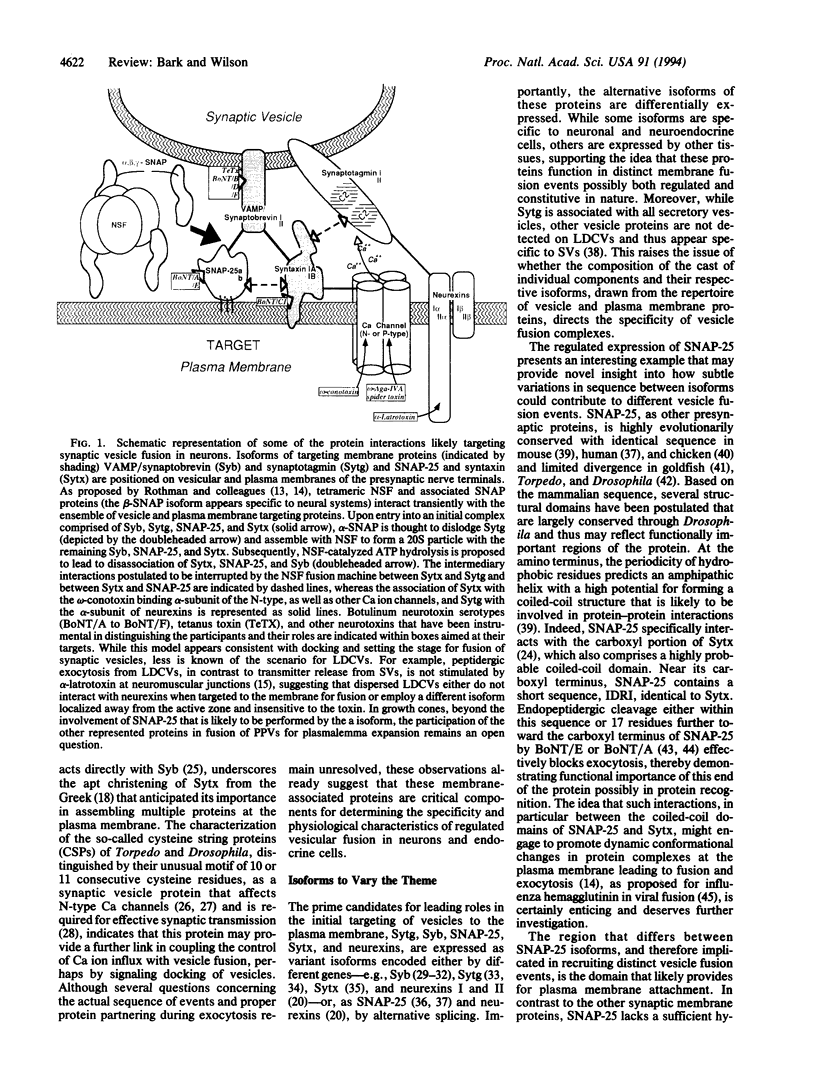
Abstract
In the past year major strides have been made toward our understanding of the molecular mechanisms involved in regulated vesicle fusion and exocytosis in neurons and neuroendocrine cells. Much of this advance has come from the identification of proteins participating in these events and of their potential roles mediated by interactions with each other, the constituent membranes, and, in some cases, Ca2+ signaling. The involvement of vesicle fusion in elongation of neuronal processes during development and release of transmitters and neuromodulatory peptides in the mature nervous system indicates, however, that refinements in the fusion machinery may be required for each of these acts. For many of the participants in synaptic membrane fusion, variant isoforms have been identified that exhibit modifications that might alter interactive properties of these proteins. We discuss the idea that diversification of isoforms, as illustrated by the expression of alternatively spliced variants of SNAP-25, is likely to be an important component in providing the detail necessary to differentiate the physiology of regulated fusion of different classes of vesicles employed in development, neurotransmission, and secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Kikuchi A., Hata Y., Isomura M., Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990 Aug 5;265(22):13007–13015. [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Neuronal Ca2+ signalling takes the local route. Curr Opin Neurobiol. 1992 Jun;2(3):302–307. doi: 10.1016/0959-4388(92)90119-6. [DOI] [PubMed] [Google Scholar]
- Bark I. C. Structure of the chicken gene for SNAP-25 reveals duplicated exon encoding distinct isoforms of the protein. J Mol Biol. 1993 Sep 5;233(1):67–76. doi: 10.1006/jmbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- Bark I. C., Wilson M. C. Human cDNA clones encoding two different isoforms of the nerve terminal protein SNAP-25. Gene. 1994 Feb 25;139(2):291–292. doi: 10.1016/0378-1119(94)90773-0. [DOI] [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., García-Arrarás J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell. 1993 Sep 10;74(5):863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz T., Blasi J., Yamasaki S., Baumeister A., Link E., Südhof T. C., Jahn R., Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem. 1994 Jan 21;269(3):1617–1620. [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993 Sep 9;365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Calakos N., Bennett M. K., Peterson K. E., Scheller R. H. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994 Feb 25;263(5150):1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993 May 21;73(4):823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- Catsicas S., Larhammar D., Blomqvist A., Sanna P. P., Milner R. J., Wilson M. C. Expression of a conserved cell-type-specific protein in nerve terminals coincides with synaptogenesis. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):785–789. doi: 10.1073/pnas.88.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- DeBello W. M., Betz H., Augustine G. J. Synaptotagmin and neurotransmitter release. Cell. 1993 Sep 24;74(6):947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- Fykse E. M., Takei K., Walch-Solimena C., Geppert M., Jahn R., De Camilli P., Südhof T. C. Relative properties and localizations of synaptic vesicle protein isoforms: the case of the synaptophysins. J Neurosci. 1993 Nov;13(11):4997–5007. doi: 10.1523/JNEUROSCI.13-11-04997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Archer B. T., 3rd, Südhof T. C. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991 Jul 25;266(21):13548–13552. [PubMed] [Google Scholar]
- Gundersen C. B., Umbach J. A. Suppression cloning of the cDNA for a candidate subunit of a presynaptic calcium channel. Neuron. 1992 Sep;9(3):527–537. doi: 10.1016/0896-6273(92)90190-o. [DOI] [PubMed] [Google Scholar]
- Hata Y., Davletov B., Petrenko A. G., Jahn R., Südhof T. C. Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron. 1993 Feb;10(2):307–315. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- Hata Y., Slaughter C. A., Südhof T. C. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993 Nov 25;366(6453):347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- Hess D. T., Patterson S. I., Smith D. S., Skene J. H. Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature. 1993 Dec 9;366(6455):562–565. doi: 10.1038/366562a0. [DOI] [PubMed] [Google Scholar]
- Hess D. T., Slater T. M., Wilson M. C., Skene J. H. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992 Dec;12(12):4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa H. P., Saisu H., Ishizuka T., Sekine Y., Tsugita A., Odani S., Abe T. A complex of rab3A, SNAP-25, VAMP/synaptobrevin-2 and syntaxins in brain presynaptic terminals. FEBS Lett. 1993 Sep 13;330(2):236–240. doi: 10.1016/0014-5793(93)80281-x. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Cell biology. Snappy exocytoxins. Nature. 1993 Sep 9;365(6442):104–105. doi: 10.1038/365104a0. [DOI] [PubMed] [Google Scholar]
- Lockerbie R. O., Miller V. E., Pfenninger K. H. Regulated plasmalemmal expansion in nerve growth cones. J Cell Biol. 1991 Mar;112(6):1215–1227. doi: 10.1083/jcb.112.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomneth R., Martin T. F., DasGupta B. R. Botulinum neurotoxin light chain inhibits norepinephrine secretion in PC12 cells at an intracellular membranous or cytoskeletal site. J Neurochem. 1991 Oct;57(4):1413–1421. doi: 10.1111/j.1471-4159.1991.tb08308.x. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo A., Parsons S. M., Zampighi G. A., Jenden D. J., Umbach J. A., Gundersen C. B. Cysteine string proteins: a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science. 1994 Feb 18;263(5149):981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- Matteoli M., Haimann C., Torri-Tarelli F., Polak J. M., Ceccarelli B., De Camilli P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Südhof T. C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993 Jul 22;364(6435):346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- O'Connor V. M., Shamotienko O., Grishin E., Betz H. On the structure of the 'synaptosecretosome'. Evidence for a neurexin/synaptotagmin/syntaxin/Ca2+ channel complex. FEBS Lett. 1993 Jul 12;326(1-3):255–260. doi: 10.1016/0014-5793(93)81802-7. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A., Catsicas M., Staple J. K., Jones K. A., Ayala G., Knowles J., Grenningloh G., Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993 Jul 29;364(6436):445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler G. A., Polli J. W., Wilson M. C., Billingsley M. L. Developmental expression of the 25-kDa synaptosomal-associated protein (SNAP-25) in rat brain. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5247–5251. doi: 10.1073/pnas.88.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Risinger C., Blomqvist A. G., Lundell I., Lambertsson A., Nässel D., Pieribone V. A., Brodin L., Larhammar D. Evolutionary conservation of synaptosome-associated protein 25 kDa (SNAP-25) shown by Drosophila and Torpedo cDNA clones. J Biol Chem. 1993 Nov 15;268(32):24408–24414. [PubMed] [Google Scholar]
- Risinger C., Larhammar D. Multiple loci for synapse protein SNAP-25 in the tetraploid goldfish. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10598–10602. doi: 10.1073/pnas.90.22.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Rossetto O., Catsicas S., Polverino de Laureto P., DasGupta B. R., Benfenati F., Montecucco C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem. 1993 Nov 15;268(32):23784–23787. [PubMed] [Google Scholar]
- Schiavo G., Santucci A., Dasgupta B. R., Mehta P. P., Jontes J., Benfenati F., Wilson M. C., Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993 Nov 29;335(1):99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Simons K., Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993 Nov;11(5):789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Czernik A. J., Kao H. T., Takei K., Johnston P. A., Horiuchi A., Kanazir S. D., Wagner M. A., Perin M. S., De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989 Sep 29;245(4925):1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993 Nov 11;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Thureson-Klein A. K., Klein R. L. Exocytosis from neuronal large dense-cored vesicles. Int Rev Cytol. 1990;121:67–126. doi: 10.1016/s0074-7696(08)60659-2. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Gray T. S., Elferink L. A., Wilson M. C., Scheller R. H. Distinct patterns of expression of two VAMP genes within the rat brain. J Neurosci. 1990 Apr;10(4):1380–1387. doi: 10.1523/JNEUROSCI.10-04-01380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Linial M., Scheller R. H. Cellular and molecular biology of the presynaptic nerve terminal. Annu Rev Neurosci. 1991;14:93–122. doi: 10.1146/annurev.ne.14.030191.000521. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. A., Petrenko A. G., Geppert M., Südhof T. C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992 Jul 3;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Verhage M., McMahon H. T., Ghijsen W. E., Boomsma F., Scholten G., Wiegant V. M., Nicholls D. G. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991 Apr;6(4):517–524. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C., Takei K., Marek K. L., Midyett K., Südhof T. C., De Camilli P., Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993 Sep;13(9):3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Oho C., Omori A., Kuwahara R., Ito T., Takahashi M. HPC-1 is associated with synaptotagmin and omega-conotoxin receptor. J Biol Chem. 1992 Dec 15;267(35):24925–24928. [PubMed] [Google Scholar]
- Zhu P. C., Thureson-Klein A., Klein R. L. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience. 1986 Sep;19(1):43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]
- Zinsmaier K. E., Eberle K. K., Buchner E., Walter N., Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994 Feb 18;263(5149):977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]