Abstract
Background
Previous studies using the California Verbal Learning Test-Children’s Version (CVLT-C) to examine effects of heavy prenatal alcohol exposure on verbal learning and memory have reported impaired information acquisition (i.e., encoding), rather than retrieval, as the primary mechanism underlying learning and memory impairment. We administered the CVLT-C to two independent cohorts to determine whether (1) effects on encoding are also seen at moderate exposure levels, using both categorical (diagnostic/exposure group) and continuous exposure measures; (2) these deficits are specific or secondary to alcohol-related impairment in IQ; and (3) effects on retrieval can be detected over and above effects on initial encoding.
Methods
We administered the CVLT-C and Wechsler Intelligence Scale for Children to 151 Cape Town heavy and nonexposed children (M=10.3 years), and 291 Detroit adolescents recruited to over-represent moderate-to-heavy prenatal alcohol exposure (M=14.4 years).
Results
Effects on encoding in the heavily exposed Cape Town cohort and on retrieval in both cohorts were significant after adjustment for IQ. Although effects on retrieval were no longer significant in Cape Town after control for initial encoding, effects on recognition memory continued to be evident in Detroit. Children with full or partial fetal alcohol syndrome were less able to use the semantic cluster encoding strategy implict in the CVLT-C.
Conclusions
Effects on verbal learning were seen primarily in the more heavily exposed Cape Town cohort; effects on recall and recognition memory were also seen at moderate exposure levels in Detroit. These effects were not attributable to alcohol-related impairment in overall intellectual competence. The finding that effects on retention continued to be evident after statistical adjustment for initial encoding in Detroit suggests that a fetal alcohol-related deficit in retrieval is not secondary to a failure to encode the initial information. These data confirm that this impairment in initial learning is mediated, in part, by failure to use the semantic cluster learning strategy.
Keywords: fetal alcohol spectrum disorders, fetal alcohol exposure, fetal alcohol syndrome, verbal learning and memory, California Verbal Learning Test-Children’s Version, IQ, moderate prenatal alcohol exposure
INTRODUCTION
Despite more than four decades of research and published prevention guidelines (ACOG, 2011), many women continue to drink heavily during pregnancy, and 6.6% report binge-drinking episodes (SAMHSA, 2013). The prevalence of fetal alcohol syndrome disorders (FASD) has been estimated at 2–5% of the school age population in the United States, Canada, and Western Europe (May et al., 2009; Chudley et al., 2005), and at 13.6–20.9% in South Africa (May et al., 2013). Fetal alcohol syndrome (FAS), the most common preventable form of mental retardation, occurs in the United States at 0.2–3.0 cases per 1000 live births—rates comparable to Down syndrome or spina bifida (Bertrand et al., 2004)—and 5.9–9.1% in the Cape Coloured (mixed ancestry) community in South Africa (May et al., 2013).
Neurobehavioral deficits characterizing FASD include lower IQ, slower processing speed, and poorer executive function, attention, and verbal learning and memory (Burden et al., 2005; Jacobson et al., 1993, 2004, 2008; Mattson et al., 1997, 1998, 2011; Rasmussen, 2005). The California Verbal Learning Test-Children’s Version (CVLT-C; Delis et al., 1994), a list learning task, has proven to be particularly sensitive to prenatal alcohol exposure. Children with heavy prenatal alcohol exposure learn the CVLT-C word lists more slowly than non-exposed children (Mattson and Roebuck, 2002, Crocker et al., 2011), recall fewer words (Vaurio et al., 2011), are less accurate at discriminating between target and distractor words on recognition trials (Mattson et al., 1998; Willoughby et al., 2008), and use less semantic clustering (Kerns et al., 1997; Roebuck-Spencer and Mattson, 2004). Several studies have suggested that impaired information acquisition (i.e., poor performance on the initial learning trials) is responsible for the impairments in verbal recall and that recall per se may be relatively spared in FASD (Mattson and Roebuck, 2002; Roebuck-Spencer and Mattson, 2004; Willoughby et al., 2008; Vaurio et al., 2011). It is not clear whether this pattern of impaired encoding and spared retention would also be seen in moderately-exposed children, whose ability to learn lists may be less affected than in heavily exposed individuals.
Effects of moderate prenatal alcohol exposure on verbal learning and memory have been examined only in the Pittsburgh longitudinal cohort. Learning and memory deficits were found at age 10 years (Richardson et al., 2002) on the Wide Range Assessment of Memory and Learning (WRAML; Sheslow and Adams, 1990) and at 14 years (Willford et al., 2004) on the Children’s Memory Scale (Cohen, 1997). By contrast to the previous studies, prenatal alcohol effects on WRAML retention continued to be significant after control for information acquisition. One explanation for this inconsistency is that their lower exposure levels may have enabled the Pittsburgh children to initially encode the information, providing a more direct test of prenatal alcohol exposure on retention. An alternative interpretation is that the WRAML stimuli do not allow for semantic categorization during learning, whereas the CVLT-C stimuli are grouped according to three semantic categories, thereby enabling the child to use an implicit encoding strategy. Thus, the observed CVLT-C alcohol-related acquisition deficit may be due to poorer use of strategy by heavily exposed children (e.g., Roebuck-Spencer and Mattson, 2004; Kerns et al., 1997).
Because the CVLT-C and IQ are often highly correlated (Mattson and Roebuck, 2002), poor CVLT-C performance could be attributable to effects of alcohol exposure on general intellectual functioning. Previous studies using IQ-equivalent controls (Vaurio et al., 2011), a special education comparison group (Coles et al., 2010), and IQ groups dichotomized to compare average vs. below-average scores (Kerns et al., 1997) or IQ scores of greater vs. less than 80 (Roebuck-Spencer and Mattson, 2004) have all reported prenatal alcohol exposure effects on learning and memory. However, these results are inconsistent with the only study which adjusted statistically for IQ (Coles et al., 2010). In that study, alcohol effects on total recall were no longer significant after control for IQ for the sample as a whole; the only difference on total recall after adjustment for IQ was between the dysmorphic and control groups. This finding suggests that a specific alcohol effect on learning and memory is observed in dysmorphic individuals (i.e., at very heavy levels of exposure), whereas impairment observed in non-dysmorphic individuals may be better accounted for by deficits in general intellectual functioning.
To date, learning and memory has been examined on the CVLT-C primarily in clinic-referred samples of children with a history of heavy prenatal alcohol exposure. Because prenatal alcohol information is not available in retrospective studies, levels of exposure at which these effects are observed were not examined. In this paper, we examine CVLT-C performance in two independent prospectively recruited cohorts: one comprised of school-aged children with heavy prenatal alcohol exposure; the other, of moderately exposed adolescents. In both cohorts, detailed maternal alcohol consumption information was obtained using a timeline follow-back interview administered during pregnancy. The aims of this study were to determine the degree to which (a) effects on learning seen at high levels of exposure are also seen at moderate levels, using both categorical (diagnostic/exposure group) and continuous measures of exposure; (b) learning and memory deficits in children are specific or secondary to alcohol-related impairment in general intellectual functioning assessed by statistical adjustment for IQ; (c) effects on recall and recognition memory are seen over and above effects on initial learning at heavy and moderate levels of exposure; and (d) effects of prenatal alcohol exposure on learning are attributable to less optimal learning strategies. This study is also the first to examine effects of prenatal alcohol exposure on learning and memory in children outside of North America and in a language other than English.
METHODS
Participants
Cape Town cohort
The sample consisted of 91 heavily exposed and 60 control children born to women from the Cape Coloured community in Cape Town, South Africa, who were recruited between July 1999 and January 2002 (Jacobson et al., 2008). At her first antenatal clinic visit (M=19.3 weeks gestation, SD=6.4), each mother was administered a 2-week timeline follow-back interview regarding her alcohol consumption at time of recruitment and around time of conception. Volume of each type of alcoholic beverage consumed was converted to oz absolute alcohol (AA). Women were invited to participate if they reported an average of at least 1.0 oz AA/day (≈2 standard drinks) or two incidents of binge drinking (>5 standard drinks/occasion) during the first trimester of pregnancy. Controls consisted of women presenting for antenatal care who reported abstaining or drinking no more than minimally during pregnancy. Timeline follow-back interviews were administered again in mid-pregnancy and at 1 month postpartum, focusing on the latter part of pregnancy. Data from the three interviews were averaged to provide a summary measure of prenatal alcohol exposure (oz AA/day). Women were also interviewed regarding drug use (marijuana, cocaine, methaqualone (“mandrax”); days/month) and smoking (cigarettes/day) during pregnancy at the three interviews. Women <18 years of age and those with diabetes, epilepsy, or cardiac problems requiring treatment were excluded, as were observant Muslims whose religious practices prohibit alcohol consumption. Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, multiple births, and seizures.
In September 2005, we organized a clinic at which each child (M age=4.8, SD=0.9) was examined for growth and fetal alcohol syndrome (FAS) anomalies by two U.S.-based expert dysmorphologists (H.E. Hoyme and L.K. Robinson) using the revised Institute of Medicine criteria (Hoyme et al., 2005). FAS, the most severe of the FASD, is characterized by distinctive facial dysmorphology (short palpebral fissures, thin vermillion, flat philtrum), small head circumference, and pre- and/or postnatal growth retardation (Stratton et al. 1996; Hoyme et al. 2005); PFAS is diagnosed when there is a history of heavy prenatal maternal drinking, the presence of two of the principal alcohol-related facial anomalies, and small head circumference, growth retardation, or cognitive and/or behavioral dysfunction. There was substantial inter-examiner agreement on assessment of dysmorphic features among the two dysmorphologists and a Cape Town-based dysmorphologist (N. Khaole), who examined 20 children who could not attend the clinic (see Jacobson et al., 2008). FAS and PFAS diagnoses were determined at case conferences by HEH, LKR, SWJ, JLJ, and CDM.
Detroit cohort
The sample consisted of 291 African-American adolescents whose mothers were recruited between September 1986 and April 1989 at their first antenatal visits (M=23.3 weeks gestation, SD=8.0), using the same timeline follow-back procedure as in Cape Town (Jacobson et al., 2002, 2004; J. Jacobson et al., 2011). To over-represent moderate-to-heavy prenatal alcohol exposure, all women who averaged at least 0.5 oz AA/day at conception were invited to participate, as was a random sample of 5% of women who abstained or drank <0.5 oz AA/day. To reduce the risk that alcohol effects would be confounded with prenatal cocaine exposure, a group of heavy cocaine (≥2 days/week) but light alcohol (<0.5 oz AA/day) users was also recruited. The timeline follow-back interview was re-administered at each prenatal clinic visit (M=5.5 visits), and prenatal alcohol exposure was averaged to measure oz AA/day. Infant exclusionary criteria were birthweight <1500g, gestational age <32 weeks, major chromosomal anomalies, neural tube defects, and multiple births.
At the 7.5-year follow-up assessment, the children (M age=7.7, SD=0.3) were examined for facial dysmorphology and growth by a trained research psychologist under the supervision of E. Bawle, who evaluated all suspect cases for FAS diagnosis (see Jacobson et al., 2004). Three children were determined to meet criteria for FAS, diagnoses that were subsequently confirmed independently in blind reviews of a large set of frontal and profile photographs of children in the cohort by two expert FAS dysmorphologists (S. Clarren, and K.L. Jones).
Materials and Procedure
The CVLT-C was administered at the 10-year follow-up assessment in Cape Town and at 14 years in Detroit. It is designed to assess cognitive processes involved in learning and memory; namely, information encoding, retrieval/recall, and recognition (Table 1). During encoding, five learning trials are administered; in each trial, the examiner reads the same 15 words (List A) in the same order, and the child is asked to recall as many as s/he can. List A consists of five words from each of three semantic categories (clothing, toys, and fruits). The examiner then administers a single trial with a new list of 15 words (List B, the distractor list), after which the child is asked to recall as many of the List A items as s/he can (short-delay free recall). After a 20-minute delay, the child is again asked to recall as many List A items as possible (long-delay free recall). Finally, the child is asked to identify which items from a 45-word list were present in List A (recognition discrimination). As in Roebuck-Spencer and Mattson (2004), semantic clustering was calculated for the Cape Town cohort using the target list as a baseline of chance expected values, as recommended by Stricker et al. (2002), but the scoring tool used in Detroit did not permit extraction of the raw semantic clustering data needed for this calculation. False-positive errors on the recognition trials (Delis et al., 2000) were examined in both cohorts.
Table 1.
CVLT-C Outcome Variables
| Variable Name | Description |
|---|---|
| Immediate learning | Number of words correctly recalled on learning trial 1 |
| Total learning | Number of words correctly recalled over the 5 learning trials |
| Short-delay recall | Number of words correctly recalled after a short-delay (i.e., after the presentation of distractor list) |
| Long-delay recall | Number of words correctly recalled after a delay of 20 minutes |
| Recognition discrimination accuracy | Number of words correctly recognized when both correct rejections and number of words presented during the recognition trial (N = 45) are taken into account. Calculated as [number of correct hits + correct rejections]/total words (Delis et al., 1994) |
| Perseveration errors | Number of perseverations (i.e., response repetition) on recall trials |
| Intrusion errors | Number of intrusions (i.e., non-target words) reported on recall trials |
| False-positive errors | Number of words incorrectly identified as target words during recognition testing. |
| Semantic cluster | Semantic clusters are observed when two consecutively recalled words come from the same semantic category (viz., either fruits, clothing, or playthings). An observed/expected ratio score is calculated for each of the free recall trials. Expected correct semantic clustering scores are calculated using the approach recommended by Stricker et al. (2002) and Delis (2002). |
| Serial cluster | Serial clusters are observed when two words are recalled in the same order in which they were originally presented. An observed/expected ratio score is calculated for each of the free recall trials. Expected correct serial clustering scores are calculated using the approach recommended by Stricker et al. (2002) and Delis (2002). |
The Wechsler Intelligence Scale for Children-IV (WISC-IV; Wechsler, 2003) was administered at the 10-year Cape Town assessment; the WISC-III (Wechsler, 1991), at 14 years in Detroit. Testing in Cape Town was conducted in Afrikaans (n=70, 46.4%) or English (n=81, 53.6%), depending on the primary language of instruction in the child’s school. The CVLT-C and WISC-IV were translated by a native Afrikaans-speaking Master’s-level child psychologist with extensive experience working with the children in this cohort and back-translated by a second fluent Afrikaans speaker. The 10-year WISC-IV IQ scores for 150 of the children were strongly correlated with 5-year IQ on the Junior South African Individual Scale (Madge et al., 1981), which is normed for South African children, r=.71, p<.001. Except in the most severe cases, examiners were blind to FASD diagnosis and prenatal alcohol exposure history.
Human subjects approval was obtained from the Wayne State University and University of Cape Town Faculty of Health Sciences ethics committees. Informed consent was obtained from mothers at recruitment and at the child assessment visits; written assent was obtained from the child. Children received a small gift; mothers received compensation consistent with guidelines from the ethics committees and a photo of the child.
Statistical Analysis
Statistical analyses were performed using SPSS, version 22.0. AA/day and marijuana (days/month) were log transformed in both cohorts, as was cocaine use (days/month) in Detroit; none of the women reported using cocaine or methaqualone during pregnancy in Cape Town. Smoking data for seven outliers (>3 SD beyond the mean) in Detroit were recoded to 1 point beyond the next most extreme value (Winer, 1971), as were data for one smoking outlier in Cape Town and three outliers on recognition discrimination in each cohort.
Six variables were considered as potential confounders: socioeconomic status (SES; Hollingshead, 2011), maternal verbal competence (Peabody Picture Vocabulary Test-Revised; Dunn and Dunn, 1981), marijuana and smoking during pregnancy, and child sex and age at testing. Prenatal cocaine exposure was also included as a potential confounder in Detroit. All control variables even weakly related to each outcome (p<.10) were adjusted statistically using analysis of covariance (ANCOVA). Because prenatal exposure to methaqualone (“mandrax”; n=4) and cocaine (n=1) were too rare for statistical adjustment in the Cape Town sample, associations with prenatal alcohol use were rerun omitting these children.
Effects of heavy and moderate exposure were examined initially by comparing the diagnostic/exposure groups in each cohort. In Cape Town we compared three diagnostic groups (FAS/PFAS, nonsyndromal heavily exposed (HE), and controls). In Detroit we divided exposure into three levels (heavy: ≥1.0 oz AA/day; moderate: 0.5–0.99 oz AA/day; and low: 0.5 oz AA/day). Groups were compared using analysis of variance (ANOVA); for learning across trials, using repeated-measures ANOVA. Post-hoc analyses were calculated with least-significant difference tests. ANCOVAs were run to control for potential confounders. Effects of heavy and moderate exposure were also examined in multiple regression analyses using a continuous measure of exposure, AA/day.
Mediation by IQ was examined using hierarchical multiple regression analyses with AA/day entered at the first step; IQ, at the second. A third set of regressions tested whether fetal alcohol-related recall and recognition deficits were attributable to impaired initial learning; AA/day was entered at the first step, number of words acquired by learning trial 5 at the second. ANCOVAs compared learning strategies by diagnostic group (FAS/PFAS, HE, and controls) in Cape Town.
RESULTS
Sample characteristics
The Cape Town mothers were more economically disadvantaged, less educated, and smoked less than the Detroit mothers (Table 2). They drank more heavily and more frequently, but drinking on a daily basis was rare in both cohorts (Jacobson et al., 2013); most women concentrated their drinking on 1–2 days/week. Although there was a significant decrease across pregnancy in drinks/occasion and frequency among drinkers, these women continued to binge drink, with frequency of binges in later pregnancy averaging approximately 1 day/week in both cohorts (Table 3). Mothers in the Cape Town control group abstained from drinking during pregnancy, except for one who drank one drink on six occasions and one who drank two drinks on three occasions; in Detroit, 49 (16.8%) abstained from drinking. In Cape Town, child age ranged from 8.7–12.2 years; in Detroit, from 13.3–16.5. Although IQ scores were low in both cohorts, they were significantly lower in Cape Town, presumably due to poorer education and the large number of children with full FAS.
Table 2.
Sample Characteristics
| Cape Town (N=151) |
Detroit (N=291) |
t or χ² | |
|---|---|---|---|
| Maternal age at delivery | 26.1 (5.6) |
27.0 (6.1) |
1.51 |
| Socioeconomic statusa | 22.0 (9.1) |
29.7 (10.2) |
7.76* |
| Maternal education (years) | 9.3 (2.3) |
12.5 (1.9) |
14.85* |
| Prenatal alcohol exposureb | |||
| AA/day (oz) | 0.9 (0.8) |
0.3 (0.7) |
5.75* |
| AA/occasion (oz) | 3.9 (2.4) |
1.9 (2.3) |
6.87* |
| Frequency (days/week) | 1.5 (0.9) |
1.0 (1.1) |
3.63* |
| Prenatal marijuana (days/month)c | 2.5 (1.9) |
3.3 (3.8) |
0.80 |
| Prenatal smoking (cigarettes/day)d | 7.5 (5.6) |
14.8 (10.4) |
7.73* |
| Child’s age at testing (years) | 10.3 (0.9) |
14.4 (0.6) |
52.21* |
| Sex (% male) | 51.0 | 57.4 | 1.60 |
| WISC IQe | 73.4 (13.8) |
79.0 (13.0) |
4.25* |
Values are mean (SD) or %.
Hollingshead Four Factor Index of Social Status Scale (Hollingshead, 2011). Cape Town: mean=low end of Hollingshead Level IV of five levels, mainly semi-skilled workers; Detroit: mean=low end of Level III, mainly skilled workers, clerical, sales.
Consumers only (Cape Town: n=85; Detroit: n=181).
Users only (Cape Town: n=14; Detroit: n=86).
Smokers only (Cape Town: n=104; Detroit: n=181).
Wechsler Intelligence Scale for Children—Full Scale IQ based on WISC-IV in Cape Town and WISC-III in Detroit.
p<.001
Table 3.
Alcohol Consumption by Pregnancy Timepoint (Abstainers Excluded)
| Cape Town (N = 72)
|
Detroit (N = 233)
|
||||||
|---|---|---|---|---|---|---|---|
| Trimester
|
|||||||
| 1 | 2 | 3 | F | At conception | Across pregnancy | t | |
| AA per day (oz) | 1.2 (1.2) |
0.8 (0.8) |
0.4 (0.5) |
27.78*,a | 1.2 (2.3) |
0.3 (0.7) |
7.01* |
| AA per occasion (oz) | 3.9 (2.7) |
3.2 (2.6) |
2.2 (2.0) |
21.05*,a | 2.5 (3.2) |
1.9 (2.3) |
4.59* |
| Frequency (days/week) | 1.9 (1.4) |
1.4 (1.1) |
0.8 (0.9) |
38.75*,a | 2.8 (1.9) |
1.0 (1.1) |
16.52* |
Values are M (SD). AA=absolute alcohol.
p < .001
Post-hoc comparisons: Trimester 1 > Trimester 2 > Trimester 3; all ps < .001
Prenatal alcohol exposure and CVLT-C performance
The Cape Town diagnostic groups differed significantly in both immediate and total learning (Table 4 and Fig. 1). Learning increased across trials for the sample as a whole, F(4,592)=218.48, p<.001, with a main effect for FASD diagnosis. The FAS/PFAS group learned fewer words than the HE and control groups, ps=.05 and <.001, respectively, and the HE group learned fewer words than controls, p<.05. The trials×diagnostic group interaction was not significant, indicating that the groups did not differ in learning rates, F(8,592)=1.45, p>.15. On both short- and long-delay recall, the FAS/PFAS group recalled fewer words than the HE and control groups, ps<.05, with no differences between HE and controls. On recognition discrimination, the FAS/PFAS group performed more poorly than controls, p<.01, and the difference between the FAS/PFAS and HE groups fell just short of statistical significance, p=.06. Effects on total learning and short- and long-delay recall persisted after control for potential confounders, although effects on immediate learning and recognition discrimination did not. Effects on all CVLT-C outcome measures persisted after removal of the four children prenatally exposed to methaqualone and the one to cocaine.
Table 4.
CVLT-C Learning and Memory Outcome Variables by Diagnotic/Exposure Group
| Cape Town
|
Detroit
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FAS/PFAS (n = 36) |
HE (n = 55) |
Control (n = 60) |
F1 | F2 | Heavy (n = 19) |
Moderate (n = 21) |
Abstainer/Light (n = 251) |
F1 | F2 | |
| Immediate learning | 5.2 (2.2) |
5.6 (1.9) |
6.2 (1.7) |
3.67* | 2.24 | 6.8 (1.8) |
6.6 (1.9) |
7.1 (1.7) |
0.89 | 0.94 |
| Total learning | 39.0 (12.0) |
43.1 (9.9) |
46.9 (8.2) |
7.45*** | 3.10* | 46.8 (7.3) |
49.4 (8.9) |
50.4 (8.1) |
1.82 | 1.72 |
| Short-delay recall | 7.1 (3.1) |
9.2 (2.9) |
9.7 (2.6) |
9.31*** | 4.91** | 9.0 (1.9) |
10.3 (2.3) |
10.5 (2.3) |
4.05* | 3.90* |
| Long-delay recall | 7.4 (3.1) |
9.7 (2.6) |
9.6 (2.6) |
9.22*** | 6.27** | 9.4 (1.8) |
10.7 (2.1) |
10.9 (2.1) |
4.87** | 3.34* |
| Recognition discrimination | 88.1 (10.2) |
91.5 (8.1) |
93.5 (7.3) |
4.79** | 0.94 | 93.4 (5.7) |
94.6 (6.0) |
96.4 (4.1) |
5.65** | 5.17** |
Values are mean (SD).
CVLT-C = California Verbal Learning Test—Children’s Version; FAS = fetal alcohol syndrome; PFAS = partial FAS; HE = heavily exposed nonsyndromal.
F1 = Unadjusted between-group comparison.
F2 = Between-group comparison controlling for confounders. Cape Town: Immediate and Total learning, Short and Long delay, Recognition discrimination–SES; Long delay—child sex; Recognition discrimination–smoking during pregnancy. Detroit: Immediate and Total learning, Short delay, Recognition discrimination–SES; Total learning, Short and Long delay—maternal PPVT; Long delay—child sex and maternal smoking during pregnancy.
p < .10,
p < .05,
p ≤ .01,
p≤.001
Figure 1.
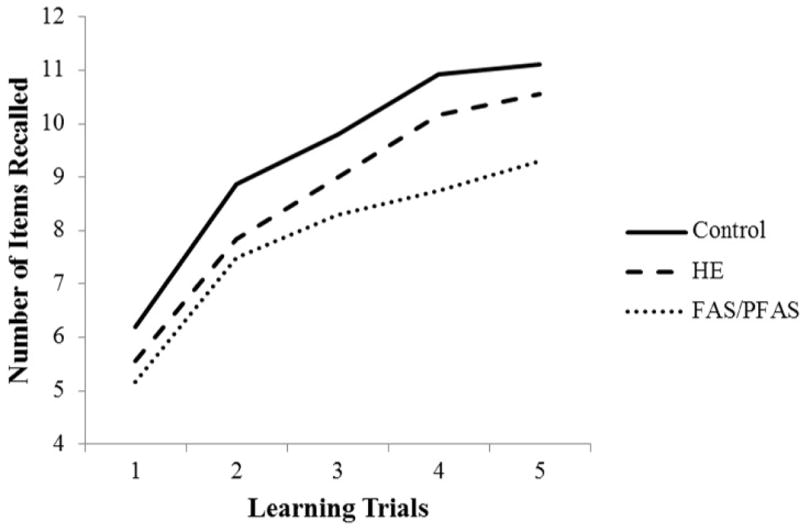
Mean recall for prenatal alcohol exposure groups across CVLT-C learning trials in Cape Town cohort
In Detroit, there was also a significant increase in learning across trials for the sample as a whole, F(4,1152)=142.44, p<.001 (Fig. 2) but no significant between-group differences for immediate or total learning (Table 4). By contrast, there was a significant group difference in short-delay recall, with the heavily exposed children recalling fewer words than the abstain/light exposure group, p<.01, and in long-delay recall, with the heavily exposed recalling fewer words than the moderate and abstain/light groups, ps<.05 and .01, respectively. The heavily exposed group also performed more poorly on recognition discrimination than the abstain/light group, p=.004, and the difference between the moderate and abstain/light groups fell short of significance, p=.06. The alcohol-related short- and long-delay and recognition discrimination deficits persisted after control for confounders.
Figure 2.
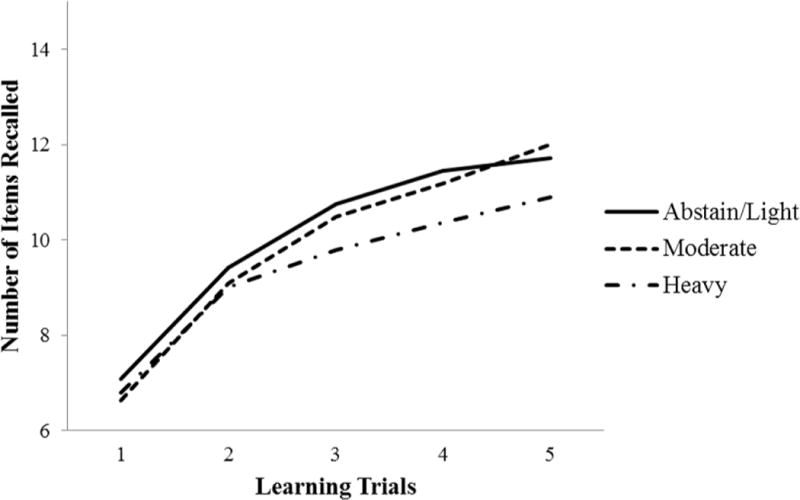
Mean recall for prenatal alcohol exposure groups across CVLT-C learning trials in Detroit cohort
The continuous measure of prenatal alcohol exposure was significantly related to all CVLT outcomes in the Cape Town cohort (Table 5). In Detroit, AA/day was not related to immediate learning but was significantly related to total learning, long-delay recall, and recognition discrimination accuracy, with an effect on short-delay just short of significance.
Table 5.
Relation of prenatal alcohol exposure to verbal learning and memory measures
| Cape Town
|
Detroit
|
|||||
|---|---|---|---|---|---|---|
| r | ß1 | ß2 | r | ß1 | ß2 | |
| Immediate learning | −.18* | −.14† | –a | −.06 | −.03 | –a |
| Total learning | −.26** | −.20** | –a | −.12* | −.09 | –a |
| Short-delay recall | −.22** | −.17* | −.04 | −.11† | −.08 | −.05 |
| Long-delay recall | −.18* | −.13† | −.01 | −.15* | −.13* | −.09† |
| Recognition discrimination | −.23** | −.17** | −.11 | −.18** | −.15** | −.14* |
p < .10,
p < .05,
p ≤ .01
r shows the relation between continuous measure of prenatal alcohol exposure (oz AA/day) and the CVLT-C outcomes.
ß1 shows effect of prenatal alcohol exposure after controlling for WISC IQ.
ß2 shows effect of prenatal alcohol exposure after controlling for total learning, the measure used to assess word acquisition at the end of the encoding period.
N/A, given that ß2 assesses effects of prenatal alcohol exposure on recall and recognition after adjustment for learning.
In regression analyses testing for mediation by IQ in Cape Town, effects on total learning, short-delay recall, and recognition discrimination remained significant after adjustment for IQ and fell short of significance on long-delay recall (Table 5). In Detroit, effects of prenatal alcohol on long-delay recall and recognition discrimination remained significant after adjustment for IQ.
In the Cape Town cohort, effects of prenatal alcohol exposure on short- and long-delay recall and recognition discrimination were no longer significant after adjusting for number of words learned by trial 5 (Table 5). By contrast, in the moderately-exposed Detroit cohort, the effect on recognition discrimination continued to be significant after adjustment for trial 5 and the effect on long-delay recall fell just short of significance.
Learning Strategies
The clearest between-group differences in learning strategies were found on semantic clustering, a measure that was available only for the Cape Town cohort (Table 6). The FAS/PFAS group used semantic clustering less frequently than the controls across all of the learning trials, ps<.05. On short-delay recall, the FAS/PFAS group employed this strategy less frequently than the HE and control groups, ps<.05, and the HE group used it less than controls, p<.05. The FAS/PFAS group also used semantic clustering less frequently on long-delay recall than the HE and control groups, ps<.05. There were no group differences in the use of serial clustering on any of the learning and memory trials. There was some evidence of increased intrusion errors in the short- and long-delay recall trials for the FAS/PFAS children. AA/day was related to increased false-positive errors on the recognition discrimination trials in both Cape Town (r=.20, p=.015) and Detroit (r=.17, p=.004).
Table 6.
CVLT-C Error Scores and Learning Strategies by Group
| Cape Town
|
||||
|---|---|---|---|---|
| FAS/PFAS (n = 36) |
HE (n = 55) |
Control (n = 60) |
F | |
| Total learning | ||||
| Perseveration errors | 6.8 (5.9) |
5.2 (4.0) |
7.2 (5.6) |
2.43† |
| Intrusion errors | 4.5 (4.3) |
3.2 (3.5) |
3.6 (3.8) |
1.25 |
| Semantic clusters | 11.7 (6.6) |
13.0 (4.9) |
14.5 (5.3) |
3.14* |
| Serial clusters | 3.9 | 4.9 (3.5) |
4.6 (4.1) |
1.01 (2.7) |
| Short-delay recall | ||||
| Perseveration errors | 1.6 (1.9) |
0.9 (1.1) |
1.2 (1.4) |
2.66† |
| Intrusion errors | 1.3 (1.2) |
0.7 (0.9) |
0.8 (1.2) |
2.91† |
| Semantic clusters | 2.5 (2.1) |
3.4 (2.0) |
4.1 (1.9) |
7.55** |
| Serial clusters | 0.6 (0.8) |
1.0 (1.4) |
0.7 (1.1) |
1.28 |
| Long-delay recall | ||||
| Perseveration errors | 0.8 (1.3) |
0.9 (1.3) |
1.1 (1.0) |
0.56 |
| Intrusion errors | 1.4 (1.5) |
0.8 (1.0) |
1.1 (1.1) |
3.37* |
| Semantic clusters | 2.7 (1.7) |
3.5 (1.9) |
3.9 (1.9) |
4.83** |
| Serial clusters | 0.8 (1.0) |
1.1 (1.6) |
0.8 (1.1) |
0.79 |
Values are mean (SD).
CVLT-C = California Verbal Learning Test—Children’s Version; FAS = fetal alcohol syndrome; PFAS = partial FAS; HE = heavily exposed nonsyndromal.
p < .05;
p ≤ .01;
p≤.001;
p < .10
DISCUSSION
Our data confirm previous reports of adverse effects of heavy prenatal alcohol exposure on verbal learning and memory assessed using the CVLT-C and extend these findings to moderately exposed children. We observed these effects using both categorical (diagnostic/exposure group) and continuous (maternal report) measures of exposure. Consistent with previous studies, prenatal alcohol exposure was associated with impairment in number of words learned across acquisition trials (Mattson and Roebuck, 2002; Vaurio et al., 2011), recalled after a delay (Coles et al., 2010; Mattson et al., 1998), and recognition memory (Crocker et al., 2011; Willoughby et al., 2008). We observed these effects in two different prospectively recruited cohorts: heavily-exposed children in Cape Town and moderately-exposed adolescents in Detroit. Significantly, this is the first study to report learning and memory impairment on the CVLT-C in South Africa and in a language other than English.
By contrast to alcohol, prenatal exposure to marijuana in both cohorts was not related to any of the outcomes (all ps>.20). Similarly, none of the outcomes were related to cocaine exposure in Detroit, where there was a sufficient number of children exposed to cocaine to assess its impact.
Because verbal learning is highly correlated with IQ, most previous studies used indirect approaches (described in the Introduction) instead of simple adjustment for IQ to assess whether the observed learning and memory deficits are attributable to alcohol-related impairment in general intellectual function. Using a standard cohort study design, we saw a specific effect of prenatal alcohol exposure on encoding after control for IQ in Cape Town. This finding extends the work of Coles et al. (2010), who found a specific effect of prenatal alcohol exposure on total recall after control for IQ when comparing FASD dysmorphic young adults with controls. We did not see an effect of prenatal alcohol on encoding after adjusting for IQ in Detroit. However, alcohol effects on short-delay recall in Cape Town and on long-delay recall in Detroit remained significant after controlling for IQ, as did the effect on recognition memory in both cohorts, indicating specific effects on recall and recognition memory. The similarities in the results seen in Cape Town and Detroit suggest that these findings are likely to be generalizable to other populations.
Several studies have concluded that the essential alcohol effect on verbal learning involves impaired information acquisition (i.e., encoding) rather than recall (i.e., retrieval). In the heavily exposed Cape Town cohort, prenatal alcohol effects on short- and long-delay recall and recognition discrimination were no longer significant after statistical adjustment for trial 5 performance. This finding of impaired encoding but spared retention is consistent with previous studies of children with heavy prenatal exposure. By contrast, data from the moderately exposed Detroit cohort, where encoding was largely unaffected, revealed an adverse effect of prenatal alcohol exposure on retention. Moreover, the effect on recognition discrimination was significant and the effect on long-delay recall just short of significant after control for trial 5 performance, suggesting a specific retention impairment in the less heavily exposed Detroit cohort.
In Detroit, only the heavily exposed group performed more poorly on short- and long-delay recall, but the threshold for the effect on recognition discrimination was somewhat lower, with the moderate group also performing more poorly than controls. It should be noted that even the moderate group concentrated their drinking on weekends, resulting in a binge drinking pattern. These findings are consistent with Willford et al.’s (2004) observation that, although effects on learning and memory were seen in children born to light-moderate drinkers in their Pittsburgh cohort, “even low to moderate drinkers engage[d] in binge drinking, a pattern of drinking associated with adverse outcomes in both the animal and human literature (p. 504).”
The pattern of impaired acquisition with spared retention in verbal learning and memory seen in several studies using the CVLT-C is not seen as consistently in studies using other verbal memory measures (such as, the WRAML) that do not incorporate a semantic clustering strategy. Our finding that, despite the availability of an implicit learning strategy in the CVLT-C, the alcohol-exposed children in Cape Town showed less frequent use of semantic clustering provides additional support for the hypothesis by Mattson and Roebuck (2002) that an inefficient learning strategy contributes to the verbal memory deficits in FASD (Kerns at al., 1997; Mattson et al., 1992; Roebuck-Spencer and Mattson, 2004). Moreover, our observation of a specific deficit in recognition memory suggests that impairment in executive functioning (viz., inefficient retrieval strategies) may contribute to poorer declarative memory in children with FASD (Manji et al., 2009).
A recent developmental study found that children with FASD displayed considerable difficulty with verbal learning and memory tasks that relied on intact working memory (Pei et al., 2008) and used strategies, such as verbal rehearsal, less consistently than controls (Rasmussen et al., 2009). These data suggest that learning and memory tasks like the CVLT-C that rely on the phonological loop, the aspect of working memory that underlies verbal rehearsal, may be particularly impaired in FASD. This interpretation is supported by an fMRI study showing that, relative to typically-developing controls, alcohol-exposed individuals showed decreased medial temporal lobe and increased left dorsolateral prefrontal activation during a verbal paired-associates test (Sowell et al., 2007), suggesting increased demands on frontal executive memory systems because medial temporally-mediated encoding is compromised. We found evidence of less efficient functioning of the phonological loop in an fMRI study of working memory in our Cape Town cohort. Whereas controls primarily activated a portion of Broca’s area known to mediate verbal rehearsal, the HE group showed extensive fronto-striatal activation and the FAS/PFAS group, extensive cerebellar and parietal activations (Diwadkar et al., 2013). In addition, Coles et al. (2011) have reported that reduced hippocampal volume partially mediates effects of prenatal alcohol exposure on recall memory performance.
Limitations
Although the CVLT-C was not normed for South African children and was administered to some of the children in Afrikaans, the similarity of the effects to those seen in the U.S. studies provides evidence for its validity in a cross-cultural context. Other limitations include between-cohort differences in the ages at which the CVLT-C, IQ, and dysmorphology examinations were administered and the version of the WISC used. Nevertheless, effects on CVLT-C performance have been documented in previous studies of children ranging from 5–16 years, the WISC-IQ test is standardized for age, and the dysmorphology examinatons were administered in early childhood in both studies. Wechsler et al. (2004) reported a correlation between WISC-III and WISC-IV of r=.87.
Conclusions
Our data confirm previous reports demonstrating adverse effects of prenatal alcohol exposure on verbal learning and memory on the CVLT-C. Although learning and memory are important components of general intellectual function, we found specific effects on encoding and short-delay recall in Cape Town, on long-delay recall in Detroit, and on recognition in both cohorts over and above IQ. In Cape Town, as in several prior studies of heavily exposed children, effects on memory retrieval were no longer significant after adjustment for initial learning. However, in the more moderately exposed sample in Detroit, where initial learning was relatively unaffected, we found a significant effect on recognition even after controlling for trial 5 performance. Thus, our data indicate that the fetal alcohol-related deficit in retrieval is not secondary to a failure to encode the initial information since a specific alcohol-related deficit in retrieval was seen in a moderately exposed cohort in which adolescents successfully encoded the information. These data also provide evidence that the fetal alcohol-related impairment in initial learning on the CVLT-C is mediated, in part, by failure to use a semantic cluster learning strategy, particularly by children with FAS and PFAS.
Acknowledgments
We thank Robert J. Sokol, who collaborated on the Detroit longitudinal research; Denis L. Viljoen, who collaborated on the Cape Town study; our University of Cape Town research staff, Maggie September, Anna Susan Marais, Julie Croxford, Mariska Pienaar and Nadine Lindinger, and our Wayne State University research staff Lisa Chiodo and Renee Sun, for their contributions to subject recruitment and data collection. We also thank H. Eugene Hoyme, M.D., Luther K. Robinson, M.D., and Nathaniel Khaole, M.D., who conducted the Cape Town dysmorphology examinations in conjunction with the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorders. We thank Sterling Clarren, M.D., and Kenneth L. Jones, M.D., for their input on the dysmorphology diagnoses of the Detroit cohort, and Erawati Bawle, M.D. Director of the Genetics Department, Children’s Hospital of Michigan, for her examination of the children. We express our appreciation to the mothers and children who participated in the longitudinal research in both Cape Town and Detroit. Funding: NIAAA R01 AA06966, R01 AA09524, R01 AA016781, U01 AA014790, U24 AA014815; University of Cape Town; and Joseph Young, Sr., Fund from the State of Michigan. Portions of this research were presented at the 2011 and 2012 meetings of the Research Society on Alcoholism.
Footnotes
The authors declare no competing financial interests.
References
- ACOG. Committee opinion no. 496: At-risk drinking and alcohol dependence: obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383–8. doi: 10.1097/AOG.0b013e31822c9906. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: Correlations with brain dysfunction. Alcohol. 2001;36:147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test–II: Findings from patients with focal frontal lesions. J Int Neuropsychol Soc. 2002;8:539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK, O’Connor M, Riley EP, Johnson KA, Cohen DE, the National Task Force on FAS/FAE . Fetal alcohol syndrome: Guidelines for referral and diagnosis. Atlanta, GA: Center for Disease Control and Prevention; 2004. [Google Scholar]
- Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Can Med Assoc J. 2005;172(5 Suppl):S1–S21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ. Children’s Memory Scale Manual. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcohol Clin Exp Res. 2010;34:897–906. doi: 10.1111/j.1530-0277.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain Cogn. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2011;35:1–8. doi: 10.1111/j.1530-0277.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test-Children’s Version. Psychological Corporation; New York: 1994. [Google Scholar]
- Delis DC, Kaplan E, Kramer J, Ober B. California Verbal Learning Test–II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, Jacobson SW, Jacobson JL. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum Brain Mapp. 2013;34:1931–1945. doi: 10.1002/hbm.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test Manual for Forms L and M. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders : Clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. 1993;64:1706–1721. [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Jacobson JL, Sokol RJ. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Jacobson JL. Invited commentary on Day and colleagues (2013): The association between prenatal alcohol exposure and behavior at 22 years of age—Adverse effects of risky patterns of drinking among low to moderate alcohol-using pregnant women. Alcohol Clin Exp Res. 2013;37:1069–1073. doi: 10.1111/acer.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Don A, Mateer CA, Streissguth AP. Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J Learn Disabil. 1997;30:685–696. doi: 10.1177/002221949703000612. [DOI] [PubMed] [Google Scholar]
- Madge EM, van den Berg AR, Robinson M, Landman J. Junior South African Individual Scales. Human Sciences Research Council; Pretoria, South Africa: 1981. [Google Scholar]
- Manji S, Pei J, Loomes C, Rasmussen C. A review of the verbal and visual memory impairments in children with foetal alcohol spectrum disorders. Dev Neurorehabil. 2009;12:239–247. doi: 10.1080/17518420902980118. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Ehlers CL, Delis DC, Jones KL, Stem C, Johnson KA, Hesselink JR, Bellugi U. Fetal alcohol syndrome: A case report of neuropsychological, MRI, and EEG assessment of two children. Alcohol Clin Exp Res. 1992;16:1001–1003. doi: 10.1111/j.1530-0277.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychol. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabl Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole N, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JR, Rinaldi CM, Rasmussen C, Massey V, Massey D. Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2008;15:44–56. [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29:1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Pei J, Manji S, Loomes C, Andrew G. Memory strategy development in children with foetal alcohol spectrum disorders. Dev Neurorehabil. 2009;12:207–214. doi: 10.1080/17518420902980126. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford JA, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Roebuck-Spencer TM, Mattson SN. Implicit strategy affects learning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:1424–1431. doi: 10.1097/01.alc.0000139826.25247.5b. [DOI] [PubMed] [Google Scholar]
- SAMHSA. The NSDUH Report: 18 percent of pregnant women drink alcohol during early pregnancy. NSDUH Report 2013 [Google Scholar]
- Sheslow D, Adams W. Manual for the Wide Range Assessment of Memory and Learning. Jastak Associates; Wilmington, DE: 1990. [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport. 2007;18:635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wixted J, Baldo JV, Delis DC. New semantic and serial clustering indices for the California Verbal Learning Test–Second Edition: Background, rationale, and formulae. J Int Neuropsychol Soc. 2002;8:425–435. doi: 10.1017/s1355617702813224. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Neuropsychological comparison of children with heavy prenatal alcohol exposure and an IQ-matched comparison group. J Int Neuropsychol Soc. 2011;17:463–473. doi: 10.1017/S1355617711000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WISC-IV Administration Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wechsler D, Kaplan E, Fein D, Kramer J, Morris R, Delis D, Maerlender A. WISC-IV Intelligence Scale for Children Fourth Edition–Integration: Technical and Interpretive Manual. San Antonio, TX: The Psychological Corporation; 2004. [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 4. McGraw-Hill; New York: 1971. [Google Scholar]


