Abstract
Purpose/Objectives
IMRT has improved the toxicity profile of chemo-radiotherapy for head and neck cancer. Long-term patient-reported outcomes beyond two years, however, remain scarcely reported. Amidst concerns of delayed-onset dysphagia and other toxicities, we evaluated long-term health-related quality-of-life (HRQOL) in two prospective studies of chemo-IMRT for oropharyngeal cancer (OPC).
Methods and Materials
69 of 93 patients with stage III/IV OPC treated on prospective studies of swallowing and salivary organ-sparing chemo-IMRT were eligible for long-term HRQOL assessment. Three validated patient-reported instruments, the Head and Neck QOL [HNQOL], University of Washington [UW]QOL, and Xerostomia Questionnaire [XQ]), previously administered from baseline through two-years in the parent studies, were re-administered at long-term follow-up along with the Short-Form 36. Long-term changes in HRQOL from pre-treatment and two-years were evaluated.
Results
40 patients (58%) with a median follow-up of 6.5 years participated, 39 of whom (97.5%) had confirmed HPV+ OPC. Long-term, no clinically significant worsening was detected in mean HRQOL scores compared with two-years, with stable or improved HRQOL from pre-treatment in nearly all domains. “Moderate” or greater severity problems were uncommon, reported by 5% of patients for eating, 5% for swallowing, and 2.5% and 5% by HNQOL and UWQOL summary scores, respectively. Freedom-from-PEG-tube dependence and stricture dilation beyond 2 years was 97.5% and 95%, respectively. 11% and 14% of patients reported “moderate” or “severe” long-term worsening in HNQOL Pain and Overall Bother domains, respectively, which were associated with mean dose to the cervical esophagus, larynx, and pharyngeal constrictors.
Conclusions
At more than 6-year median follow-up, OPC patients treated with swallowing and salivary organ-sparing chemo-IMRT reported stable or improved HRQOL in nearly all domains compared to both pre-treatment and two-year follow-up. New late toxicity after two years was uncommon. Further emphasis on sparing the swallowing organs may yield additional HRQOL gains for long-term OPC survivors.
Introduction
Concomitant chemoradiotherapy (CRT) is considered standard-of-care treatment for organ-preservation in patients with locally advanced head and neck cancer (HNC). Traditionally, CRT using conventional radiotherapy was associated with significant morbidity. Xerostomia and dysphagia in particular have been identified as the primary determinants of health-related quality-of-life (HRQOL) in long term survivors of HNC (1, 2). Advances in radiotherapy planning with intensity-modulated radiation therapy (IMRT) in recent years have enabled selective sparing of structures critical for salivary production and swallowing (3, 4), which have translated into improvements in xerostomia, dysphagia, and global HRQOL (5–8).
We previously reported two-year results of a prospective study of IMRT designed to spare the swallowing structures in patients with locally advanced oropharyngeal cancer (OPC) receiving CRT, which demonstrated very low rates of patient- and observer-rated dysphagia and PEG-tube dependence while maintaining high rates of locoregional control and overall survival (8). While the two-year outcomes are encouraging, long-term HRQOL after IMRT remains scarcely reported, and concerns remain regarding radiotherapy-related complications that may manifest several years after CRT and thereby compromise HRQOL in long-term survivors (9, 10). Herein, we report long-term HRQOL outcomes in patients with locally advanced OPC treated with definitive CRT using IMRT specifically aimed to spare the salivary and swallowing structures.
Materials and Methods
Patients and Treatment
Between May 2003 and January 2011, 93 patients were enrolled on two consecutive prospective Institutional Review Board-approved studies of swallowing and salivary organ-sparing chemo-IMRT for locally advanced OPC. Patient eligibility and treatment in both protocols were as previously detailed (8, 11). Patients with newly diagnosed, non-metastatic stage III or IV OPC without posterior pharyngeal wall involvement or distant metastases were eligible for the original parent studies. Patients who remained alive and HNC-free >2 years after CRT were eligible for the present study.
All patients received IMRT with prescription doses of 70 Gy to the primary and nodal gross tumor volumes (GTV), and 56–63 Gy to medium and low-risk clinical target volumes (CTVs), delivered over 35 daily fractions with concurrent weekly carboplatin (AUC=1) and paclitaxel (30 mg/m2). GTVs and CTVs were uniformly expanded 3–5 mm to create planning target volumes. All patients received bilateral neck radiotherapy. IMRT treatment planning was intended to minimize dose to the parotid glands, oral cavity, glottic and supraglottic larynx (GSL), esophagus, and the pharyngeal constrictor muscles (PCMs), with organ-at-risk (OAR) contours, dosimetric goals, and optimization algorithms as previously detailed (3, 5).. Patients with clinical suspicion for residual neck disease on PET-CT after CRT underwent adjuvant neck dissection.
Human papillomavirus (HPV) and p16 expression testing was performed on primary tumor specimens, as previously described (12).
Quality-of-life assessment
HRQOL was assessed by three validated instruments: the HNQOL questionnaire (13), University of Washington QOL (UWQOL) questionnaire (14), and Xerostomia Questionnaire (XQ) (4). During the initial study period, patients completed each questionnaire pre-treatment and at 1, 3, 6, 12, 18, and 24 months post-CRT. Invitations to participate in the present long-term assessment study were mailed in September 2013 to all patients who were alive and without cancer recurrence, and consisted of informed consent, the previously administered HRQOL instruments, and the Short Form-36 (SF-36) questionnaire (15). Patients also provided information regarding their current health habits, hospitalizations, procedures since CRT, which were verified within the medical record. HNQOL, UWQOL, and XQ instrument responses were normalized on a linear 100-point scale, with 0 representing no toxicity and 100 representing maximum toxicity. Scores of 0, 25, 50, 75, and 100 approximated responses of “none”, “mild/slight”, “moderate”, “severe”, and “extreme”, respectively. A difference of ≥10 points on the 0–100 point scale was considered clinically meaningful (16). SF-36 instrument responses were normalized with 0 representing maximum and 100 representing no disability; SF-36 domain scores for the study cohort were compared with previously published United States population mean scores (15).
Statistical analysis
Pre-treatment characteristics and 2-year HRQOL instrument domain and summary scores were compared between study participants and non-participants using independent samples T-test, Chi-squared test, and Fisher’s exact test. Paired T-tests were used to compare scores between time-points. Logistic regression analysis was performed to identify factors associated with clinically meaningful late declines in HRQOL (i.e. ≥10-point increase in domain score from 2-years to long-term assessment). All patient, disease, and treatment variables were tested for univariable association with the outcome of interest. Spearman’s rank correlation (ρ) tested correlations between patient, disease, and treatment characteristics. Multivariable analysis was not performed due to limited number of events and presence of multicollinearities between treatment-related variables (17). For all statistical comparisons, two-sided p-values ≤0.05 were considered significant. Analyses were performed using MedCalc (v13.1.2.0, MedCalc Software, Mariakerke, Belgium).
Results
Patient characteristics
Sixty-nine patients who were alive and without recurrence/second primary cancer met eligibility for the present study. Forty (58%) responded to the mailed questionnaires. Of the non-respondents, 12 were unable to be reached and 17 elected to not participate. A comparison of T-and N-stages of patients in the parent studies, those eligible for the present study, and those who participated in the present study is provided in Table S1. Possible attrition bias in long-term respondents was assessed by comparing 2-year HRQOL and baseline characteristics between long-term survey respondents and non-respondents, and revealed no differences in HRQOL domain and summary scores (p>0.5 for all comparisons) or tumor characteristics, including T-classification, N-classification, or oropharyngeal subsite (p>0.20). Non-respondents were younger (mean age 54 vs. 57 years old, p=0.04), and were more commonly smokers at diagnosis (38% vs 13%, p=0.02) and treated with adjuvant neck dissection after chemoradiation (41% vs. 18%, p=0.055). Baseline patient, tumor, and treatment characteristics of participants in the present study did not differ (p>0.10) from the 93 patients enrolled in the parent studies, apart from less patients with T4 tumors in the present long-term study(p=0.07), consistent with higher rates of cancer recurrence in these patients (Table S1). All 40 long-term respondents had pre-therapy HRQOL data, and 37/40 (93%) had 2-year HRQOL data.
Patient characteristics for the 40 respondents are described in Table 1. All had stage III-IV OPC and nearly all tumors (97.5%) were HPV-positive. Median follow-up from completion of chemoradiation was 78 months (range 31–123 months; interquartile range 59–100 months), with 98% having follow-up of more than 3 years and 75% more than 5 years.
Table 1.
Patient Characteristics
| Characteristic | Result |
|---|---|
| N | |
| Age at time of diagnosis (median [range]) | 58 (44–76) |
| Age at time of present study (median [range]) | 63 (52–80) |
| Male (N[%]) | 34 (85) |
| Tumor site (N[%]) | |
| Tonsil/ | 22 (55) |
| Base of Tongue | 18 (45) |
| T-classification (N[%]) | |
| T1 | 8 (20) |
| T2 | 20 (50) |
| T3 | 8 (20) |
| T4 | 4 (10) |
| N-classification (N[%]) | |
| N0 | 1 (3) |
| N1 | 6 (15) |
| N2a | 2 (5) |
| N2b | 19 (48) |
| N2c | 8 (20) |
| N3 | 4 (10) |
| AJCC stage (N[%]) | |
| III | 6 (15) |
| IV | 34 (85) |
| Smoking status at time of diagnosis (N[%]) | |
| Current | 5 (13) |
| Former | 18 (45) |
| Never | 17 (43) |
| Smoking pack-years (among smokers) (median [range]) | 21 (1–60) |
| HPV status (N[%]) | |
| Positive | 39 (98) |
| Negative | 0 (0) |
| N/A | 1 (3) |
| Neck Dissection after Chemoradiation (N[%]) | 7 (18) |
| Time to Neck Dissection from Completion of CRT (months) (median [range]) | 3.2 (1.6–6.5) |
| Time since completion of CRT (months) (median [range]) | 78 (31 – 123) |
Long-Term Global Head and Neck-Related QOL Assessment
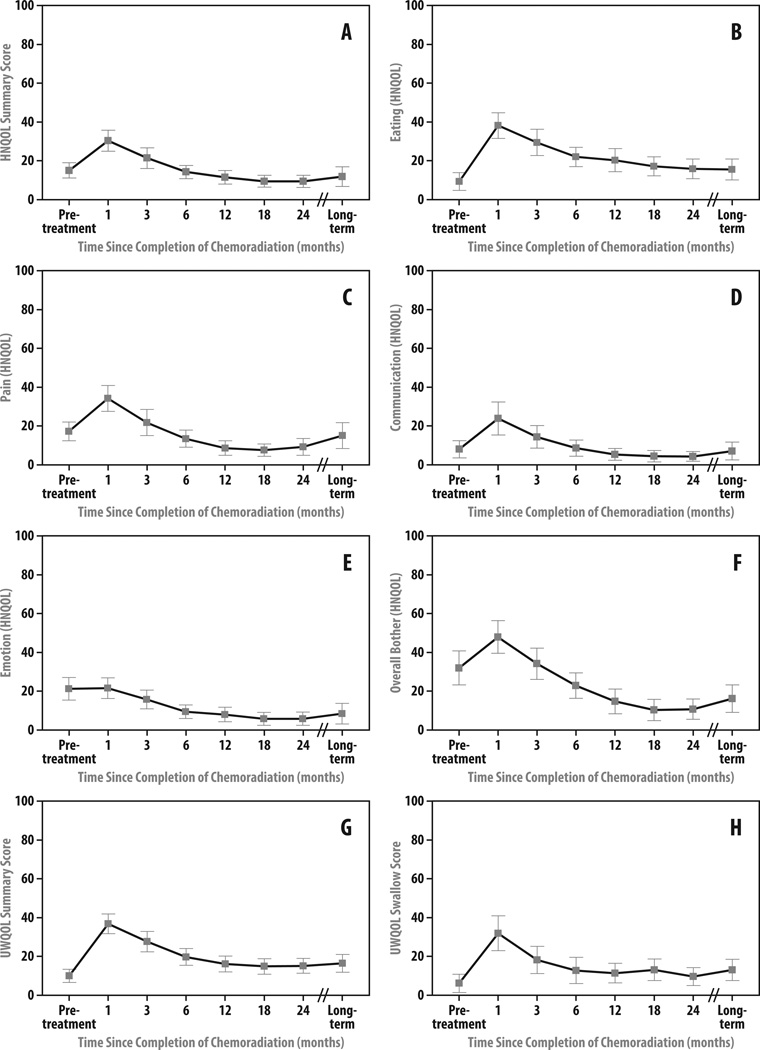
At long-term follow-up, global HN-related QOL remained stable compared to the 2-year assessments as measured by both HNQOL and UWQOL summary scores (Δmean score=+1.0, p=0.52 and Δ=+0.5, p=0.72, respectively) (Table 2; Figures 1A and 1G). Clinically meaningful declines (≥10 point change) in global HRQOL from 2-year assessment were reported by 8% and 14% of patients by HNQOL and UWQOL summary scores, respectively, with similar proportions reporting meaningful improvements in global HN-related QOL (8% and 11% by each instrument, respectively) (Table 3). 84% and 75% of patients reported stable global HRQOL at long-term compared with 2-years by each instrument, respectively.
Table 2.
Patient-reported QOL Following Organ Sparing Chemo-IMRT
| Measure |
Pre-Treatment1 Mean (95% CI) |
24-month1 Mean (95% CI) |
Long-term1 Mean (95% CI) |
Δ long-term vs. 24 months2 Mean (95% CI) p |
Δ long-term vs. pre-treatment2 Mean (95% CI) p |
|---|---|---|---|---|---|
| HNQOL Instrument | |||||
| Summary Score | 15.1 (11.2 to 19.0) |
9.5 (6.3 to12.6) |
11.9 (6.9 to 16.9) |
+1.0 (−2.2 to 4.2) p=0.52 |
−3.2 (−8.1 to 1.7) p=0.20 |
| Eating | 9.4 (4.8 to 13.9) |
15.8 (10.7 to 20.9) |
15.5 (10.1 to 20.9) |
−1.4 (−5.3 to 2.4) p=0.44 |
+6.2 (0.1 to 12.3) p=0.05 |
| Pain | 17.3 (12.5 to 22.1) |
9.3 (5.0 to 13.6) |
15.2 (8.5 to 21.8) |
+5.1 (−0.9 to 11.1) p=0.09 |
−2.1 (−8.6 to 4.3) p=0.51 |
| Communication | 8.3 (3.9 to 12.7) |
4.6 (2.1 to 7.1) |
7.3 (2.8 to 11.9)) |
+1.3 (−2.4 to 5.0) p=0.48 |
−0.9 (−6.7 to 4.9) p=0.75 |
| Emotion | 21.3 (15.5 to 27.1) |
5.9 (2.4 to 9.3) |
8.5 (3.2 to 13.8) |
+1.2 (−2.4 to 4.9) p=0.49 |
−12.8 (−18.3 to −7.2) p<0.001 |
| Overall Bother | 32.1 (23.3 to 40.8) |
10.8 (5.6 to 16.0) |
16.3 (9.1 to 23.4) |
+4.2 (−0.7 to 9.0) p=0.09 |
−15.4 (−24.0 to −6.7) p<0.001 |
| UWQOL Instrument | |||||
| Summary Score | 10.0 (6.6 to 13.4) |
15.2 (11.3 to 19.0) |
16.5 (11.9 to 21.1) |
+0.5 (−2.4 to 3.4) p=0.72 |
+6.5 (1.6 to 11.4) p=0.01 |
| Swallowing | 6.3 (1.5 to 11.0) |
9.7 (5.1 to 14.4) |
13.1 (7.7 to 18.6) |
+2.1 (−2.1 to 6.3) p=0.49 |
+6.9 (−0.1 to 13.9) p=0.05 |
| XQ Summary Score | 6.4 (2.2 to 10.6) |
29.0 (20.4 to 37.7) |
29.9 (21.2 to 38.5) |
−0.8 (−5.7 to 4.1) p=0.74 |
+23.4 (14.8 to 32.1) p<0.001 |
includes all patients with responses at study time-point
includes only patients with responses at both time-points
Figure 1.
Head and Neck Quality of Life (HNQOL) Instrument (a) Summary and (b–f) Domain Mean Scores and University of Washington Quality of Life (UWQOL) Instrument (g) Summary and (h) Swallowing Mean Scores after Organ-Sparing Chemo-IMRT. Bars represent 95% confidence intervals. All scores are represented on a linear 100-point scale, with 0 representing no toxicity and 100 representing maximum toxicity.
Table 3.
Patients Experiencing Clinically Meaningful Changes1 in Long-Term Quality of Life
| Measure | Long Term vs. 24 months | Long Term vs. Pre-Treatment | ||||
|---|---|---|---|---|---|---|
| Worsening | No Change | Improvement | Worsening | No Change | Improvement | |
| HNQOL Instrument | ||||||
| Summary Score | 8% (3/37) | 84% (31/37) | 8% (3/37) | 8% (3/40) | 65% (26/40) | 28% (11/40) |
| Eating | 14% (5/36) | 61% (22/36) | 25% (9/36) | 30% (12/40) | 58% (23/40) | 13% (5/40) |
| Pain | 31% (11/36) | 61% (22/36) | 8% (3/36) | 23% (9/40) | 40% (16/40) | 38% (15/40) |
| Communication | 14% (5/36) | 75% (27/36) | 11% (4/36) | 15% (6/40) | 68% (27/40) | 18% (7/40) |
| Emotion | 8% (3/37) | 86% (32/37) | 2% (2/37) | 5% (2/40) | 48% (19/40) | 48% (19/40) |
| Overall Bother | 26% (9/35) | 66% (23/35) | 9% (3/35) | 10% (4/39) | 41% (16/39) | 49% (19/39) |
| UWQOL Summary Score | 14% (5/36) | 75% (27/36) | 11% (4/36) | 30% (12/40) | 65% (26/40) | 5% (2/40) |
| UWQOL Swallowing | 14% (5/36) | 83% (30/36) | 3% (1/36) | 35% (14/40) | 53% (21/40) | 13% (5/40) |
| XQ Summary Score | 14% (5/37) | 68% (25/37) | 19% (7/37) | 63% (25/40) | 35% (14/40) | 3% (1/40) |
Change defined as clinically ≥10 point change on 100 point normalized scale
Compared with pre-treatment, mean long-term global HRQOL for the overall cohort remained stable by HNQOL summary score (Δ= −3.2, p=0.20) and was statistically, but not clinically meaningfully, worse by UWQOL summary score (Δ=+6.5, p=0.01) (Table 2). Clinically meaningful declines (≥10 points) in global HRQOL from pre-treatment were reported by 8% and 30% of patients by HNQOL and UWQOL summary scores, respectively (Table 3), though long-term HNQOL and UWQOL summary scores corresponding to “moderate” or greater severity were reported by only 5% and 2.5% of patients, respectively.
On the SF-36 questionnaire, long term overall physical and mental health mean scores for the cohort were comparable in each HRQOL domain to the US population norms (15) (Table 4).
Table 4.
Long-term Global Health Measures by SF-36
| Category | Survey Mean (stdv) |
Population Mean (stdv)* |
|---|---|---|
| Physical health | ||
| Physical functioning | 88.6 (20.3) | 84.2 (23.3) |
| Role limitations due to physical health | 85.9 (31.8) | 80.9 (34.0) |
| Bodily pain | 83.8 (22.4) | 75.2 (23.7) |
| General health | 75.5 (22.4) | 71.9 (20.3) |
| Mental health | ||
| Vitality | 66.9 (24.6) | 60.9 (20.9) |
| Social functioning | 91.9 (17.6) | 83.3 (22.7) |
| Role limitations due to emotional problems | 93.2 (23.2) | 81.3 (33.0) |
| General mental health | 84.9 (12.4) | 74.7 (18.1) |
Reference 19.
Long-Term Patient-Reported Dysphagia
Compared with assessments at 2 years, patient-reported dysphagia at long-term assessment remained unchanged on average as measured by both HNQOL Eating domain and UWQOL Swallowing question (Δ=−1.4, p=0.44 and Δ=2.1, p=0.49, respectively) (Table 2; Figures 1B and 1H). Clinically meaningful improvement in HNQOL Eating domain from 2 years was reported by 25% of patients at long-term follow-up. Worsening dysphagia from 2-year assessment was reported by 5 patients (14%) at long-term follow-up by both HNQOL and UWQOL assessment (Table 3), though all were of only “mild/slight” severity by HNQOL Eating domain score with one patient reporting “moderate” severity swallowing problems by UWQOL assessment. Among these 5 patients, two underwent prior stricture dilation within 2 years of CRT completion, one of whom required additional dilations beyond 5 years and eventually developed PEG-tube dependence 8 years after CRT due to progressive stricture. Only one patient required new stricture dilation more than 2 years after CRT completion. Freedom-from-PEG-dependence beyond 2 years was 97.5%. Of all patients who participated in the long-term follow-up study, only 5% reported ≥“moderate” severity symptoms on each of the HNQOL Eating domain and UWQOL Swallowing question, including the aforementioned patient who required late PEG-tube placement.
Compared with pre-treatment, long-term mean HNQOL Eating domain and UWQOL Swallowing scores worsened statistically, though not clinically meaningfully, at long-term follow-up (Δ=+6.2, p=0.048 and Δ=+6.9, p=0.054, respectively) (Table 2).
Other Long-Term HRQOL Outcomes and Patient-Reported Sequelae
Trends toward statistical, but not clinically meaningful, worsening from 2 years were observed in mean Pain (Δ=+5.1, p=0.093) and Overall Bother (Δ=+4.2, p=0.088) domain scores (Table 2, Figures 1C–F). Clinically significant long-term worsening in HNQOL Pain domain compared to assessment at 2 years was reported by 31% of patients (Table 3), though only 11% reported the pain to be ≥“moderate” severity. Similar proportions of patients (17–29%) reported clinically meaningful long-term worsening compared with 2 years for each of the three pain-related UWQOL instrument questions, but with fewer pain responses of ≥“moderate” severity (3–9%). Worsening in HNQOL Overall Bother domain at long-term compared with 2 years was reported by 26% of patients, with 14% reporting worsening of ≥“moderate” severity. Patients who experienced late worsening in both Pain and Overall Bother domains at long-term follow-up had higher (worse) mean scores in each respective domain at 12 months, compared with those without late worsening (19.3 vs 4.7, p<0.01, and 25.0 vs 9.4, p<0.01, respectively).
Both mean Emotion and Overall Bother HNQOL domains scores improved at long-term follow-up compared with pre-treatment (Δ=−12.8, p<0.01 and Δ= −15.4, respectively, p<0.01) (Table 2); improvements in each domain were reported by 50% of individual patients (Table 3). “Moderate” or greater severity adverse outcomes for the HNQOL Communication and Emotion domains were reported by only 2.5% and 5% of patients, respectively.
While XQ scores worsened in most patients at long-term compared with pre-therapy, the worsening was mostly confined to the early period after therapy: only 14% reported worse xerostomia (while 19% reported improved xerostomia) at long-term, compared with 2 years (Table 3).
Hospitalization related to HNC treatment beyond 2 years after CRT was rare and reported by only 2 patients (5%), involving spinal accessory neuropathy from fibrosis-related nerve compression and mandibular reconstruction for osteoradionecrosis after tooth extraction from irradiated mandible.
Predictors of Late Worsening in Pain and Overall Bother HRQOL Domains
Given the unexpected frequency of worsening in Pain and Overall Bother domains observed after 2 years, we analyzed factors associated with late worsening of these domains. Analyses for worsening in other endpoints were not performed given the small number of patients experiencing late worsening. On univariate regression, T-classification, primary GTV, time since CRT completion, and mean radiation dose to the PCMs, cervical esophagus, GSL, oral cavity, and submandibular glands, were significantly associated with clinically meaningful worsening in either Overall Bother (Table 5) or Pain domain scores (Table S2). Mean doses to swallowing-related OARs, including oral cavity, PCMs, GSL, and cervical esophagus, were moderately-to-strongly inter-correlated, as were mean doses between swallowing-related structures and submandibular glands, T-stage, and primary GTV (ρ=0.48–0.76, all p<0.001) (Table S3). Earlier year of CRT was moderately-to-strongly correlated with mean dose to swallowing structures and submandibular glands (ρ=0.47–0.71, all p<0.001). The limited number of patients and multiple correlations between treatment-related variables precluded additional multivariable analysis.
Table 5.
Univariate Logistic Regression Analysis of Worsening Overall Bother From 24 Months to Long-Term Follow-up after Organ-Sparing Chemo-IMRT
| Variable | Odds ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Age | 0.96 | 0.86 to 1.07 | 0.44 |
| T-classification | 2.62 | 1.01 to 6.79 | 0.048 |
| N-classification | 1.60 | 0.29 to 8.76 | 0.59 |
| Tumor Site (Base of Tongue vs. Tonsil) | 3.75 | 0.64 to 22.0 | 0.14 |
| Primary Tumor GTV (per ml) | 1.02 | 1.0 to 1.04 | 0.11 |
| Lymph Node GTV (per ml) | 1.02 | 0.99 to 1.05 | 0.25 |
| Smoking Pack-Years (per pack-year) | 0.95 | 0.88 to 1.02 | 0.18 |
| Post-CRT Neck Dissection (yes vs. no) | 12.50 | 1.69 to 92.3 | 0.023 |
| Months since CRT Completion (per month) | 1.06 | 1.01 to 1.11 | 0.021 |
| Total Pharyngeal Constrictor Mean Dose* | 6.12 | 1.47 to 25.5 | 0.013 |
| Superior Pharyngeal Constrictor Mean Dose* | 6.19 | 1.08 to 35.4 | 0.041 |
| Middle Pharyngeal Constrictor Mean Dose* | 4.03 | 1.11 to 14.6 | 0.034 |
| Inferior Pharyngeal Constrictor Mean Dose* | 2.95 | 1.23 to 7.16 | 0.016 |
| Cervical Esophagus Mean Dose* | 3.80 | 1.52 to 9.50 | 0.0044 |
| Supraglottic & Glottic Larynx Mean Dose* | 3.90 | 1.43 to 10.7 | 0.0079 |
| Oral Cavity Mean Dose* | 2.08 | 0.90 to 4.81 | 0.088 |
| Parotid Gland Mean Dose* | 1.66 | 0.76 to 3.60 | 0.20 |
| Submandibular Gland Mean Dose* | 7.58 | 1.29 to 44.6 | 0.025 |
| Baseline Overall Bother Domain Score^ | 1.01 | 0.98 to 1.04 | 0.52 |
CRT = chemoradiation; GTV = gross tumor volume.
Odds ratio calculated per 10 Gy increase in mean dose to each structure
Per point on 0–100 scale
Discussion
The present study demonstrates that chemo-IMRT intended to spare the swallowing and salivary structures in patients with locally advanced OPC can achieve highly encouraging long-term patient-reported HRQOL, with overall and most HN-specific symptoms showing stability or continued improvement after 2-years. Compared with pre-treatment, the overall cohort experienced clinically meaningful improvement in HNQOL Emotion and Overall Bother domain scores at long-term follow-up, stable HNQOL summary scores, and clinically non-significant declines in HNQOL Eating domain and UWQOL Swallowing and summary scores. Notably, despite treatment with intensive chemoradiation, long-term global HRQOL in both physical and mental health domains (as assessed by SF-36) is comparable to US population norms.
These findings build on our earlier reports of 2-year HRQOL outcomes following organ-sparing chemo-IMRT for OPC, in which HRQOL initially declined after completion of therapy, improved from 3-months to 1-year, and thereafter remained stable through 2 years (2, 8). The low frequency of late adverse HRQOL outcomes in the present study contrasts with prior reports demonstrating frequent severe long-term swallowing toxicity after HN chemoradiation using conventional radiotherapy techniques (18), 3D-conformal techniques (1), and IMRT without specific sparing of the swallowing structures (19). Wang et al. in a pooled systematic review, for example, reported pharyngoesophageal stricture formation in 17% of 648 patients treated with IMRT for HNC (19). In contrast, at more than 6 years median follow-up, only 2.5% and 5% of patients in our study reported PEG-tube dependence and ≥moderate severity swallowing dysfunction. Recent large retrospective series of IMRT for OPC suggest an improvement in the rate of PEG-tube dependence compared with previous series of 2D or 3D RT (20, 21). While the low rates of PEG-tube dependence even among patients receiving concurrent chemo-IMRT without specific sparing of the swallowing structures are reassuring, the lackof patient-reported outcomes preclude more detailed comparisons with our series.
Recent studies have also suggested that de novo late toxicity may develop years after chemoradiation for HNC, with dysphagia as a common presenting symptom (9, 10). By comparison, in the present study of patients treated with CRT using swallowing- and salivary-structure-sparing IMRT techniques, of whom 75% had follow-up of ≥5 years, de novo late onset toxicity, late dysphagia or other adverse HRQOL effects of ≥“moderate” severity, and hospitalization for HN-related problems beyond 2 years, were uncommon. Furthermore, in our study, worsening HRQOL domain scores long after 2 years were correlated with worsening in the same domains already at 1 year, rather than appearing de novo in later years, suggesting late progression of symptoms that appeared soon after therapy, but very little new symptoms developing in the long run. Hutcheson et al. reported a similar lack of de novo late toxicity among 39 long-term survivors of OPC treated with induction chemotherapy and predominantly IMRT or chemo-IMRT (22). Ackerstaff et al. also reported that HRQOL for most domains improved within first year after completion of CRT and remained stable through 5-year follow-up; however, 10% of patients were still dependent on PEG tubes (23).
The long-term retention of good HRQOL scores is of particular import in the present era, in which a growing majority of OPC patients have HPV-associated disease (24). Given the younger age, lower comorbidity, and superior prognosis of HPV+ OPC patients (25), avoidance of late treatment-related toxicity and maximal preservation of HRQOL is critical, motivating calls for de-intensification of multimodality therapy in this population (26). Our long-term results in a population of nearly all HPV+ OPC patients provide reassurance regarding long-term toxicity and HRQOL outcome of full-intensity chemo-IMRT sparing the swallowing structures.
The present study did reveal patients who experienced late worsening in Pain and Overall Bother domains occurring beyond 2 years after CRT, though only 11% and 14% of patients, respectively, experienced worsening of ≥“moderate” severity. Late worsening in both these HRQOL domains was most strongly associated with radiation dose to the swallowing structures and time since completion of CRT, as well as with post-CRT neck dissection for Overall Bother. As time since CRT completion was also moderately-to-strongly correlated with mean dose to all OARs, we suspect that the relationship between time since RT and late worsening pain is likely attributable to improved technical proficiency in sparing the swallowing structures over the course of the 8-year study period, rather than to a prolonged latency period to development of late toxicity. Irrespectively, the associations of dose to the swallowing structures and neck dissection with late worsening in QOL domains add to the accumulating evidence that sparing the swallowing structures during HN IMRT and avoidance of post-CRT neck dissection, where possible, are critical to optimizing HRQOL (1, 3, 5, 27–31).
A primary weakness of this study include substantial attrition of patients at long-term follow up, which may have limited the study’s statistical power to detect late worsening in HRQOL. While PROs were available for almost all patients pre-therapy and at 2 years, only 58% of eligible patients returned their long-term questionnaires. Between long-term respondents and nonrespondents there were no significant differences among baseline tumor characteristics (p>0.20) nor were there differences in HRQOL at 2-years (p>0.50). However, due to difference in smoking status, the use of adjuvant neck dissection, and potential unmeasured or undetected confounding between long-term respondents and non-respondents, non-random participation bias remains a possibility (i.e., patients with better HRQOL more likely to return the questionnaires than patients with worse HRQOL). Substantial attrition is the rule in longitudinal studies assessing PROs, with 60% compliance at 2 years typical of large studies (32), which is comparable to the 58% participation rate at much longer follow-up in our study. In RTOG studies, for example, compliance as early as 12 months was only 36% when paper forms were used (33). Overall, the proportion of patients with advanced T and N stages participating in our original protocols are identical to those reported in recent series of HPV-related OPC (12, 25, 34). As expected with long-term followup, patients with advanced tumors, especially T4, recurred more often, resulting in a lower proportion of such patients in the long-term study. This phenomenon is expected in all long-term QOL studies. Another factor that potentially limited the generalizability of our findings include the exclusion of patients with posterior pharyngeal wall involvement, as sparing of the pharyngeal constrictors is not feasible in such patients. However, the prevalence of T4 disease (23%) in the original cohort is similar to other HPV+ OPC series (12, 20, 25, 34), suggesting that these exclusion criteria affected very few patients.
In conclusion, at 6.5 years median follow-up, the majority of locally advanced OPC survivors treated with swallowing and salivary structure-sparing chemo-IMRT who participated in the present study continued to experience HRQOL essentially unchanged from earlier evaluation at 2-years with stable or improved HRQOL in most domains as compared to pre-treatment. De novo late toxicity was infrequently observed. Further improvements may be achieved by increased sparing of the swallowing structures through careful dose de-escalation studies for patients predicted to do well (26), and by reducing xerostomia, which correlates with patient-reported dysphagia (35), by sparing submandibular and oral minor salivary glands in addition to the parotid glands (36).
Supplementary Material
Summary.
In this longitudinal study of patients with locally advanced HPV-associated oropharyngeal cancer treated on two prospective studies of swallowing and salivary organ-sparing chemo-IMRT, health-related (HR)QOL at a median of 6.5 years follow-up remained stable or improved in nearly all HRQOL domains compared to pre-treatment and 2-year post-treatment assessments. A minority of patients (10–14%) reported long-term worsening in pain and overall bother, which were associated with mean dose to the cervical esophagus, larynx, and pharyngeal constrictors.
Acknowledgments
Supported in part by NIH grant CA59827 and the Newman Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 56th Annual Meeting of ASTRO, Sep 14–16 2014, San Francisco, CA and at Best of ASTRO 2014, Oct 16–17 2014. Miami Beach, FL
The authors report no actual or potential conflicts of interest
Contributor Information
Jeffrey M. Vainshtein, Department of Radiation Oncology, University of Michigan Medical School, Ann Arbor, MI.
Dominic H. Moon, University of Michigan Medical School, Ann Arbor, MI.
Felix Y. Feng, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Douglas B. Chepeha, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Avraham Eisbruch, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Matthew H. Stenmark, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
References
- 1.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401–408. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 2. xxxx. [Google Scholar]
- 3. xxx. [Google Scholar]
- 4. xxx. [Google Scholar]
- 5. xxx. [Google Scholar]
- 6. xxx. [Google Scholar]
- 7.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. xxx. [Google Scholar]
- 9.Payakachat N, Ounpraseuth S, Suen JY. Late complications and long-term quality of life for survivors (>5 years) with history of head and neck cancer. Head Neck. 2013;35:819–825. doi: 10.1002/hed.23035. [DOI] [PubMed] [Google Scholar]
- 10.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–5799. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. xxx. [Google Scholar]
- 12.Vainshtein JM, Spector ME, McHugh JB, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral Oncol. 2014;50:513–519. doi: 10.1016/j.oraloncology.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrell JE, Nanavati KA, Esclamado RM, et al. Head and neck cancer-specific quality of life: instrument validation. Arch Otolaryngol Head Neck Surg. 1997;123:1125–1132. doi: 10.1001/archotol.1997.01900100101014. [DOI] [PubMed] [Google Scholar]
- 14.Hassan SJ, Weymuller EA. Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15:485–496. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 16.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 17.Tu YK, Clerehugh V, Gilthorpe MS. Collinearity in linear regression is a serious problem in oral health research. Eur J Oral Sci. 2004;112:389–397. doi: 10.1111/j.1600-0722.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- 18.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JJ, Goldsmith TA, Holman AS, et al. Pharyngoesophageal stricture after treatment for head and neck cancer. Head Neck. 2012;34:967–973. doi: 10.1002/hed.21842. [DOI] [PubMed] [Google Scholar]
- 20.Bhayani MK, Hutcheson KA, Barringer DA, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck. 2013;35:1634–1640. doi: 10.1002/hed.23200. [DOI] [PubMed] [Google Scholar]
- 21.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–298. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheson KA, Lewin JS, Holsinger FC, et al. Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2014;36:474–480. doi: 10.1002/hed.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackerstaff AH, Rasch CR, Balm AJ, et al. Five-year quality of life results of the randomized clinical phase III (RADPLAT) trial, comparing concomitant intra-arterial versus intravenous chemoradiotherapy in locally advanced head and neck cancer. Head Neck. 2012;34:974–980. doi: 10.1002/hed.21851. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quon H, Forastiere AA. Controversies in treatment deintensification of human papillomavirus-associated oropharyngeal carcinomas: should we, how should we, for whom? J Clin Oncol. 2013;31:520–522. doi: 10.1200/JCO.2012.46.7746. [DOI] [PubMed] [Google Scholar]
- 27. xxx. [Google Scholar]
- 28.Vlacich G, Spratt DE, Diaz R, et al. Dose to the inferior pharyngeal constrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother Oncol. 2014;110:435–440. doi: 10.1016/j.radonc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen HR, Jensen K, Aksglaede K, et al. Late dysphagia after IMRT for head and neck cancer and correlation with dose-volume parameters. Radiother Oncol. 2013;107:288–294. doi: 10.1016/j.radonc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Christianen ME, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105:107–114. doi: 10.1016/j.radonc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93:539–544. doi: 10.1016/j.radonc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Bottomley A, Tridello G, Coens C, et al. An international phase 3 trial in head and neck cancer: quality of life and symptom results: EORTC 24954 on behalf of the EORTC Head and Neck and the EORTC Radiation Oncology Group. Cancer. 2014;120:390–398. doi: 10.1002/cncr.28392. [DOI] [PubMed] [Google Scholar]
- 33.Movsas B, Hunt D, Watkins-Bruner D, et al. Can electronic web-based technology improve quality of life data collection? Analysis of Radiation Therapy Oncology Group 0828. Pract Radiat Oncol. 2014;4:187–191. doi: 10.1016/j.prro.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 35.Vainshtein JM, Schipper M, DeYoung J, et al. Impact of Xerostomia on Patients’ Perception of Dysphagia After Chemo-IMRT for Oropharyngeal Cancer (OPC) Int J Radiat Oncol Biol Phys. 2014;90:S100. [Google Scholar]
- 36.Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83:1007–1014. doi: 10.1016/j.ijrobp.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.