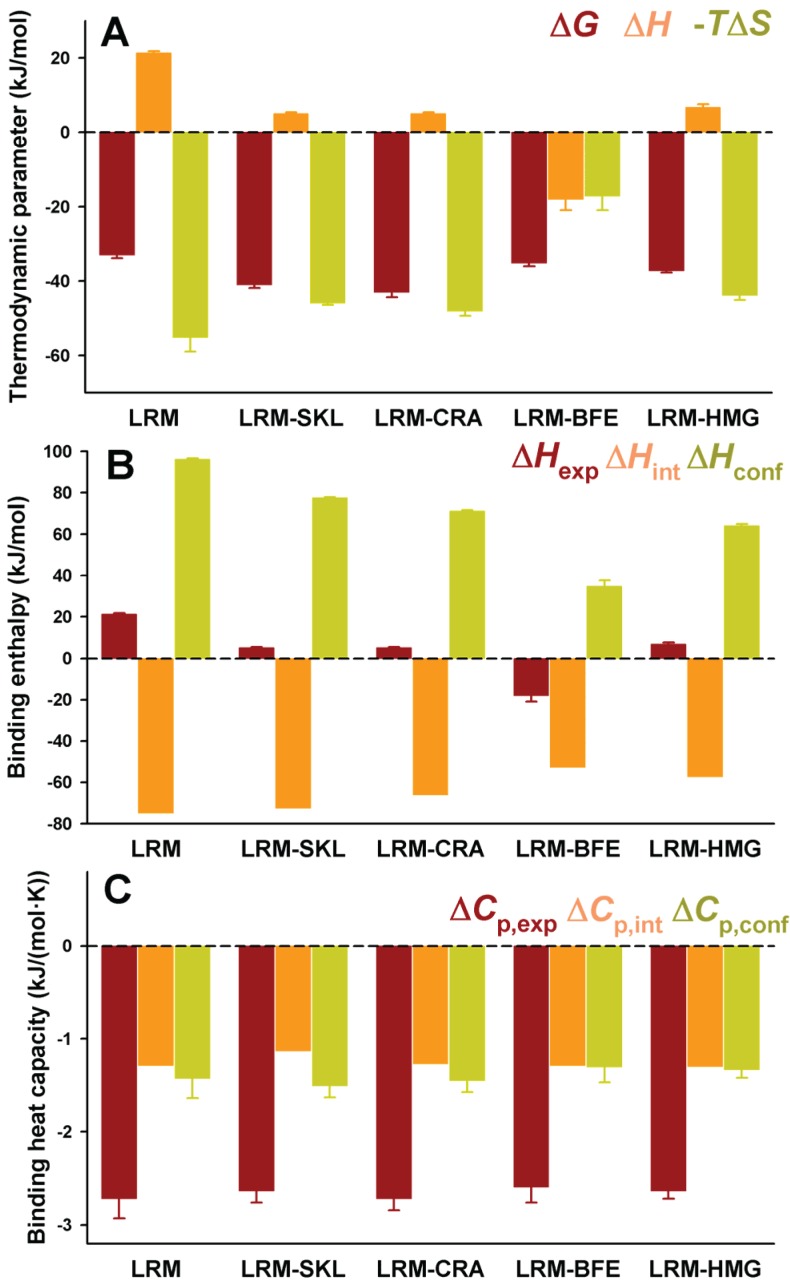
Figure 4.
Thermodynamic binding properties for the interaction of protein variants on the AGT-LRM background with Pex5p-pbd. (A) Free energy (ΔG), enthalpy (ΔH) and entropy (−TΔS) contributions to the binding reaction; (B,C) Thermodynamic dissection of binding enthalpies (B) and heat capacity changes (ΔCp, C) into their intrinsic contribution (estimated from polar and apolar ΔASA using the crystal structure for the complex; Figure 1) and the contribution from conformational changes. In (A,B), data are mean ± s.d. for three independent experiments at 25 °C.