by ELISA and results are expressed as the ratio of collagen 4 binding to VWF:Ag. (A) VWF:CB4/VWF:Ag ratios for human plasma samples from healthy controls (n = 246) and type 3 VWD subjects (n = 24) as well as type 2A (n = 43) and type 2B subjects (n = 19) lacking high-molecular-weight multimers. (B) VWF:CB4/VWF:Ag ratios for recombinant VWF constructs expressed in HEK293T cells with either full-length human VWF sequence or VWF containing the indicated sequence variation (n = 3 separate transfections for each construct). Error bars show mean ± 1 SD.
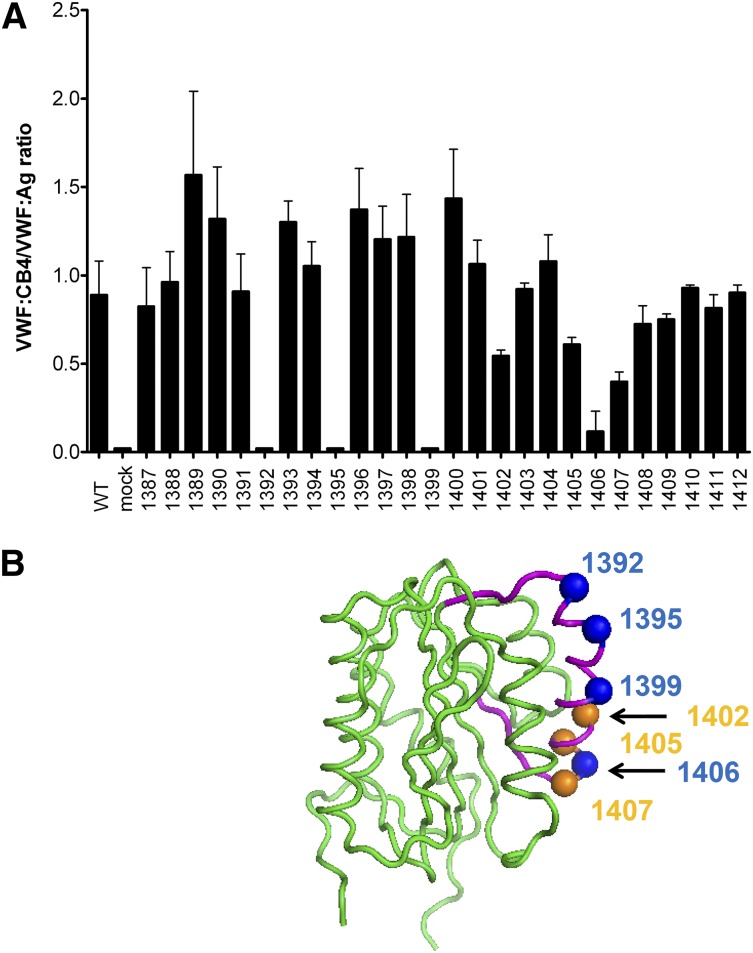
Given the apparent involvement of the VWF A1 domain on binding to collagen 4, additional VWF A1 domain variants were studied. Scanning alanine mutagenesis was performed with VWF A1 domain residues 1387 through 1412, to include the region where sequence variations affecting collagen 4 binding had previously been noted in either patient or recombinant VWF samples, as described previously. All residues studied had equivalent VWF:Ag, collagen 3 binding, and multimer distribution compared with WT rVWF (data not shown). Analysis of collagen 4 binding revealed a number of key VWF A1 domain residues (Figure 3A). These localized to 1 face of the VWF A1 domain crystal structure (Figure 3B).30
Figure 3.
VWF A1 domain contains key residues for collagen 4 binding. Scanning alanine mutagenesis was performed for VWF A1 domain residues 1387 to 1412. (A) VWF:CB4/VWF:Ag ratios for each alanine mutant as listed on the x-axis. Error bars show mean ± 1 SD. (B) Location of key residues with absent binding (blue spheres) or reduced binding (orange spheres) to collagen 4 in a drawing of the VWF A1 domain crystal structure.30 Residues that were changed in the scanning alanine mutagenesis are shown in purple. An 11-amino-acid deletion that also affected collagen 4 binding is not shown for clarity.
VWF-mediated platelet binding to collagen 4 under flow conditions
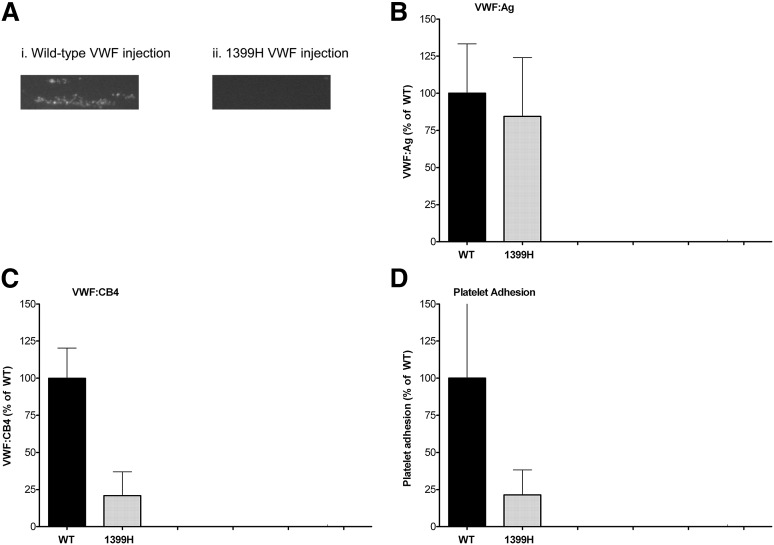
To investigate the relationship between VWF and collagen 4 under flow conditions, an in vitro flow model using the Venaflux system (Cellix) was used.24,25 VWF-mediated platelet adhesion to collagen 4 was observed for a human healthy control sample (data not shown), and for WT mice, but no adhesion was observed in VWF-deficient mouse plasma (Figure 4A), suggesting that VWF is required for this interaction under flow conditions. The p.R1399H sequence variation was chosen as an example VWF with a collagen 4 binding defect and introduced into murine VWF by hydrodynamic tail-vein injection. This resulted in normal VWF expression (Figure 4B) but reduced static binding to collagen 4 (Figure 4C) and reduced platelet adhesion compared with control mice (Figure 4D). Although there was a decrease in high-molecular-weight multimers for both WT and p.1399H VWF, as has been seen previously with the hydrodynamic tail-vein injection model, there was no difference in multimer distribution between the two (data not shown), thus enabling comparison of collagen binding despite the multimer defect.
Figure 4.
Murine 1399H VWF demonstrates defect in shear-induced collagen 4 binding. VWF-deficient mice were injected with either WT or p.1399H murine VWF DNA to achieve temporary correction of their VWF levels. Blood samples were obtained 24 hours postinjection. Binding to collagen 4 under flow conditions was assessed using the Venaflux system at 37°C using a shear rate of 1111 s−1. Platelets were labeled with mepacrine for visualization. (A) Platelet adhesion after 180 seconds for a representative sample from a mouse injected with WT VWF (panel 1) and a mouse injected with 1399H VWF (panel 2). Images were obtained using a Zeiss Axio Observer.A1 microscope at ×10 original magnification and a Hamamatsu Orca R2 camera. (B) VWF:Ag levels obtained 24 hours postinjection for WT (n = 4) and 1399H (n = 4) mice. (C) VWF:CB4 levels obtained 24 hours postinjection for WT (n = 4) and 1399H (n = 4) mice. (D) Platelet adhesion as measured by number of platelet aggregates for 1399H-injected mice (n = 3) compared with WT-injected mice (n = 4).
Discussion
The interaction of VWF with collagen is crucial for initiation of clot formation at sites of injury. The presence of multiple different intravascular collagens, however, complicates analysis of this interaction. Initial workup of VWD rarely includes analysis of collagen binding and, when performed, such workups generally use collagen 1 or collagen 3, which are available in standardized commercial assays. We have previously shown that collagen 6 can bind VWF, and we now show that collagen 4 binds VWF as well. Collagens 4 and 6 appear to bind VWF via the VWF A1 domain, and binding is altered by some (but not all) VWF A1 domain sequence variations. Many of the sequence variations cluster on one side of the VWF A1 domain.
The clinical data from Zimmerman Program subjects was confirmed by the in vitro data generated using recombinant VWF. The prevalence of sequence variations affecting collagen 4 binding was approximately 5% in type 1 subjects and 27% in type 2M subjects, for an overall combined prevalence in those groups of 6%. In the type 2M subjects, the mean bleeding score was higher in subjects with both a platelet-binding and a collagen-binding defect compared with subjects with only a platelet-binding defect. The p.R1399H sequence variation was found in ∼1% of the subjects reported here, including both healthy controls and type 1 subjects.
The classification of those subjects with low VWF levels, initially diagnosed as type 1 VWD, who were also found to have a collagen-binding defect, is problematic. Although they have normal VWF:RCo/VWF:Ag ratios, they do have a functional defect in VWF more consistent with a diagnosis of type 2M VWD. However, heterozygosity for p.R1399H alone was seen in healthy controls and most likely does not represent a hemorrhagic phenotype. In conjunction with a second mutation, or a null allele, p.R1399H may result in additional symptoms. Collagen-binding variants may be viewed as a risk factor for bleeding, and their presence could necessitate a change in treatment.
Classification of VWD variants is important because it may serve to guide treatment of affected patients. Therefore we favor classifying patients with normal multimer distribution as type 1 VWD if all VWF activity assays are commensurate with VWF:Ag, but as type 2M if there is a specific defect in activity, either reduced VWF:RCo/VWF:Ag or VWF:CB/VWF:Ag. Type 2M patients may respond to desmopressin but may also require use of VWF-containing concentrates. It is possible that some collagen-binding defects may have inadequate clinical response to desmopressin and require further therapy with VWF-containing concentrates as well.
Variation in VWF levels and bleeding score was seen across subjects despite the presence of the same variant. This was true for p.R1399H, as discussed previously, but also for p.R1315C. Heterogeneity in both symptoms and laboratory findings has been demonstrated for other VWF variants.33 The classification of p.R1315C is presently unclear; Ribba and colleagues reported on a series of patients with this variant who all lacked high-molecular-weight multimers and would thus be considered type 2A, but not all of the subjects in our study had multimer defects.28 The presence of other modifying factors apart from either VWF levels or VWF genetics cannot be excluded and highlights the importance of individual consideration with regard to diagnosis and treatment.
Collagen 4 binding also relies on the presence of intact high-molecular-weight VWF multimers, with decreased collagen 4 binding seen in both type 2A and type 2B VWD subjects. However, in our studies, collagen 3 appears to be more sensitive to the loss of high-molecular-weight multimers, as evidenced by the data from type 2A VWD subjects, where VWF:CB4/VWF:Ag ratios were higher than VWF:CB3/VWF:Ag ratios. Therefore, it is unlikely that collagen 4 binding would be able to replace analysis of VWF multimer distribution. However, there are significant differences between collagen 3 and collagen 4, because they bind different VWF domains. Collagen 3 and collagen 1 each bind VWF via the A3 domain. Although an alternate binding site in the VWF A1 domain has been postulated,34 our data under static conditions did not demonstrate the ability of an intact A1 domain to compensate for A3 domain variants in collagen 3 binding. Collagens 4 and 6, alternately, bind VWF exclusively via the A1 domain and do not appear to bind the VWF A3 domain. Although this interaction depends on the presence of high-molecular-weight multimers, sequence variations in the A3 domain or other VWF domains did not affect collagen 4 binding.
Binding of VWF to collagen 4 was demonstrated under both static and flow conditions. Collagen 4 is present in the basement lamina8 and could conceivably be exposed after significant vessel injury. Defects in VWF that reduce or eliminate its ability to bind collagen 4 might therefore incur a risk of clinically significant bleeding. Such defects would potentially go undetected based on the current VWD diagnostic testing used at most centers, but it might explain bleeding in patients without a definitive diagnosis or in those who have mild deficiency of VWF but bleeding symptoms beyond that expected for their VWF level. Furthermore, defects in the VWF A1 domain that could affect collagen 4 or 6 binding are more common than defects in the VWF A3 domain (of which only 5 have been reported to date).4-6,35
Our work has demonstrated that the VWF A1 domain plays a critical role in binding to type 4 collagen. More work is required to elucidate the role of VWF in binding different vascular collagens at sites of injury, and the utility of collagen binding with different collagen types in VWD diagnosis. These data suggest that defects in the ability of VWF to bind collagen 4 exist in VWD and present an additional risk factor for bleeding in affected patients.
Acknowledgments
The authors acknowledge Patricia Morateck and Nereida Sotelo for assistance with collagen-binding assays; the Zimmerman Program investigators (see Appendix), staff, and subjects for their participation; and Drs Darryl Stafford (University of North Carolina–Chapel Hill) and Billy Hudson (Vanderbilt University) for helpful discussions.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute K08 grant HL102260 (V.F.), program project grant HL081588 (R.R.M.), and grants HL33721 and HL044612 (R.R.M.); and by the Midwest Athletes Against Childhood Cancer.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.H.F. designed and supervised the study and wrote the manuscript; A.C.S. and P.M.J. performed the murine experiments including hydrodynamic tail-vein injection, murine VWF assays, and studies of VWF under flow; S.L.H. supervised the murine experiments and edited the manuscript; T.L.S. performed the recombinant VWF experiments; D.B.B. performed and analyzed the VWF sequencing; P.A.C. collected Zimmerman Program subject information and edited the manuscript; K.D.F., J.C.G., S.L.H., and D.B.B. assisted with Zimmerman Program subject classification and edited the manuscript; R.G.H. performed the statistical analysis; and R.R.M. supervised the Zimmerman Program subject classification and edited the manuscript.
Conflict-of-interest disclosure: J.C.G. is a consultant for Baxter, Bayer, and CSL Behring. R.R.M. is a consultant for AstraZeneca, Baxter, Bayer, Biogen Idec, CSL Behring, Grifols, Immucor, and Octapharma. The remaining authors declare no competing financial interests.
Correspondence: Veronica H. Flood, Comprehensive Center for Bleeding Disorders, 8739 Watertown Plank Rd, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: vflood@mcw.edu.
Appendix
The Zimmerman Program for the Molecular and Clinical Biology of Von Willebrand Disease includes the following investigators:
Directors of the primary centers: T. Abshire, A. Dunn, C. Bennett, Emory University School of Medicine, Atlanta, GA; J. Lusher, M. Rajpurkar, Wayne State University, Detroit, MI; D. Brown, University of Texas Health Science Center at Houston, Houston, TX; A. Shapiro, Indiana Hemophilia & Thrombosis Center, Indianapolis, IN; S. Lentz, University of Iowa, Iowa City, IA; J. Gill, Comprehensive Center for Bleeding Disorders, Milwaukee, WI; C. Leissinger, Tulane University Health Sciences Center, New Orleans, LA; M. Ragni, University of Pittsburgh, Pittsburgh, PA.
In addition, numerous secondary centers contributed to subject recruitment: J. Hord, Akron Children's Hospital, Akron, OH; M. Manco-Johnson, Mountain States Regional Hemophilia and Thrombosis Center, Aurora, CO; J. Strouse, Johns Hopkins Children's Center, Baltimore, MD; A. Ma, University of North Carolina Chapel Hill, Chapel Hill, NC; L. Valentino, L. Boggio, Rush University Medical Center, Chicago, IL; A. Sharathkumar, Children's Memorial Hospital, Chicago, IL; R. Gruppo, Cincinnati Children's Hospital, Cincinnati, OH; B. Kerlin, Nationwide Children's Hospital, Columbus, OH; J. Journeycake, UT Southwestern, Dallas, TX; R. Kulkarni, Michigan State University, East Lansing, MI; D. Green, Northwestern University, Evanston, IL; D. Mahoney, Baylor College of Medicine, Houston, TX; L. Mathias, A. Bedros, Loma Linda University Medical Center, Loma Linda, CA; C. Diamond, University of Wisconsin Madison, Madison, WI; A. Neff, Vanderbilt University, Nashville, TN; D. DiMichele and P. Giardina, Weill Cornell Medical College, New York, NY; A. Cohen, Newark Beth Israel Medical Center, Newark, NJ; M. Paidas, Yale School of Medicine, New Haven, CT; E. Werner, Children's Hospital of the King's Daughters, Norfolk, VA; A. Matsunaga, Children's Hospital & Research Center Oakland, Oakland, CA; M. Tarantino, Comprehensive Bleeding Disorders Center, Peoria, IL; F. Shafer, Drexel University College of Medicine, Philadelphia, PA; B. Konkle and A. Cuker, University of Pennsylvania, Philadelphia, PA; P. Kouides, Rochester General Hospital, Rochester, NY; D. Stein, Toledo Children's Hospital, Toledo, OH.
References
- 1.Weiss HJ, Sussman II, Hoyer LW. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J Clin Invest. 1977;60(2):390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohri H, Fujimura Y, Shima M, et al. Structure of the von Willebrand factor domain interacting with glycoprotein Ib. J Biol Chem. 1988;263(34):17901–17904. [PubMed] [Google Scholar]
- 3.Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987;262(28):13835–13841. [PubMed] [Google Scholar]
- 4.Ribba AS, Loisel I, Lavergne JM, et al. Ser968Thr mutation within the A3 domain of von Willebrand factor (VWF) in two related patients leads to a defective binding of VWF to collagen. Thromb Haemost. 2001;86(3):848–854. [PubMed] [Google Scholar]
- 5.Riddell AF, Gomez K, Millar CM, et al. Characterization of W1745C and S1783A: 2 novel mutations causing defective collagen binding in the A3 domain of von Willebrand factor. Blood. 2009;114(16):3489–3496. doi: 10.1182/blood-2008-10-184317. [DOI] [PubMed] [Google Scholar]
- 6.Flood VH, Lederman CA, Wren JS, et al. Absent collagen binding in a VWF A3 domain mutant: utility of the VWF:CB in diagnosis of VWD. J Thromb Haemost. 2010;8(6):1431–1433. doi: 10.1111/j.1538-7836.2010.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flood VH, Gill JC, Christopherson PA, et al. Critical von Willebrand factor A1 domain residues influence type VI collagen binding. J Thromb Haemost. 2012;10(7):1417–1424. doi: 10.1111/j.1538-7836.2012.04746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bornstein P, Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- 9.Saelman EU, Nieuwenhuis HK, Hese KM, et al. Platelet adhesion to collagen types I through VIII under conditions of stasis and flow is mediated by GPIa/IIa (alpha 2 beta 1-integrin). Blood. 1994;83(5):1244–1250. [PubMed] [Google Scholar]
- 10.Henrita van Zanten G, Saelman EU, Schut-Hese KM, et al. Platelet adhesion to collagen type IV under flow conditions. Blood. 1996;88(10):3862–3871. [PubMed] [Google Scholar]
- 11.Gould DB, Phalan FC, van Mil SE, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354(14):1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 12.Jeanne M, Labelle-Dumais C, Jorgensen J, et al. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am J Hum Genet. 2012;90(1):91–101. doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng YC, Sonni A, Labelle-Dumais C, et al. COL4A1 mutations in patients with sporadic late-onset intracerebral hemorrhage. Ann Neurol. 2012;71(4):470–477. doi: 10.1002/ana.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould DB, Phalan FC, Breedveld GJ, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308(5725):1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 15.Howard MA, Sawers RJ, Firkin BG. Ristocetin: a means of differentiating von Willebrand’s disease into two groups. Blood. 1973;41(5):687–690. [PubMed] [Google Scholar]
- 16.Brown JE, Bosak JO. An ELISA test for the binding of von Willebrand antigen to collagen. Thromb Res. 1986;43(3):303–311. doi: 10.1016/0049-3848(86)90150-7. [DOI] [PubMed] [Google Scholar]
- 17.Favaloro EJ. Evaluation of commercial von Willebrand factor collagen binding assays to assist the discrimination of types 1 and 2 von Willebrand disease. Thromb Haemost. 2010;104(5):1009–1021. doi: 10.1160/TH10-06-0360. [DOI] [PubMed] [Google Scholar]
- 18.Flood VH, Gill JC, Morateck PA, et al. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116(2):280–286. doi: 10.1182/blood-2009-10-249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellissimo DB, Christopherson PA, Flood VH, et al. VWF mutations and new sequence variations identified in healthy controls are more frequent in the African-American population. Blood. 2012;119(9):2135–2140. doi: 10.1182/blood-2011-10-384610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–2065. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 21.Flood VH, Gill JC, Christopherson PA, et al. Comparison of type I, type III and type VI collagen binding assays in diagnosis of von Willebrand disease. J Thromb Haemost. 2012;10(7):1425–1432. doi: 10.1111/j.1538-7836.2012.04747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flood VH, Gill JC, Friedman KD, et al. Zimmerman Program Investigators. Collagen binding provides a sensitive screen for variant von Willebrand disease. Clin Chem. 2013;59(4):684–691. doi: 10.1373/clinchem.2012.199000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruss CM, Golder M, Bryant A, Hegadorn C, Haberichter S, Lillicrap D. Use of a mouse model to elucidate the phenotypic effects of the von Willebrand factor cleavage mutants, Y1605A/M1606A and R1597W. J Thromb Haemost. 2012;10(5):940–950. doi: 10.1111/j.1538-7836.2012.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J Thromb Haemost. 2012;10(8):1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philipose S, Konya V, Sreckovic I, et al. The prostaglandin E2 receptor EP4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol. 2010;30(12):2416–2423. doi: 10.1161/ATVBAHA.110.216374. [DOI] [PubMed] [Google Scholar]
- 26.Sadler JE, Ginsburg D For the Consortium on von Willebrand Factor Mutations and Polymorphisms and the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. A database of polymorphisms in the von Willebrand factor gene and pseudogene. Thromb Haemost. 1993;69(2):185–191. [PubMed] [Google Scholar]
- 27.Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ, Jr, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69(6):1691–1695. [PubMed] [Google Scholar]
- 28.Ribba AN, Hilbert L, Lavergne JM, et al. The arginine-552-cysteine (R1315C) mutation within the A1 loop of von Willebrand factor induces an abnormal folding with a loss of function resulting in type 2A-like phenotype of von Willebrand disease: study of 10 patients and mutated recombinant von Willebrand factor. Blood. 2001;97(4):952–959. doi: 10.1182/blood.v97.4.952. [DOI] [PubMed] [Google Scholar]
- 29.Favaloro EJ. Toward a new paradigm for the identification and functional characterization of von Willebrand disease. Semin Thromb Hemost. 2009;35(1):60–75. doi: 10.1055/s-0029-1214149. [DOI] [PubMed] [Google Scholar]
- 30.Emsley J, Cruz M, Handin R, Liddington R. Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J Biol Chem. 1998;273(17):10396–10401. doi: 10.1074/jbc.273.17.10396. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso DJ, Kroner PA, Christopherson PA, Vokac EA, Gill JC, Montgomery RR. Type 2M:Milwaukee-1 von Willebrand disease: an in-frame deletion in the Cys509-Cys695 loop of the von Willebrand factor A1 domain causes deficient binding of von Willebrand factor to platelets. Blood. 1996;88(7):2559–2568. [PubMed] [Google Scholar]
- 32.Larsen DM, Haberichter SL, Gill JC, Shapiro AD, Flood VH. Variability in platelet- and collagen-binding defects in type 2M von Willebrand disease. Haemophilia. 2013;19(4):590–594. doi: 10.1111/hae.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castaman G, Federici AB, Tosetto A, et al. Different bleeding risk in type 2A and 2M von Willebrand disease: a 2-year prospective study in 107 patients. J Thromb Haemost. 2012;10(4):632–638. doi: 10.1111/j.1538-7836.2012.04661.x. [DOI] [PubMed] [Google Scholar]
- 34.Bonnefoy A, Romijn RA, Vandervoort PA, VAN Rompaey I, Vermylen J, Hoylaerts MF. von Willebrand factor A1 domain can adequately substitute for A3 domain in recruitment of flowing platelets to collagen. J Thromb Haemost. 2006;4(10):2151–2161. doi: 10.1111/j.1538-7836.2006.02111.x. [DOI] [PubMed] [Google Scholar]
- 35.Keeling D, Beavis J, Marr R, Sukhu K, Bignell P. A family with type 2M VWD with normal VWF:RCo but reduced VWF:CB and a M1761K mutation in the A3 domain. Haemophilia. 2012;18(1) doi: 10.1111/j.1365-2516.2011.02676.x. e33-2516.2011.02676.x. Epub 2011 Oct 17. [DOI] [PubMed] [Google Scholar]