Abstract
ATP7A is a P-type ATPase in which diverse mutations lead to X-linked recessive Menkes disease or occipital horn syndrome. Recently, two previously unknown ATP7A missense mutations, T994I and P1386S, were shown to cause an isolated distal motor neuropathy without clinical or biochemical features of other ATP7A disorders. These mutant alleles cause subtle defects in ATP7A intracellular trafficking, resulting in preferential plasma membrane localization compared with wild-type ATP7A. We reported previously that ATP7AP1386S causes unstable insertion of the eighth and final transmembrane segment, preventing proper position of the carboxyl-terminal tail in a proportion of mutant molecules. Here, we utilize this and other naturally occurring and engineered mutant ATP7A alleles to identify mechanisms of normal ATP7A trafficking. We show that adaptor protein (AP) complexes 1 and 2 physically interact with ATP7A and that binding is mediated in part by a carboxyl-terminal di-leucine motif. In contrast to other ATP7A missense mutations, ATP7AP1386S partially disturbs interactions with both APs, leading to abnormal axonal localization in transfected NSC-34 motor neurons and altered calcium-signaling following glutamate stimulation. Our results imply that AP-1 normally tethers ATP7A at the trans-Golgi network in the somatodendritic segments of motor neurons and that alterations affecting the ATP7A carboxyl-terminal tail induce release of the copper transporter to the axons or axonal membranes. The latter effects are intensified by diminished interaction with AP-2, impeding ATP7A retrograde trafficking. Taken together, these findings further illuminate the normal molecular mechanisms of ATP7A trafficking and suggest a pathophysiological basis for ATP7A-related distal motor neuropathy.
Introduction
ATP7A is one member of the family of P1B-type ATPases, most of which are heavy metal transporters that play important roles in regulating the intracellular metabolism of heavy metals such as Cu, Zn, Co, Cd and Pb (1). ATP7A and another P1B-type ATPase, ATP7B, are copper transporters and, together with the copper uptake protein, hCTR1, and cytosolic copper chaperones, regulate intracellular copper homeostasis (2). ATP7A has eight-transmembrane domains with a bulky N-terminus and short C-terminal tail located on the cytosolic aspect. The N-terminus of ATP7A harbors six copper-binding domains, which accept copper atoms from the copper chaperone ATOX1 (3). Models based on the crystal structure of the bacterial homolog of ATP7A, CopA, predict that the second transmembrane domain forms a platform to accept copper from amino-terminal copper-binding domains (4). Copper is subsequently conveyed into a transmembrane channel and pumped to the other side.
At basal intracellular copper concentrations (0.5 μm), ATP7A normally locates in the trans-Golgi network (TGN) of cells. When environmental copper increases, intracellular copper concentrations rise correspondingly and, upon receipt of copper from its cytosolic chaperone ATOX1, ATP7A migrates from the TGN to the plasma membrane (PM) and pumps excess copper out of the cell. On release of copper, ATP7A is then retrieved to the TGN (5,6). Previous work has focused on the study of elements, motifs or domains within the ATP7A molecule, including a 38-amino acid segment in the third transmembrane domain associated with movement from the endoplasmic reticulum (ER) to the TGN (7); the metal-binding sites in the N-terminus which influence movement from the TGN to the PM (8–10); the C-terminal di-leucine motif (positions 1487–1488) which affects endosomal retrieval of ATP7A from the PM (11,12); a phosphorylation motif (DKTG; positions 1044–1047) which regulates copper-induced re-localization from post-Golgi vesicles to the PM, a phosphatase domain (LITGEA; positions 873–878) which also contributes to endosomal retrieval of ATP7A from the PM (13); a carboxyl-terminal PDZ motif (DTAL; positions 1497–1500) which mediates specific basolateral (as opposed to apical) localization of ATP7A in polarized cells (14) and a CPC motif in transmembrane domain 6 (positions 1000–1002) which enables copper-induced re-localization from post-Golgi vesicles to the PM (15).
Recent studies have focused on exploring the components and regulators of the ATP7A trafficking machinery. Pascale et al. reported that endosomal trafficking of ATP7A was mediated by vesicles containing the Rab7 and Rab5 GTPases (16). Holloway et al. described the role of ADP-ribosylation factor (Arf1) in the biogenesis of copper-induced ATP7A trafficking (17). Further work suggested that ATP7A trafficking is dependent on multiple membrane-associated factors, including clathrin, adaptor protein (AP) complexes and Rab22 (18). While the cumulative prior work has contributed to the understanding of ATP7A mobility, understanding of the precise molecular details remains unresolved.
ATP7A is located on the X-chromosome, and mutations in this gene lead to Menkes disease (OMIM 309400), occipital horn syndrome (OMIM #304150) or, as recently described, an isolated distal motor neuropathy resembling Charcot–Marie–Tooth disease, type 2 (3,19). This latter phenotype is caused by two unique missense mutations in ATP7A, T994I and P1386S. Affected patients have no clinical or biochemical stigmata of Menkes disease, manifesting normal childhood neurodevelopment, normal serum copper and ceruloplasmin levels, and normal plasma neurochemical ratios (19). The latter are typically abnormal in subjects with Menkes disease and occipital horn syndrome owing to partial deficiency of dopamine-beta-hydroxylase, a copper-dependent enzyme. Electrophysiological testing revealed normal nerve conduction velocities and, often, normal sensory action potentials in combination with markedly decreased motor action potentials (19). Yeast complementation analyses documented near-normal (70–80%) copper transport by these mutant alleles (19,20). Taken together, the findings suggested a length-dependent metabolic axonopathy and an underlying molecular mechanism distinct from the copper deficiency implicated in Menkes disease and occipital horn syndrome (3).
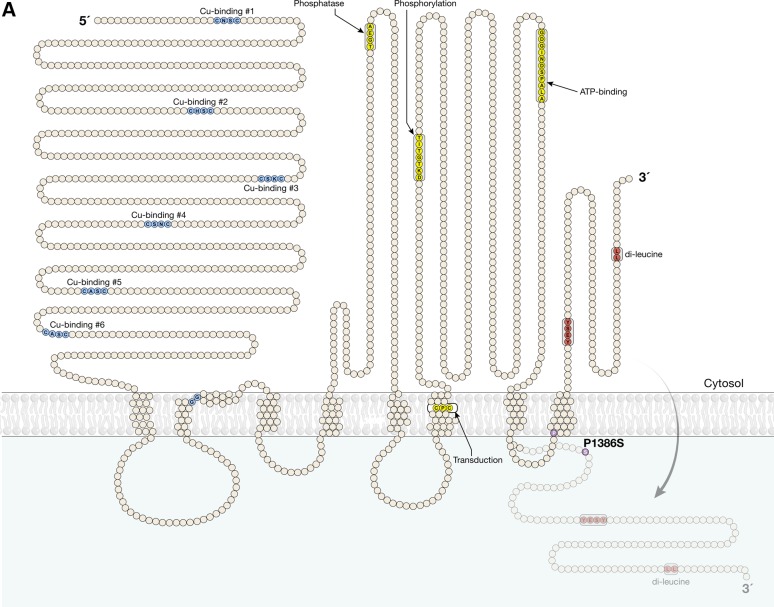
We previously identified abnormal PM localization of the T994I and P1386S ATP7A mutants in cells cultured in normal (0.5 µm) concentrations of copper. We documented abnormal interactions between ATP7AT994I and p97/valosin-containing protein, a molecule implicated in the molecular basis of amyotrophic lateral sclerosis and another autosomal dominant form of motor neuron degeneration (20). In addition, we showed that the P1386S mutation destabilized insertion of the eighth and final transmembrane segment of ATP7A, owing to the helix breaker effect of substituting for proline (20). An important topological consequence of this mutation is mis-positioning of the ATP7A carboxyl-terminal tail with respect to the membrane in which it embeds (Fig. 1A).
Figure 1.
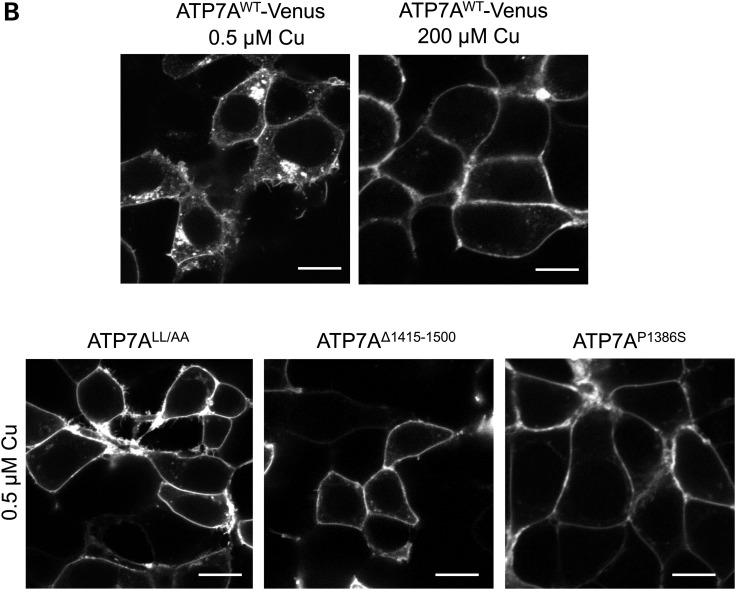
ATP7A mutations affecting the C-terminal di-leucine motif result in PM localization. (A) Model of ATP7AP1386S based on previous experimental analyses (20) illustrating partial malposition of the C-terminal di-leucine and Yxxϕ signals (red) owing to destabilized membrane insertion of the eighth transmembrane segment of ATP7A. This topological change is caused by proline to serine substitution (lavender) precisely at the normal membrane insertion site. (B) Localization of wild-type ATP7A-Venus in a perinuclear pattern under basal (0.5 µm) copper concentration, and redistribution to the PM under high (200 μm) copper concentration (top panels). In contrast, ATP7ALL/AA, ATP7AΔ1415-1500 and ATP7AP1386S all manifest nearly exclusive PM localization at basal (0.5 µm) copper concentrations (bottom panels). HEK293T cells were transfected with Venus-tagged wild-type ATP7A, ATP7ALL/AA, ATP7AΔ1415-1500 or ATP7AP1386S and imaged by confocal microscopy (Zeiss 510). Scale bars = 10 μm.
Adaptor protein complexes play important roles in the regulation of intracellular vesicular trafficking, in particular clathrin-coated vesicle (CCV)-mediated trafficking (21,22). There are five AP complexes discovered to date, AP-1, AP-2, AP-3, AP-4 and AP-5 (22). These complexes each possess four subunits, including one small subunit, named σ1–σ5 respectively; one medium subunit (μ1–μ5) and two large subunits, β1–β5, respectively, and γ for AP-1, α for AP-2, δ for AP-3, ε for AP-4 and ζ for AP-5 (22). AP-1 has been associated with basolateral targeting of specific cargo proteins in polarized epithelial cells (23,24). This process appears to be regulated by Rab8 GTPase and dependent on myosin VI (25,26). AP-1/σ1B was reported to mediate endosomal synaptic vesicle recycling, learning and memory (27). More recent work revealed the role of AP-1 in selective sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons (28). In contrast, AP-2 normally locates on the PM and is believed to guide cargo proteins into endosomes during internalization (22). AP-2 has also been reported to associate with various neuronal functions, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking and hippocampal long-term potentiation, synaptic vesicle fusion and recycling via synaptotagmin binding and postsynaptic membrane trafficking (29–31).
Various forms of neurodegeneration have been reported in relation to functional defects in AP-1 and AP-2. For instance, mutations in the sigma 1A subunit of AP-1 (AP1S1) cause a neurocutaneous syndrome in zebrafish (32) and MEDNIK syndrome in humans (33). MEDNIK represents an acronym denoting the multisystem effects manifest in individuals with this autosomal recessive condition: mental retardation, enteropathy, congenital deafness, neuropathy, ichthyosis and keratodermia.
As ATP7A contains motifs that specifically associate with AP complexes in other transmembrane proteins (3,23,24,34), we examined the putative roles of AP-1 and AP-2 in intracellular ATP7A trafficking while pursuing an explanation for one form of ATP7A-related isolated distal motor neuropathy, caused by ATP7AP1386S.
Results
ATP7A mutants that alter carboxyl-terminus structure or motifs mimic the effects of P1386S
ATP7A is an eight-transmembrane domain protein, and the P1386S mutation is located in the fourth lumenal loop, precisely at the insertion of the final transmembrane segment (Fig. 1A). Thus, the cytosolic position of the ATP7A carboxyl-terminal tail relies on proper insertion of the eighth transmembrane segment. Our previous work revealed abnormal PM localization of ATP7AP1386S and that, in some percentage of the mutant molecules, the carboxyl-terminal tail resides outside the PM (20). The ATP7A carboxyl-terminal tail harbors both YxxΦ (Y1415ESY1418) and di-leucine (L1477L1478) motifs (Fig. 1A), sequences generally associated with binding of AP complexes in the process of intracellular trafficking (34).
To further evaluate the importance of the ATP7A carboxyl-terminal tail, the YxxΦ motif and the di-leucine motif, we generated three additional mutants:
ATP7AYESY/AESA in which the YxxΦ motif (Y1415ESY1418) was mutated to AESA,
ATP7ALL/AA in which the di-leucine motif was mutated to di-alanine and
ATP7AΔ1415-1500 in which the carboxyl-terminal was truncated at residue 1414, removing both motifs.
We studied the localization of these three mutants in relation to the ATP7AP1386S mutation. Both ATP7ALL/AA and ATP7AΔ1415-1500 localized on the PM instead of the TGN under normal copper conditions, recapitulating the abnormal localization of ATP7AP1386S (Fig. 1B). In contrast, the ATP7AYESY/AESA construct showed normal localization and trafficking (Supplementary Material, Fig. S1). It is known that wild-type ATP7A normally traffics to the PM to extrude copper from cell when intracellular concentrations become elevated and recycles back to TGN when normal intracellular copper concentrations return (3). The default PM localization of ATP7ALL/AA, ATP7AΔ1415-1500 and ATP7AP1386S suggested that the ATP7A carboxyl-terminal di-leucine motif is required for TGN localization and/or involved in the retrieval of ATP7A from the PM.
Adaptor protein complex 1 regulates ATP7A localization under basal copper concentrations
Based on these data and previous suggestive studies (18,33,35), we speculated that AP complex 1 (AP-1) and 2 (AP-2) were integral to proper ATP7A localization and trafficking and physically interact with this copper transporter. AP-1 is believed to bring cargo proteins to clathrin-coated vesicles budding from the TGN and transport to endosomal vesicles, and vice-versa (21,27,34). Other single-molecule adaptors such as GGA1, GGA2 and GGA3 are also reported to participate in anterograde trafficking of CCVs from the TGN (35).
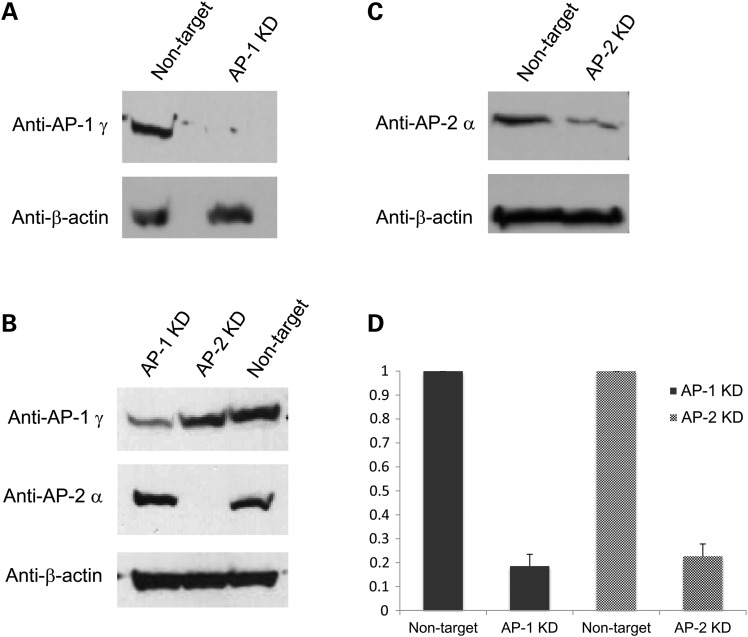
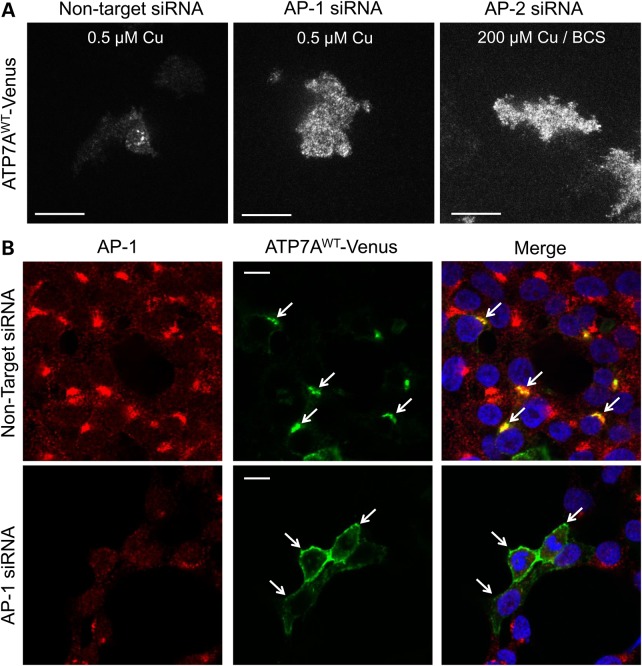
To confirm the direct roles of AP-1 and AP-2 in ATP7A trafficking, we knocked down these complexes and studied the effects on ATP7A localization. To obtain maximal silencing, we simultaneously introduced two sets of small interfering RNAs targeting (for subunits σ1 and γ in the case of AP-1, and subunits σ2 and α for AP-2) into HEK293T or HeLa cells. We achieved ≈80% knockdown on average for AP-1 as measured in western blots with anti-AP-1γ, and ≈75% mean knockdown of AP-2 as detected by anti-AP-2α (Figure 2A–D). After knocking down AP-1 or AP-2 for 48 h, we transfected cells with Venus-tagged wild-type ATP7A (ATP7A-Venus). Total internal reflection fluorescence (TIRF) imaging revealed impaired anterograde trafficking of ATP7A after AP-1 knockdown in HeLa cells and impaired retrograde trafficking of ATP7A after AP-2 knockdown (Fig. 3A). Similarly, confocal imaging revealed that ATP7A localized on the PM of AP-1 knockdown HEK293T cells at basal copper concentration (Fig. 3B, bottom row, middle panel), compared with TGN localization in non-knocked down controls (Fig. 3B, upper row, middle panel). We also knocked down two other single-molecule adaptors, GGA2 and GGA3. In contrast to AP-1, neither GGA2- or GGA3- knock down affected ATP7A localization, nor did it alter anterograde or retrograde ATP7A trafficking in response to changes in copper concentration (Supplementary Material, Figs S2 and S3). These combined data demonstrate the potential specific role of AP-1 in tethering ATP7A at the TGN.
Figure 2.
SiRNA silencing of AP complexes 1 and 2 (AP-1 and AP-2). HEK293T cells were treated with 200 nM of AP-1 siRNA (sigma 1 and gamma subunits) or AP-2 siRNA (sigma 2 and alpha subunits) or non-target siRNA, by Lipofectamine 2000 (Invitrogen). After 48 h, the cells were transfected with Venus-tagged ATP7A. (A and B) Expression of AP-1 in non-target siRNA and AP-1 siRNA-transfected cells, illustrated by representative western blots of AP-1 gamma subunit. (B and C) Expression of AP-2 in non-target siRNA and AP-2 siRNA-transfected cells, illustrated by representative western blots of AP-2 alpha subunit; (D) Summary of densitometric analyses of five western blots. Error bars = SEM. KD, knock down.
Figure 3.
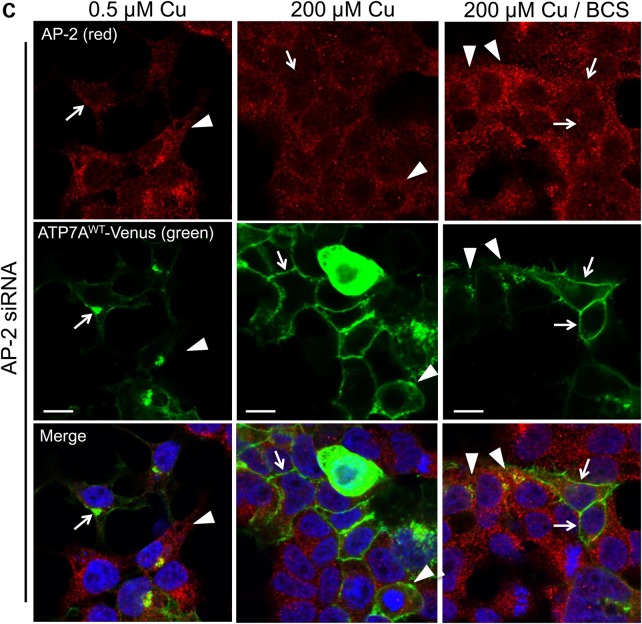
Impaired wild-type ATP7A trafficking associated with AP-1 or AP-2 knockdown. (A) TIRF microscopy after AP-1 or AP-2 knock down reveals enhanced wild-type ATP7A-Venus signal at the PM of HeLa cells (used instead of HEK293T cells owing to superior attachability to glass cover slips). Under normal copper concentrations (0.5 µm), non-target siRNA-treated cells show minimal PM signal as expected (left panel), whereas AP-1 siRNA (middle panel) results in abundant TIRF signal. To demonstrate the effect of AP-2 siRNA on wild-type ATP7A localization, we exposed siRNA-treated, transfected cells to 200 µm copper followed by copper chelation with BCS, conditions that normally induce retrieval of ATP7A from the PM (20). However, AP-2 knockdown resulted in persistent retention of ATP7A at the PM (right panel). (B) Immunofluorescence confocal microscopy confirms altered localization of ATP7A in AP-1 siRNA-transfected HEK293T cells under normal copper concentrations (0.5 µm). Red signal denotes AP-1, green signal denotes ATP7A and blue signal denotes DAPI nuclear counterstain. Non-target siRNA (top panels) does not disturb the proper trans-Golgi (perinuclear) localization of ATP7A (arrows). In contrast, cells in which both AP-1 knockdown (lower panels) and transfection with ATP7AWT-Venus occurred, preferential accumulation at the PM is evident (arrows). (C) Trafficking assay of wild-type ATP7A in AP-2 siRNA-transfected cells. Red signal denotes AP-2, green signal denotes ATP7A and blue signal denotes DAPI nuclear counterstain. In normal copper concentration (0.5 µm), cells with (arrows) or without (arrow-heads) AP-2 knockdown display proper trans-Golgi localization of ATP7A (left panels). In high copper (200 µm), cells with (arrows) or without (arrow-heads) AP-2 knockdown display the usual PM re-localization of ATP7A (middle panels). However, after copper chelation with BCS (right panels), cells with AP-2 knockdown (arrows) retain ATP7A at the PM, whereas cells without non-knockdown (arrow-heads) manifest return of ATP7A to the trans-Golgi (perinuclear) compartment. Scale bars = 10 µm.
Adaptor protein complex 2 regulates ATP7A retrieval from the PM
We observed that AP-2 knockdown did not influence the normal TGN localization of ATP7A at basal copper concentration (Fig. 3C, left column) nor interfere with TGN to PM trafficking of ATP7A in response to increased intracellular copper (Fig. 3C, middle column). However, on removal of excess copper by treatment with the copper chelator bathocuproine-disulfonate (BCS), ATP7A remained on the PM in AP-2 knocked down cells (Fig. 3C, right column, arrows) instead of being retrieved to the TGN, as occurred in cells with normal AP-2 levels (Fig. 3C, right column, arrow-heads). As with the TIRF data on AP-2 knockdown under the same conditions (Fig. 3A, right panel), these data suggest the role of AP-2 in endocytic retrieval or retrograde trafficking of ATP7A from the PM.
ATP7A physically interacts with AP-1 and AP-2 through its carboxyl-terminal tail
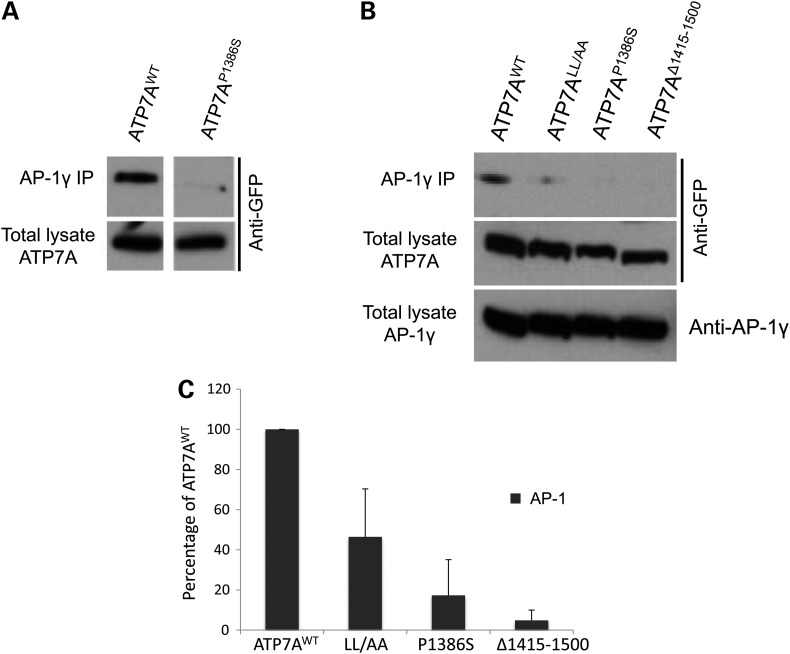
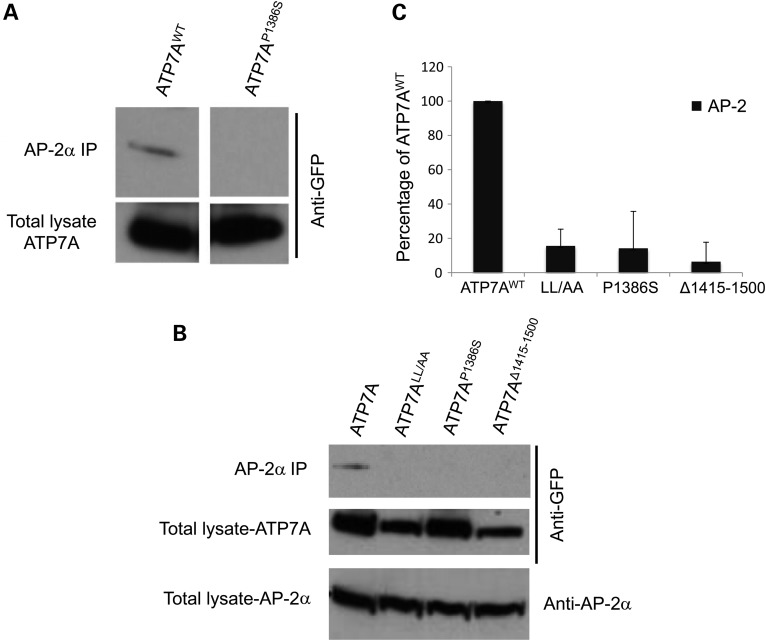
Next, we studied the physical interaction between ATP7A and AP-1 or AP-2 by using immunoprecipitation methods. We overexpressed Venus-tagged wild-type ATP7A, ATP7ALL/AA, the distal motor neuropathy-inducing mutant ATP7AP1386S and the carboxyl-terminus-truncated mutant ATP7AΔ1415-1500 in HEK293T cells, pulled down AP-1 by anti-AP-1γ subunit antibody or pulled down AP-2 by anti-AP-2α subunit antibody and evaluated for the presence of the wild-type ATP7A and mutants in the pulled-down protein pool (Figs 4 and 5). Through these assays (and the converse immunoprecipitation experiments, Supplementary Material, Fig. S4), we confirmed that wild-type ATP7A interacted with both AP-1 and AP-2, whereas for ATP7ALL/AA, ATP7AP1386S and ATP7AΔ1415-1500, these interactions were significantly diminished (Figs 4 and 5). The L1477L1478/AA mutation of ATP7A retained somewhat greater interaction with AP-1 than the other mutant alleles (Fig. 4C). This suggests that the di-leucine motif is necessary but not sufficient for this interaction and that other motifs in the C-terminal tail may also be involved. The same effect was not observed in interaction of this allele with AP-2, as ATP7ALL/AA blocked AP-2/ATP7A immunoprecipitation almost entirely, comparable with that found for the other two mutations (Fig. 5C). Notably, physical interactions with AP-1 and AP-2 were preserved in ATP7A mutations (G727R, T994I) not expected to affect the carboxyl terminus or di-leucine motif (Supplementary Material, Fig. S5). These combined data imply that while the ATP7A carboxyl-terminal tail is crucial for binding both AP-1 and AP-2, the mechanisms of interaction may be distinct. The AP-2/ATP7A interaction appears more directly dependent on the di-leucine motif.
Figure 4.
The distal motor neuropathy mutation ATP7AP1386S, an ATP7A C-terminus truncation (ATP7AΔ1415-1500) and a di-leucine ATP7A mutation (L1477L1478/AA) diminish physical interaction with AP-1. HEK293T cells were transfected with wild-type ATP7A-Venus, ATP7ALL/AA-Venus, ATP7AP1386S-Venus, or ATP7AΔ1415-1500-Venus. Transfected cells were immunoprecipitated (IP) by an AP-1 gamma subunit antibody and pull-down products detected by anti-GFP (Venus) antibody. (A and B) ATP7AWT consistently immunoprecipitated AP-1, with interaction variably impaired by the different mutations. (C) Densitometric summary of AP-1 IP blots (N = 11 for ATP7AWT, N = 2 for ATP7ALL/AA, N = 10 for ATP7AP1386S and N = 3 for ATP7AΔ1415-1500). Error bars = SD.
Figure 5.
The distal motor neuropathy mutation ATP7AP1386S, an ATP7A C-terminus-truncation (ATP7AΔ1415-1500) and a di-leucine ATP7A mutation (L1477L1478/AA) diminish physical interaction with AP-2. HEK293T cells were transfected with wild-type ATP7A-Venus, ATP7ALL/AA-Venus, ATP7AP1386S-Venus, or ATP7AΔ1415-1500-Venus. Transfected cells were immunoprecipitated (IP) by an AP-2 alpha subunit antibody and pull-down products detected by anti-GFP (Venus) antibody. (A and B) ATP7AWT consistently immunoprecipitated AP-2, with interaction markedly impaired by the different mutations. (C) Densitometric summary of AP-2 IP blots (N = 10 for ATP7AWT, N = 4 for ATP7ALL/AA, N = 6 for ATP7AP1386S and N = 3 for ATP7AΔ1415-1500). Error bars = SD.
ATP7A locates in the somatodentritic area of motor neurons and traffics to axons in response to elevated intracellular copper
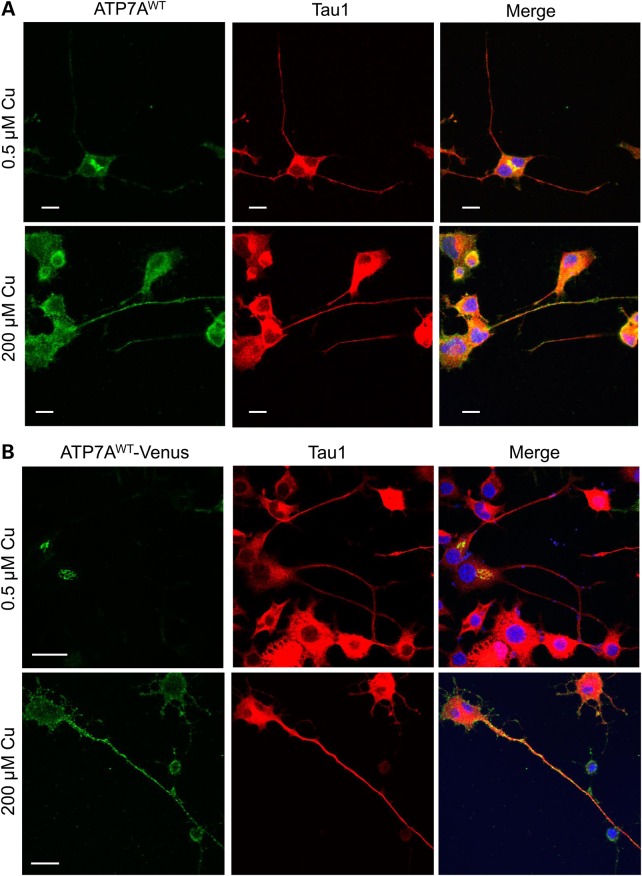
There are two classical types of polarized cells, epithelia and neurons. It was reported that ATP7A specifically translocates to the basolateral aspect of polarized MDCK epithelial cells upon copper treatment (14); however, copper-induced translocation movement in neurons has not been studied in detail. We therefore studied the localization of ATP7A in differentiated NSC-34 neurons, which are derived from fusion of motor neuron-enriched, embryonic mouse spinal cord cells with mouse neuroblastoma cells (36,37). Differentiated NSC-34 neurons are a reasonable surrogate for motor neurons in the study of function and subcellular trafficking of various neuronal receptors or proteins, and useful as an experimental model for study of motor neuron degeneration (38–40). In our immunofluorescence images, we observed that, under basal copper concentrations, endogenous ATP7A predominately located in the somatodendritic area of differentiated NSC-34 neurons, with very little ATP7A located in axonal areas, as identified by microtubule-associated protein Tau1 (Fig. 6A, top row, merge panel and Supplementary Material, Fig. S6). ATP7A relocated to axons in response to increased copper concentrations however (Fig. 6A, bottom row, merge panel). Our localization/trafficking experiments with exogenous Venus-tagged ATP7A confirmed these results (Fig. 6B).
Figure 6.
High copper triggers trafficking of endogenous and exogenous ATP7A from the somatodendritic region into the axons of differentiated NSC-34 motor neurons. (A) Differentiated NSC-34 motor neurons at basal (0.5 μm) or high (200 μm) copper concentrations were permeabilized and stained with rabbit anti-ATP7A (C-terminus) antibody and an antibody for the somatodendritic and axonal marker, Tau1 (Millipore). Note axonal distribution of ATP7A in response to copper (lower panel). (B) Differentiated NSC-34 motor neurons were transfected with ATP7AWT-Venus. Transfected cells at basal copper concentration (0.5 μm) or high copper (200 μm) were permeabilized and stained with Tau1. Redistribution of ATP7AWT-Venus axonally in response to copper is evident (lower panel). Scale bars = 10 μm.
The P1386S mutation releases ATP7A protein to axons at basal copper concentrations
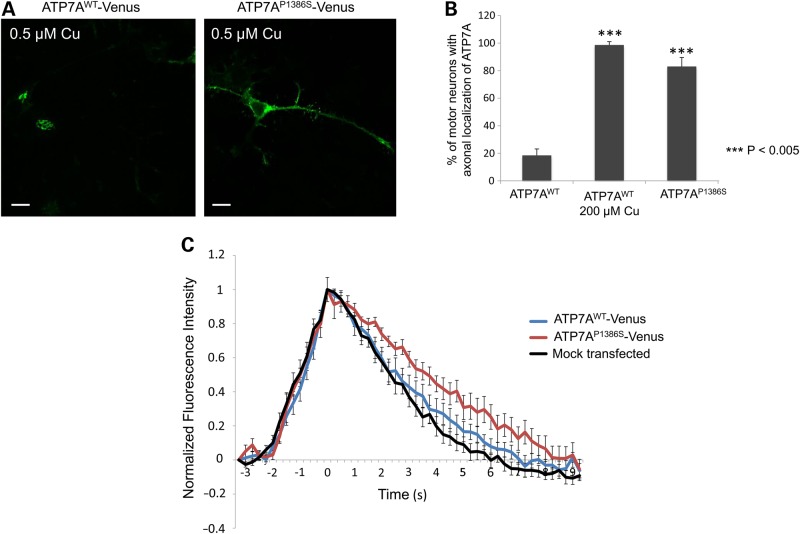
The P1386S mutation in ATP7A destabilizes insertion of the eighth transmembrane segment affecting the position of the carboxyl-terminal tail and impeding its interaction with AP-1 (20). This consequence is predicted to reduce its localization in the TGN region and also to block its interaction with AP-2, which presumably assists in ATP7A retrieval to TGN from the PM. We speculated that, as with the abnormal trafficking seen in transfected HEK293T cells, reduced interactions with AP-1 and AP-2 would result in abnormal patterns of ATP7AP1386S localization in differentiated NSC-34 motor neurons. We confirmed this hypothesis by demonstrating localization of ATP7AP1386S in both the somatodendritic and axonal areas of transfected NSC-34 cells under normal copper concentrations (Fig. 7A, right panel). Statistical analyses revealed that only 20% of motor neurons transfected with wild-type ATP7A showed signal axonally, whereas >80% of ATP7AP1386S -transfected motor neurons illustrated the mutant protein signal in their axons (Fig. 7A,B). This phenotype of the mutant allele under normal copper conditions recapitulated the effect of elevated copper concentration on wild-type ATP7A (Fig. 6A,B).
Figure 7.
ATP7AP1386S locates axonally as well as in the somatodendritic region under basal copper concentration and alters the calcium-signaling pattern after glutamate stimulation. Differentiated NSC-34 cells were transfected with wild-type ATP7A-Venus, ATP7AP1386S-Venus or were mock-transfected and imaged 24 h post-transfection. (A) Localization of ATP7A-Venus and ATP7AP1386S-Venus in differentiated NSC-34 motor neurons at basal copper concentration (0.5 μm). Scale bars = 10 μm. (B) Percentage of motor neurons with axonally localized ATP7A among all ATP7AWT-Venus or ATP7AP1386S-Venus transfected cells (N > 200 cells per construct/condition). Error bars = SD. (C) Differentiated NSC-34 motor neurons were transfected with ATP7A-Venus or ATP7AP1386S-Venus, loaded with the calcium sensor, Fluo-4 AM (Invitrogen), and stimulated with 1.33 μm glutamate. The calcium-signaling response was determined by time-lapse imaging at excitation wavelength 488λ, using a Zeiss 510 confocal microscope. ATP7A-Venus (blue line); ATP7AP1386S-Venus (red line); mock transfection (black line). Release of calcium during repolarization from 1.75 to 7.25 s was significantly slower for neurons transfected with ATP7AP1386S than that for ATP7AWT or after mock-transfection. Error bars = SEM.
Retention of ATP7AP1386S in axonal area subtly alters the calcium-signaling pattern after glutamate stimulation in neurons
As questions remain concerning how the abnormal axonal localization of ATP7APP1386S induces the gradual onset distal motor neuropathy, we investigated the response of differentiated NSC-34 motor neurons to glutamate stimulation. Upstream firing signals such as glutamate stimulation normally trigger increases of intracellular calcium through three pathways: influx of extracellular calcium via ionotropic receptors, voltage-gated calcium channels, through the sodium calcium exchanger (NCX), and release of calcium from the ER and mitochondria (41). Post-stimulation intraneuronal calcium should be removed by pumping across the axonal membrane through a calcium ATPase (PMCA), or pumped into the ER compartment by sarcoplasmic reticulum calcium-transporting ATPase (SERCA), or pumped into mitochondria by NCX. In our calcium-signaling experiments, neuronal cells transfected with ATP7AP1386S showed delayed calcium removal compared with wild-type ATP7A-transfected neurons [(P = 0.0107–0.0395 at ten 0.25-s intervals between 1.75 and 7.25 s of the repolarization phase (Figure 7C)].
Discussion
The precise mechanisms underlying intracellular movement of the copper transporting, ion-motive pump, ATP7A, remain obscure despite considerable scientific scrutiny over the past two decades. Given the association between mutations in ATP7A and human neurological illness, including motor neuron degeneration (3), ATP7A trafficking is likely important for proper neuronal functioning. ATP7A contains motifs that specifically associate with AP complexes in other transmembrane proteins (3,23,24,34), which led us to examine the putative roles of AP-1 and AP-2 in intracellular ATP7A trafficking. Here, for the first time, we identify physical interactions between ATP7A and these AP complexes and show that the interactions are impaired by malpositioning, mutation or removal of an ATP7A carboxyl-terminal di-leucine motif. In motor neuron cells, the missense mutation ATP7AP1386S shifts distribution of ATP7A under normal copper concentrations and subtly alters calcium signaling. Our findings provide mechanistic insight about ATP7A trafficking and suggest that influences on axonal ion balance underlie or contribute to ATP7AP1386S-related distal motor neuropathy.
We previously reported that ATP7AP1386S could alter the carboxyl-terminal tail position because the substitution is located at the precise point of membrane insertion of the final transmembrane domain (Fig. 1A) (20). We now better understand the consequence of this topological change, showing here that ATP7AP1386S nearly completely abrogates proper binding with AP-1 and AP-2. The interactions documented in AP-1 and AP-2 immunoprecipitation assays for ATP7AP1386S were minimal, equivalent to that of the full carboxyl-terminus-truncation mutant, ATP7AΔ1415-1500. These results are consistent with our previous finding that the P1386S mutation affects the position of the ATP7A C-terminal tail in some percentage of the mutant molecules.
We also demonstrate that AP-1 and AP-2 play key roles in ATP7A trafficking and selective somatodendritic area localization in motor neurons and that the motor neuron degeneration-inducing mutation, P1386S, results in ATP7A escape from the normal system of regulated neuronal localization enforced by the two AP complexes. We document reduced calcium removal from motor neurons during repolarization following glutamate stimulation, providing a novel clue to understanding the underlying mechanism of isolated distal motor neuropathy induced by ATP7AP1386S. Delayed calcium removal indicates possible interference with the functions of other ion channel proteins (e.g. PMCA, SERCA or NCX), details of which require further study.
ATP7A follows an interesting intracellular itinerary, controlled at least in part, by intracellular copper concentrations. However, the detailed mechanisms underlying the mobility of this protein have remained obscure. In addition to the roles of AP-1 and AP-2 described here, there is evidence that a single domain PDZ protein, AIPP1, interacts with the class I PDZ binding motif at the ATP7A C-terminus (42) and may influence trafficking. In addition, the sorting nexin-27 (SNX27)-containing retromer complex, an important regulator of multiple endosome recycling pathways, may perform this latter function in the case of ATP7A, based on a recent unbiased proteomics survey (43).
AP-1 and AP-2 were discovered as adaptors that connect cargo proteins to clathrin and are therefore involved in CCV-mediated trafficking. AP-1 is located in the TGN and endosomes, regulating CCV trafficking between these two compartments. AP-2 locates in early endosomes and the PM and regulates CCV-dependent endocytosis. Thus, the current work, by illustrating direct physical interactions between AP-1/AP-2 and ATP7A, and cell biological correlations in knockdown experiments, provides compelling evidence for critical mechanistic aspects of normal ATP7A intracellular trafficking.
Aberrant PM localization of ATP7A in patients with the MEDNIK syndrome, owing to mutations in the AP1S1 gene encoding σ1A subunit of AP-1, has been reported (33). Co-localization of ATP7A and AP-1 in CCVs and alteration of this co-localization by copper treatment has also been described (35). Other studies suggested a dependence on AP-1, in cooperation with AP-2, for retrograde trafficking of ATP7A from the PM to the TGN (18). For example, in AP-1-depleted cells, ATP7A showed a somewhat dispersed intracellular pattern, implying the possibility of localization in small vesicles (18). The present findings, demonstrating localization of ATP7AP1386S in both the somatodendritic and axonal compartments of transfected NSC-34 cells under normal copper concentrations with prevalent location toward the cell periphery, are not inconsistent with this phenomenon. The dispersed pattern implies a dynamic function of the AP-1 pathway in recycling from endosome to TGN, and vice-versa.
AP-1 was documented to regulate basolateral localization of the LDL and transferrin receptors in polarized epithelial cells (23,24). Thus, disturbance of AP-1 function and regulation could lead to abnormal localization of other basolateral transmembrane protein residents. Basolateral targeting also requires myosin VI, v-SNARE cellubrevin and small GTPase Rab8 (26,44).
Motor neurons represent a specialized type of polarized cell, and proteins targeted to the basolateral membrane in epithelia, such as LDLR and TfR, localize to the somatodendritic area in neurons (45). In contrast, apical membrane-resident proteins such as VSV G-protein and NgCAM localize to axonal area in neurons (46). Farias et al. reported recently that AP-1 mediates the somatodendritic sorting of the glutamate receptor proteins NR2A and NR2B (28). We noted in our experiments that wild-type ATP7A exclusively locates in the TGN of the somatodendritic area and translocates to the axon only after a rise in intracellular copper concentration. This result supports a somatodendritic sorting function of AP-1 for ATP7A, and a working model in which AP-1 physically binds to ATP7A to retain it in the TGN.
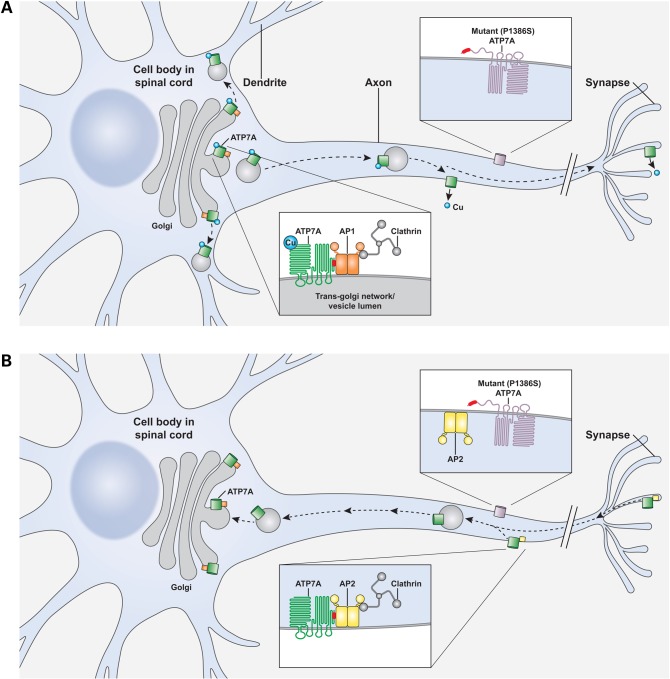
As summarized in the model depicted in Figure 8A, we speculate that elevated copper concentrations induce filling of ATP7A metal-binding domains, which may contribute to breaking down the interaction between AP-1 and ATP7A and allow the copper transporter to escape from the somatodendrite into the axon. After copper atoms are removed from the N-terminal metal-binding domains, ATP7A at the axonal membrane could re-conform to allow binding access to AP-2. In this fashion, endocytosis and retrieval from the axon to the TGN in the somatodendrite would occur (Fig. 8B). Further biochemical and structural studies are needed to explain the mechanisms through which copper binding and release at the ATP7A metal-binding domains may disrupt and foster interactions with AP complexes.
Figure 8.
Speculative models of neuronal ATP7A trafficking regulated by AP-1 and AP-2 and working hypotheses of distal motor neuropathy mechanism. (A) Proposed model of AP-1 interaction with clathrin and ATP7A in motor neurons in which AP-1 (orange) tethers ATP7A (green) at the trans-Golgi network until copper triggers release from this compartment and anterograde ATP7A trafficking. Loss of physical interaction with AP-1 at the carboxyl-terminus (red) of ATP7A owing to unstable insertion of the eighth transmembrane helix alters the normal ratio of somatodendritic: axonal localization in the case of P1386 mutant alleles (lavender), some of which may escape the trans-Golgi without copper, due to loss of the tether. (B) Analogously, endocytic retrieval of P1386S mutant alleles from the axonal membrane is impaired owing to loss of interaction between the C-terminal regions (red) with AP-2 (yellow). Chronic mislocalization of this ion-motive ATPase may interfere with proper axonal ion balance and contribute to the isolated distal motor neuron degeneration associated with this unique missense mutation.
In neuronal cells, copper modulates the function of N-methyl-d-aspartate (NMDA) receptors, AMPA receptor, γ-aminobutyric acid type A (GABAA) receptors and voltage-gated Ca2+ channels (VGCA) (47). Recently, it was reported that elevated copper in the synapses of PC12 cells regulated the large dense core vesicles (LDCVs) secretory pathway by modulating the levels of synaptophysin, SNAP-25 and syntaxin-5 in synaptic-like microvesicles (SLMVs) and LDCVs (48). Correspondingly, ATP7A was enriched in synaptic vesicles, suggesting that ATP7A may be involved in synaptic vesicle secretion by influencing the synaptic fusion machinery (49).
Our previous yeast complementation assays revealed 60–70% normal copper transport function by ATP7AP1386S, suggesting that a mechanism other than copper deficiency may underlie the distal motor neuropathy induced by P1386S (19,20). We noted that the P1386S mutation at the point of insertion of the last transmembrane domain of ATP7A could randomly destabilize insertion of the last transmembrane domain of ATP7A and mislocate the protein's carboxyl-terminal tail (20). Our current results indicate that this mutation impairs interaction with AP-1 and AP-2 and partially excludes ATP7AP1386S from the normal trafficking machinery, with at least some of the mutant molecules embedded in the axonal membrane of polarized motor neurons (Fig. 8B). We hypothesize that this abnormal localization could disturb the function of nearby proteins on the axonal membrane, including the synaptic fusion machinery, NMDA receptors, AMPA receptor and VGCA, as implied by our calcium-signaling results (Fig. 7C). Calcium that enters motor neurons during action potentials normally is removed speedily, because calcium accumulation damages mitochondria and alters motor neuron function (50). The slower than normal calcium release documented in ATP7AP1386S-transfected NSC-34 cells may be a consequence of disturbed axonal copper transport related to preferential PM localization of this mutant allele. In normal rat hippocampal neurons, exposure to increased copper was associated with decreased intracellular calcium levels after NMDA receptor activation, suggesting that neuronal handling of these two elements may be linked (51).
In summary, we demonstrate here that (1) AP complexes 1 and 2 of the CCV traffic machinery physically interact with ATP7A, (2) that the ATP7A carboxyl-terminal tail containing a di-leucine motif (L1477L1478) is crucial for this interaction and (3) that the P1386S mutation in ATP7A abrogates these interactions and leads to abnormal axonal localization in motor neuron cells. Taken together, our results suggest that AP-1 normally retains ATP7A at the TGN in the somatodendritic segment of motor neurons and that disruption of this interaction by the P1386S mutation results in release of ATP7A to the axons or axonal membranes. The latter effect is intensified by loss of interaction with AP-2, thus disturbing ATP7A-mediated copper transport in polarized motor neurons and predisposing to motor neuron disease. Further studies in motor neurons obtained from animal models harboring an atp7a mutation analogous to ATP7AP1386S should provide additional insight as to the precise pathophysiologic mechanism(s) and potential remedies for ATP7A-related motor neuron degeneration.
Materials and Methods
Cell culture
HEK293T human embryonic kidney cells, HeLa human epithelial cancer cells and NSC-34 motor neurons were cultured in Dulbecco's modified Eagle medium (DMEM) with 10% fetal calf serum, antibiotics and l-glutamine under standard sterile culture conditions in a 5% CO2 incubator at 37°C. NSC-34 cells were also cultured in neuronal differentiation medium consisting of high-glucose DMEM/Ham's F-12 (1 : 1) with 1% fetal calf serum and 1% non-essential amino acids for 6 days before transfections.
Cell transfections and confocal microscopy
Full-length ATP7A and a version with the carboxyl-terminus truncated at amino acid residue 1414 (ATP7AΔ1415-1500) were constructed by reverse transcription polymerase chain reaction using total RNA as template. Site-directed mutagenesis was used to generate the P1386S and 1487,1488/AA ATP7A mutant alleles. After sequence fidelity was confirmed, the cDNAs were inserted between the SalI and Apa I sites of pVenus-C1 and the constructs used to transfect HEK293T cells, NSC-34 cells and differentiated NSC-34 motor neurons. Transfections were mediated by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions and performed in triplicate or greater. The following primary antibodies were used: purified mouse anti-adaptin gamma for AP-1 gamma subunit (BD Biosciences), purified mouse anti-adaptin alpha for AP-2 alpha subunit (BD Biosciences), purified mouse anti-human GGA2 (BD Biosciences), purified mouse anti-human GGA3 (BD Biosciences) and mouse monoclonal anti-Tau-1, clone PC1C6 (Millipore). Secondary antibodies used included goat anti-mouse or goat anti-rabbit IgG coupled to Alexa Fluor 488 or Alexa Fluor 647 (Invitrogen; used at 1:4000 dilution). Wild-type and mutant ATP7A-transfected cells were examined by confocal microscopy (Zeiss 510) and images captured using META software.
RNA-mediated interference
RNA-mediated interference of AP-1 (gamma and sigma subunits), AP-2 (alpha and sigma subunits), GGA-2 and GGA-3 was performed using on-targetplus SmartPool siRNAs (Dharmacon). HEK293T cells or HeLa cells were transfected twice at 48-h intervals with 200 pmol of the siRNAs by using Oligofectamine (Invitrogen). After 72 h of siRNA treatment, the cells were transfected as described earlier for the expression of Venus-tagged ATP7A before further immunofluorescence assays, immunoprecipitation assays or western blotting.
Immunoprecipitation assays and western blotting
HEK293T cells were transfected with the respective EYFP-tagged ATP7A (Venus tag) alleles. Twenty-four hours post-transfection, cells were rinsed with PBS and cross-linked with 5 mm DTBP in PBS at RT for 30 min. Crosslinking was then quenched by addition of 1 m glycine to a final concentration of 100 mm, and the samples were incubated at 4°C for 5 min. The cells were then collected and lysed in lysis buffer [50 mm Tris–HCl, pH 7.4, 150 mm NaCl, 1 mm ethylenediaminetetraacetic acid, 1% Triton X-100, 10% glycerol and protein inhibitor mixture (Roche)] on ice, for 20 min. The lysate was centrifuged at 10 000 g for 10 min, and supernatants used for immunoprecipitation with purified mouse anti-adaptin gamma for AP-1 gamma subunit, purified mouse anti-adaptin alpha for AP-2 alpha subunit (BD Biosciences) and high capacity protein A/G agarose beads (Thermo Scientific). Immunoprecipitates were denatured with 5% β-mercaptoethanol/2% SDS loading buffer and electrophoresed through a 10% polyacrylamide gel (Invitrogen) and transferred to polyvinylidine fluoride membranes. The membranes were incubated at 4°C overnight in Tris-buffered saline blocking buffer [50 mm Tris–HCl, pH 7.5, 150 mm NaCl, 0.1% Tween 20 v/v] containing 5% (w/v) nonfat milk. Blots were washed with Tris-buffered saline and incubated for 3 h with a 1 : 2000 dilution of rabbit anti-GFP antibody (Clontech).
Densitometry
Relevant bands on western blots from knockdown and IP experiments were digitized and quantitated by densitometry using the ImageJ program (http://rsb.info.nih.gov/ij) with correction for background, as previously described (20). Multiple blots were analyzed for each experiment, as indicated in the legends to Figures 2, 4 and 5.
TIRF microscopy
HeLa cells were grown on 0.15-mm-thick, 25-mm-diameter cover slips in six-well plates. Following AP-1 or AP-2 siRNA knockdown and Venus-tagged ATP7A transfection, the cells were fixed with 4% paraformaldehyde at RT for 10 min, washed twice with PBS, and TIRF images collected with an Olympus TIRF microscope at 488-nm laser with ×100 objective lens.
Endocytic retrieval studies
HEK293T cells were transfected with WT ATP7A-EYFP (“Venus” enhanced yellow fluorescent protein) as described earlier and pulsed with 200 μM CuCl2 for 3 h to localize ATP7A to the PM and replicate the primary location of ATP7A in cells transfected with T994I and P1386S under basal copper conditions. The cell cultures were then simultaneously exposed to 50 µM BCS (to chelate Cu) and 10 mm cycloheximide (to inhibit protein synthesis) for 4 h prior to confocal imaging.
Calcium signaling
Differentiated NSC34 in four-well chambers (Lab-Tek) were loaded with 5 μm Fluo-4 (Invitrogen F14201) acetoxymethyl ester in calcium imaging buffer (10 mm Hepes, 130 mm NaCl, 20 mm d-(+)-Glucose, 2 mm MgCl2, 2.4 mm KCl, 2 mm CaCl2, pH 7.4) at room temperature for 30 min, washed with Ca2+ washing buffer for 10 min and cells kept in 0.5 ml calcium imaging buffer. The four-well chambers containing Fluo-4 AM-loaded NSC34 cells were mounted on Zeiss 510 40× objective lens, time-lapse imaging set up with 100 frames at 0.25-s intervals (frame pixel 128 × 128) and cells stimulated with 0.1 ml of 10 mm glutamate in calcium imaging buffer (final glutamate concentration was 1.33 mm) at the 10th frame. Calcium-signaling data from ATP7AP1386S (n = 19), ATP7Awild type (n = 23) and mock-transfected (n = 12) motor neurons were analyzed with Image J software.
Statistical analyses
Data from siRNA, confocal microscopy and calcium signaling were analyzed by one- or two-tailed Student's t-tests. P-values of ≤0.05 were considered statistically significant.
Supplementary Material
Funding
This research was supported by the NIH Intramural Research Program (IRP).
Supplementary Material
Acknowledgements
We thank the other members of our laboratory for their advice and support in this work.
Conflict of Interest statement. None declared.
References
- 1.Palmgren M.G., Nissen P. (2011) P-type ATPases. Ann. Rev. Biophys, 40, 243–266. [DOI] [PubMed] [Google Scholar]
- 2.Lutsenko S. (2010) Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol., 14, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaler S.G. (2011) ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol., 7, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gourdon P., Liu X.Y., Skjørringe T., Morth J.P., Møller L.B., Pedersen B.P., Nissen P. (2011) Crystal structure of a copper-transporting PIB-type ATPase. Nature, 475, 59–64. [DOI] [PubMed] [Google Scholar]
- 5.Petris M.J., Mercer J.F., Culvenor J.G., Lockhart P., Gleeson P.A., Camakaris J. (1996) Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J., 15, 6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 6.Pase L., Voskoboinik I., Greenough M., Camakaris J. (2004) Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem. J., 378, 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis M.J., Jones E.E., Levy E.R., Ponnambalam S., Chelly J., Monaco A.P. (1998) A Golgi localization signal identified in the Menkes recombinant protein. Hum. Mol. Genet., 7, 1245–1252. [DOI] [PubMed] [Google Scholar]
- 8.Goodyer I.D., Jones E.E., Monaco A.P., Francis M.J. (1999) Characterization of the Menkes protein copper-binding domains and their role in copper-induced protein relocalization. Hum. Mol. Genet., 8, 1473–1478. [DOI] [PubMed] [Google Scholar]
- 9.Strausak D., La Fontaine S., Hill J., Firth S.D., Lockhart P.J., Mercer J.F. (1999) The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J. Biol. Chem., 274, 11170–11177. [DOI] [PubMed] [Google Scholar]
- 10.Voskoboinik I., Strausak D., Greenough M., Brooks H., Petris M., Smith S., Mercer J.F., Camakaris J. (1999) Functional analysis of the N-terminal CXXC metal-binding motifs in the human Menkes copper-transporting P-type ATPase expressed in cultured mammalian cells. J. Biol. Chem., 274, 22008–22012. [DOI] [PubMed] [Google Scholar]
- 11.Petris M.J., Camakaris J., Greenough M., LaFontaine S., Mercer J.F. (1998) A C-terminal di-leucine is required for localization of the Menkes protein in the trans-Golgi network. Hum. Mol. Genet., 7, 2063–2071. [DOI] [PubMed] [Google Scholar]
- 12.Francis M.J., Jones E. E., Levy E.R., Martin R.L., Ponnambalam S., Monaco A.P. (1999) Identification of a di-leucine motif within the C terminus domain of the Menkes disease protein that mediates endocytosis from the plasma membrane. J. Cell Sci., 112, 1721–1732. [DOI] [PubMed] [Google Scholar]
- 13.Petris M.J., Voskoboinik I., Cater M., Smith K., Kim B.E., Llanos R.M., Strausak D., Camakaris J., Mercer J.F. (2002) Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J. Biol. Chem., 277, 46736–46742. [DOI] [PubMed] [Google Scholar]
- 14.Greenough M., Pase L., Voskoboinik I., Petris M.J., O'Brien A.W., Camakaris J. (2004) Signals regulating trafficking of Menkes (MNK; ATP7A) copper-translocating P-type ATPase in polarized MDCK cells. Am. J. Physiol. Cell Physiol., 287, C1463–C1471. [DOI] [PubMed] [Google Scholar]
- 15.Lenartowicz M., Grzmil P., Shoukier M., Starzyński R., Marciniak M., Lipiński P. (2012) Mutation in the CPC motif-containing 6th transmembrane domain affects intracellular localization, trafficking and copper transport efficiency of ATP7A protein in mosaic mutant mice—an animal model of Menkes disease. Metallomics, 4, 197–204. [DOI] [PubMed] [Google Scholar]
- 16.Pascale M.C., Franceschelli S., Moltedo O., Belleudi F., Torrisi M.R., Bucci C., La Fontaine S., Mercer J.F., Leone A. (2003) Endosomal trafficking of the Menkes copper ATPase ATP7A is mediated by vesicles containing the Rab7 and Rab5 GTPase proteins. Exp. Cell Res., 291, 377–385. [DOI] [PubMed] [Google Scholar]
- 17.Holloway Z.G., Grabski R., Szul T., Styers M.L., Coventry J.A., Monaco A.P., Sztul E. (2007) Activation of ADP-ribosylation factor regulates biogenesis of the ATP7A-containing trans-Golgi network compartment and its Cu-induced trafficking. Am. J. Physiol. Cell Physiol., 293, C1753–1767. [DOI] [PubMed] [Google Scholar]
- 18.Holloway Z.G., Velayos-Baeza A., Howell G.J., Levecque C., Ponnambalam S., Sztul E., Monaco A.P. (2013) Trafficking of the Menkes copper transporter ATP7A is regulated by clathrin-, AP-2-, AP-1- and Rab22-dependent steps. Mol. Biol. Cell., 24, 1735–1748, S1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennerson M.L., et al. (2010) Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am. J. Hum. Genet., 86, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi L., Donsante A., Kennerson M.L., Mercer J.F.B., Garbern J.Y., Kaler S.G. (2012) Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Hum. Mol. Genet., 21, 1794–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson M.S. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol., 14, 167–174. [DOI] [PubMed] [Google Scholar]
- 22.Canagarajah B.J., Ren X., Bonifacino J.S., Hurley J.H. (2013) The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci., 22, 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folsch H., Ohno H., Bonifacino J.S., Mellman I. (1999) A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell, 99, 189–198. [DOI] [PubMed] [Google Scholar]
- 24.Folsch H., Pypaert M., Schu P., Mellman I. (2001) Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol., 152, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ang A.L., Folsch H., Koivisto U.M., Pypaert M., Mellman I. (2003) The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J. Cell Biol., 163, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au J.S., Puri C., Ihrke G., Kendrick-Jones J., Buss F. (2007) Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J. Cell Biol., 177, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glyvuk N., Tsytsyura Y., Geumann C., D'Hooge R., Hüve J., Kratzke M., Baltes J., Boening D., Klingauf J., Schu P. (2010) AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J., 29, 1318–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farias G.G., Cuitino L., Guo X., Ren X., Jarnik M., Mattera R., Bonifacino J.S. (2012) Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron, 75, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C.J., Bardoni R., Tong C.K., Engelman H.S., Joseph D.J., Magherini P.C., MacDermott A.B. (2002) Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron, 35, 135–146. [DOI] [PubMed] [Google Scholar]
- 30.Walther K., Diril M.K., Jung N., Haucke V. (2004) Functional dissection of the interactions of stonin 2 with the adaptor complex AP-2 and synaptotagmin. Proc. Natl Acad. Sci. USA, 101, 964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racz B., Blanpied T.A., Ehlers M.D., Weinberg R.J. (2004) Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci., 7, 917–918. [DOI] [PubMed] [Google Scholar]
- 32.Montpetit A., et al. (2008) Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet., 4, e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinelli D., et al. (2013) MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain, 136, 872–881. [DOI] [PubMed] [Google Scholar]
- 34.Braulke T., Bonifacino J.S. (2009) Sorting of lysosomal proteins. Biochim. Biophys. Acta, 1793, 605–614. [DOI] [PubMed] [Google Scholar]
- 35.Hirst J., Borner G.H., Antrobus R., Peden A.A., Hodson N.A., Sahlender D.A., Robinson M.S. (2012) Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol., 22, 1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cashman N.R., Durham H.D., Blusztajn J.K., Oda K., Tabira T., Shaw I.T., Dahrouge S., Antel J.P. (1992) Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn., 194, 209–221. [DOI] [PubMed] [Google Scholar]
- 37.Durham H.D., Dahrouge S., Cashman N.R. (1993) Evaluation of the spinal cord neuron X neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicology, 14, 387–395. [PubMed] [Google Scholar]
- 38.Matusica D., Fenech M.P., Rogers M.L., Rush R.A. (2008) Characterization and use of the NSC-34 cell line for study of neurotrophin receptor trafficking. J. Neurosci. Res., 86, 553–565. [DOI] [PubMed] [Google Scholar]
- 39.Prause J., et al. (2013) Altered localization, abnormal modification and loss of function of Sigma receptor-1 in amyotrophic lateral sclerosis. Hum. Mol. Genet., 22, 1581–1600. [DOI] [PubMed] [Google Scholar]
- 40.Maier O., Bohm J., Dahm M., Brück S., Beyer C., Johann S. (2013) Differentiated NSC-34 motoneuron-like cells as experimental model for cholinergic neurodegeneration. Neurochem. Int., 62, 1029–1038. [DOI] [PubMed] [Google Scholar]
- 41.Cross J.L., Meloni B.P., Bakker A.J., Lee S., Knuckey N.W. (2010) Modes of neuronal calcium entry and homeostasis following cerebral ischemia. Stroke Res. Treat, 2010, 316862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephenson S.E., Dubach D., Lim C.M., Mercer J.F., La Fontaine S. (2005) A single PDZ domain protein interacts with the Menkes copper ATPase, ATP7A. A new protein implicated in copper homeostasis. J. Biol. Chem., 280, 33270–9. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J., Tavaré J.M., Cullen P.J. (2013) A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell. Biol., 15, 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fields I.C., Shteyn E., Pypaert M., Proux-Gillardeaux V., Kang R.S., Galli T., Fölsch H. (2007) v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J. Cell. Biol., 177, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda S., Miura E., Matsuda K., Kakegawa W., Kohda K., Watanabe M., Yuzaki M. (2008) Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron, 57, 730–745. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda S., Yuzaki M. (2009) Polarized sorting of AMPA receptors to the somatodendritic domain is regulated by adaptor protein AP-4. Neurosci. Res., 65, 1–5. [DOI] [PubMed] [Google Scholar]
- 47.Gaier E.D., Eipper B.A., Mains R.E. (2013) Copper signaling in the mammalian nervous system: synaptic effects. J. Neurosci. Res., 91, 2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duncan C., et al. (2013) Copper modulates the large dense core vesicle secretory pathway in PC12 cells. Metallomics, 5, 700–714. [DOI] [PubMed] [Google Scholar]
- 49.Gaier E.D., Miller M.B., Ralle M., Aryal D., Wetsel W.C., Mains R.E., Eipper B.A. (2013) Peptidylglycine alpha-amidating monooxygenase heterozygosity alters brain copper handling with region specificity. J. Neurochem., 127, 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vos K.J., Mórotz G.M., Stoica R., Tudor E.L., Lau K.F., Ackerley S., Warley A., Shaw C.E., Miller C.C. (2012) VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet., 21, 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlief M., West T., Craig A.M., Holtzman D.M., Gitlin J.D. (2006) Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl Acad. Sci. USA, 103, 14919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.