Preface
Copper and palladium catalysts are critically important for numerous commercial chemical processes. Improvements in the activity, selectivity, and scope of these catalysts have the potential to dramatically reduce the environmental impact and increase the sustainability of chemical reactions. One rapidly emerging strategy to achieve these goals is to exploit “high-valent” copper and palladium intermediates in catalysis. This review describes exciting recent advances involving both the fundamental chemistry and the applications of these high-valent metal complexes in numerous synthetically useful catalytic transformations.
Introduction
Homogeneous copper and palladium-catalyzed reactions are widely used for the construction of important organic molecules, including pharmaceuticals,1–3 commodity chemicals,4 and polymers.5 The development of copper and palladium catalysis has been inextricably linked as both metals have found extensive utility in the construction of similar types of carbon–carbon and carbon–heteroatom bonds. Furthermore, advancements and insights in copper chemistry have often spurred improvements in palladium-catalyzed processes and vice versa, leading to a wealth of robust, synthetically valuable, and often complementary synthetic methods. This review focuses particularly on an area that has seen tremendous progress and advancements over the past decade - the use of high-valent Cu and Pd complexes in catalysis (see Text box for definitions of ‘high-valent Cu and Pd’).
Text Box.
Definitions
In the context of Pd and Cu, high-valent compounds include Pd in the +3 or +4 oxidation state (abbreviated PdIII and PdIV throughout this review) and Cu in the +3 oxidation state (abbreviated CuIII). Organometallic complexes of these high-valent metals are defined as molecules that contain Cu– or Pd–carbon bonds. “High-valent Cu/Pd catalysis” is defined as a catalytic reaction in which the metal is oxidized to form a high-valent organometallic intermediate during the catalytic cycle.
Key issues in the field of high-valent Cu chemistry
Exciting advances in the area of high-valent Cu chemistry have enabled researchers to address a number of critical issues, including: i) the synthetic accessibility of organo–CuIII complexes, ii) the viability of carbon–carbon and carbon–heteroatom bond formation from discrete organo–CuIII species, iii) the catalytic relevance of CuIII complexes, and iv) the ability to exploit high-valent Cu intermediates to improve catalytic reactions and/or discover new reactivity modes.
Key issues in the field of high-valent Pd chemistry
There has been a renaissance in high-valent Pd chemistry over the past decade that has provided key insights into the following scientific issues: i) the synthetic accessibility of high-valent PdIII or PdIV organometallic complexes, ii) the ability of these species to participate in stoichiometric carbon–carbon and carbon–heteroatom bond-forming reactions, iii) the catalytic relevance of these high-valent Pd species, andiv) the advantages of high-valent Pd catalysis (in terms of enhanced substrate scope, milder reaction conditions, and improved and chemo-, regio-, and/or site-selectivity) versus more common low-valent analogues. A representative high-valent palladium catalytic cycle is shown in the figure.
History and Importance of Copper Catalysis
Copper is an inexpensive, earth-abundant, and non-toxic metal that has found widespread application in homogeneous catalysis. For example, Cu-catalyzed cross-coupling reactions have been extensively explored since their discovery at the turn of the 20th century.6,7 These serve as versatile methods for synthesizing biaryl linkages as well as for constructing the carbon–heteroatom bonds of aryl amines, aryl ethers, and aryl thioether derivatives. 89 The alkylation of carbon electrophiles with organometallic copper compounds (also known as organocuprates) is another classic transformation in organic synthesis.3 Diverse C–C bond-forming reactions of organocuprates have been developed over the past 70 years, and these transformations are featured in most introductory undergraduate organic textbooks.
High-valent Cu intermediates (i.e., organometallic CuIII species) have long been proposed to play a role in both Cu-catalyzed cross-coupling and organocuprate reactions.10 In particular, C–C and/or C–heteroatom bond formation from an organo-CuIII species has been invoked as the product-releasing step of many of these transformations.8,9,11,12, However, the proposed high-valent Cu intermediates have eluded detection for decades, and, as a result, there has been considerable controversy over the mechanistic details of both organocuprate additions and Cu-catalyzed cross-coupling.11 Indeed, until very recently, these two transformations were among the least mechanistically understood synthetic methods in organometallic chemistry. These mechanistic questions and controversy have provided tremendous motivation for probing the accessibility and reactivity of organo-CuIII species. A deeper mechanistic understanding of their chemistry promises to enable the development of improved Cu catalysts for known reactions as well as to inspire novel Cu-catalyzed transformations. Section I presents exciting recent progress to address many of the vital questions in this area (discussed in detail in the Text Box).
History and Importance of Palladium Catalysis
Although Cu-mediated cross-coupling methods were the first of their kind, today cross-coupling has become synonymous with a different metal – palladium. Well-defined Pd-catalyzed cross-coupling reactions were first developed in the 1970’s, and they quickly surpassed Cu-based methods in both popularity and scope. These reactions have transformed the way organic chemists approach the construction of bonds in complex molecules,1,5,13 and the wide-ranging impact of this methodology was recognized by the 2010 Nobel Prize.
The rapid success of Pd-catalyzed cross-coupling is due, in large part, to extensive and systematic investigations of reaction mechanism. Mechanistic analysis has revealed that nearly all of these processes involve catalysis by “low-valent” Pd (i.e., with the metal in the 0 or +2 oxidation states). For many reactions, Pd0 and PdII catalytic intermediates have been well-characterized and the steric and electronic influence of supporting ligands on catalysis has been studied in detail.14 Such mechanistic studies have been crucial for the development of new catalyst structures and novel transformations with wide scope and mild reaction conditions.1,13,14
While low-valent Pd catalysis is ubiquitous and extremely synthetically useful, it has several important limitations that stem from the fundamental properties of organo-PdII complexes. These include limited reactivity toward forming certain important types of chemical bonds (e.g., carbon–halogen and carbon–CF3 linkages) as well as a high susceptibility to decomposition pathways like β-hydride elimination. These challenges have provided motivation to study high-valent Pd catalysis as a potentially complementary mechanistic manifold. While the first 30 years of Pd chemistry was dominated by low-valent Pd (Pd0 and PdII), over the past decade, the unique reactivity of PdIII and PdIV intermediates has increasingly been recognized and exploited in catalysis. Section II presents recent advances in the field that demonstrate the relevance and utility of high-valent palladium in diverse catalytic applications (discussed in detail in the Text Box).
I. High-Valent Copper
Until the last decade, high-valent organometallic Cu complexes were rare. The sporadic examples reported in the literature were stabilized by rigid, chelating, and/or perfluorinated ligands, as exemplified by structures 1–3 in Figure 1a.15–17 While complexes 1–3 are structurally interesting, they do not exhibit the characteristic reactivity that has been attributed to CuIII in catalysis. Specifically, they are all inert toward carbon–carbon and carbon–heteroatom bond-forming reactions. As such, these compounds were largely considered curiosities, whose relevance to Cu catalysis was tenuous at best. The past ten years have seen tremendous developments in this area with the observation and detailed investigation of catalytically relevant organo-CuIII species in both carbon–carbon and carbon–heteroatom bond formation. In this section we will specifically focus on two representative areas: high-valent Cu intermediates in C–C bond-forming reactions of organocuprates and high-valent Cu intermediates in C–N and C–O couplings.
Figure 1.
High-valent copper complexes (a) Early examples of isolable organometallic CuIII complexes. (b) CuIII intermediates of organocuprate reactions detected at −100 °C using rapid injection-NMR. [Et = ethyl, TMS = trimethylsilyl]
An early advance in the area of high-valent Cu chemistry came from investigations of C–C bond-forming reactions of organocuprates with enones, alkyl halides, and allylic electrophiles. Computational studies of these transformations implicated a CuIII intermediate in the key carbon–carbon bond-forming step.18–20 However, for many years little experimental evidence was available to support this hypothesis, as the putative CuIII compounds proved too transient for detection using standard spectroscopic techniques.21 In 2007 Bertz and Ogle pioneered the use of rapid injection NMR spectroscopy (RI-NMR) to observe directly CuIII species like 4–7 in real time (Figure 1b). Remarkably, when generated using this technique, the CuIII adducts could be detected and fully characterized at −100 °C.22–26 Furthermore, upon warming, these discrete organo-CuIII intermediates underwent carbon–carbon bond-forming reductive elimination.22
While this field is still in its infancy, the ability to directly study the reactivity of high-valent organo-CuIII species has begun to provide mechanistic insights of direct relevance to Cu catalysis. For example, Lewis basic additives such as cyanide, phosphines, pyridines, and amines have been known for decades to improve the yield and/or rate of organocuprate conjugate addition reactions.27,28 Some researchers have proposed that the primary role of these additives is to enhance the solubility of copper starting materials/intermediates.28 In contrast, other groups have speculated that these Lewis bases play a more intimate role in the reaction mechanism by binding to Cu and tuning its reactivity toward oxidative addition and/or C–C bond-forming reductive elimination.19,27
RI-NMR has provided a means to directly interrogate these possibilities. A series of CuIII complexes of general structure (CH3CH2)(CH3)2CuIII(LB) [LB = Lewis basic ligand] were prepared using this technique and evaluated as models for conjugate addition intermediates.29 The nature of LB was found to have a profound influence on the stability of these species. For instance, with LB = pyridine, the CuIII complex 8 was a short-lived intermediate at −100 °C (0.5 h to maximum concentration) (Figure 2). At this temperature, 8 underwent facile ligand exchange [to form CuIII complex (CH3CH2)(CH3)3CuIIILi] as well as C–C bond-forming reductive elimination to release propane and (CH3)3CuI2Li. In contrast, under otherwise analogous conditions the dimethylaminopyridine complex 9 was very stable at −100 °C (Figure 2). Very little (<10%) reductive elimination was detected, and ligand exchange to form (CH3CH2)(CH3)3CuIIILi was not observed in this system.
Figure 2.

RI-NMR studies of the effect of Lewis bases on the reactivity of CuIII complexes 8 and 9. (i) Pyridine-containing intermediate 8 is short lived and undergoes ligand exchange to form (CH3CH2)(CH3)3CuIIILi as well as C–C bond-forming reductive elimination. (ii) In contrast, dimethylaminopyridine-containing intermediate 9 is stable at −100 °C under analogous conditions.
This study clearly shows that Lewis basic ligands dramatically influence the relative and absolute rates of carbon-carbon coupling at CuIII centers. Moving forward, more quantitative RI-NMR analysis of reaction kinetics and ligand electronic/steric effects should provide further insights about the rate- and selectivity-determining steps of conjugate addition and other organocuprate reactions. Such studies will also be invaluable for establishing the role of CuIII in the reactions of organocuprates with other electrophiles (e.g., acyl halides, carbonyl compounds, and cyclopropanes). Furthemore, they will provide a mechanistic platform for rationally designing new synthetic methods in this area.
The ability to use RI-NMR to observe and study organo-CuIII intermediates also has important implications for emerging areas of Cu catalysis. For example, Gaunt recently reported the Cu-catalyzed C–H arylation of anilides with diphenyliodonium triflate, [Ph2I]OTf.30 In these reactions C–C bond-formation occurs meta to the amide substituent, site selectivity that is complementary to both Pd-catalyzed C–H arylation methods31 and classical electrophilic aromatic substitution. The mechanism of this reaction remains controversial. DFT calculations have implicated the intermediacy of a CuIII–Ph complex;32 however, the accessibility of such intermediates has yet to be confirmed experimentally. Alternative mechanisms such as Lewis acid catalysis are also plausible. RI-NMR would serve as a powerful technique for detecting CuIII (if present) during catalysis and/or for interrogating stoichiometric reactions of CuI model complexes with [Ph2I]OTf. Such studies could help to clarify the mechanism of this novel transformation as well as to probe the origin of the meta selectivity.
A second key advance in high-valent Cu catalysis has come in the study of carbon–heteroatom bond formation from CuIII intermediates. As representative examples, we highlight recent investigations of the Cu-catalyzed amination of aryl bromides and of Cu-catalyzed C–H bond amination and oxygenation. Cu catalysts are well-known to promote the amination of aryl boronic acids,33,34 aryl halides,35,36 and carbon–hydrogen bonds.37–42 Common nitrogen heterocycles such as pyrazole, pyridone, and phthalimide are particularly effective coupling partners, and the reactions often proceed under mild conditions. Many researchers have proposed the intermediacy of CuIII in these transformations.18,43,44 However, this hypothesis has been the subject of significant controversy, and others have argued that single-electron transfer, halide atom transfer, or σ-bond metathesis mechanisms at low-valent CuI or CuII are more likely.45,46 Until very recently no CuIII catalytic intermediates had been detected, and C–N bond-formation from a CuIII complex had not been directly observed.
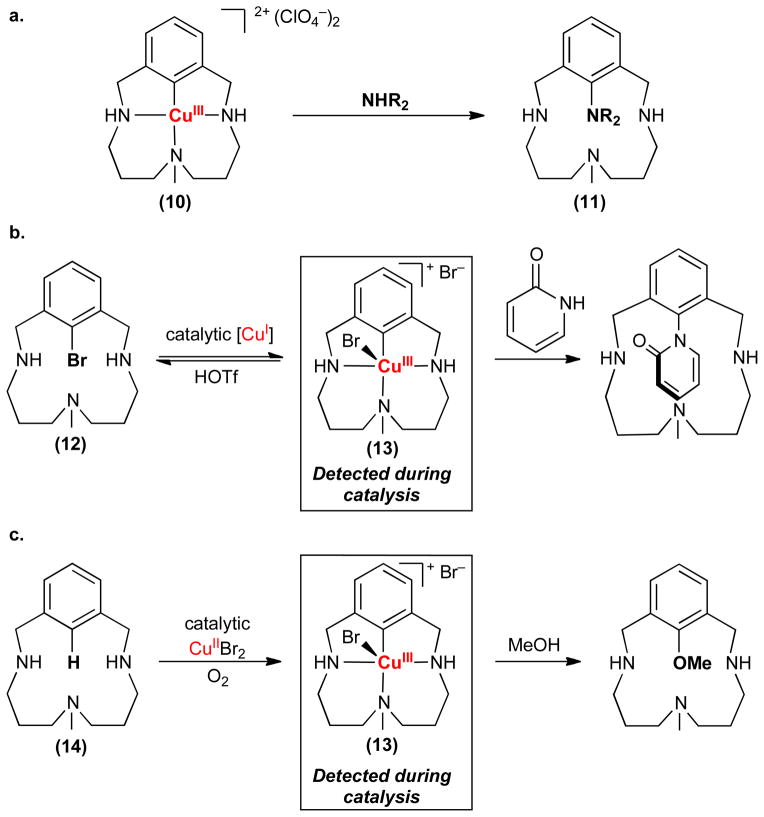
The synthesis of the first isolable CuIII–monoaryl species (10, Figure 3a) in 2002 was a turning point for this field.47 Like many of the early examples of organometallic CuIII compounds (e.g., 2,3 in Figure 1), complex 10 is stabilized by an electron donating macrocylic ligand. However, unlike its predecessors, 10 is remarkably reactive toward C–N bond-formation. For example, Stahl demonstrated that 10 reacts stoichiometrically with amines like pyridone, oxazolidinone, and acetanilide (collectively abbreviated NHR2) to form aminated products (11, Figure 3a).43 Further study showed that less basic amines reacted faster, implicating deprotonation of NHR2 at or before the rate-determining step of this sequence. Notably, in the absence of amine, 13 was shown to undergo C–Br bond-forming reductive elimination to release aryl bromide 12 when triflic acid is present.
Figure 3.
High-valent copper complexes involved in carbon–heteroatom bond formation (a) Stoichiometric carbon–nitrogen bond-formation from an isolated organo-CuIII [NHR2 = pyridone, oxazolidinone, acetanilide]. (b) In situ observation of an organo-CuIII intermediate in the coupling of aryl bromide 12 with pyridone. (c) In situ observation of an organo-CuIII intermediate in the oxygenation of C–H bonds.
The next key question was whether CuIII complex 10 and analogues thereof are relevant to catalytic C–N coupling reactions. To test this possibility, Ribas and Stahl studied the Cu-catalyzed amination of aryl bromide 12 with pyridone.35 Remarkably, they were able to detect a steady state concentration of CuIII intermediate 13 during catalysis via UV-vis and NMR spectroscopy. The observation of 13 is consistent with its participation in the turnover-limiting step of the catalytic reaction. While studies of this one system do not resolve the mechanistic controversy surrounding all Cu-catalyzed cross-coupling reactions, they provide the first definitive demonstration that CuIII can be catalytically relevant in these transformations.
Pioneering studies have also recently established a role for high-valent Cu in certain Cu-catalyzed carbon–hydrogen bond functionalization reactions.37,48 Prior to this work, there was considerable mechanistic uncertainty surrounding these transformations.38–42,49–53 Radical pathways initiated by single electron transfer from amine, enolate, and electron-rich arene substrates have frequently been proposed.42,52 However, a growing number of examples have been reported with substrates (e.g., alkynes,50,51 electron-deficient arenes40,42,52,53) that are unlikely to participate in such a mechanism. Very recently, Stahl demonstrated the catalytic aerobic C–H oxygenation of macrocycle 14 with methanol (Figure 3c).37 In situ UV-vis spectroscopic studies revealed the build-up and subsequent decay of CuIII complex 13 during the catalytic reaction, implicating this species as a catalytically relevant intermediate. Further kinetic studies suggested that the rates of CuII-mediated C–H cleavage and of C–O bond-formation from CuIII are closely matched, which would explain the observed concentration profile of intermediate 13 during catalysis.37
In summary, at the beginning of this decade little was known about the stability and reactivity of high-valent copper complexes. The past ten years have seen considerable progress, with the first observation and study of carbon–carbon and carbon–heteroatom bond formation from discrete organo-CuIII species in stoichiometric and catalytic transformations. Fundamental studies of organo-CuIII are clearly beginning to provide greater understanding of mechanism, which in turn should enable the rational development of new synthetic methods.54
II. High-Valent Palladium
Sporadic reports over the past 50 years have proposed the intermediacy of PdIV in catalysis.55,56,57 However, these proposals were frequently viewed with skepticism due to a lack of evidence supporting the viability of such species. Thus a first key challenge was to determine whether it was possible to form, detect, and isolate PdIII and/or PdIV complexes from the reactions of PdII precursors with oxidants. Early work by Canty and Cotton established the viability of this approach, and demonstrated that electron-donating, rigid, and multidentate supporting ligands can be used to stabilize high-valent Pd products. For example, in 1988, Canty prepared organometallic PdIV complex 16 (Figure 4) via the reaction of 15 (containing the rigid, bidentate 2,2′-bipyridine ligand) with CH3I.58 Similarly, Cotton generated organo-PdIII dimer 18 by reacting 17 (containing bidentate, electron-donating cyclometalated phosphines) with PhICl2.59 These seminal discoveries have inspired extensive efforts to exploit related intermediates in catalysis. In this section we specifically focus on progress in two areas: (i) high-valent Pd intermediates in C–halogen bond-formation and (ii) high-valent Pd intermediates in C–CF3 coupling reactions.
Figure 4.
Early examples of PdIII and PdIV organometallic complexes. [Ph = phenyl]
The formation of carbon–halogen bonds has been an important target reaction for high-valent Pd catalysis. Halogenated molecules are valuable starting materials for many organic transformations including nucleophilic substitutions, metal catalyzed cross-couplings, and Friedel-Crafts alkylations. Notably, carbon–halogen bond-forming reductive elimination is both thermodynamically unfavorable and kinetically slow from most PdII complexes.60 As a result, most PdII/0-catalyzed transformations of aryl/alkyl halides involve breaking carbon–halogen bonds rather than forming them (Figure 5a).61 In marked contrast, recent work has shown that many high-valent Pd complexes promote the facile formation of carbon–halogen bonds.62–64 Initial studies in this area focused on generating high-valent organometallic Pd halide complexes via the stoichiometric two electron oxidation of PdII precursors with electrophilic halogenating reagents [for example, Cl2, PhICl2, N-chlorosuccinimide, and XeF2 (collectively abbreviated “X+” in Figure 5a)].65–67 Depending on the structure of the PdII starting material, these reactions afford either monomeric PdIV complexes like 19,68 20,69 and 2170 or dimeric PdIII species like 2271 (Figure 4). Many of these high-valent Pd compounds are isolable at room temperature. However, upon heating, they all undergo kinetically fast and highly thermodynamically favorable C–X bond-forming reductive elimination to release halogenated organic products (Figure 5a).
Figure 5.
High-valent Pd complexes involved in carbon–halogen bond formation (a) Carbon–halogen bond-forming reductive elimination is thermodynamically unfavorable from most PdII species but not from high-valent Pd complexes like 19–22. (b) Select examples of Pd-catalyzed C–H halogenation reactions [L = L-type ligand, R = aryl, X= halogen, o-Ns = ortho-Nosyl, Tf = triflate, t-Bu = tert-butyl.]
The stoichiometric studies shown in Figure 5a have informed the development of new Pd-catalyzed halogenation reactions that involve high-valent intermediates. One particularly well-studied example involves the ligand-directed halogenation of arene and alkane C–H bonds (Figure 5b).62–64,72,73 Electrophilic halogenating reagents (X+) are used to promote the formation of high-valent Pd intermediates during catalysis. Depending on the structure of “X+”, diverse carbon–halogen bonds can be formed. For example, N-fluoropyridinium salts generate carbon–fluorine bonds, N-chlorosuccinimide promotes the formation of C–Cl bonds, and acetyl hypoiodite provides access to iodinated products.
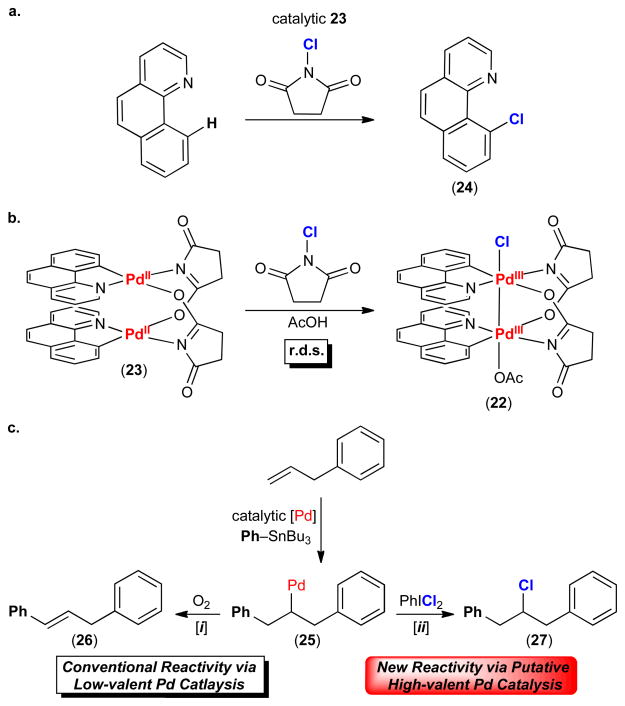
Detailed mechanistic studies of the Pd-catalyzed C–H chlorination of benzo[h]quinoline with N-chlorosuccinimide support the intermediacy of a high-valent Pd species (Figure 6a).71 The resting state of the catalyst was determined to be the dinuclear succinate-bridged PdII complex 23 (Figure 6b). This compound is a kinetically competent catalyst in the presence of added acetate. Furthermore, rate studies of the 23-catalyzed C–H chlorination reaction show a 1st order dependence on this PdII/II dimer and a 1st order dependence on oxidant. These data are consistent with rate-limiting two-electron oxidation of 23 to generate the high-valent PdIII/III dimer 22 (Figure 6b). This PdIII/III intermediate could not be observed under the catalytic conditions, as to be expected when oxidation is rate limiting. However, dimer 22 could be synthesized independently at −78 °C. Upon warming to 23 °C, 22 underwent C–Cl bond-forming reductive elimination to release chlorinated product 24 in 84% yield, further supporting its intermediacy in catalysis.
Figure 6.
Pd-catalyzed chlorination (a) Complex 23 is an efficient catalyst for the Pd-catalyzed C–H chlorination of benzo[h]quinoline. (b) The rate determining step (r.d.s.) of this Pd-catalyzed reaction is oxidation of 23 by N-chlorosuccinimide to form the PdIII/III dimer 22. (c) High-valent Pd-catalyzed 1,2-arylchlorination (ii) is complementary to low-valent Pd-catalyzed reactions (i) of α-olefins.
High-valent Pd catalysis has also been exploited for the halofunctionalization of alkenes, as exemplified by Figure 6b.74–76 The combination of a Pd catalyst, alkene, and an aryl-metal reagent like Bu3SnPh is well-known to produce a PdII–alkyl intermediate like 25 (Figure 6b). However, the fate of this intermediate and the ultimate organic product of the reaction vary dramatically depending on the choice of oxidant. For example, with oxidants like O2 (which has low kinetic reactivity with most PdII complexes), 25 undergoes β-hydride elimination to release styrene product 26 via a conventional low-valent PdII/0 manifold (Figure 6b, i).77 In contrast, kinetically reactive Cl+ oxidants like PhICl2 can rapidly intercept 25 to generate putative high-valent Pd intermediates. These can then undergo C–Cl bond-forming reductive elimination to release 1,2-arylchlorinated compound 27 (Figure 6b, ii).76 While the intermediacy of PdIII and/or PdIV in these arylchlorination reactions has not yet been definitively confirmed, the observed reactivity (favoring C–Cl bond-formation over β-hydride elimination) is consistent with such a mechanism. This highlights another key complementarity between low-valent Pd catalysis (where square planar PdII-alkyl intermediates typically undergo fast decomposition via β-hydride elimination) versus high-valent Pd catalysis (where β-hydride elimination is disfavored due to the lack of open coordination sites at octahedral PdIII and/or PdIV-alkyl complexes).62,63,64,72 Notably, many related Pd-catalyzed alkene difunctionalization reactions have been reported over the past 6 years that also likely proceed via high-valent Pd pathways.63,64
Another challenging and desirable chemical target for high-valent Pd catalysis has been the generation of C–CF3 linkages. Trifluoromethyl groups appear in numerous commercial pharmaceuticals and drug candidates and can dramatically enhance the metabolic stability and bioavailability of biologically active molecules.78 Despite the prevalence of these groups in medicinal chemistry, efficient approaches for introducing CF3 into organic compounds under mild conditions are limited. Metal-catalyzed methods are particularly rare79,80 and would constitute powerful synthetic tools to complement currently available chemical processes.
Many previous efforts to develop catalytic trifluoromethylation reactions have been hampered by the kinetic inertness of most metal–CF3 complexes toward C–CF3 bond-formation.79 For example, C–CF3 coupling at PdII centers requires specialized phosphine ligands to proceed efficiently (Figure 7a).79,80 In contrast, PdIV complexes containing simple bidentate nitrogen donor ligands undergo facile C–CF3 bond-forming reductive elimination. For example, the stoichiometric reaction of (N~N)PdII(Aryl)(CF3) complexes with N-fluoropyridinium oxidants affords isolable high-valent (N~N)PdIV(Aryl)(CF3) intermediates (N~N = bidentate nitrogen donor ligand). These compounds participate in rapid C–CF3 coupling at temperatures as low as 25 °C.81
Figure 7.
Pd-catalyzed trifluoromethylation (a) Carbon–CF3 bond-formation from PdII requires specialized phosphine ligands. (b) Using a high-valent Pd strategy, catalytic C–H trifluoromethylation has been developed via putative PdIV intermediate 28. [TES = triethylsilyl, Cy= cyclohexyl, Ac = acetyl, i-Pr = iso-propyl]
A related approach has been utilized to achieve catalytic ligand-directed trifluoromethylation of aromatic C–H bonds. In this system, electrophilic trifluoromethylating reagents (CF3+) were used to promote the formation of high-valent Pd intermediates, which decompose to afford aryl-trifluoromethlayed products.82 Remarkably, these reactions proceed efficiently with simple Pd salts as catalysts, and no external ligands (other than substrate) are required. Subsequent mechanistic studies suggested that the PdIV complex 28 might be a catalytic intermediate (Figure 7b),83 as it serves as a kinetically competent catalyst under the reaction conditions. This methodology represents a transformation (ligand-directed conversion of C–H → C–CF3) that is not currently accessible using any other transition metal catalyst, again highlighting the power of high-valent Pd chemistry to achieve novel chemistry.
In addition to the carbon-halogen and carbon-trifluoromethyl bond-forming reactions discussed above, high-valent Pd intermediates have also been implicated in the selective transformation of alkane and arene C–H bonds into C–O, C–C, C–N, and C–S linkages. Detailed mechanistic investigations of catalytic C–H acetoxylation84 and arylation31 have provided evidence consistent with the formation of high-valent Pd intermediates in these reactions as well.
In summary, this section has described exciting recent progress in high-valent Pd catalysis. Over the past decade, numerous organometallic PdIV and PdIII complexes have been synthesized by the reaction of PdII starting materials with strong oxidants. A wide scope of C–C and C–heteroatom bond-forming reductive elimination reactions can be achieved from these species, and the selectivity, reactivity, and mechanisms of these transformations have been studied in detail. Furthermore, a number of these species have been demonstrated as kinetically competent catalysts for C–H bond halogenation, trifluoromethylation, and other reactions. These results have firmly established the feasibility and synthetic utility of high oxidation state Pd catalysis in organic synthesis, and this field has a vibrant and exciting future.
III. Comparison and Contrast
There are two main common features of the high-valent Cu chemistry described in Section I and the high-valent Pd chemistry discussed in Section II of this review. First, while the specific transformations that have been selected for detailed mechanistic investigation at each metal vary significantly, CuIII and PdIII/PdIV species have both been shown to participate in closely related carbon–carbon and carbon–heteroatom bond-forming reductive elimination reactions. One particularly striking example is the accessibility of C–halogen bond-forming reductive elimination from CuIII complex 13 as well as from PdIV/PdIII complexes 19–22. Similar ligand environments have been shown to stabilize high-valent complexes of both metals. In particular, rigid multidentate ligands (like the macrocyles of Cu complexes 2, 10, and 13 and the cyclometalated benzoquinoline of PdIV species 21 and 28 and PdIII complex 22) tend to slow competing reductive elimination processes. Furthermore, the presence of multiple highly electron-donating σ-aryl/alkyl ligands (as in CuIII complexes 4–9 and PdIV complexes 16 and 19–21) facilitate the detection/isolation of high-valent species of both Cu and Pd.
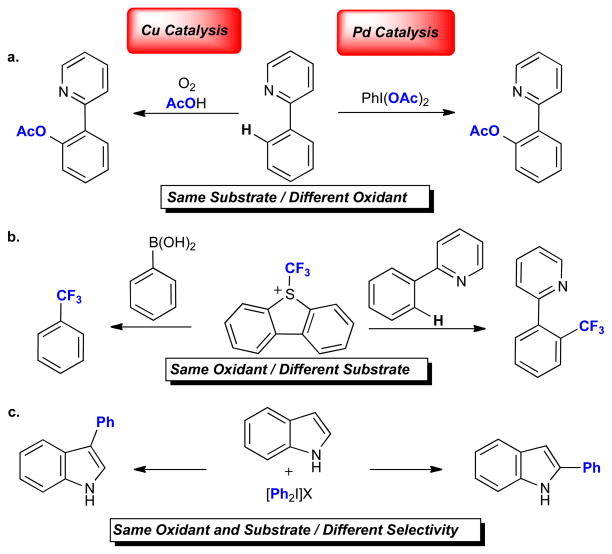
Recent examples of Cu/Pd-catalyzed oxidation reactions reveal additional intriguing similarities. There are a multitude of catalytic C–C and C–heteroatom coupling reactions that share the following features: (i) a Cu or Pd catalyst, (ii) an oxidant, and (iii) an organic substrate that is a precursor to a metal–carbon bond (such as an aryl halide, C–H bond, or transmetallating reagent). Three examples of such transformations are shown in Figure 8. While detailed mechanistic analysis will be required to establish firmly the pathway for each system, it seems likely that many (if not all) of these reactions proceed via high-valent Cu/Pd manifolds. In the first example, Cu and Pd catalyze the same overall reaction, the ligand directed C–H acetoxylation of 2-phenylpyridine (Figure 8a).52,85 In the second, the same oxidant (S-(trifluoromethyl) dibenzothiophenium) is used to effect the trifluoromethylation of two different organic substrates (Figure 8b).82,86 Finally, in the third example, both metals catalyze the C–H arylation of indole with diaryliodonium salts (Figure 8c).87,88
Figure 8.
Oxidative bond-forming reactions catalyzed by Cu and Pd that exemplify similarities and differences between these two metals.
The reactions in Figure 8 not only illustrate key similarities but also highlight key differences/complementarities between the oxidative chemistry of Pd and Cu. For example, the Pd-catalyzed C–H acetoxylation of 2-phenylpyridine (Figure 8a) requires the use of PhI(OAc)2 as the terminal oxidant.85 This reagent is quite expensive and generates an equivalent of iodobenzene waste with each catalytic turnover. In marked contrast, the Cu-catalyzed acetoxylation employs abundant and atom economical dioxygen (O2) as the oxidant.52 The ability to generate high-valent Cu using O2 is currently a distinct advantage of high-valent Cu catalysis.40,42,53 Although dioxygen is thermodynamically capable of oxidizing PdII to PdIV, most organo-PdII intermediates are kinetically inert to oxidation by O2.73 However, two very exciting recent reports have shown that aerobic Pd-catalyzed ligand-directed C–H oxygenation is possible (potentially via high-valent Pd intermediates) suggesting a promising future in this area.89,90
As shown in Figure 8b, both Cu and Pd catalyze carbon–CF3 bond-forming reactions with S-(trifluoromethyl) dibenzothiophenium.82,86 Catalytically competent PdIV intermediates have been observed and isolated in the C–H trifluoromethylation reaction (Figure 7b).83 In contrast, mechanisms involving CuIII(Aryl)(CF3) intermediates have been proposed but remain to be confirmed experimentally for the Cu-catalyzed trifluoromethylation of boronic acids.86 These two examples demonstrate another key complementarity between Pd and Cu catalysis. High-valent Pd-catalysis has been used for transforming C–H substrates into many different functional groups (with trifluoromethyl being just one example), and Pd-catalyzed C–H oxidation is an extremely common, general, and well-studied reaction.73 In marked contrast, high-valent Cu catalysis has predominantly focused on pre-functionalized substrates like aryl boronic acids and aryl halides (Figure 8b). At present, Cu-catalyzed C–H bond oxidation reactions are comparatively rare, and have significantly narrower substrate scope than analogous Pd-catalyzed reactions.50–53 For example, the Cu-catalyzed functionalization of unactivated alkane C–H bonds remains highly challenging while such transformations are increasingly common at Pd.73 Moving forward, the development of more robust and general methods for C–H bond oxidation via high-valent Cu catalysis is likely to be a major thrust of research in this field.
Finally, Cu and Pd both catalyze the arylation of indole with diaryliodonium salts; however, under some conditions, the site selectivities of these two reactions are orthogonal (Figure 8c). Whereas the palladium-catalyzed reaction results in selective arylation at the 2-position,87 the Cu-catalyzed methods can be tuned to give exclusive arylation at the 3-position.88 Site-selectivity is one of the most difficult challenges in the field of C–H functionalization. As such, the ability to tune selectivity as a function of the metal is of great potential synthetic utility as well as mechanistically fascinating. Thus far neither of these transformations has been the subject of detailed mechanistic analysis, but the generation of CuIII and PdIV intermediates has been suggested in both cases. Overall, all of the examples in Figure 8 demonstrate the tremendous opportunities available in the concurrent development of high-valent Cu- and Pd-catalysis.
IV. Looking Forward
Moving forward the fields of high-valent Pd and Cu chemistry are sure to have a bright and rapidly expanding future. It will be critical to increase understanding and enhance the chemo-, regio-, and stereoselectivity of catalytic processes involving high-valent Cu and Pd intermediates. In many cases, the coordination sphere of these high-valent metal centers contains multiple possible partners for reductive bond-forming reactions. The ability to control the chemoselectivity of the bond-forming event is of central importance for achieving efficient and high yielding catalytic transformations. In addition, the identification of chiral ligands that are compatible with high-valent Cu and/or Pd-catalysis could potentially enable novel asymmetric conjugate addition, aryl–aryl coupling, C–H oxidation, and/or alkene difunctionalization reactions, which would all be of great value for organic synthesis.
Additional future work in this field will focus on expanding the scope of the fundamental organometallic reactions that are possible at high-valent Cu and Pd centers. Despite the impressive progress described above, the synthetic power of high-valent organometallic intermediates has thus far been explored quite narrowly, with an almost exclusive focus on reductive bond-forming reactions. We anticipate that the design of new ancillary ligands that even better stabilize high-valent Pd and Cu will facilitate the study and application of C–H activation, σ-bond metathesis, migratory insertion, and nucleopalladation reactions at these metal centers. Such reactions could potentially proceed with novel patterns of reactivity and selectivity relative to analogous transformations at low-valent analogues. For example, several exciting preliminary reports have suggested that C–H activation occurs with completely different site selectivity at PdIV versus PdII centers.91–93,94
Finally, a number of recent reports suggest that high-valent organometallic complexes of other late transition metals can catalyse reactions similar to those discussed for CuIII and PdIII/IV above. For example, complexes of NiIII, NiIV, AgII, and AgIII have been observed and/or implicated in carbon–halogen and carbon–nitrogen bond-forming processes.95–100 Further exploration of these is likely to uncover many additional applications for high-valent late transition metals in catalysis.
Acknowledgments
The work from the MSS group described herein was supported by the NIH-NIGMS (GM073836) and by the NSF (CHE-0545909 and CHE-1111563).
References
- 1.Magano J, Dunetz JR. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 2.Evano G, Blanchard N, Toumi M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem Rev. 2008;108:3054–3131. doi: 10.1021/cr8002505. [DOI] [PubMed] [Google Scholar]
- 3.Krause N, editor. Modern Organocopper Chemistry. Wiley-VCH; Weinheim: 2002. [Google Scholar]
- 4.Eckert M, Fleischmann G, Jira R, Bolt HM, Golka K. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2006. Acetaldehyde. [Google Scholar]
- 5.Corbet JP, Mignani G. Selected patented cross-coupling reaction technologies. Chem Rev. 2006;106:2651–2710. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]
- 6.Ullmann F. On a new formation of diphenylamine derivatives. Ber Dtsch Chem Ges. 1903;36:2382–2384. [Google Scholar]
- 7.Ullmann F. Over a new preparation manner of phenylethersalicylic acid. Ber Dtsch Chem Ges. 1904;37:853–854. [Google Scholar]
- 8.Beletskaya IP, Cheprakov AV. Copper in cross-coupling reactions: The post-Ullmann chemistry. Coord Chem Rev. 2004;248:2337–2364. [Google Scholar]
- 9.Monnier F, Taillefer M. Catalytic C–C, C–N, and C–O Ullmann-type coupling reactions. Angew Chem Int Ed. 2009;48:6954–6971. doi: 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]
- 10.Ribas X, Casitas A. The bioinorganic chemistry and organometallic chemistry of copper(III) In: Pignataro B, editor. Ideas in Chemistry and Molecular Sciences: Where Chemistry Meets Life. Wiley-VCH; Weinheim: 2010. pp. 31–57. This is a thorough review on high-valent Cu chemistry. [Google Scholar]
- 11.Sperotto E, van Klink GPM, van Koten G, de Vries JG. The mechanism of the modified Ullmann reaction. Dalton Trans. 2010;39:10338–10351. doi: 10.1039/c0dt00674b. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura E, Mori S. Wherefore art though copper? Structures and reaction mechanisms of organocuprate clusters in organic chemistry. Angew Chem Int Ed. 2000;39:3750–3771. doi: 10.1002/1521-3773(20001103)39:21<3750::aid-anie3750>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Torborg C, Beller M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv Synth Catal. 2009;351:3027–3043. [Google Scholar]
- 14.Hartwig JF. Electronic effects on reductive elimination to form carbon–carbon and carbon–heteroatom bonds from palladium(II) complexes. Inorg Chem. 2007;46:1936–1947. doi: 10.1021/ic061926w. [DOI] [PubMed] [Google Scholar]
- 15.Willert-Porada MA, Burton DJ, Baenziger NC. Synthesis and x-ray structure of bis(trifluoromethyl)(N,N-diethyldithiocarbamato)-copper; a remarkably stable perfluoroalkylcopper(III) complex. J Chem Soc, Chem Commun. 1989:1633–1634. [Google Scholar]
- 16.Furuta H, Maeda H, Osuka A. Doubly N-confused porphyrin: A new complexing agent capable of stabilizing higher oxidation states. J Am Chem Soc. 2000;122:803–807. [Google Scholar]
- 17.Santo R, et al. Diamagnetic-paramagnetic conversion of tris(2-pyridylthio)methylcopper(III) through a structural change from trigonal bipyramidal to octahedral. Angew Chem Int Ed. 2006;45:7611–7614. doi: 10.1002/anie.200603127. [DOI] [PubMed] [Google Scholar]
- 18.Cohen T, Wood J, Dietz AG. Organocopper intermediates in the exchange reaction of aryl halides with salts of copper(I). The possible role of copper(III) Tetrahedron Lett. 1974;40:3555–3558. [Google Scholar]
- 19.Dorigo AE, Wanner J, Schleyer PR. Computational evidence for the existence of CuIII intermediates in addition and substitution reactions with dialkylcuprates. Angew Chem Int Ed. 1995;34:476–478. [Google Scholar]
- 20.Snyder JP. Mechanism of lithium cuprate conjugate addition: Neutral tetracoordinate CuI cuprates as essential intermediates. J Am Chem Soc. 1995;117:11025–11026. [Google Scholar]
- 21.Karlström ASE, Bäckvall JE. Experimental evidence supporting a CuIII intermediate in cross-coupling reactions of allylic esters with diallylcuprate species. Chem Eur J. 2001;7:1981–1989. doi: 10.1002/1521-3765(20010504)7:9<1981::aid-chem1981>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Bertz SH, Cope S, Murphy M, Ogle CA, Taylor BJ. Rapid injection NMR in mechanistic organocopper chemistry. Preparation of the elusive copper(III) intermediate. J Am Chem Soc. 2007;129:7208–7209. doi: 10.1021/ja067533d. This paper reports the first observation of CuIII intermediates in conjugate addition reactions using RI-NMR. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Snyder JP. Organocuprate conjugate addition: The square-planar “CuIII” intermediate. J Am Chem Soc. 2007;129:7210–7211. doi: 10.1021/ja0675346. [DOI] [PubMed] [Google Scholar]
- 24.Bertz SH, Cope S, Dorton D, Murphy M, Ogle CA. Organocuprate cross-coupling: The central role of the copper(III) intermediate and the importance of the copper(I) precursor. Angew Chem Int Ed. 2007;46:7082–7085. doi: 10.1002/anie.200703035. [DOI] [PubMed] [Google Scholar]
- 25.Gärtner T, Henze W, Gschwind RM. NMR detection of Cu(III) intermediates in substitution reactions of alkyl halides with Gilman cuprates. J Am Chem Soc. 2007;129:11362–11363. doi: 10.1021/ja074788y. [DOI] [PubMed] [Google Scholar]
- 26.Bartholomew ER, Bertz SH, Cope S, Murphy M, Ogle CA. Preparation of σ- and π-allylcopper(III) intermediates in SN2 and SN2′ reactions of organocuprate(I) reagents with allylic substrates. J Am Chem Soc. 2008;130:11244–11245. doi: 10.1021/ja801186c. [DOI] [PubMed] [Google Scholar]
- 27.Bertz SH, Miao G, Eriksson M. It’s on lithium! An answer to the recent communication which asked the question: “If the cyano ligand is not on copper, then where is it?”. Chem Commun. 1996:815–816. [Google Scholar]
- 28.Alexakis A, Vastra J, Mangeney P. Acceleration of the conjugate addition of diethyl zinc to enones by either Cu(OTf)2 or trivalent phosphorous ligands. Tetrahedron Lett. 1997;38:7745–7748. [Google Scholar]
- 29.Bartholomew ER, Bertz SH, Cope S, Dorton DC, Murphy M, Ogle CA. Neutral organocopper(III) complexes. Chem Commun. 2008:1176–1177. doi: 10.1039/b717290g. [DOI] [PubMed] [Google Scholar]
- 30.Phipps RJ, Gaunt MJ. A meta-selective copper-catalyzed C–H bond arylation. Science. 2009;323:1593–1597. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]
- 31.Deprez NR, Sanford MS. Synthetic and mechanistic studies of Pd-catalyzed C-H arylation with diaryliodonium salts: Evidence for a bimetallic high oxidation state Pd intermediate. J Am Chem Soc. 2009;131:11234–11241. doi: 10.1021/ja904116k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Hou XL, Li YX, Wu YD. Mechanistic understanding of the unexpected meta selectivity in copper-catalyzed anilide C–H bond arylation. J Am Chem Soc. 2011;133:7668–7671. doi: 10.1021/ja201425e. [DOI] [PubMed] [Google Scholar]
- 33.Das P, Sharma D, Kumar M, Singh B. Copper promoted C–N and C–O type cross-coupling reactions. Curr Org Chem. 2010;14:754–783. [Google Scholar]
- 34.Chan DMT, Lam PYS. Recent advances in copper-promoted C-heteroatom bond cross-coupling reactions with boronic acids and derivatives. In: Hall DG, editor. Boronic Acids. Wiley-VCH; Weinheim: 2005. pp. 205–240. [Google Scholar]
- 35.Casitas A, King AE, Parella T, Costas M, Stahl SS, Ribas X. Direct observation of CuI/CuIII redox steps relevant to Ullmann-type coupling reactions. Chem Sci. 2010;1:326–330. [Google Scholar]
- 36.Kunz K, Scholz U, Ganzer D. Renaissance of Ullmann and Goldberg Reactions – Progress in copper catalyzed C-N-, C-O-, and C-S-coupling. Synlett. 2003;15:2428–2439. [Google Scholar]
- 37.King AE, et al. Copper-catalyzed aerobic oxidative functionalization of an arene C–H bond: Evidence for an aryl-copper(III) intermediate. J Am Chem Soc. 2010;132:12068–12073. doi: 10.1021/ja1045378. This paper demonstrates a CuIII-aryl complex as a catalytically relevant intermediate in a C–H bond amination and methoxylation reaction. [DOI] [PubMed] [Google Scholar]
- 38.Brasche G, Buchwald SL. C-H functionalization/C-N bond formation: Copper-catalyzed synthesis of benzimidazoles from amidines. Angew Chem Int Ed. 2008;47:1932–1934. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]
- 39.Ueda S, Nagasawa H. Synthesis of 2-arylbenzoxazoles by copper-catalyzed intramolecular oxidative C-O coupling of benzanilides. Angew Chem Int Ed. 2008;47:6411–6413. doi: 10.1002/anie.200801240. [DOI] [PubMed] [Google Scholar]
- 40.Hamada T, Ye X, Stahl SS. Copper-catalyzed aerobic oxidative amidation of terminal alkynes: efficient synthesis of ynamides. 2008;130:833–835. doi: 10.1021/ja077406x. [DOI] [PubMed] [Google Scholar]
- 41.Uemura T, Imoto S, Chatani N. Amination of the ortho C–H bonds by the Cu(OAc)2-mediated reaction of 2-phenylpyridines with anilines. Chem Lett. 2006;35:842–843. [Google Scholar]
- 42.Mizuhara T, Inuki S, Oishi S, Fujii N, Ohno H. Cu(II)-mediated oxidative intermolecular ortho C–H functionalisation using tetrahydropyrimidine as the directing group. Chem Commun. 2009:3413–3415. doi: 10.1039/b905586j. [DOI] [PubMed] [Google Scholar]
- 43.Huffman LM, Stahl SS. Carbon–nitrogen bond formation involving well-defined aryl-copper(III) complexes. J Am Chem Soc. 2008;130:9196–9197. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]
- 44.Tye JW, Weng Z, Johns AM, Incarvito CD, Hartwig JF. Copper complexes of anionic nitrogen ligands in the amidation and imidation of aryl halides. J Am Chem Soc. 2008;130:9971–9983. doi: 10.1021/ja076668w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones GO, Liu P, Houk KN, Buchwald SL. Computational explorations of mechanisms and ligand-directed selectivities of copper-catalyzed Ullmann-type reactions. J Am Chem Soc. 2010;132:6205–6213. doi: 10.1021/ja100739h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu HZ, Jiang YY, Fu Y, Liu L. Alternative mechanistic explanation for ligand-dependent selectivities in copper-catalyzed N- and O-arylation reactions. J Am Chem Soc. 2010;132:18078–18091. doi: 10.1021/ja104264v. [DOI] [PubMed] [Google Scholar]
- 47.Ribas X, et al. Aryl C–H activation by CuII to form an organometallic aryl-CuIII species: A novel twist on copper disproportionation. Angew Chem Int Ed. 2002;41:2991–2994. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6. This paper reports an early example of an isolable, well-characterized CuIII-aryl complex. [DOI] [PubMed] [Google Scholar]
- 48.Wendlandt AE, Suess AM, Stahl SS. Copper-catalyzed aerobic oxidative C–H functionalizations: Trends and mechanistic insights. Angew Chem Int Ed. 2011;50:11062–11087. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]
- 49.Ueda S, Nagasawa H. Copper-catalyzed synthesis of benzoxazoles via a regioselective C-H functionalization/C-O bond formation under an air atmosphere. J Org Chem. 2009;74:4272–4277. doi: 10.1021/jo900513z. [DOI] [PubMed] [Google Scholar]
- 50.Siemsen P, Livingston RC, Diederich F. Acetylenic coupling: A powerful tool in molecular construction. Angew Chem Int Ed. 2000;39:2632–2657. [PubMed] [Google Scholar]
- 51.Gao Y, et al. Copper-catalyzed aerobic oxidative coupling of terminal alkynes with H-phosphates leading to alkynylphosphonates. J Am Chem Soc. 2009;131:7956–7957. doi: 10.1021/ja9023397. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Hao XS, Goodhue CE, Yu JQ. Cu(II)-catalyzed functionalizations of aryl C–H bonds using O2 as an oxidant. J Am Chem Soc. 2006;128:6790–6791. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]
- 53.Wang W, Luo F, Zhang S, Cheng J. Copper(II)-catalyzed ortho-acyloxylation of the 2-arylpyridines sp2 C–H bonds with anhydrides, using O2 as terminal oxidant. J Org Chem. 2010;75:2415–2418. doi: 10.1021/jo1000719. [DOI] [PubMed] [Google Scholar]
- 54.Casitas A, Canta M, Solá M, Costas M, Ribas X. Nucleophilic aryl fluorination and aryl halide exchange mediated by a CuI/CuIII catalytic cycle. J Am Chem Soc. 2011;133:19386–19392. doi: 10.1021/ja2058567. [DOI] [PubMed] [Google Scholar]
- 55.Heck RF. Aromatic haloethylation with palladium and copper halides. J Am Chem Soc. 1968;90:5538–5542. [Google Scholar]
- 56.Fahey DR. The coordination-catalyzed ortho-halogenation of azobenzene. J Organomet Chem. 1971;27:283–292. [Google Scholar]
- 57.Tremont SJ, Rahman Hu. Ortho-alkylation of acetanilides using alkyl halides and palladium acetate. J Am Chem Soc. 1984;106:5759–5760. [Google Scholar]
- 58.Byers PK, Canty AJ, Skelton BW, White AH. Oxidative addition of iodomethane to [PdMe2(bpy)] and the x-ray structure of the organopalladium(IV) product fac-[PdMe3(bpy)I] (bpy=2,2′bipyridyl) J Chem Soc, Chem Commun. 1986:1722–1724. [Google Scholar]
- 59.Cotton FA, et al. High yield syntheses of stable, singly bonded Pd26+ compounds. J Am Chem Soc. 2006;128:13674–13675. doi: 10.1021/ja0656595. [DOI] [PubMed] [Google Scholar]
- 60.Roy AH, Hartwig JF. Directly observed reductive elimination of aryl halides from monomeric arylpalladium(II) halide complexes. J Am Chem Soc. 2003;125:13944–13945. doi: 10.1021/ja037959h. [DOI] [PubMed] [Google Scholar]
- 61.Collman JP, Hegedus LS, Norton JR, Finke RG. Principles and Applications of Organotransition Metal Chemistry. 2. University Science Books; Mill Valley, CA: 1982. pp. 322–333. [Google Scholar]
- 62.Canty AJ. Organopalladium and platinum chemistry in oxidizing milieu as models for organic synthesis involving the higher oxidation states of palladium. Dalton Trans. 2009;47:10409–10417. doi: 10.1039/b914080h. [DOI] [PubMed] [Google Scholar]
- 63.Muñiz K. High-oxidation-state palladium catalysis: New reactivity for organic synthesis. Angew Chem Int Ed. 2009;48:9412–9423. doi: 10.1002/anie.200903671. This is a thorough review on high-valent Pd chemistry. [DOI] [PubMed] [Google Scholar]
- 64.Xu LM, Li BJ, Yang Z, Shi ZJ. Organopalladium(IV) chemistry. Chem Soc Rev. 2010;39:712–733. doi: 10.1039/b809912j. [DOI] [PubMed] [Google Scholar]
- 65.Vincente J, Arcas A, Julia-Hernandez F, Bautista D. Synthesis, isolation, and characterization of an organometallic triiodopalladium(IV) complex. Quantitative and regioselective synthesis of two C–I reductive elimination products. Inorg Chem. 2011;50:5339–5341. doi: 10.1021/ic2006869. [DOI] [PubMed] [Google Scholar]
- 66.Arnold PL, Sanford MS, Pearson SM. Chelating N-heterocyclic carbene alkoxide as a supporting ligand for PdII/IV C-H bond functionalization catalysis. J Am Chem Soc. 2009;131:13912–13913. doi: 10.1021/ja905713t. [DOI] [PubMed] [Google Scholar]
- 67.Ball ND, Sanford MS. Synthesis and reactivity of a mono-σ-aryl palladium(IV) fluoride complex. J Am Chem Soc. 2009;131:3796–3797. doi: 10.1021/ja8054595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alsters PL, et al. Rigid five- and six-membered C,N,N′-bound aryl, benzyl, and alkyl organopalladium complexes: sp2 vs sp3 C-H activation during cyclopalladation and palladium(IV) intermediates in oxidative addition reactions with dihalogens and alkyl halides. Organometallics. 1993;12:1831–1844. [Google Scholar]
- 69.Whitfield SR, Sanford MS. Reactivity of Pd(II) complexes with electrophilic chlorinating reagents: Isolation of Pd(IV) products and observation of C-Cl bond-forming reductive elimination. J Am Chem Soc. 2007;129:15142–15143. doi: 10.1021/ja077866q. [DOI] [PubMed] [Google Scholar]
- 70.Furuya T, et al. Mechanism of C–F reductive elimination from palladium(IV) fluoride. J Am Chem Soc. 2010;132:3793–3807. doi: 10.1021/ja909371t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Powers DC, Xiao DY, Geibel MAL, Ritter T. On the mechanism of palladium-catalyzed aromatic C–H oxidation. J Am Chem Soc. 2010;132:14530–14536. doi: 10.1021/ja1054274. This paper describes mechanistic studies implicating a PdIII dimer intermediate in a Pd-catalyzed C–H chlorination reaction described in ref. Error! Bookmark not defined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sehnal P, Taylor RJK, Fairlamb IJS. Emergence of palladium(IV) chemistry in synthesis and catalysis. Chem Rev. 2010;110:824–889. doi: 10.1021/cr9003242. [DOI] [PubMed] [Google Scholar]
- 73.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. This is a comprehensive review of Pd-catalyzed ligand-directed C–H functionalization reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu T, Yin G, Liu G. Palladium-catalyzed intramolecular aminofluorination of unactivated alkenes. J Am Chem Soc. 2009;131:16354–16355. doi: 10.1021/ja9076588. [DOI] [PubMed] [Google Scholar]
- 75.Michael FE, Sibbald PA, Cochran BM. Palladium-catalyzed intramolecular chloroamination of alkenes. Org Lett. 2008;10:793–796. doi: 10.1021/ol702922c. [DOI] [PubMed] [Google Scholar]
- 76.Kalyani D, Satterfield AD, Sanford MS. Palladium-catalyzed oxidative arylhalogenation of alkenes: Synthetic scope and mechanistic insights. J Am Chem Soc. 2010;132:8419–8427. doi: 10.1021/ja101851v. This paper describes high-valent Pd-catalyzed arylhalogenation reactions of α-olefins and demonstrates complementarity to low-valent methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oestreich M, editor. The Mizoroki-Heck Reaction. Wiley; Hoboken: 2009. [Google Scholar]
- 78.Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 79.Grushin VV. The organometallic fluorine chemistry of palladium and rhodium: studies toward aromatic fluorination. Acc Chem Res. 2010;43:160–171. doi: 10.1021/ar9001763. [DOI] [PubMed] [Google Scholar]
- 80.Cho EJ, et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science. 2010;328:1679–1681. doi: 10.1126/science.1190524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ball ND, Gary JB, Ye Y, Sanford MS. Mechanistic and computational studies of oxidatively-induced bond-formation at Pd: Rational design of room temperature trifluoromethylation. J Am Chem Soc. 2011;133:7577–7584. doi: 10.1021/ja201726q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Truesdale L, Yu J-Q. Pd(II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter. J Am Chem Soc. 2010;132:3648–3649. doi: 10.1021/ja909522s. This paper reports a novel C–H trifluoromethylation reaction catalyzed by putative high-valent Pd. [DOI] [PubMed] [Google Scholar]
- 83.Ye Y, Ball ND, Kampf JW, Sanford MS. Oxidation of catalytically relevant palladium dimer with “CF3+”: Formation and reactivity of a monomeric palladium(IV) aquo product. J Am Chem Soc. 2010;132:14682–14687. doi: 10.1021/ja107780w. This paper provides evidence supporting the possibility of a high-valent PdIV intermediate in the C–H trifluoromethylation reaction described in ref. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Powers DC, Geibel MAL, Klein JEMN, Ritter T. Bimetallic palladium catalysis: Direct observation of Pd(III)-Pd(III) intermediates. J Am Chem Soc. 2009;131:17050–17051. doi: 10.1021/ja906935c. [DOI] [PubMed] [Google Scholar]
- 85.Dick AR, Hull KL, Sanford MS. A highly selective catalytic method for the oxidative functionalization of C-H bonds. J Am Chem Soc. 2004;126:2300–2301. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 86.Xu J, et al. Copper-catalyzed trifluoromethylation of aryl boronic acids using a CF3+ reagent. Chem Commun. 2011;47:4300–4302. doi: 10.1039/c1cc10359h. [DOI] [PubMed] [Google Scholar]
- 87.Deprez NR, Kalyani D, Krause A, Sanford MS. Room temperature palladium-catalyzed 2-arylation of indoles. J Am Chem Soc. 2006;128:4972–4973. doi: 10.1021/ja060809x. [DOI] [PubMed] [Google Scholar]
- 88.Phipps RJ, Grimster NP, Gaunt MJ. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J Am Chem Soc. 2008;130:8172–8174. doi: 10.1021/ja801767s. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J, Khaskin E, Anderson NP, Zavalij PY, Vedernikov AN. Catalytic Aerobic Oxidation of Substituted 8-Methylquinolines in PdII-2,6-Pyridine Dicarboxylic Acid Systems. Chem Commun. 2008:3625–3627. doi: 10.1039/b803156h. [DOI] [PubMed] [Google Scholar]
- 90.Zhang YH, Yu JQ. Pd(II)-Catalyzed Hydroxylation of Arenes with 1 atm O2 or Air. J Am Chem Soc. 2009;131:14654–14655. doi: 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]
- 91.Hull KL, Lanni EL, Sanford MS. Highly regioselective catalytic oxidative coupling reactions: Synthetic scope and mechanistic insights. J Am Chem Soc. 2006;128:14047–14049. doi: 10.1021/ja065718e. [DOI] [PubMed] [Google Scholar]
- 92.Rosewall CF, Sibbald PA, Liskin DV, Michael FE. Palladium-catalyzed carboamination of alkenes promoted by N-fluorobenzenesulfonimide via C–H activation of arenes. J Am Chem Soc. 2009;131:9488–9489. doi: 10.1021/ja9031659. [DOI] [PubMed] [Google Scholar]
- 93.Hickman AJ, Sanford MS. Catalyst control of site selectivity in the PdII/IV-catalyzed direct arylation of naphthalene. ACS Catal. 2011;1:170–174. [Google Scholar]
- 94.Racowski JM, Ball ND, Sanford MS. C–H bond activation at palladium(IV) centers. J Am Chem Soc. 2011;133:18022–18025. doi: 10.1021/ja2051099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grove DM, van Koten F, Zoet R, Murrall NW, Welch AJ. Unique stable organometallic nickel(III) complexes; syntheses and the molecular structure of [Ni[C6H3(CH2NMe2)2-2,6]I2] J Am Chem Soc. 1983;105:1379–1380. [Google Scholar]
- 96.Ceder RM, Granell J, Muller G. Preparation of five-membered nickelacycles with anionic C-N-N′ terdentate ligands. X-ray crystal structure of [NiCl{2-(CH=NCH2CH2NMe2)-3-ClC6H3}] Organometallics. 1996;15:4618–4624. [Google Scholar]
- 97.Higgs AT, Zinn PJ, Simmons SJ, Sanford MS. Oxidatively induced carbon-halogen bond-forming reactions at nickel. Organometallics. 2009;28:6142–6144. [Google Scholar]
- 98.Muñiz K, Streuff J. Exploring the nickel-catalyzed oxidation of alkenes: A diamination by sulfanamide transfer. Angew Chem Int Ed. 2007;46:7125–7127. doi: 10.1002/anie.200702160. [DOI] [PubMed] [Google Scholar]
- 99.Lin BL, Clough CR, Hillhouse GL. Interactions of aziridines with nickel complexes: Oxidative-addition and reductive-elimination reactions that break and make C-N bonds. J Am Chem Soc. 2002;124:2890–2891. doi: 10.1021/ja017652n. [DOI] [PubMed] [Google Scholar]
- 100.Tang P, Furuya T, Ritter T. Silver-catalyzed late-stage fluorination. J Am Chem Soc. 2010;132:12150–12154. doi: 10.1021/ja105834t. [DOI] [PMC free article] [PubMed] [Google Scholar]