Abstract
Objective:
To study the main clinical and histopathological features of 12 patients with Hodgkin’s lymphoma (HL) diagnosed primarily from bone marrow (BM) involvement.
Methods:
We included 12 acquired immunodeficiency syndrome (AIDS) patients with HL assisted in the F. J. Muñiz Infectious Diseases Hospital since January 2002 to December 2013. The diagnosis of HL with primary BM involvement in patients was confirmed by clinical, histopathological, and immunohistochemical findings.
Results:
All patients presented “B” symptoms and pancytopenia. All of them had stage IV neoplasm disease because of BM infiltration. The median of CD4+ T-cell counts was 114 cells/μL, and mixed cellularity (MC) was the most frequent histopathological subtype of 92% cases.
Conclusion:
When other causes are excluded, BM biopsy should be performed in AIDS patients with “B” symptoms and pancytopenia to evaluate BM infiltration by atypical lymphocytes.
Keywords: Acquired immunodeficiency syndrome (AIDS), Hodgkin’s lymphoma (HL), bone marrow (BM)
Introduction
The risk of Hodgkin’s lymphoma (HL) is significantly increased in acquired immunodeficiency syndrome (AIDS) patients. Patients with human immunodeficiency virus (HIV) have a 5- to 15-fold relative risk compared with the general population. The most frequent subtypes of HL in HIV/AIDS patients are mixed cellularity (MC) and lymphocyte depletion (LD), whereas the nodular sclerosis (NS) subtype predominates in the general population1,2. Primary extranodal presentation of HL is rare and includes less than 0.25% of patients3. Moreover, the incidence of bone marrow (BM) involvement in HL varies with the histological subtype. In this paper, we report a group of 12 patients with HL associated with HIV infection diagnosed primarily from BM involvement.
Materials and methods
We retrospectively reviewed the records of patients in the F. J. Muñiz Infectious Diseases Hospital, a reference hospital for infectious diseases, to search for all the HIV-positive patients with lymphoma with associated confirmation of the case by histological examination. Only adult patients who were hospitalized between 2002 and 2013 were considered. The inclusion criterion for this specific analysis was the diagnosis of HL with isolated BM involvement. Data on demographic and clinical profiles, HIV characteristics, laboratory profiles including lactate dehydrogenase (LDH) levels, CD4+ T-cell count, presenting symptoms at the time of neoplasm diagnosis, and hepatitis C virus (HCV) serological status were recorded. This study was approved by the ethics committee at the F. J. Muñiz Infectious Diseases Hospital.
All the patients underwent chest X-ray and complete tomography scan of head, neck, thorax, abdomen, and pelvis with oral and intravenous contrast as part of the initial staging work-up. Diagnosis was confirmed by histopathological examination of the trephine BM biopsy performed on the posterior iliac crest (unilateral). The core of BM biopsy was fixed in Bouin’s solution. Afterward, all smears were decalcified using an EDTA-based solution, embedded in paraffin, and then sectioned. To detect the presence of opportunistic infections, all smears were stained in hematoxylin and eosin, Giemsa, PAS, Ziehl-Neelsen, and Grocott methenamine silver stain. HL diagnosis was confirmed through the identification of classical Reed-Sternberg (R-S) cells or their variants in an adequate cellular background and was made according to the World Health Organization classification of hematological malignancies. Immunohistochemical stains with CD30, CD15, leukocyte common antigen (LCA), CD45, CD20, and CD3 antibodies were obtained from DAKO Diagnosis (Denmark). The presence of Epstein-Barr virus (EBV)-associated latent membrane protein-1 (LMP-1) by immunohistochemistry was analyzed in BM sections.
Statistical analysis
All data were tabulated using Microsoft Excel® 2007. A descriptive statistical analysis was performed. Measures of central tendency and variability were calculated. When possible, Student t-test and correlation tests were completed. All statistical tests were performed using SPSS® 22.0.
Results
Between January 2002 to December 2013, 115 HIV-positive patients with lymphoma were identified, and 38 of these patients had HL (33%). Of the 38 patients with HL, 12 had isolated BM involvement without adenopathy or other solid organ infiltration. Thus, 12 patients with isolated BM involvement were included in our descriptive analysis.
During the period of the study, we performed 429 BM biopsies; 12 (2.7%) had diagnosis of HL with primarily and isolated BM involvement. Most patients were men (n=11; 92%). The mean age was 35.91±8.69 years (range, 26-51 years), with a nearly normal distribution. Intravenous drug use (IVDU) was the main HIV infection risk factor (66.6%). Median time from diagnosis of HIV infection to HL was 10 years. All patients presented “B” symptoms, such as prolonged and unexplained fever, weight loss (median 5 kg), night sweats, and pancytopenia. No evidence of concomitant opportunistic infection was found in the microbiological analysis.
Pancytopenia was demonstrated in all patients. Median red cell count was 2.2×106 cells/µL (range, 1.89-3.54 ×106 cells/µL). Median values of hemoglobin levels and hematocrit were 6 g/dL (range, 5.5-10.9 g/dL) and 20% (range, 17%-34%), respectively. White cell count was below normal values in all cases (median count: 3,100 cells/µL, range, 1,100-3,700 cells/µL). Platelet count was abnormal in almost all patients (median count: 94,000 cells/µL; range, 41,000-189,000 cells/µL).
The median CD4+ T-cell count was 114 cells/µL (range, 17 to 282 cells/μL) with significant heterogeneity among individual patients. In only two subjects, CD4+ T-cell count was above 200 cells/µL. Eight patients were also infected with HCV, and all of them had a history of IVDU. Initial values of LDH were available for six patients and were elevated in four of them (normal value up to 350 U/L). Computed tomography scans of chest, abdomen, and pelvis demonstrated negative results for lymphadenopathies and liver or spleen involvement. No gallium or positron emission tomography (PET) scans were performed. Demographic, clinical, and histopathological findings are listed in Table 1.
Table 1.
Demographic, clinical, and histopathological findings in 12 patients with HL diagnosed primarily in BM
| Patient | Sex | Age (years) | Risk factor | CD4 | HCV | LDH |
|---|---|---|---|---|---|---|
| #1 | M | 26 | IVDU | ND | Pos | ND |
| #2 | F | 29 | IVDU | ND | Pos | ND |
| #3 | M | 27 | IVDU | 195 | Pos | ND |
| #4 | M | 34 | IVDU | 17 | Pos | ND |
| #5 | M | 26 | IVDU | 39 | Pos | ND |
| #6 | M | 31 | IVDU | 123 | Pos | ND |
| #7 | M | 49 | UHSC | 52 | Neg | 600 |
| #8 | M | 40 | IVDU | 56 | Pos | 796 |
| #9 | M | 35 | UHSC | 104 | Neg | 318 |
| #10 | M | 41 | UHSC | 237 | ND | 390 |
| #11 | M | 51 | UHSC | 282 | Neg | 638 |
| #12 | M | 42 | IVDU | 198 | Pos | 360 |
M, male; F, female; HCV, hepatitis C virus; LDH, lactate dehydrogenase; IVDU, intravenous drug user; UHSC, unprotected heterosexual contact; Pos, positive; Neg, negative; ND, no data.
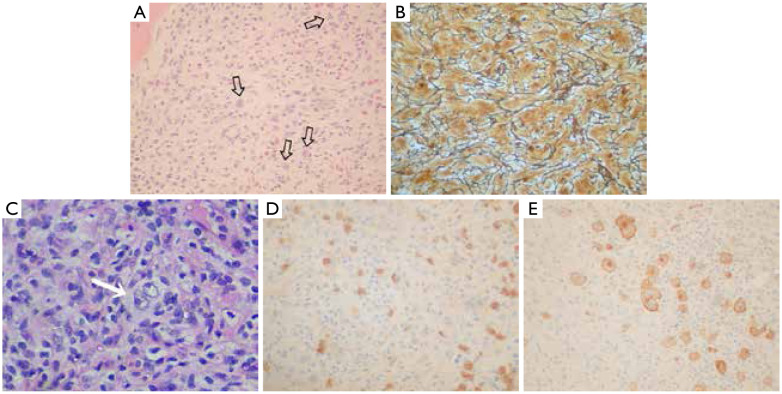
All cases presented the minimum requirement of HL diagnosis, including identification of the classical R-S cells or their variants in an adequate cellular background (Figure 1A,B). The type and extent of BM involvement, the percent of biopsy involved, the type of infiltration pattern, the immunostaining reactivity of R-S cells, the characteristics of the accompanying lymphocytes, and the EBV-associated LMP-1 results in patients in which this information were available are described in Table 2.
Figure 1.
(A) Diffuse involvement of bone marrow by HL with the identification of Reed-Sternberg cells (arrows) in an appropriate background (H&E, ×10); (B) Diffuse bone marrow fibrosis observed with methenamine-silver stain (×100); (C) Focal pattern of BM involvement in HL with the presence of a large binucleated Reed-Sternberg cell (arrow) (H&E, ×100); (D) Immunohistochemistry show some CD15+ Reed-Sternberg cells (IHC,×40); (E) Immunohistochemistry with expression of CD30 antigen in an adequate cellular background by Reed-Sternberg cells (IHC, ×100).
Table 2.
Histological features in 12 patients with Hodgkin’s lymphoma diagnosed primarily in the bone marrow
| Patient | Infiltration pattern | Infiltration (%) | Reed Sternberg cells |
Background lymphocytes | |||
|---|---|---|---|---|---|---|---|
| CD30 | CD15 | CD20 | LMP1 | ||||
| #1 | NDA | NDA | NDA | NDA | NDA | NDA | NDA |
| #2 | NDA | NDA | NDA | NDA | NDA | NDA | NDA |
| #3 | NDA | NDA | NDA | NDA | NDA | NDA | NDA |
| #4 | Nodular | 40 | Positive | Positive | Negative | Negative | CD3 |
| #5 | Diffuse | 100 | Positive | Negative | Negative | NP | CD3 |
| #6 | Nodular | 40 | Positive | Positive | Negative | NP | CD3 |
| #7 | Nodular | 35 | Positive | Positive | Negative | NP | CD3 |
| #8 | Diffuse | 80 | Positive | Positive | Negative | NP | CD3 |
| #9 | Diffuse | 90 | Positive | Positive | Negative | Positive | CD3 |
| #10 | Nodular | 40 | Positive | Positive | Negative | NP | CD3 |
| #11 | Diffuse | 80 | Positive | Positive | Negative | Positive (weak) | CD3 |
| #12 | Diffuse | 50 | Positive | Positive | Negative | NP | CD3 |
NDA, no data available; NP, not performed.
Discussion
HL is the most common non-AIDS-defining malignancy in patients infected with HIV. BM infiltration is rare as the initial or the only site of involvement in HL. In the general population, the involvement of the BM ranges from 2% to 32%, with an average of 10%4. In addition, the incidence of BM involvement is less than 1% in patients with early stage of the disease5.
In a large group of patients with BM involvement associated with HL, the incidence was 4.8% in all stages of neoplasm disease. When the authors included only the patients with stage IV neoplasm disease, 32% of them had BM infiltration5.
Trephine biopsy is the best method to evaluate BM involvement in HL. The aspirate is not indicated for the detection of BM involvement in HL because of the elevated number of false negative cases, which is probably due to the focal infiltration pattern and the accompanying fibrosis6. The infiltration of BM by HL may indicate both the vascular dissemination of the disease and/or a primary BM-HL that originated from BM. BM involvement varies according to the histological subtype: 10% in classical MC, 1% in LP, and 3% in NS subtype. LD is the rarest subtype that includes the involvement of BM in clinical presentation7–9.
A few cases of HL diagnosed primarily from BM biopsy have been reported in the literature10. The infiltration of BM as the initial site of the disease is more common in HIV patients compared with the general population. Diagnosis of HL in BM biopsy is more frequent in HIV-seropositive patients with stage IV of the disease11. Ponzoni et al.12 described only six patients with BM involvement as the only site involvement at the diagnosis of the neoplasm disease in a group of 42 HIV patients with HL.
In HIV patients, BM biopsy is performed in subjects with pancytopenia or prolonged fever of unknown origin. Ponzoni et al.12 reported that all their patients presented fever, blood cytopenias, and severe CD4-T LD, which we also observed in our patients. In our experience, BM biopsy is also useful in patients with B symptoms in which HL is not suspected. In these patients, disseminated tuberculosis, atypical mycobacteria, and systemic mycosis are often suspected clinically. The presence of nonspecific B symptoms may conceal an infectious disease, although the patients have a lymphoma. An elevated number of AIDS patients have B symptoms and pancytopenia, and BM biopsy should be performed when other etiologies are excluded.
The presence of R-S cells in the histopathological study of BM biopsy is definitive and confirmative on the diagnosis of BM infiltration. Moreover, other patterns of BM infiltration by HL include a global hypocellularity with “scattered” cells, fibrosis (Figure 1B), and necrosis13,14.
The pattern of infiltration can be diffused or focal. Diffused infiltration is more frequent and includes extensive areas between bone trabeculae (Figure 1A,B). The focal pattern is less frequent and shows small or nodular patches that surround the normal tissue (Figure 1C)13,15.
R-S cells have a characteristic immunohistochemical profile that can be seen in BM biopsy. In addition, the immunohistochemical study of BM biopsy in patients with HL is very useful to complete diagnostic information. With adequate immunohistochemical studies, the diagnosis of HL can be obtained in the majority of cases. The typical immunophenotypic pattern of classic HL includes the expression of CD15 and CD30 (Figure 1D,E) and the negativity of LCA, CD20, and CD3 antibodies16.
The histological subclassification of HL on BM alone includes some discrepancies between lymph nodes and BM histology17. Classic and nodular LP HL could be easily differentiated based on morphological and immunohistochemical studies18. The presence of tissue eosinophilia seems to be an important morphological feature, and the quantification of eosinophils should be useful in pathological description in patients with classic HL19,20. The differential diagnosis of BM involvement in HL includes peripheral T-cell lymphomas and ALK lymphoma. Other differential diagnoses that should be considered are metastatic carcinoma, chronic lymphocytic leukemia in Ritcher transformation, and immune disorders including HIV infection21.
Recent studies suggest that PET is an alternative method to detect BM involvement in HL22. However, false negative cases of BM involvement in HL have been reported using PET scan23,24. For this reason, BM biopsy is the gold standard to detect BM infiltration in HL15.
The gold standard therapy for HL associated with AIDS is the combination of highly active antiretroviral therapy (HAART) plus chemotherapy. The introduction of HAART improved the prognosis for these patients. Before the HAART era, the median survival of patients with HIV-HL was less than 20 months. In the post-HAART era, Hentrich et al.16 demonstrated significantly better 2-year overall survival in patients receiving HAART plus chemotherapy compared with those not receiving HAART (74% vs. 30%, P<0.001). Complete remission and HAART response were significantly associated with better survival.
This study is a single hospital analysis of HL primary diagnosis in BM in HIV-seropositive patients, so our findings should not be generalized. Several strengths of our groups should be mentioned, such as the multidisciplinary approach by oncologists, pathologists, and infectologists in a highly specialized hospital. The limitations of this study include its retrospective nature, the absence of a follow up, the small size of the cohort, and the characteristics of a single center of infectious diseases. The small size of our cohort, including some patients of the pre-HAART era, represents a limitation in evaluating the impact of HAART plus chemotherapy in the survival of these patients. However, to the best of our knowledge, this is the largest series of HL in AIDS patients with isolated BM involvement.
In conclusion, BM may be the only or the initial site of HL in AIDS patients. Having a high index of suspicion in these patients and performing a BM trephine biopsy to observe the changes of Hodgkin’s lesions in the BM, especially in patients with pancytopenia or systemic B symptoms, are necessary.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Hessol NA, Katz MH, Liu JY, Buchbinder SP, Rubino CJ, Holmberg SD. Increased incidence of Hodgkin disease in homosexual men with HIV infection. Ann Intern Med. 1992;117:309–311. doi: 10.7326/0003-4819-117-4-309. [DOI] [PubMed] [Google Scholar]
- 2.Glaser SL, Clarke CA, Gulley ML, Craig FE, DiGiuseppe JA, Dorfman RF, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988-1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 3.Fried G, Ben Arieh Y, Haim N, Dale J, Stein M. Primary Hodgkin’s disease of the bone. Med Pediatr Oncol. 1995;24:204–207. doi: 10.1002/mpo.2950240312. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian R, Basu D, Badhe B, Dutta TK. Role of bone marrow trephine biopsy in the diagnosis of marrow involvement in Hodgkin’s disease. Indian J Pathol Microbiol. 2007;50:640–643. [PubMed] [Google Scholar]
- 5.Howell SJ, Grey M, Chang J, Morgenstern GR, Cowan RA, Deakin DP, et al. The value of bone marrow examination in the staging of Hodgkin’s lymphoma: a review of 955 cases seen in a regional cancer centre. Br J Haematol. 2002;119:408–411. doi: 10.1046/j.1365-2141.2002.03842.x. [DOI] [PubMed] [Google Scholar]
- 6.Bain BJ. Bone marrow trephine biopsy. J Clin Pathol. 2001;54:737–742. doi: 10.1136/jcp.54.10.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siebert JD, Stuckey JH, Kurtin PJ, Banks PM. Extranodal lymphocyte predominance Hodgkin’s disease. Clinical and pathologic features. Am J Clin Pathol. 1995;103:485–491. doi: 10.1093/ajcp/103.4.485. [DOI] [PubMed] [Google Scholar]
- 8.Poppema S, Delsol G, Pileri S, Stein H, Swerdlow S, Warnke RA, et al. In: WHO Classification of Tumors of haemopoietic and Lymphoid Tissues. 4th edition. Swerdlow S, Campo E, Harris N, Jaffe ES, Pileri S, Stein H, et al., editors. Lyon, France: IARC; 2008. Nodular lymphocyte predominant Hodgkin lymphoma; pp. 323–325. [Google Scholar]
- 9.Munker R, Hasenclever D, Brosteanu O, Hiller E, Diehl V. Bone marrow involvement in Hodgkin’s disease: an analysis of 135 consecutive cases. German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 1995;13:403–409. doi: 10.1200/JCO.1995.13.2.403. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi Y, Hasegawa Y, Nakazawa M, Yoda Y, Abe T, Mori N. Hodgkin’s disease diagnosed from the numerous Reed-Sternberg cells in the bone marrow. Rinsho Ketsueki. 1989;30:731–735. [PubMed] [Google Scholar]
- 11.Karcher DS. Clinically unsuspected Hodgkin disease presenting initially in the bone marrow of patients infected with the human immunodeficiency virus. Cancer. 1993;71:1235–1238. doi: 10.1002/1097-0142(19930215)71:4<1235::aid-cncr2820710411>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Ponzoni M, Fumagalli L, Rossi G, Freschi M, Re A, Viganò MG, et al. Isolated bone marrow manifestation of HIV-associated Hodgkin lymphoma. Mod Pathol. 2002;15:1273–1278. doi: 10.1097/01.MP.0000037311.56159.13. [DOI] [PubMed] [Google Scholar]
- 13.O’Carroll DI, McKenna RW, Brunning RD. Bone marrow manifestations of Hodgkin’s disease. Cancer. 1976;38:1717–1728. doi: 10.1002/1097-0142(197610)38:4<1717::aid-cncr2820380445>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Corti M, Soto I, Villafañe MF, Minué G, Campitelli A, Narbaitz M. Bone marrow necrosis as clinical presentation of plasmablastic lymphoma in an AIDS patient: First case reported and literature review. J Hematol Malig. 2013;3:57–62. [Google Scholar]
- 15.Franco V, Tripodo C, Rizzo A, Stella M, Florena AM. Bone marrow biopsy in Hodgkin’s lymphoma. Eur J Haematol. 2004;73:149–155. doi: 10.1111/j.1600-0609.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 16.Hentrich M, Maretta L, Chow KU, Bogner JR, Schürmann D, Neuhoff P, et al. Highly active antiretroviral therapy (HAART) improves survival in HIV-associated Hodgkin’s disease: results of a multicenter study. Ann Oncol. 2006;17:914–919. doi: 10.1093/annonc/mdl063. [DOI] [PubMed] [Google Scholar]
- 17.Lukes RJ. Criteria for involvement of lymph node, bone marrow, spleen, and liver in Hodgkin’s disease. Cancer Res. 1971;31:1755–1767. [PubMed] [Google Scholar]
- 18.Steimle-Grauer SA, Tinguely M, Seada L, Fellbaum C, Hansmann ML. Expression patterns of transcription factors in progressively transformed germinal centers and Hodgkin lymphoma. Virchows Arch. 2003;442:284–293. doi: 10.1007/s00428-002-0735-5. [DOI] [PubMed] [Google Scholar]
- 19.Axdorph U, Porwit-MacDonald A, Grimfors G, Björkholm M. Tissue eosinophilia in relation to immunopathological and clinical characteristics in Hodgkin’s disease. Leuk Lymphoma. 2001;42:1055–1065. doi: 10.3109/10428190109097726. [DOI] [PubMed] [Google Scholar]
- 20.Enblad G, Sundstrom C, Glimelius B. Infiltration of eosinophils in Hodgkin’s disease involved lymph nodes predicts prognosis. Hematol Oncol. 1993;11:187–193. doi: 10.1002/hon.2900110404. [DOI] [PubMed] [Google Scholar]
- 21.Ree HJ, Strauchen JA, Khan AA, Gold JE, Crowley JP, Kahn H, et al. Human immunodeficiency virus-associated Hodgkin’s disease. Clinicopathologic studies of 24 cases and preponderance of mixed cellularity type characterized by the occurrence of fibrohistiocytoid stromal cells. Cancer. 1991;67:1614–1621. doi: 10.1002/1097-0142(19910315)67:6<1614::aid-cncr2820670624>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Moog F, Bangerter M, Kotzerke J, Guhlmann A, Frickhofen N, Reske SN. 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol. 1998;16:603–609. doi: 10.1200/JCO.1998.16.2.603. [DOI] [PubMed] [Google Scholar]
- 23.Bangerter M, Moog F, Buchmann I, Kotzerke J, Griesshammer M, Hafner M, et al. Whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin’s disease. Ann Oncol. 1998;9:1117–1122. doi: 10.1023/a:1008486928190. [DOI] [PubMed] [Google Scholar]
- 24.Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose compared to standard procedures for staging patients with Hodgkin’s disease. Haematologica. 2001;86:266–273. [PubMed] [Google Scholar]