Abstract
The following, from the 12th OESO World Conference: Cancers of the Esophagus, includes commentaries on the role of the nurse in preparation of esophageal resection (ER); the management of patients who develop high-grade dysplasia after having undergone Nissen fundoplication; the trajectory of care for the patient with esophageal cancer; the influence of the site of tumor in the choice of treatment; the best location for esophagogastrostomy; management of chylous leak after esophagectomy; the optimal approach to manage thoracic esophageal leak after esophagectomy; the choice for operational approach in surgery of cardioesophageal crossing; the advantages of robot esophagectomy; the place of open esophagectomy; the advantages of esophagectomy compared to definitive chemoradiotherapy; the pathologist report in the resected specimen; the best way to manage patients with unsuspected positive microscopic margin after ER; enhanced recovery after surgery for ER: expedited care protocols; and long-term quality of life in patients following esophagectomy.
Keywords: esophageal resection, Nissen fundoplication, esophagogastrostomy, esophagectomy, chemoradiotherapy, OESO
Concise summary
The nurse plays a major role in the implementation of the selected treatment and ensures compliance at all phases of the protocol. The nursing care before esophagectomy aims to maintain an optimal nutritional status for the patient submitted to parenteral nutrition and to reduce the risk of infection by immuno-nutrition. The nurse will also have to control the patient’s respiratory function and tolerance to adjuvant therapy. In the perioperative period, multiple categories of nurses are also involved: the circulating nurses, the scrub nurse, the endocopy nurse, and the anesthesiology nurse.
Esophagectomy has been commonly recommended when high-grade dysplasia (HGD) is discovered. More recently, however, multiple studies have confirmed the safety and efficacy of both endoscopic mucosal resection (EMR) and radiofrequency ablation (RFA) techniques in the management of these patients. Endoscopic therapy can be safely recommended as a first step in dealing with the disease, though esophagectomy may be necessary if this fails to fully eliminate the dysplastic mucosa.
A succinct review is made of the current surgical management of biopsy-proven esophageal cancer (beyond T1a lesions) at the Mayo Clinic. Resectability, usually considered first, is predominantly determined by tumor stage, by fine-needle aspiration guided and performed at the time of the endoscopic ultrasound (EUS). Combined positron emission tomography and computed tomography (PET-CT) scanning using fluorodeoxyglucose is currently the most useful adjunctive test for uncovering otherwise occult distant metastases. When early-stage esophageal cancer is identified (T0–T2 and N0), upfront surgical resection is advocated in patients who fit the criteria for operability. For patients with locally advanced esophageal cancer (T3/N0 or any T with positive lymph nodes), neoadjuvant chemotherapy with concomitant radiation therapy is recommended, with restaging of the patient following approximately 6 weeks to rule out unresectable disease. Esophagectomy will be performed, unless the identifiable malignancy has extended beyond the confines of the planned resection. Operability can then be broken down into quantifying the operative risk and identifying opportunities to mitigate this risk. A temporary feeding jejunostomy is performed at the time of esophageal resection (ER). All patients are extubated immediately following the procedure.
The primary goal of surgery in patients with a resectable adenocarcinoma of the gastroesophageal junction (GEJ) should be a complete en bloc resection of the tumor and its lymph nodes, with a microscopically tumor-free resection margin. For a Siewert type I tumor, an en bloc esophagectomy with resection of the proximal stomach and reconstruction with a gastric tube seems to be the preferable approach. In type III tumors, a total gastrectomy with a transhiatal resection of the distal esophagus is the treatment of first choice. For the treatment of a Siewert type II adenocarcinoma of the GEJ, the optimal surgical approach remains controversial. No clear evidence for the superiority of esophagectomy with proximal hemigastrectomy or total gastrectomy with resection of the distal esophagus has been provided for treatment of these tumors.
The location for esophagogastrostomy depends on the ease of anastomosis, tension on the repair, the incidence and severity of leaks, the ability to diagnose and manage these problems, and oncologic issues. Recent guidelines would suggest that a high intrathoracic anastomosis above the azygous vein or cervical anastomoses are acceptable alternatives. The mechanical circular stapled and hand-sewn techniques for the esophagogastric anastomosis have equivalent outcomes. Three-field lymph node dissection may be considered with either squamous cell or adenocarcinoma, although there is little adoption of this approach outside of Japan. Total minimally invasive esophagectomy or its hybrid versions are acceptable alternatives to open approaches where institutional expertise is available. Whenever possible, a low intrathoracic anastomosis should be avoided.
Anatomical variations of the thoracic duct are present in up to 40% of the cases, and chylothorax is associated with life-threatening metabolic, immunologic, and respiratory complications. Prophylactic supradiaphragmatic duct ligation during transthoracic esophagectomy has been recommended in order to prevent inadvertent damage and postoperative chylous fistula. Since a spontaneous resolution of the chylous fistula is possible, a 2-week conservative trial with total parenteral nutrition and pleural drainage appears to be justified in patients with a chyle output of less than 1000 mL/day. If medical treatment fails, patients should undergo reoperation and ligation of the thoracic duct. The introduction of video-assisted thoracic surgery has offered a safe and effective alternative for treatment. In some circumstances, such as previously failed transthoracic procedures, transabdominal ligation of the cisterna chyli via laparotomy or laparoscopy represents a viable alternative to the thoracic approach.
Guidelines for the optimal management of intrathoracic leak have yet to be established. Management begins intraoperatively. Additional prophylactic interventions such as omental reinforcement are advocated by many surgeons with significant decrease in anastomotic leak rates. Adequate prophylactic drainage, therefore, is a key principle for management of anastomotic leak. If the leak appears well-contained, endoscopic examination is indicated for both diagnostic and therapeutic management. Drain manipulation and anastomotic dilation can be used successfully for early management of well-contained leaks. Endoscopic stent placement at the time of the initial endoscopic evaluation is increasingly used for management of these cases, providing immediate coverage of the defect and enabling earlier oral intake. Surgical intervention is reserved for patients with symptomatic or uncontained intrathoracic leaks and those for whom conservative management has failed. It is rare that the anastomosis requires revision and revision is rarely successful. If, however, the conduit is non-viable, conduit take-down and esophageal diversion should be performed.
In large series of patients with cancer of the proximal part of the stomach and the abdominal esophageal segment, there was no difference in survival rate between the groups of transthoracic and transhiatal approaches, in correlation with the overall low frequency of tumor dissemination in mediastinal lymph nodes. Five-year overall and relapse-free survival was slightly higher in the group that underwent the transthoracic approach than in the group that underwent the transhiatal approach, parallel to more frequent nonradical resections of the esophagus and recurrent mediastinal metastases following transhiatal resection.
Robot-assisted thoracolaparoscopic esophagectomy facilitates a complex minimally invasive procedure with a stable, enlarged, three-dimensional field of view and articulated instruments that facilitate dissection with seven degrees of freedom. Manipulation is enhanced so that a very precise dissection can be performed, even on moving targets like the aorta and pericardium. Thereby, the oncologic results might improve, while maintaining the advantages of minimally invasive surgery. Compared to conventional open transthoracic esophagectomy, robot-assisted thoracolaparoscopic esophagectomy is accompanied by reduced blood loss, reduction of intensive care–unit stay, and a lower percentage of cardiopulmonary complications. Until now, the level of evidence for robot-assisted minimally invasive thoracolaparoscopic esophagectomy has been suboptimal and based on case series or expert opinions only. A monocenter randomized controlled trial to compare robot-assisted minimally invasive thoracolaparoscopic esophagectomy with conventional open transthoracic esophagectomy is currently underway in Utrecht.
As thoracic surgeons may lack experience with laparoscopic procedures, the same goes for gastrointestinal (GI) surgeons lacking experience with thoracoscopic procedures. It can therefore be safely asserted that, although minimally invasive surgery for esophageal cancer is feasible and perhaps superior to open esophagectomy, the majority of esophageal surgery is done by means of an open procedure that remains the gold standard for most surgical teams. The high complexity of the totally minimally invasive procedure necessitates technical skills that provide a threshold for most surgeons.
Late toxicity is an important issue that could impair quality of life after chemoradiotherapy (CRT), and previous studies showed that the negative impact of esophagectomy on quality of life. The role of adding neoadjuvant CRT to surgery has been extensively investigated in the past, and meta-analyses have shown that it was associated with complete tumor response and significant survival benefit. Investigations on the role of definitive CRT in patients with squamous esophageal carcinoma demonstrated that esophagectomy performed after neoadjuvant therapy provides better local control of the disease, but does not prolong overall survival compared with definitive CRT. Concurrent CRT is a potentially curative nonsurgical option for locally advanced esophageal cancer, with pathological complete response (pCR) ranging from 13% to 49%, but the rate of persistent and recurrent disease within the esophagus remains high and surgical treatment of these tumors may improve disease-free survival (DFS).
Pathology reports should encompass all important information regarding the tumor and the quality of the surgical procedure. The definition of the proximal and distal resection margin is clear. Involvement of one of these margins is associated with bad clinical outcome. Regarding the circumferential resection margin of esophagectomy specimens, there are different definitions among the major pathology schools. The current edition of the American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) TNM classification provides a detailed classification of lymph node metastase (LNMs), which now parallels gastric cancer. Several tumor-regression grading systems are described in the literature, which aim to categorize the amount of regressive changes after cytotoxic treatment. Mostly they refer to the percentage of residual tumor in relation to the previous tumor site or they estimate the amount of therapy-induced fibrosis in relation to residual tumor. A high number of original papers offer additional data concerning potential prognostic parameters that might be also considered for implementation in future standard reporting. For conventional CRT, at present no validated markers for response prediction are used in practice, although the deregulation of many molecules has been shown to be associated with later treatment response. It should be noted that a very promising study showed the successful immunohistochemical application of a three-gene panel in esophageal adenocarcinomas (EACs), which confirmed previous findings from an independent cohort.
The circumferential resection margin status has an impact on survival, and patients with tumor present at the resection margin have significantly worse median overall survival rates compared to those with a confirmed negative margin. Neoadjuvant treatment has been shown to consistently reduce circumferential margin rates for locally advanced esophageal tumors. Regional nodal disease should guide adjuvant treatment decisions, but there is a lack of evidence to support adjuvant treatment for patients with positive circumferential resection margin status.
ER provides a particular challenge when developing streamlined, standardized care. Enhanced recovery describes systems of care delivery to reduce the physiological stress response and optimize postoperative recovery, involving a multimodal approach to perioperative management. The goal is to improve quality of care with reduced length of stay, improved patient satisfaction, and reduced cost. Approaches to these systems are centered on interventions in the preoperative, intraoperative, and postoperative phases of care. ER pathways have demonstrated improvements in critical care and total inpatient stay, as well as improving patient satisfaction and conferring some cost savings.
As a result of surgical and oncological improvements, more people are surviving both esophageal cancer surgery and esophageal cancer itself. Quality of life after treatment has therefore become more important than ever, and its measurement by tools such as the EORTC QLQ-C30 and its add-on module OG25 has become an integral outcome for many trials. Most symptom scores reach a peak in the immediate postoperative period following esophagectomy and can take months or years to improve. Likewise, the functional scores show an immediate dip after surgery and slowly improve back to preoperative levels by a year. Minimally invasive esophagectomy appears to offer the potential to improve short-term effects on quality of life but is unlikely to affect long-term issues.
1. Role of the nurse in preparation of transhiatal resection of esophageal cancer without thoracotomy
Renè Lambert
lambert@iarc.fr
In the preparation of the patient for surgery of esophageal cancer, with optimal nutritional conditions and a wellness program, the role of the nurse also includes assessment of tolerance to neoadjuvant therapy before surgery. Of course, in a preliminary step, the medical staff, including nurses, should identify those cases not able to complete a surgical protocol. When the surgical option is adopted, the nurse plays a major role in the implementation of the selected treatment and ensures compliance at all phases of the protocol. The nurse’s advocacy role concerns feasibility of surgery, informed consent of the patient, and documentation of all clinical events. In reference to the perioperative period, multiple categories of nurses are involved: the circulating nurses take care of the nursing during the intervention, with scrub nurses working directly with the surgeon and a nurse endoscopist when upper GI endoscopy is part of the protocol. The nurse endoscopist should have enough experience and have participated in a required number of procedures of digestive endoscopy. In addition, anesthetic nurses will control the sedation, with or without the presence of an anesthesiologist. In synthesis, the nursing care before esophagectomy aims to maintain the nutritional status of the patient, control his ventilation and respiratory function, and control his tolerance to adjuvant therapy.
As a rule, the surgical resection of the tumor is the adapted decision to be taken if esophageal cancer is confirmed; this means resection of the thoracic esophagus. A squamous cell cancer develops in the squamous epithelium of the upper, middle, or distal sections of the esophagus. A recent decrease in the incidence of squamous cell cancer has been observed in most countries, including in Asia and South America. An adenocarcinoma develops on the metaplastic mucosa of a Barrett’s esophagus (BE). For many years, the risk of cancer was believed to be linked to the presence of intestinal metaplasia. Recently, it has been shown that gastric metaplasia with simple columnar cells is a more typical precursor.1 The well-known method of esophagectomy with thoracotomy for the resection of the esophagus was associated with a high toll of pulmonary complications. In 1980,2 it was replaced by a less aggressive intervention: the transhiatal resection of the esophagus with a short laparotomy without thoracotomy and completed by an incision in the left of the neck. With this technique, the toll of pulmonary complications is much lower and the 5-year survival after resection is slightly higher.3,4 Recently, the surgical procedure was made still less traumatic when video laparoscopy replaced laparotomy. As shown in a recent publication,5 the nursing care in the period before esophagectomy helps to maintain an optimal nutritional status for the patient submitted to parenteral nutrition and to reduce the risk of infection by feeding him through immunonutrition based on administration of glutamine, arginine, and polyunsaturated omega-3 fatty acids. During the same period, the nursing care also ensures the control of his ventilation and respiratory function and the control of his tolerance to adjuvant therapy, which combines radiotherapy and chemotherapy with cisplatin and paclitaxel.
2. How do we manage the patient who develops HGD after having undergone Nissen fundoplication?
Roger P. Tatum
rtatum@uw.edu
Many studies have confirmed the efficacy of Nissen fundoplication in patients with BE. In fact, regression of BE has been observed in approximately 14–33% of patients after antireflux surgery.6–10 Nonetheless, a small percentage of patients have been observed to develop progression of the disease to either HGD or, less commonly, esophageal cancer in follow-up after fundoplication.11
Because HGD is associated with a rate of progression to EAC of around 25% within 2–3 years of diagnosis,12,13 esophagectomy has been commonly recommended when HGD is discovered. More recently, however, multiple studies have confirmed the safety and efficacy of both EMR and RFA techniques in the management of HGD.13,14 One recent retrospective analysis comparing the outcomes of endoscopic treatment (ablation and/or EMR) with esophagectomy for HGD (40 patients) or intramucosal adenocarcinoma (61 patients) by Zehetner and colleagues found similar 3-year survival rates (94%) between the two treatment strategies, with significantly lower morbidity in the patients undergoing endoscopic therapy, as one might expect. Patients in the endoscopic group underwent a median of three treatment sessions each. There was a 10% failure rate among the HGD patients undergoing endoscopic therapy (two of 22 patients); however, these patients subsequently received an esophagectomy for persistent HGD.14 Notably, EMR and ablation techniques are both feasible and effective in patients who have previously undergone fundoplication.13
Another important consideration in addressing this question is the possible cause for progression of BE despite the presence of an antireflux procedure. Multiple studies have suggested that patients who exhibit progression have abnormal pH studies or other evidence of a failed fundoplication.7,11 In another study of 75 patients with BE who had 5-year follow-up after antireflux surgery by Zehetner et al., patients with a failed fundoplication were seven times more likely to exhibit progression to HGD or EAC than those with an intact fundoplication.11 Therefore, not only is continued surveillance of BE recommended after fundoplication,15 but it also stands to reason that continued acid suppression is important over time.
Given the above evidence and the availability of endoscopic techniques that are effective alternatives to esophagectomy in patients with HGD, it appears that a combined approach to the patient who presents with progression to HGD after antireflux surgery is warranted. Endoscopic therapy can be safely recommended as a first step in dealing with the disease, though esophagectomy may be necessary if this fails to fully eliminate the dysplastic mucosa. It is also important to determine whether or not the patient is continuing to experience pathologic acid exposure and whether or not they are symptomatic. A re-do fundoplication should be performed if there is evidence of fundoplication failure, though this should only be done once eradication of HGD (and ideally of all esophageal metaplasia) has been confirmed.14
3. Surgical management of esophageal cancer: how we do it
Stephen D. Cassivi
cassivi.stephen@mayo.edu
Background
Esophageal cancer, the focus of the 12th OESO World Conference in 2013, is the eighth most common cause of cancer death worldwide.16 The incidence of adenocarcinoma of the esophagus, usually of the distal esophagus or GEJ, continues to increase dramatically and accounts for the majority of cases in North America and Western Europe.17 Surgical resection has been a mainstay of curative-intent treatment of esophageal cancer for over a century. Its role, applicability, and how it is fits into the overall treatment of esophageal cancer has evolved significantly. This article presents a succinct review of current surgical management of biopsy-proven esophageal cancer (beyond T1a lesions) at the Mayo Clinic.
Surgical work-up
Two evocative initials can summarize the surgical work-up of a patient with a diagnosis of any cancer, including esophageal cancer: O and R. O is used to connote operability and refers to patient factors. R is used to connote resectability and implicates tumor-related factors.
Resectability, usually considered first, is predominantly determined by tumor stage. In the case of esophageal cancer, staging is accomplished by history and physical examination followed by deliberately focused investigations emanating from those initial combined modalities. Beyond this, T status is best determined by esophageal EUS. Currently, the most accurate means to determine N status is fine-needle aspiration (FNA) guided and performed at the time of the EUS. Combined PET/CT scanning using18 fluorodeoxyglucose (FDG) is currently the most useful adjunctive test for uncovering otherwise occult distant metastases.
With the introduction of the 7th edition of AJCC staging for esophageal cancer, we now have much more accurate prognostic information available.18,19 For esophageal cancer, clinical staging also has material importance, as it assists in guiding treatment options. Our surgical algorithm follows the pathway summarized in Figure 1. When early-stage esophageal cancer is identified (T0–T2 and N0), we advocate for upfront surgical resection in patients who fit the criteria for operability detailed below.20 For patients with locally advanced esophageal cancer (T3/N0 or any T with positive lymph nodes), we recommend neoadjuvant chemotherapy with concomitant radiation therapy.21
Figure 1.

Surgical algorithm for esophageal cancers.
Following a recovery period of approximately 4 weeks, patients are restaged. Since it is virtually impossible to ascertain whether the induction therapy has achieved a complete eradication of the malignancy, and knowing that even in instances of cPR only a 50% 5-year survival is observed, our goal at this point is to rule out unresectable disease (i.e., local progression to unresectability or the appearance of distant metastases).22 Therefore, it is our strategy that, following neoadjuvant therapy, we will proceed to ER unless the identifiable malignancy has extended beyond the confines of the planned resection. For that reason, our restaging work-up is limited to a history and physical examination coupled with PET/CT.
Operability, as the second component of patient evaluation, is predicated on patient-related factors and is therefore specific to each individual patient considered for ER. Operability can be broken down into quantifying the operative risk and identifying opportunities to mitigate this risk. A number of tools are available or can be adapted to assist the surgeon in quantifying operative risk, such as the Thoracic Revised Cardiac Risk Index for predicting the risk of major cardiac complications.23 Depending on the specific patient situation, various opportunities are available to mitigate operative risk. Among these options are preoperative pulmonary rehabilitation, tobacco cessation, use of less invasive surgical approaches (thoracoscopy/laparoscopy), aggressive postoperative pulmonary physiotherapy, and others. Once resectability and operability have been assessed, this allows for an informed discussion with the patient regarding the apparent risk/benefit profile for that specific patient.
Surgical approach
We endeavor to individualize the operative approach to the specific patient and tumor characteristics.20 One size does not fit all purposes in the realm of ER. Regardless of the operative approach ultimately chosen, a number of fundamental principles are, however, generalizable. We aim to have all our patients extubated immediately following the procedure in an effort to improve on early physical mobilization and pulmonary ablutionary care. This is achieved in over 98% of cases. We also have adopted the practice of placing a temporary feeding jejunostomy at the time of ER. This is integral to our postoperative nutritional strategy of avoiding early oral feeding, as summarized in Figure 2.24
Figure 2.
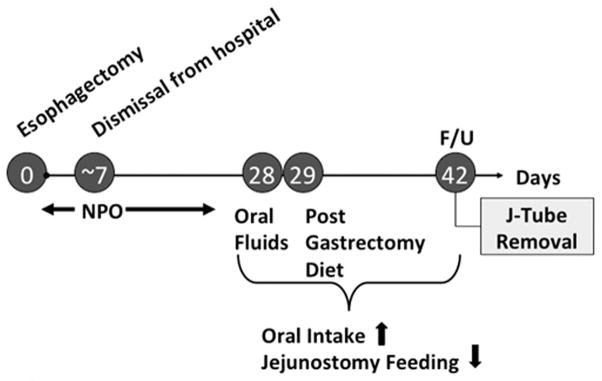
Postoperative nutritional strategy of avoiding early oral feeding.
Follow-up
We see our patients back in follow-up 6 weeks after ER in a multidisciplinary esophageal cancer clinic, where we assess their postoperative progress, remove their temporary feeding jejunostomy tube, review their pathology findings, and, in this context, discuss prognosis and follow-up/further treatment recommendations. Barring any significant complicating issues, we plan on seeing the patient back every 6 months for the first 2 years for a clinical examination coupled with a CT scan of the chest and upper abdomen. Ancillary testing is only ordered as clinically indicated. The interval between follow-up visits is extended to 12 months after the 2-year postoperative anniversary.
4. How do esophagogastric junction tumors differ from esophageal and gastric tumors?
Leonie Haverkamp, Kevin Parry, and Richard van Hillegersberg
L.Haverkamp@umcutrecht.nl
Siewert and colleagues developed a classification system to categorize adenocarcinomas of the GEJ into three different types according to their anatomic and topographic location.25 Type I arises from 1 to 5 cm proximal to the anatomic GEJ (tumor of the distal esophagus), type II arises from 1 cm proximal to 2 cm distal to the GEJ (true cardiacarcinoma), and type III arises from 2 to 5 cm distal to the GEJ (subcardial gastric carcinoma).
The primary goal of surgery in patients with a resectable adenocarcinoma of the GEJ should be a complete en bloc resection of the tumor and its lymph nodes, with a microscopically tumor-free resection margin (R0 resection). At the same time, a maximal postoperative quality of life must be achieved. The most important factor in predicting long-term survival in patients with GEJ tumors is considered to be a R0 resection.
To achieve this primary goal, several surgical modalities are being used in the treatment of GEJ tumors. For a type I tumor, an en bloc esophagectomy (preferably transthoracic) with resection of the proximal stomach and reconstruction with a gastric tube seems to be the preferable approach. In type III tumors, a total gastrectomy with a transhiatal resection of the distal esophagus is the treatment of first choice.26,27 The optimal surgical approach of type II remains controversial. The choice of the surgical approach is mainly based on the preference of the surgeon. Some centers prefer an esophagectomy with a proximal hemigastrectomy,28 while others prefer a total gastrectomy with resection of the distal esophagus (extended gastrectomy).27 However, no surgical therapy has yet been proven superior in the treatment of type II tumors.
A review of the literature did not provide evidence for a better outcome in 5-year survival for either esophagectomy or gastrectomy, nor was there a difference in the R0 resection rate. There might be a higher rate of mortality and morbidity after esophagectomy and a better quality of life after a gastrectomy. Further research is needed to determine the optimal surgical strategy. This should preferably consist of a multicenter randomized controlled trial comparing esophagectomy versus gastrectomy in type II tumors.
In conclusion, no clear evidence for the superiority of esophagectomy or gastrectomy for the treatment of a Siewert type II adenocarcinoma of the GEJ has been provided.
5. What is the best location for esophagogastrostomy?29–40
Mark J. Krasna
mkrasna@meridianhealth.com
Introduction
The choice of location for esophagogastrostomy after esophagectomy depends on several key factors. These include ease of anastomosis; tension on the repair; the incidence and severity of leaks, if they occur; the ability to diagnose problems; and the ease of management of these problems. Finally, the decision may be affected by oncologic issues that may favor one approach over the other. Ultimately, the surgeon must decide whether to perform the anastomosis in the neck or the chest. The following discussion is based on the recent Society of Thoracic Surgeons guidelines.
After esophagogastrectomy, the goals should include achieving adequate margins. Resection margins measured in the patient in the OR of 8–10 cm proximally and 7 cm distally should be obtained to achieve an R0 resection (recommendation class IIB, level of evidence C). Likewise, the resected material should be adequately handled to assess and report on circumferential radial margin status (recommendation class IIB, level of evidence C). Either location, chest or neck, would achieve this goal. Next, the surgeon must choose between the available conduit choices before completing the anastomosis. The choice of conduit includes stomach and large and small intestine. Currently, a gastric tube is the preferred esophageal substitute (recommendation class IIA, level of evidence C). This conduit can be used with either technique. The gastric tube probably has the best ease of use, least tension, and longest-term conduit survival of the available choices. Anastomotic technique must be considered next. The mechanical circular stapled and hand-sewn techniques for the esophagogastric anastomosis have equivalent outcomes (recommendation class IIA, level of evidence A). Despite many studies prospective and retrospective, no clear-cut advantage has been shown for one technique over the other. The semi-mechanical technique, recently popularized by Orringer, is an acceptable alternative (recommendation IIA, level of evidence B). These anastomotic techniques can be done with either technique. The surgeon next needs to decide on the need for a gastric drainage procedure. Drainage of the gastric conduit by pyloroplasty or myotomy is recommended after esophagectomy (recommendation class I, level of evidence B). This can be performed either when the anastomosis is in the chest and in the neck. If there is a difference in the leak rate and its severity, this may affect the choice of anastomotic location as well. In general, it is found that cervical anastomoses have a higher leak and stricture rate but lower mortality. In the past, thoracic anastomosis was thought to be associated with lower leak rate but with leaks that were more difficult to manage when they occur. Mortality after intrathoracic leak has been improving in recent years.
One of the most important oncologic issues recently recognized in esophageal surgery is the ability to perform a complete lymph node dissection (LND). In fact, recent data support that the number of lymph nodes (LN) harvested correlates with ultimate outcomes including overall and DFS. Lymph node dissection is recommended as a key component of ER (class I, level of evidence B). To optimize staging in T1, T2, and T3 tumors, at least 10 lymph nodes for T1 and 20–30 lymph nodes for T2 and above should be resected (class IIA, level evidence B). For patients with distal tumors, transthoracic esophagectomy (TTE) with two-field lymphadenectomy leads to better survival (class IIA, level of evidence B). For GEJ tumors, transhiatal esophagectomy (THE) and TTE have equal benefit provided a two-field lymphadenectomy is performed (class IIA, level of evidence B). Three-field LND may be considered with either squamous cell or adenocarcinoma, although there is little adoption of this approach outside of Japan (class IIB, level of evidence C). When using the third field, this requires a neck anastomosis.
The last factor the surgeon must consider is the surgical approach. Open transthoracic and transhiatal approaches are acceptable alternatives for esophagectomy (class IIA, level of evidence B). Total minimally invasive esophagectomy (MIE) or its hybrid versions are acceptable alternatives to open approaches where institutional expertise is available (class IIA, level of evidence B). The location of the anastomosis depends on the choice of approach, although a high intrathoracic anastomosis can be done for TTE with either the open or MIE approach.
Recent guidelines would suggest that a high intrathoracic anastomosis above the azygous vein or cervical anastomoses are acceptable alternatives. Whenever possible, a low intrathoracic anastomosis should be avoided (class IIB, level of evidence C).
Conclusion
The location for esophagogastrostomy depends on the ease of anastomosis, tension on the repair, the incidence and severity of leaks, the ability to diagnose and manage these problems, and oncologic issues. Recent guidelines would suggest that a high intrathoracic anastomosis above the azygous vein or cervical anastomoses are acceptable alternatives. Whenever possible, a low intrathoracic anastomosis should be avoided (class IIB, level of evidence C).
6. How should we manage chylous leak after esophagectomy?
Luigi Bonavina
luigi.bonavina@unimi.it
Chylothorax is a rare adverse event of esophagectomy associated with life-threatening metabolic, immunologic, and respiratory complications. A recent systematic review of 12 studies including 9794 patients submitted to esophagectomy for cancer between 1982 and 2012 showed that the incidence of postoperative chylothorax was 2.6% (range 0.9–9%). In addition, the authors reviewed their own institutional series of 1856 patients and found that the incidence of chylothorax was 2.1%. A Cox regression analysis showed that neoadjuvant therapy and squamous cell histology were the main risk factors. Reoperation was performed in 64.1% of these patients at a mean interval of 23 days after esophagectomy, and the 30-day mortality rate was 1.6%.41 Injury to the thoracic duct may occur during either transthoracic or transhiatal ER, but it appears to be more frequent with the first approach.42
The thoracic duct usually arises from the cisterna chyli and emerges in the right chest to the right of the aorta and posterior to the esophagus. On CT, the cisterna chyli can be mistaken for a retrocrural lymph node, but in about 15% of patients it can be visualized by magnetic resonance imaging at the level of the L1–L2 vertebral body.43 Anatomical variations of the thoracic duct (i.e., division into two or more branches) are present in up to 40% of the cases, and the course of the duct in the chest before entering the left subclavian vein is not constant. Since iatrogenic injury of the thoracic duct is rarely recognized at operation, prophylactic supradiaphragmatic duct ligation during transthoracic esophagectomy has been recommended in order to prevent inadvertent damage and postoperative chylous fistula.44
Chylothorax can easily be diagnosed at bedside due to the milky appearance of fluid in the chest drainage. However, in the fasting postoperative patient, only a high-volume output from the chest drain or a recurrent pleural effusion may be noted. In such circumstances, the diagnosis can be confirmed by assessment of tryglyceride levels in the fluid drainage and by administering cream per os or via the nasogastric tube. Delayed recognition of chyle loss may occur after removal of the chest drain and return to oral feeding; in such circumstances, the patient can be readmitted with dyspnea and pleural effusion showing the typical milky appearance. Atypical presentation with severe hemodynamic instability from mediastinal chylocele is unusual.45
Since a spontaneous resolution of the chylous fistula is possible, a 2-week conservative trial with total parenteral nutrition and pleural drainage appears to be justified in patients with a chyle output of less than 1000 mL/day to allow optimal healing of the intrathoracic anastomosis.46,47 Earlier operative management in these patients has been advocated in an attempt to reduce the postoperative morbidity and mortality, based on the evidence that T cell depletion occurs within 8 days of chyle drainage despite optimal supportive care.48
If medical treatment fails, patients should undergo reoperation and ligation of the thoracic duct. Before reoperation, a gastrographin-swallow study or an upper-digestive endoscopy should be performed to exclude the presence of an anastomotic leak that should also be addressed. The surgical approach consists of a right thoracotomy or thoracoscopy. In some circumstances, a transabdominal approach to the cisterna chyli can be considered. Administration of cream per os or through a nasogastric tube 6–12 hours before surgery helps to identify the site of the leak at the time of surgical exploration. When the duct is not clearly identifiable, mass ligation of the tissue between the aorta, azygous vein, and spine should be performed. Although the overall incidence of chylothorax in esophageal surgery is low, it has been reported that about 90% of patients undergoing esophagectomy and presenting with this complication require reoperation.49
Thoracic duct ligation was first performed by Lampson in 1948 in a patient with traumatic chylothorax.50 Despite the excellent results and the significant decrease of hospital mortality, the enthusiasm to proceed with early surgical intervention has been tempered by the morbidity of thoracotomy. The introduction of video-assisted thoracic surgery has offered a safe and effective alternative for the treatment of chylothorax. The thoracoscopic procedure is usually performed on the left lateral decubitus using a 30° scope and three trocars, one in the drainage site, one in the sixth intercostal space on the mid-axillary line, and one in the seventh intercostal space on the posterior axillary line. Identification of the site of the injured duct is attempted by gently pushing the gastric tube in the medial direction and by noting release of chyle in the posterior costophrenic angle. Sutures and clips can be initially used to seal the thoracic duct, but this is likely to be unsuccessful. Supradiaphragmatic en bloc encircling of the azygos vein and periaortic fat tissue between the spine and the gastric tube using a conventional right-angled clamp and firing a single blue cartridge of an articulating endostapler is an effective approach.42
In some circumstances, such as previously failed transthoracic procedures, transabdominal ligation of the cisterna chyli via laparotomy or laparoscopy represents a viable alternative to the thoracic approach.45,51,52 After gentle left displacement of the gastric tube at the level of the diaphragmatic hiatus, multiple suture ligations of the cisterna chyli and the thoracic duct at its origin are performed transhiatally on the right side of the aorta.
7. Optimal approach to the management of intrathoracic esophageal leak following esophagectomy
Lara W. Schaheen and Katie S. Nason
nasonks@upmc.edu
Over the past several decades, there has been a shift in the management of intrathoracic anastomotic leaks from aggressive surgical intervention to conservative management and, more recently, to endoscopic interventions. Currently described strategies include prophylactic drain placement, omental reinforcement, conservative management (strict NPO, intravenous antibiotics, and drainage), endoscopic management, and early surgical exploration. Although a multitude of interventions have been studied, guidelines for the optimal management of intrathoracic leak have yet to be established. However, efforts to minimize leak rates and optimize management remain critically important owing to the impact of anastomotic leak, whether intrathoracic or cervical, on postoperative morbidity, need for subsequent interventions, protracted hospitalization, and decreased quality of life.53
Management begins intraoperatively with efforts to minimize the complication. Tenets such as minimal handling of the conduit, avoiding injury to the vascular pedicle, and careful contouring of the conduit to preserve collateral blood flow are well described and universally accepted. Additional prophylactic interventions such as omental reinforcement and drain placement at the time of esophagectomy are advocated by many surgeons. Reinforcement of the anastomosis with a pedicled omental flap is theoretically appealing; the procedure involves creation of a two- or three-vessel omental pedicle on the greater curvature of the stomach during conduit mobilization to encircle the anastomosis and buttress the suture line. This flap contains endoluminal contents if breakdown of the anastomosis occurs. This has been studied in two randomized controlled trials and a retrospective review; all found a significant decrease in anastomotic leak rates.54–56 In addition, the review by Sepsi and colleagues showed that the need for re-intervention after esophagectomy was decreased in the flap cohort. In their series, mobilization of the omentum from the transverse colon and creation of the pedicle added approximately 20 minutes to the operative time, and did not demonstrate any additional morbidity.56 Although the studies performed by Bhat and Dai demonstrated a reduced incidence of anastomotic leak, they did not demonstrate a significant difference in hospital mortality, incidence of postoperative complications, anastomotic strictures, or duration of hospitalization. These studies suggest that routine reinforcement of the thoracic anastomosis with pedicled omental flap may reduce the incidence of anastomotic leak.
Regardless of whether an omental flap is used, however, use of appropriately positioned drainage provides an additional measure of safety if a leak does occur. Mortality in the setting of an inadequately drained leak has mortality rates as high as 80%.57 Prophylactic placement of a drainage tube adjacent to the anastomosis at time of surgical resection allowed for shorter time to diagnosis, shorter time to resolution (23.4 days vs. 80.7 days) and shorter time to return to oral intake (32.2 days vs. 98 days) in a review of 414 patients by Teng and colleagues.58 Additionally, the presence of an operatively placed drain allowed patients to avoid subsequent interventions including drain placement while also lessening the severity of clinical symptoms at time of initial diagnosis.58 Adequate prophylactic drainage, therefore, is a key principle for management of anastomotic leak.
Once intraoperative management has been optimized, management of the intrathoracic anastomotic leak transitions in the postoperative setting. A high degree of suspicion, early diagnosis, and immediate intervention are required. Initial management includes strict NPO, intravenous antibiotics, parenteral or enteral nutrition, and adequate drainage. In a stable patient, radiographic assessment for mediastinitis should be performed to help guide subsequent intervention. If radiographic assessment demonstrates gross mediastinal or thoracic contamination, operative drainage is needed. If, however, the leak appears well-contained, endoscopic examination is indicated for both diagnostic and therapeutic management of the leak. Initial assessment confirms viability of the conduit, defect size, and drain position. Depending on the findings, endoscopic intervention may be possible.
In general, patients with extensive devitalization of esophageal anatomy, large leaks, or a nonviable conduit are not suitable for endoscopic management. In many circumstances, such as a completely contained and well-drained dehiscence with otherwise healthy and viable conduit, endoscopic drain manipulation and anastomotic dilation can be used successfully for early management. Esophageal stricture is common in these patients and repeated dilation is often required during and following complete healing of the leak.53 A new and potentially useful option for endoscopic management of large dehiscence has been described by Schorsch and colleagues. They have performed transnasal placement of an endoscopic wound vacuum for anastomotic leaks with excellent results.59
Endoscopic stent placement at the time of the initial endoscopic evaluation is increasingly used for management of well-contained leaks, providing immediate coverage of the defect and enabling earlier oral intake. Endoscopic stenting can be considered for leaks involving <30% of the anastomotic circumference and with a viable conduit. This approach requires close follow-up and, often, repeat interventions. Serial surveillance radiography is required to monitor for stent migration and evidence of inadequate drainage, including new pneumothorax or undrained pleural effusion. The surgeon must exercise vigilance and be prepared for more aggressive surgical intervention if the leak is not contained. If the problem is due to stent migration, repositioning of the stent can be accomplished in most patients. Finally, endoscopic removal of the stent is the only definitive method to assess healing of the defect. Dai and colleagues demonstrated that repeat stenting was necessary in 33 of 40 patients, the mean number of stents per patient was 3.2, and the mean time to healing was 30 days.55 Freeman and colleagues demonstrated that 82% of patients were able to resume oral intake within 72 hours of stent placement.60 Although multiple studies have demonstrated the successful use of endoscopic stenting for the management of anastomotic leaks, these studies are limited by small study populations with heterogeneous patient selection, lack of randomized trials, use of a variety of stent types, varying management algorithms, and, importantly, different underlying pathology.
There are patients for whom endoscopic management fails and surgical intervention may still be required. However, as the mortality of patients who require reoperation is as high as 40%, surgical intervention is reserved for patients with symptomatic or uncontained intrathoracic leaks and those for whom conservative management has failed.61 Patients with undrained pneumothorax or sepsis (empyema or mediastinitis), require video-assisted thoracoscopic drainage and decortication at a minimum. Early surgical intervention rapidly eliminates the source of sepsis and allows for placement of additional drains, is well-tolerated, and can be lifesaving. It is very rare that the anastomosis requires revision and revision is rarely successful. If, however, the conduit is non-viable, conduit take-down and esophageal diversion should be performed without hesitation.
Optimal management of thoracic anastomotic leak begins with careful operative technique and a high index of suspicion to facilitate early diagnosis. Once identified, intravenous antibiotics, adequate drainage, and nutritional support are critical. Patients without significant mediastinal contamination may be amenable to endoscopic management with drain manipulation, anastomotic dilation, or endoscopic stent placement. Stent placement enhances early return to oral intake, but the surgeon must exercise diligence in monitoring for signs of inadequate stent sealing or migration to avoid additional mediastinal contamination. Surgical intervention should be reserved for patients with uncontained intrathoracic anastomotic leaks and non-viable conduits and those in whom conservative or endoscopic management has failed.
Acknowledgment
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number 5K07CA151613 (KSN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
8. The choice for operational approach in gastric cancer with transition to the esophagus
Igor N. Turkin and Maksat A. Ibraev
inturkin@mail.ru
From January 1, 1990 to December 31, 2010, a total of 575 patients with gastric cancer that had transitioned to the esophagus underwent surgical operations in the Thoracic–Abdominal Department of the Blokhin Russian Cancer Research Center.
When tumors infiltrated the esophagus, 11.2% of gastric cancers disseminated into mediastinal lymph nodes (LNs). The metastasis rate increased with tumor dissemination along the esophagus: over 6.1% when the tumor affected an abdominal segment, 12.8% for supradiaphragmatic segments and 42.9% for retropericardial segments of the esophagus (P < 0.001). The higher in the esophagus the tumor infiltrated, the more frequent dissemination was not only to the supradiaphragmatic and lower paraesophageal LNs, but also to those in the tracheal bifurcation; while no metastases with infiltration of the abdominal segment were noted, 2.7% had infiltration of the supradiafragmal segment and 21.4% had tumor dissemination into the retropericardial segment of the esophagus (P < 0.001).
The total volume of tumor infiltration of the stomach had no correlation with the dissemination rate to the mediastinal LNs: the metastatic rate was 12.7% in cancer of the cardia with transition to the esophagus, 11.3% in cancer of the subcardia with transition to the esophagus, and 12.1% in gastroesophageal cancer (P > 0.05). Unlike mediastinal metastases, the rate of intra-abdominal LN metastases increased in correlation with the progression of gastric tumors (P < 0.01).
Only the rate of esophageal tumor infiltration correlated with the number of mediastinal LN metastases. If the tumor infiltrated the abdominal esophageal segment, the dissemination spread to the supradiaphragmal LNs, with the tumor affecting the supradiaphragmal segment––metastases spread mostly to the supradiaphragmal and lower paraesophageal LNs (97.3%)––and with tumor infiltration of the retropericardial segment, dissemination spread to all groups of lower mediastinal LNs, including 21.4% of bifurcational LNs.
The successful operation of gastric cancer with transition to the esophagus is determined mainly by the surgical approach, which is the clue to treatment implications. The transthoracic approach in R0 operations of functionally safe patients with cancer types Siewert II and Siewert III, regardless of the level of transition to the esophagus, achieved reliably better outcomes.
In patients with cancer of the proximal part and body of the stomach with transition to the abdominal esophageal segment, there was no difference in the survival rate between the groups of transthoracic and transhiatal approaches. This was connected with overall low frequency of tumor dissemination to mediastinal LNs (6.1%) and no metastases in paraesophageal and bifurcation LNs, while both approaches could easily reach supradiaphragmal LNs.
In patients with cancer of the proximal part and body of the stomach with transition to supradiaphragmal esophageal segments, 5-year overall and relapse-free survival was slightly higher in the group that underwent the transthoracic approach than the group that underwent the transhiatal approach (39.4% and 35.6% vs. 27.7% and 20.8%, respectively, P > 0.05). This was associated with more frequent non-radical resections of the esophagus as well as recurrent metastases in mediastinal LNs after transdiaphragmal interventions.
In patients with subtotal and total gastric cancer with transition to the abdominal and supradiaphragmal esophageal segments, both approaches were equivalent in terms of survival. This patient group has the poorest prognosis and the highest rate of relapses.
The choice of surgical approach should consider not only the topography and anatomy allowing complete planned intervention, but also the specific character of surgical trauma taking into account the patient’s functional status.62,63 In elderly patients and those with low ventilation function, the method of choice seems to be the transhiatal approach. Modern circular staplers form high anastomoses in the mediastinum. A number of authors note a low (<1%) rate of suture failure of anastomoses and scar strictures.64,65
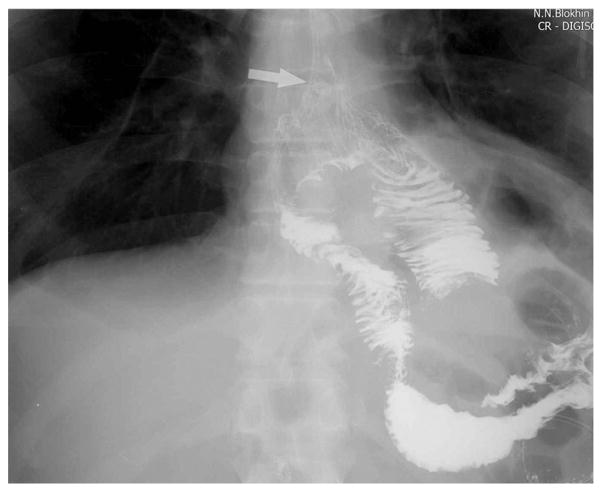
In 2012–2013, we carried out a small pilot study of 30 cases to evaluate the reliability of high machine anastomosis (Figs. 3 and 4). No lethal outcomes were registered. On day 7, one patient had an esophageal anastomosis fistula, which was cured conservatively.
Figure 3.
Endoscopic control of anastomosis (indicated by the arrow) during the operation.
Figure 4.
Contrasting imagining of the anastomosis. The anastomosis is indicated by the arrow.
The following conclusions were reached after our first experience of high machine anastomosis. The level of the formed anastomosis corresponds to the level achieved by the transthoracic approach. R0 resection in the advanced tumor infiltration into the subdiaphragmal esophageal segment cannot be fulfilled by machine anastomosis. The transhiatal approach manages adequate mediastinal lympodissection from the diaphragm to the trachea bifurcation; however, the transthoracic approach is more convenient because of the narrow and extended operation field over the diaphragm. Functional disorders during manipulations are high in the mediastinum. No marked rhythm disorders or significant cardiac output, which could interfere with surgery, were registered. The average time of transhiatal operation was 185 ± 24 min versus 241 ± 28 min of transthoracic operation (P < 0.05). Intraoperative blood loss was 620 ± 488 mL with the transhiatal approach, and 1.300 ± 625 mL (P < 0.001) with the transthoracic approach. The total number of postoperative complications was significantly lower after the transhiatal approach than the transthoracic approach (31.7% vs. 47.8%).
Thus, disease prognosis, planning the radical extension of the operation, the availability of approaches to achieve radical and safe intervention over the diaphragm, age, history of other open lung surgery, and diseases of the pleura determine the surgeon’s responsibility in estimating all risks when making a choice of surgical approach in gastric cancer with transition to the esophagus.
9. Robot-assisted thoracoscopic esophagectomy for esophageal cancer
Peter C. van der Sluis, Sylvia van der Horst, and Jelle P. Ruurda
j.p.ruurda@umcutrecht.nl
Neoadjuvant therapy followed by radical esophagectomy is the curative treatment for patients with esophageal cancer. Even though conventional open transthoracic esophagectomy is associated with significant morbidity, predominantly consisting of cardiopulmonary complications (50–70%), it is the preferred surgical approach worldwide, allowing for en bloc resection of the tumor with the locoregional lymph nodes.66,67
MIE was designed to reduce the rates of morbidity and mortality. A review of the literature concerning MIE shows a substantial decrease in blood loss, postoperative complications, and days of hospital stay, without prejudice to oncologic results.68 Conventional (thoraco)scopic surgery has some important limitations, such as a two-dimensional view, disturbed eye–hand coordination, and limited degrees of freedom. A recent survey demonstrated that it is not widely adapted.66 Robot-assisted thoracolaparoscopic esophagectomy (RATE) facilitates a complex minimally invasive procedure with a stable, enlarged, three-dimensional field of view and articulated instruments that facilitate dissection with seven degrees of freedom.69,70
The enlarged, very stable image facilitates dissection close to organs that need to be preserved. Manipulation is enhanced so that a very precise dissection can be performed, even on moving targets like the aorta and pericardium. Thereby, the oncologic results might improve, while maintaining the advantages of minimally invasive surgery, such as diminished pulmonary complications.69
From our first experiences with RATE, we concluded that it is a feasible and safe technique.70,71 Compared to conventional open transthoracic esophagectomy from the literature, RATE was accompanied by reduced blood loss, reduction of intensive care–unit stay, and a lower percentage of cardiopulmonary complications.67,71 In hospital mortality, hospital stay and lymph node retrieval were comparable.2,6
These results are in concordance with a systematic review, which included nine articles (130 cases) related to robot-assisted esophagectomy.72 In this review, it was shown that compared to open esophagectomy, RATE was accompanied by reduced blood loss and reduced intensive care–unit stay. Pulmonary complication rate, hospital stay, perioperative mortality, and oncological outcomes were equivalent to open esophagectomy.72
Until now, the level of evidence for robot-assisted minimally invasive thoraco-laparoscopic esophagectomy has been suboptimal and based on case series or expert opinions only (Level 4 or 5).72 There is a need for well-conducted randomized controlled trials and large prospective cohort studies with long-term survival to prove the superiority of RATE over the worldwide current standard of open transthoracic esophagectomy.
Therefore, we have started a monocenter randomized controlled trial (NCT01544790) to compare robot-assisted minimally invasive thoracola-paroscopic esophagectomy with conventional open transthoracic esophagectomy.73 This monocenter randomized controlled superiority trial can provide further evidence supporting RATE as superior treatment for resectable esophageal cancer.
10. Is there still a place for open esophagectomy?
Donald L. van der Peet, Barbara M. Zonderhuis, and Miguel A. Cuesta
d.vdpeet@vumc.nl
Minimally invasive surgery (MIS) for ER has been gradually introduced and has become an accepted treatment modality. The apparent advances of the MIS are thought to be applicable to esophageal operations. Despite the similarities, several unique factors involved in this type of surgery play important roles in the acceptance of MIS for esophageal cancer. Treatment has changed from primarily surgical to neoadjuvant chemoradiation followed by surgery. Adenomatous carcinoma has become the main type of esophageal cancer. The spectrum of patient characteristics has changed accordingly. Obesity and elderly issues have become a routine part of taking care of these patients. Also the anatomical characteristics of the esophagus may have an impact upon the advance of MIS. As thoracic surgeons may lack experience with laparoscopic procedures, the same goes for GI surgeons lacking experience with thoracoscopic procedures.
As Low tried to frame the issue concerning the best approach, a lack of randomized studies was a major problem.74 Since then, one randomized trial was published concerning minimally invasive versus open esophagectomy. The so-called TIME trial was conducted to find out how the minimally invasive approach relates to open procedures.31 With respiratory complications as the primary endpoint, it was found that these occurred significantly less after MIS than after open esophagectomy (29% vs. 9%). Other studies are underway but address different questions. The MIRO trial by Briez et al. tries to depict the value of the laparoscopic versus open abdominal phase in combination with an open thoracotomy.75 Another study by van der Sluis et al. aimed primarily at the use of robotic surgery in esophageal cancer.73
What type of surgery for esophageal cancer is common practice?
From an international survey done by Boone et al., it is made clear that the majority of surgeons favor open esophagectomy (78%).66 The same has been shown by studies from the United Kingdom with a slow uptake of minimally invasive techniques by surgical groups.76 It can therefore be safely concluded that, although MIS for esophageal cancer is feasible and perhaps superior to open esophagectomy, the majority of esophageal surgery is done by means of an open procedure.
Conclusions
As of 2014, the open esophagectomy remains the gold standard for most surgical teams. The high complexity of the totally minimally invasive procedure necessitates technical skills that provide a threshold for most surgeons. It has been shown that, in experienced hands, the minimally invasive procedure offers a viable alternative that to some extent may benefit the patient when compared to open surgery.
11. SCC: What are the advantages of esophagectomy compared to definitive chemoradiotherapy?
Valter Nilton Felix
v.felix@terra.com.br
The optimal management of esophageal cancer is still controversial. Traditionally, surgery is the gold standard in the treatment of squamous esophageal carcinoma. However, the 5-year survival remains poor, as most patients become only symptomatic with advanced disease.19
Literature data suggest that for some populations with early-stage disease and long-term life expectancy, surgery is the best option for treatment. Some cases really can survive after chemoradiotherapy (CRT) without esophagectomy, but local recurrence still develops in many responders and some need a salvage resection.
Late toxicity is an important issue that could impair quality of life (QOL) after CRT,77 and previous studies showed that the negative impact of esophagectomy on QOL was transient for patients who survive for at least 2 years and that the QOL of the 2-year survivors was significantly better than that of other patients.78,79 A few reports, like that of Yamashita et al., found that the QOL of the CRT and OP groups was similar and that the QOL of the CRT group regarding symptoms such as diarrhea, appetite loss, and eating problems was significantly superior to that of the OP group.
Prophylactic irradiation covering the area treated by radical esophagectomy in all patients with T1N0M0 tumors may be controversial, because of the incidence of severe toxicities associated with CRT. Acute toxicity includes respiratory failure, hyperglycemia, anemia, granulocytopenia, thrombo-cytopenia, general fatigue, and allergy. On the other hand, the accuracies of lymph node metastasis of early-stage esophageal cancer by CT scan or PET-CT are 60–70% as a result of the low sensitivity, maybe because the size of lymph node metastasis in these cases is small. EUS, which has proved to have good sensitivity in evaluating regional lymph node metastasis, is considered to be superior to CT.80 Assessing lymph node metastasis by both EUS and CT may reduce the number of false-negative findings.
The role of adding neoadjuvant CRT to surgery has been extensively investigated in the past, and meta-analyses have shown that neoadjuvant CRT was associated with a significant survival benefit.81 A complete tumor response was frequently observed after neoadjuvant CRT, and this has prompted investigations on the role of definitive CRT in patients with squamous esophageal carcinoma (Table 1), demonstrating that esophagectomy performed after neoadjuvant CRT provides better local control of the disease, but does not prolong overall survival compared with definitive CRT, in most studies.
Table 1.
Chemoradiotherapy (CRT) × surgery (S) for esophageal squamous cell carcinoma (ESCC)
| Author | Year | n | Type of the study | Random | Type of tumor | Survival (2–5 years) approximate rates > better than < worse than |
|---|---|---|---|---|---|---|
| Kato et al.82 | 2004 | 67 T4 | retrosp | N | SCC | CRT = S (20% × 36%) |
| Stahl et al.83 | 2005 | 172 T3–4N0–1M0 | prosp | Y | SCC | CRT = S (24% × 31%) CRT < S for local recurrence |
| Nagata et al.84 | 2006 | 74 T1–3 N0–1M0 | retrosp | N | SCC | CRT = S (44% × 51%) CRT < S for local recurrence |
| Toh et al.85 | 2006 | 49 T1 | retrosp | N | SCC | CRT < S (37% × 75%) |
| Adams et al.86 | 2007 | 330 (CRT for the worst cases) | prosp | N | SCC + Adenoca | CRT = S (53% × 50%) |
| Bedenne et al.87 | 2007 | 259 T3N0–1M0 | prosp | Y | SCC + Adenoca | CRT = S (40% × 34%) |
| Yamashita et al.88 | 2008 | 82 (CRT for the worst cases) | retrosp | N | SCC | CRT = S (48% × 65%) |
| Ariga et al.89 | 2009 | 99 T1–3N0–1M0 | prosp | Y | SCC | CRT = S (50%) |
| Yamashita et al.90 | 2009 | 138 stage II and III | retrosp | N | SCC + Adenoca | CRT = S (36% × 51%) CRT > S for QOL |
| Salek et al.91 | 2011 | 986 | retrosp | N | SCC + Adenoca | CRT = S (50%) |
| Morita et al.92 | 2012 | 81 T4 | retrosp | N | SCC | CRT< CRT + S (19% × 42%) |
| Motoori et al.93 | 2012 | 173 T1bN0M0 (102 S + 71 CRT) | retrosp | N | SCC | CRT < S (77% × 68%) CRT < S for local recurrence |
| Teoh et al.94 | 2013 | 81 (most of T3) | prosp | Y | SCC | CRT = S (50% × 30%) CRT < S for morbidity |
Note: retrosp, retrospective; prosp, prospective; Adenoca, adenocarcinoma; QOL, quality of life.
There are advantages and disadvantages to each modality, and surgeons and oncologists might have different opinions about which modality to recommend, especially in clinical stage II or III. The 5-year global survival rate after surgery remains relatively modest, around 50%, in these cases, but it is uncertain whether definitive CRT achieves treatment outcomes comparable to surgery, because there are few reported prospective randomized studies trying to compare morbidity and overall survival rates, and the reported studies lack well-designed series, almost all mixing stages and types of tumor.
Furthermore, the performance of these clinical trials is quite difficult to analyze because of the peculiar treatment characteristics and different follow-up periods. The surgical procedure (transhiatal or transthoracic) to be performed has been decided in accordance with the site of the tumor, perceived radiological stage, and the physiological fitness (after anesthetic assessment, relating comorbidities and past medical history) of the patient, with transhiatal resection reserved for those patients with impaired respiratory reserve. During the neoadjuvant phase of treatment, 5-fluorouracil (5-FU) is given as a continuous infusion of different doses and schedules, with or without cisplatin, via a central venous catheter, and a variable fractioned radiotherapy schema is used. Full CRT typically consists of high-dose (50–66 Gy) external beam radiotherapy concurrent with variable schema including 5-FU and cisplatin, and in some studies the CRT is selected for medically unfit cases or followed by salvage surgery.
T3 or T4 squamous cell carcinomas and lymph node–positive disease are associated with worsened prognosis. Concurrent CRT is a potentially curative nonsurgical option for locally advanced esophageal cancer, with pathological complete response ranging from 13% to 49%, but the rate of persistent and recurrent disease within the esophagus remains high at 40–60% and surgical treatment of these tumors may improve DFS. However, these patients are often medically unfit and have much more expressive postoperative morbidity and mortality rates if submitted to neoadjuvant CRT. We studied a series of 20 T3–T4a (resectable tumor – AJCC Cancer Staging Manual. 7th ed, 2010) patients, divided into two groups, operated on with or without use of neoadjuvant CRT (daily regimen consisted of a 24-hour continuous infusion of 250 mg/m2 of 5-FU combined with a 1-hour infusion of 6 mg/m2 of cisplatin, while radiation was concurrently administered at 1.6 Gy) performed 3–4 weeks before surgery. Much more elevated postoperative morbidity and mortality rates were observed in the first group (63% and 14% respectively against 31% and 5% of the other group; P < 0.05). Pulmonary infection and respiratory failure were the mean postoperative complications. Global overall survival did not surpass 18 months in either group. It is worth observing that every case related as complete tumor response after neoadjuvant CRT had tumoral cells in the pathological analysis of the resected esophagus.
Summarizing, neoadjuvant CRT, carefully employed to avoid severe collateral effects, seems to be useful even in the early stage of ESCC. When the tumor is more advanced, the theoretical advantages of adding CRT to the treatment of esophageal cancer include potential tumor downstaging before surgery, as well as targeting micrometastases and, thus, decreasing the risk of distant metastasis. Cisplatin and 5-FU–based regimes are used worldwide. CRT could be considered as an option for stage II and III resectable tumors and for patients who are medically or technically inoperable. Although neoadjuvant CRT followed by surgery or salvage surgery after definitive CRT is a practical treatment, judicious patient selection is crucial. It is important to have a thorough understanding of these therapeutic modalities to assist these patients, and certainly we have to enhance our therapeutic methods to overcome actual results.
12. What should the pathologist report in the resected specimen?
Rupert Langer
rupert.langer@pathology.unibe.ch
Pathology reports should encompass all important information regarding the tumor and the quality of the surgical procedure. The major pathology communities, such as the College of American Pathologists (CAP)95 and the Royal College of Pathologists (RCP),96 as well as numerous other national guidelines, cover these issues. Based on main sources, such as the regularly updated WHO classification of tumors (i.e., WHO classification of tumors of the digestive tract97), or the AJCC/UICC TNM classification,18,98 pathology reports should at least encompass the following items: specimen/procedure; tumor site; distance of tumor center from GEJ and relationship of tumor to GEJ; histologic type (WHO classification); histologic grade (WHO classification); tumor size; microscopic tumor extension; lymphatic and/or vascular invasion; perineural invasion; LNMs; concluding pathologic/postoperative staging (pTNM); involvement/distance to margins (oral, aboral, circumferential; including dysplastic conditions); treatment effect after neoadjuvant treatment; additional pathologic findings; and ancillary studies (if applicable).
A special emphasis of this session was set on the items R0 resection definition, lymph node involvement and location, response to neoadjuvant therapy, and genetic markers:
Resection status
The definition of the proximal and distal resection margin is clear. Involvement of one of these margins is associated with bad clinical outcome. In mucosectomy specimens, the deep resection margin is also clearly defined. Regarding the circumferential resection margin of esophagectomy specimens, there are different definitions among the major pathology schools and the UICC/AJCC. The CAP and UICC/AJCC define R0 as non-involvement of the (inked) resection margin,18,95,98 whereas the RCP requests a distance of greater than 1 mm to the resection margin as criterion for an R0 situation.96 These discrepancies have their basis in the results of different studies. In a recent meta-analysis, the CAP criteria differentiated a higher-risk group of patients than the RCP criteria. The RCP criterion was considered to give important additional information. However, an international consensus regarding the most accurate and prognostically important definition of circumferential resection margin (CRM) involvement would be welcomed; in the interim, arguably the exact nearest distance of the tumor from the CRM should form part of routine pathology reporting.99
Lymph node involvement
The current edition of the AJCC/UICC TNM classification provides a more detailed classification of LNMs for esophageal carcinomas and carcinomas of the GEJ, which now encompasses the categories N1 (1–2 regional LNM), N2 (2–6 regional LNM), and N3 (7 or more LNM). This classification now parallels gastric cancer, similar to the T category.18,98 Recent data about the presence of extratumoral extension of LNMs suggest an unfavorable influence of this finding.100 Localization of LNM above or below the diaphragm may also have prognostic impact.101 These items may be considered in future classification after confirmation in larger, independent cohorts. The Japanese Classification of Cancer recognizes three lymph node categories that also follow anatomic localizations but not the number of LNMs (group 1: cervical LN; group 2: thoracic LN; group 3: abdominal LN; N1 metastasis involving only group 1 lymph nodes; N2 metastasis to group 2 lymph nodes, regardless of involvement of group 1 lymph nodes; N3 metastasis to group 3 lymph nodes, regardless of involvement of group 1 or 2 lymph nodes; N4 metastasis to distant lymph nodes).102
Effects of treatment
Several tumor-regression grading (TRG) systems are described in the literature, and aim to categorize the amount of regressive changes after cytotoxic treatment. Mostly they refer to the percentage of residual tumor in relation to the previous tumor site or they estimate the amount of therapy-induced fibrosis in relation to residual tumor. Most of these systems have been shown to provide highly accurate prognostic value, which may even exceed the impact of the TNM staging systems. Currently, however, there is no international consensus concerning which of the TRG systems should be used in general. However, the implementation of TRG (any) is widely recommended and may also be part of future staging systems.103
Genetic markers
A high number of original papers offer additional data concerning potential prognostic parameters that might be also considered for implementation in future standard reporting. The determination of Her2 status is mandatory for prediction of therapy response to treatment with trastuzumab, which has been approved for the treatment of metastatic gastric and gastroesophageal adenocarcinomas in case of Her2 positivity.104 It can be expected that, owing to the introduction of other targeted, molecular-based therapies, similar predictive tests will enter the clinic and pathologic practice. For conventional CRT, at present no validated markers for response prediction are used in practice, although the deregulation of many molecules has been shown to be associated with later treatment response. The same accounts for the usage of potentially prognostic biomarkers, where currently no biomarker is regarded to constantly provide prognostic value for risk stratification of esophageal cancer patients. However, a very promising study showed the successful immunohistochemical application of a three-gene panel in EAC, which confirmed previous findings from an independent cohort.105 For squamous cell carcinomas, cyclin-D1, p53, E-cadherin, and vascular endothelial growth factor (VEGF) seem to have the strongest potential to serve as prognostic markers.106 Novel technologies such as next-genome sequencing or determination of miRNA profiles are expected to detect further potentially useful markers. It can be expected that molecular characterization of tumors may also be part of future pathology reports.
13. What is the best way to manage the patient with an unsuspected positive microscopic margin after ER?
Peter C. Wu, Edgar Figueredo, and Zhao Ming Dong
pcwu@uw.edu
The prognostic significance of a close resection margin (<1 mm) has been well described in rectal cancer surgery,107 leading the RCP to define a positive circumferential margin for esophageal cancer at or within 1 mm of the cut margin, while the CAP strictly defines a positive margin as tumor present at the cut margin. There is ongoing controversy over which definition is more clinically significant and most reliably predicts patient outcome.
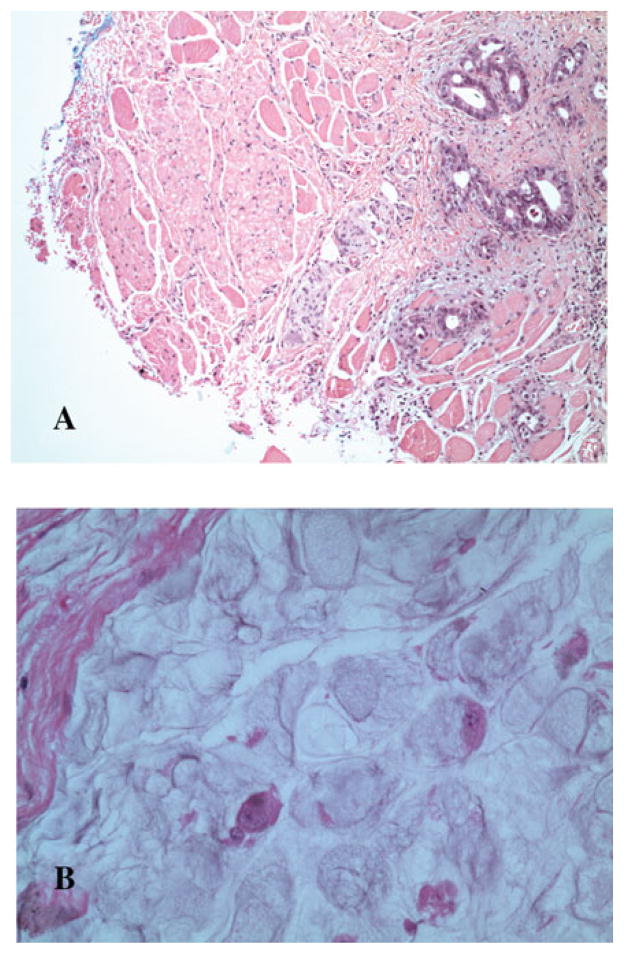
Figure 5 provides two case examples of positive margin status following esophagectomy. Patient A underwent transhiatal esophagectomy for a locally advanced distal esophageal adenocarcinoma following neoadjvuant CRT and was found to have mul-tifocal intramuscular islands of tumor cells at the proximal cervical ER margin. Patient B was diagnosed with a locally advanced GEJ adenocarcinoma, underwent a transthoracic esophagectomy following induction CRT, and was found to have large mucin pools containing scattered carcinoma cells present at the distal gastric resection margin.
Figure 5.
(A) Patient A: proximal cervical esophageal margin (inked blue) with tumor foci present within the muscle wall. (B) Patient B: mucinous signet ring adenocarcinoma consisting of mucin pools with scattered rare viable carcinoma cells involving the distal stomach resection margin.
A meta-analysis99 of 14 studies involving nearly 2500 patients who underwent curative esophagectomy for esophageal cancer was performed to study the impact of CRM on survival. Margin criteria as defined by the RCP and CAP were compared, and outcomes showed that tumors present at the cut margin defined a higher-risk group compared to those with close margin status. This study also showed that neoadjuvant treatment significantly lowered positive margin rates in both groups, and lymph node status was a stronger predictor of negative outcome compared to CRM status. A compilation of 10 studies reporting outcomes for T3 tumors showed an overall positive CRM rate of 15% by CAP criteria and 43% by RCP criteria. Five studies included patients who had received neoadjvuant CRT and showed lower positive CRM rates of 11% and 32% according to CAP and RCP criteria, respectively.
Table 2 summarizes results from two high-volume U.S. centers108,109 and one each from the Netherlands110 and Ireland111 specifically comparing outcomes of positive cut margins (CAP) with close resection margins (RCP) in locally advanced T3 tumors. Patients with tumor present at the resection margin had significantly worse median overall survival rates compared to those with a confirmed negative margin. On the other hand, there was no significant difference in survival observed in patients with close versus negative margins. Centers using neoadjuvant treatment protocols reported lower positive margin rates.
Table 2.
Significance of CRM status in pT3 tumors
| Institution | N | % ypT3 | CAP (−) % median OS | CAP (+) % median OS | RCP (−) % median OS | RCP (+) % median OS |
|---|---|---|---|---|---|---|
| Virginia Mason (USA) | 135 | 44% | 88% 30 months |
12% 8 months |
39% 29 months P < 0.001 |
61% 22 months P = 0.14 |
| Utrecht (the Netherlands) | 132 | 0% | 80% 22 months | 20% 9 months P = 0.000 |
33% 21 months |
67% 16 months P = 0.144 |
| MD Anderson (USA) | 160 | 100% | 95% 28 months |
5% 8 months P = 0.01 |
74% 28 months |
26% 50 months P = 0.31 |
| St. James (UK) | 157 | 52% | 82% 37 months |
18% 21 months P = 0.023 |
40% 38 months |
60% 27 months P = 0.168 |
Among the largest single-institutional series of transhiatal esohagectomies,112 the University of Michigan team reported 26 patients out of 1044 cases with distal esophageal or gastric cardia tumors that were found to have a positive gastric resection margin. Among these patients, 80% died with distant metastatic disease. Nine patients received adjuvant treatment which neither improved survival nor dysphagia from local recurrence.
We propose the following guidelines with regard to the unsuspected positive microscopic margin following curative resection for esophageal cancer. A minimum gastric margin of 5 cm should be ideally maintained from the palpable tumor. Based upon current evidence, it remains controversial whether a close esophageal cancer resection margin should be treated the same as close rectal cancer resection margins. Neoadjuvant treatment has been shown to consistently reduce circumferential margin rates for locally advanced esophageal tumors. Regional nodal disease should guide adjuvant treatment decisions, but there is a lack of evidence to support adjuvant treatment for the CRM+ patients. Finally, surgical reexploration in the perioperative period including en bloc resection of the esophagogastric anastomosis and gastric conduit with bowel interposition could only be tolerated in a few highly selected patients, with expected high morbidity. Multidisciplinary evaluation by an experienced esophageal cancer team is recommended for all patients to minimize complications and optimize outcomes.
14. Enhanced recovery after surgery: expedited care protocols
Piers A.C. Gatenby, William H. Allum, and Shaun R. Preston
p.gatenby@ucl.ac.uk
Introduction
Enhanced recovery, fast-track pathway, standardized clinical protocol, expedited-care program, and other terms have been used to describe systems of care delivery to reduce the physiological stress response and optimize postoperative recovery, involving a multimodal approach to perioperative management within a framework for goal-directed therapy with consistency of care. The goal is to improve quality of care with reduced length of stay, improved patient satisfaction, and reduced cost.113 These systems have grown in popularity in recent years (with an increasing drive to improve the quality of services within the constraints of limited resources and increasing demands114).
Enhanced recovery principles
Approaches to these systems are centered around interventions in the preoperative (pre-admission counseling, fluid and carbohydrate loading, avoidance of prolonged fasting, antibiotic prophylaxis, and thromboprophylaxis), intraoperative (short-acting anesthetic agents, avoidance of drains, goal-directed fluid therapy, maintenance of normothermia) and postoperative (regional anesthesia/analgesia and non-opioid analgesia, early removal of nasogastric tubes and urinary catheters, prevention of nausea and vomiting, early enteral nutrition, early mobilization and stimulation of gut motility) phases of care.115
Enhanced recovery in ER
ER provides a particular challenge when developing streamlined, standardized care. Patients are frequently elderly and frail; many have undergone neoadjuvant therapy and require extensive surgery on multiple body cavities that is performed in relatively small numbers, even in busy units, when compared to other surgical specialties such as joint replacement or colorectal surgery.
ER pathways have been undertaken for esophageal surgery since 1991,116 far ahead of the popularity gained in other surgical specialties in the last decade.117 Table 3 demonstrates the components of enhanced recovery programs in recent studies (adapted from Ref. 115).
Table 3.
Components of ER pathways in published studies
| Number (ER/ control) |
Preadmission counseling |
Intraoperative fluid restriction |
Thoracic epidural analgesia |
Early extubation |
Avoidance/ early removal of drains |
Avoidance/ early removal of NGT |
Early enteral/ oral feeding |
Early mobilization |
Early discharge criteria |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Brodner et al. 1998 | 42/49 | * | * | * | ||||||
| Neal et al. 2003 | 56 | * | * | * | * | * | * | |||
| Cerfolio et al. 2004 | 90 | * | * | * | * | * | * | * | * | |
| Low et al.116 | 340 | * | * | * | * | * | * | * | * | * |
| Jiang et al. 2009 | 114 | * | * | * | * | * | * | * | ||
| Munitiz et al. 2010 | 74/74 | * | * | * | * | * | * | * | ||
| Gatenby et al.114 | 9/16 | * | * | * | * | * | * | * | * | * |
| Preston et al. 2013 | 12/12 | * | * | * | * | * | * | * | * | |
| Tang et al. 2013 | 36/27 | * | * | * | * | * | * | * | * | * |
| Blom et al. 2013 | 103/78 | * | * | * | * | * | * | * | * |
Table 4 shows the clinical outcomes from ER pathways in published studies (adapted from Ref. 115).
Table 4.
Clinical outcomes of ER pathways in published studies
| Number (ER/control) | ICU stay | Total stay | Complications | Mortality | |
|---|---|---|---|---|---|
| Brodner et al. 1998 | 42/49 | 1.7/4.0 | 0%/10.2% | ||
| Neal et al. 2003 | 56 | 1 | 10 | 18% | 0% |
| Cerfolio et al. 2004 | 90 | 1 | 7 | 26.6% | 4.4% |
| Low et al.116 | 340 | 2.2 | 11.5 | 45% | 0.3% |
| Jiang et al. 2009 | 114 | 7 | 16.6% | 2.6% | |
| Munitiz et al. 2010 | 74/74 | 9/13 | 31%/38% | 1%/5% | |
| Gatenby et al.114 | 9/16 | 12.0/10.9 | 17/20.5 | 22%/13% | 11%/0% |
| Preston et al. 2013 | 12/12 | 3/4 | 7/17 | 33%/75% | 0%/0% |
| Tang et al. 2013 | 36/27 | 11/15 | 16.7%/25.9% | 5.6%/3.7% | |
| Blom et al. 2013 | 103/78 | 1/1 | 14/15 | 71%/98% | 4%/1% |
Discussion
The pathways have demonstrated improvements in critical care and total inpatient stay as well as improving patient satisfaction and conferring some cost savings. It is impossible to completely disaggregate which components of the pathway are most important to achieve and sustain improvement in quality and outcomes, and any standardized pathway of care may help to achieve improvements.
From their own experience, the authors would like to highlight the following key considerations. First, buy-in from all clinicians involved in the study and their involvement in development and implementation of the program is vital for success. Similarly, patients must all subscribe to the program, and their expectation and understanding of the pathway are of key importance. Second, specific areas which are of probable high yield for expeditious recovery are early mobilization (with elimination of typical impediments to free movement such as monitoring equipment, drains, catheters, and nasogastric tubes), consideration of early enteral nutrition, judicious fluid management, and optimized delivery of analgesia. Third, there is a need to measure and sustain results. This requires robust data collection, ideally covering compliance with the pathway components as well as main outcomes. This should help to promote a culture of continued improvement, with refinement of the pathway and avoidance of deteriorating drift of outcomes following the initial improvements.
15. Long-term quality of life after esophagectomy
Michael P.N. Lewis and Justin C.R. Wormald
Michael.Lewis@nnuh.nhs.uk
The treatment of esophageal cancer, like many other cancers, has seen improved outcomes over the last 20 years. This has come as a result of improvements in neoadjuvant treatment, but also as a result of improved staging, surgery, and perioperative care. The most recent English national audit published in 2013 has shown a postoperative mortality of only 1.7% following esophagectomy.118 The CROSS study, a recent large study of neoadjuvant chemoradiotherapy in esophageal cancer, reported a 5-year survival of 47% in the treatment arm.119 However, it is also worth noting the impressive 34% 5-year survival in the control (surgery only) group.
As a result of surgical and oncological improvements, more people are surviving both esophageal cancer surgery and esophageal cancer itself. QOL after treatment has therefore become more important than ever.
Measurement of QOL by tools such as the EORTC QLQ-C30 and its add-on module OG25 has become an integral outcome for many trials in esophageal cancer. There is some prognostic value to the scores recorded by such tools, with high pretreatment fatigue and dyspnea scores and low physical function scores being associated with decreased cancer survival.120 There are a number of important issues that affect the QOL of esophageal cancer patients after treatment, such as patient demographic and comorbid factors, disease stage, and disease recurrence. Surgical factors include the type of surgery, site of anastomosis, type of conduit, and postoperative complications.
Most symptom scores reach a peak in the immediate postoperative period following esophagectomy and can take months or years to improve. Likewise, the functional scores show an immediate dip after surgery and slowly improve back to pre-operative levels by a year. Patients who do not survive beyond 2 years as a result of disease recurrence have a persistently low QOL score after surgery, until death.78 Several studies have examined the long-term impact of esophagectomy on QOL. Specific long-term symptom issues associated with esophagectomy include gastroesophageal reflux, pain, dysphagia, and dumping.121 A further study on 87 patients at 3 years post-esophagectomy reported reductions in many aspects of QOL compared to a reference population.122 Conversely, a longer follow-up study in 50 patients showed comparable QOL to the reference population after either thoracotomy or THE.123
Surgical factors
The aim of esophagectomy for esophageal cancer should primarily be to achieve a complete pathological resection (R0). Secondary aims of surgery should include limiting the physiological insult as much as possible, achieving an adequate lymph node yield for prognosis, replacing the esophagus with a functioning conduit, and realizing a good QOL for the patient after discharge from the hospital. There have been a number of different surgical operations developed in order to achieve these aims and there have been randomized trials to compare them.
The type of conduit is plainly important to long-term aspects such as reflux and swallowing issues. An interesting randomized trial of a narrow gastric tube versus whole-stomach conduit in 104 patients revealed, perhaps not surprisingly, better QOL in the narrow gastric tube group at 6 and 12 months compared to the whole-stomach group, with impaired pulmonary function and worse reflux in the whole-stomach group.124 De Boers’ randomized study of THE versus thoracotomy reported on QOL factors: the impact of avoiding a thoracotomy in the transhiatal group was reflected by better physical activity and fewer physical symptoms at 3 months postoperative, although these effects lessened with time and the long-term effects have not been compared fully.125 A comparison of anastomotic site (cervical anastomosis versus high mediastinal anastomosis) at a median postoperative period of 6 years in 62 patients failed to show a difference in QOL, both between groups and when compared to a reference population.126 However, an earlier study using the MOS SF-36 tool and informal symptom reporting suggested less reflux in patients treated with a cervical anastomosis, though again, comparable QOL to reference populations.78 The role of pyloroplasty on long-term QOL has yet to be fully identified. Minimally invasive esophagectomy appears to offer the potential to improve short-term effects on QOL but is unlikely to affect long-term issues. QOL is a vital aspect of patient treatment and should be measured at important points in the patient journey. While cancer cure should be the main aim of surgery, the impact of treatment on the patient’s QOL should remain a major priority of the clinician.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Takubo K, Aida J, Naomoto Y, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol. 2009;40:65–74. doi: 10.1016/j.humpath.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg. 1978;76:643–654. [PubMed] [Google Scholar]
- 3.Camilo Barreto J, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World J Gastroenterol. 2010;18:3804–3810. doi: 10.3748/wjg.v16.i30.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ovrebo KK, Lie SA, Laerum OD, et al. Long term survival from adenocarcinoma of the esophagus after transthoracic and transhiatal esophagectomy. World J Surg Oncol. 2012;10:130–140. doi: 10.1186/1477-7819-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logue B, Griffin S. Road Map to esophagectomy for nurses. Crit Care Nurse. 2011;31:69–86. doi: 10.4037/ccn2011426. [DOI] [PubMed] [Google Scholar]
- 6.Desai KM, Soper NJ, Frisella MM, et al. Efficacy of laparoscopic antireflux surgery in patients with Barrett’s esophagus. Am J Surg. 2003;186:652–659. doi: 10.1016/j.amjsurg.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 7.O’Riordan JM, Byrne PJ, Ravi N, et al. Long-term clinical and pathologic response of Barrett’s esophagus after antireflux surgery. Am J Surg. 2004;188:27–33. doi: 10.1016/j.amjsurg.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Oelschlager BK, Barreca M, Chang L, et al. Clinical and pathologic response of Barrett’s esophagus to laparoscopic antireflux surgery. Ann Surg. 2003;238:458–64. doi: 10.1097/01.sla.0000090443.97693.c3. discussion 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaninotto G, Cassaro M, Pennelli G, et al. Barrett’s epithelium after antireflux surgery. J Gastrointest Surg. 2005;9:1253–1260. doi: 10.1016/j.gassur.2005.09.027. discussion 60–61. [DOI] [PubMed] [Google Scholar]
- 10.Biertho L, Dallemagne B, Dewandre JM, et al. Laparoscopic treatment of Barrett’s esophagus: long-term results. Surg Endosc. 2007;21:11–15. doi: 10.1007/s00464-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 11.Zehetner J, DeMeester SR, Ayazi S, et al. Long-term follow-up after anti-reflux surgery in patients with Barrett’s esophagus. J Gastrointest Surg. 2010;14:1483–1491. doi: 10.1007/s11605-010-1322-8. [DOI] [PubMed] [Google Scholar]
- 12.Levine DS. Management of dysplasia in the columnar-lined esophagus. Gastroenterol Clin North Am. 1997;26:613–634. doi: 10.1016/s0889-8553(05)70318-6. [DOI] [PubMed] [Google Scholar]
- 13.Roorda AK, Marcus SN, Triadafilopoulos G. Early experience with radiofrequency energy ablation therapy for Barrett’s esophagus with and without dysplasia. Dis Esophagus. 2007;20:516–522. doi: 10.1111/j.1442-2050.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 14.Zehetner J, DeMeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:39–47. doi: 10.1016/j.jtcvs.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 15.Abbas AE, Deschamps C, Cassivi SD, et al. Barrett’s esophagus: the role of laparoscopic fundoplication. Ann Thorac Surg. 2004;77:393–396. doi: 10.1016/S0003-4975(03)01352-3. [DOI] [PubMed] [Google Scholar]
- 16.Melhado RE, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers. 2010;2:1379–1404. doi: 10.3390/cancers2031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York: Springer-Verlag; 2009. pp. 103–115. [Google Scholar]
- 19.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 20.Tomaszek S, Cassivi SD. Esophagectomy for the treatment of esophageal cancer. Gastroenterology Clinics of North America. 2009;38:169–181. doi: 10.1016/j.gtc.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Ku GY, Ilson DH. Preoperative therapy for esophageal cancer. Gastroenterology Clinics of North America. 2009;38:135–152. doi: 10.1016/j.gtc.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–398. doi: 10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunelli A, Cassivi SD, Fibla J, et al. External validation of the recalibrated thoracic revised cardiac risk index for predicting the risk of major cardiac complications after lung resection. Ann Thorac Surg. 2011;92:445–448. doi: 10.1016/j.athoracsur.2011.03.095. [DOI] [PubMed] [Google Scholar]
- 24.Tomaszek SC, Cassivi SD, Allen MS, et al. An alternative postoperative pathway reduces length of hospitalization following oesophagectomy. Eur J Cardiothorac Surg. 2010;37:807–813. doi: 10.1016/j.ejcts.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 26.von Rahden BH, Stein HJ, Siewert JR. Surgical management of esophagogastric junction tumors. World J Gastroenterol. 2006;12:6608–6613. doi: 10.3748/wjg.v12.i41.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the new union internationale contre le Cancer/American joint cancer committee staging system. Ann Surg. 2011;253:689–698. doi: 10.1097/SLA.0b013e31821111b5. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasoma P, Wickramasinghe K, Ma Y, De-Meester T. Adenocarcinomas of the distal esophagus and “gastric cardia” are predominantly esophageal carcinomas. Am J Surg Pathol. 2007;31:569–575. doi: 10.1097/01.pas.0000213394.34451.d2. [DOI] [PubMed] [Google Scholar]
- 29.Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: a meta-analysis. Ann Surg. 2011;254:894–906. doi: 10.1097/SLA.0b013e3182263781. [DOI] [PubMed] [Google Scholar]
- 30.Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc. 2010;24:1621–1629. doi: 10.1007/s00464-009-0822-7. [DOI] [PubMed] [Google Scholar]
- 31.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 32.Groth SS, Virnig BA, Whitson BA, et al. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the surveillance epidemiology and end results database. J Thorac Cardiovasc Surg. 2010;139:612–620. doi: 10.1016/j.jtcvs.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50. doi: 10.1097/SLA.0b013e3181b2f6ee. [DOI] [PubMed] [Google Scholar]
- 34.Rao VSR, Yeung MMY, Cooke J, et al. Comparison of circumferential resection margin clearance criteria with survival after surgery for cancer of esophagus. J Surg Oncol. 2012;105:745–749. doi: 10.1002/jso.23006. [DOI] [PubMed] [Google Scholar]
- 35.Urschel JD, Blewett CJ, Bennett WF, et al. Handsewn or stapled esophagogastric anastomoses after esophagectomy for cancer: meta-analysis of randomized controlled trials. Dis Esophagus. 2001;14:212–217. doi: 10.1046/j.1442-2050.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 36.Honda M, Kuriyama A, Noma H, et al. Hand-sewn versus mechanical esophagogastric anastomosis after asophagectomy: a systematic review and meta-analysis. Ann Surg. 2012;257:238–248. doi: 10.1097/SLA.0b013e31826d4723. [DOI] [PubMed] [Google Scholar]
- 37.Wang W-P, Qiang G, Wang K-N, et al. A prospective randomized controlled trial of semi-mechanical versus hand-sewn or circular stapled esophagogastrostomy for prevention of anastomotic stricture. World J Surg. 2013;37:1043–1050. doi: 10.1007/s00268-013-1932-x. [DOI] [PubMed] [Google Scholar]
- 38.Lanuti M, DeDelva P, Morse CR, et al. Management of delayed gastric emptying after esophagectomy with endoscopic balloon dilatation of the pylorus. Ann Thorac Surg. 2011;91:1019–1024. doi: 10.1016/j.athoracsur.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell JD, Krasna MJ. The society of thoracic surgeons esophageal cancer guideline series. Ann Thorac Surg. 2013;96:7. doi: 10.1016/j.athoracsur.2013.02.068. [DOI] [PubMed] [Google Scholar]
- 40.Varghese TK, Hofstetter WL, Rizk NP, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg. 2013;96:346–356. doi: 10.1016/j.athoracsur.2013.02.069. [DOI] [PubMed] [Google Scholar]
- 41.Kranzfelder MK, Gertler R, Hapfelmeier A, et al. Chylothorax after esophagectomy for cancer: impact of the surgical approach and neoadjuvant treatment. Systematic review and institutional analysis. Surg Endosc. 2013;27:3530–3538. doi: 10.1007/s00464-013-2991-7. [DOI] [PubMed] [Google Scholar]
- 42.Bonavina L, Saino G, Bona D, et al. Thoracoscopic management of chylothorax complicating esophagectomy. J Laparoendosc Adv Surg Techn. 2001;11:367–369. doi: 10.1089/10926420152761888. [DOI] [PubMed] [Google Scholar]
- 43.Pinto PS, Sirlin CB, Andrade-Barreto OA, et al. Cisterna chyli at routine abdominal MR imaging: a normal anatomic structure in the retrocrural space. Radiographics. 2004;24:809–817. doi: 10.1148/rg.243035086. [DOI] [PubMed] [Google Scholar]
- 44.Lai FC, Chen L, Tu JR, et al. Prevention of chylothorax complicating extensive esophageal resection by mass ligation of thoracic duct: a random control study. Ann Thorac Surg. 2011;91:1770–1774. doi: 10.1016/j.athoracsur.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 45.Rottoli M, Russo IS, Bernardi D, Bonavina L. Atypical presentation and transabdominal treatment of chylothorax complicating esophagectomy for cancer. J Cardiothorac Surg. 2012;7:9. doi: 10.1186/1749-8090-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merrigan B, Winter D, O’Sullivan G. Chylothorax Br J Surg. 1997;84:15–21. [PubMed] [Google Scholar]
- 47.Dugue L, Sauvanet A, Farges O, et al. Output of chyle as an indicator of treatment for chylothorax complicating esophagectomy. Br J Surg. 1998;85:1147–1149. doi: 10.1046/j.1365-2168.1998.00819.x. [DOI] [PubMed] [Google Scholar]
- 48.Breaux J, Marks C. Chylothorax causing reversible T-cell depletion. J Trauma. 1998;28:705–707. doi: 10.1097/00005373-198805000-00029. [DOI] [PubMed] [Google Scholar]
- 49.Cerfolio R, Allen M, Deschampes C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg. 1996;112:1361–1365. doi: 10.1016/S0022-5223(96)70152-6. [DOI] [PubMed] [Google Scholar]
- 50.Lampson R. Traumatic chylothorax: a review of the literature and report of a case treated by mediastinal ligation of the thoracic duct. J Thorac Surg. 1948;17:778–791. [PubMed] [Google Scholar]
- 51.Mason PF, Ragoowansi RH, Thorpe JA. Post-thoracotomy chylothorax-a cure in the abdomen? Eur J Cardiothorac Surg. 1997;11:567–570. doi: 10.1016/s1010-7940(96)01120-7. [DOI] [PubMed] [Google Scholar]
- 52.Icaza OJ, Andrews K, Kuhnke M. Laparoscopic ligation of the thoracic duct in management of chylothorax. J Laparoendosc Adv Surg Tech. 2002;12:129–133. doi: 10.1089/10926420252939673. [DOI] [PubMed] [Google Scholar]
- 53.Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery. 2010;148:831–838. doi: 10.1016/j.surg.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 54.Bhat MA, Dar MA, Lone GN, Dar AM. Use of pedicled omentum in the esophagogastric anastomosis for prevention of anastomotic leak. Ann Thorac Surg. 2006;82:1857–1862. doi: 10.1016/j.athoracsur.2006.05.101. [DOI] [PubMed] [Google Scholar]
- 55.Dai JG, Zhang ZY, Min JX, et al. Wrapping of the omental pedicle flap around anastomosis after esophagectomy for esophageal cancer. Surgery. 2011;149:404–410. doi: 10.1016/j.surg.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Sepesi B, Raymond DP, Peters JH. Esophageal perforation: surgical, endoscopic and medical management strategies. Curr Opin Gastroenterol. 2010;26:379–383. doi: 10.1097/MOG.0b013e32833ae2d7. [DOI] [PubMed] [Google Scholar]
- 57.Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg. 2004;16:124–132. doi: 10.1053/j.semtcvs.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Tang H, Xue L, Hong J, et al. A method for early diagnosis and treatment of intrathoracic esophageal anastomotic leakage: prophylactic placement of a drainage tube adjacent to the anastomosis. J Gastrointest Surg. 2012;16:722–727. doi: 10.1007/s11605-011-1788-z. [DOI] [PubMed] [Google Scholar]
- 59.Schorsch T, Mueller C, Loske G. Endoscopic vacuum therapy of anastomotic leakage and iatrogenic perforation in the esophagus. Surg Endosc. 2013;27:2040–2045. doi: 10.1007/s00464-012-2707-4. [DOI] [PubMed] [Google Scholar]
- 60.Freeman RK, Ascioti AJ, Wozniak TC. Postoperative esophageal leak management with the Polyflex esophageal stent. J Thorac Cardiovasc Surg. 2007;133:333–338. doi: 10.1016/j.jtcvs.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Kauer WK, Stein HJ, Dittler HJ, Siewert JR. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc. 2008;22:50–53. doi: 10.1007/s00464-007-9504-5. [DOI] [PubMed] [Google Scholar]
- 62.Peracchia A, Bonavina L, Incarbone R. Results of surgical therapy in patients with adenocarcinoma of the esophagus and cardia. Gastric Cancer. 1999;2:89–94. doi: 10.1007/s101200050029. [DOI] [PubMed] [Google Scholar]
- 63.Steup WH, De Leyn P, Deneffe G, et al. Tumor of the esophagogastric junction: long-term survival in relation to the pattern of lymph node metastasis and a critical analysis of accuracy or inaccuracy of pTNM classification. J Thor Cardiovasc Surg. 1996;111:85–95. doi: 10.1016/S0022-5223(96)70404-X. [DOI] [PubMed] [Google Scholar]
- 64.Hirai T, Yoshida K, Hihara J, et al. Clinicopatological results of gastric cancer involving the esophagus and indication of transhiatal esophagectomy. Proceedings of the 3rd International Gastric Cancer Congress; Seoul. 1999. pp. 513–518. [Google Scholar]
- 65.Nomura S, Sasako M, Katai H, et al. Decresing complication rates with stapled esophagoejunostomy following a learning curve. Gastric Cancer. 2000;3:97–101. doi: 10.1007/pl00011703. [DOI] [PubMed] [Google Scholar]
- 66.Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus. 2009;22:195–202. doi: 10.1111/j.1442-2050.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- 67.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. NEJM. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 68.Verhage RJ, Hazebroek EJ, Boone J, Van Hillegersberg R. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir. 2009;64:135–146. [PubMed] [Google Scholar]
- 69.Ruurda JP, van Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl. 2002;84:223–226. doi: 10.1308/003588402320439621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc. 2006;20:1435–1439. doi: 10.1007/s00464-005-0674-8. [DOI] [PubMed] [Google Scholar]
- 71.Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. British Journal of Surgery. 2009;96:878–886. doi: 10.1002/bjs.6647. [DOI] [PubMed] [Google Scholar]
- 72.Clark J, Sodergren MH, Purkayastha S, et al. The role of robotic assisted laparoscopy for oesophagogastric oncological resection; an appraisal of the literature. Dis Esophagus. 2011;24:240–250. doi: 10.1111/j.1442-2050.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 73.van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial) Trials. 2012;13:230. doi: 10.1186/1745-6215-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Low DE. Open versus minimally invasive esophagectomy: what is the best approach? Frame the issue. J Gas-trointest Surg. 2011;15:1497–1499. doi: 10.1007/s11605-011-1559-x. [DOI] [PubMed] [Google Scholar]
- 75.Briez N, et al. Open versus laparoscopically-assisted esophagectomy for cancer: a multi centre randomized controlled phase III trial- the MIRO-trial. BMC Cancer. 2011 Jul 23;11:310. doi: 10.1186/1471-2407-11-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mamidanna R, et al. Short-term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population-based national study. Ann Surg. 2012;255:197–203. doi: 10.1097/SLA.0b013e31823e39fa. [DOI] [PubMed] [Google Scholar]
- 77.Ishikura S, Nihei K, Ohtsu A, et al. A Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–2702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 78.Blazeby JM, Farndon JR, Donovan J, et al. A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer. 2000;88:1781–1787. [PubMed] [Google Scholar]
- 79.Bonnetain F, Bouche O, Michel P, et al. A comparative longitudinal quality of life study using the Spitzer quality of life índex in a randomized multicenter phase III trial (FFCD 9102): chemoradiation followed by surgery compared with chemoradiation alone in locally advanced squamous resectable thoracic esophageal cancer. Ann Oncol. 2006;17:827–834. doi: 10.1093/annonc/mdl033. [DOI] [PubMed] [Google Scholar]
- 80.Choi J, Kim SG, Kim JS, et al. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380–1386. doi: 10.1007/s00464-009-0783-x. [DOI] [PubMed] [Google Scholar]
- 81.Kranzfelder M, Schuster T, Geinitz H, et al. Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg. 2011;98:768–783. doi: 10.1002/bjs.7455. [DOI] [PubMed] [Google Scholar]
- 82.Kato H, Fukuchi M, Manda R, et al. The effectiveness of planned esophagectomy after neoadjuvant chemoradiotherapy for advanced esophageal carcinomas. Anticancer Res. 2004;24:4091–4096. [PubMed] [Google Scholar]
- 83.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 84.Nagata M, Yamamoto H, Takiguchi N, et al. Neoadjuvant chemoradiotherapy followed by esophagectomy versus definitive chemoradiotherapy in resectable stage II/III (T1–3N0, 1M0) esophageal squamous cell carcinoma. Esophagus. 2006;3:105–111. [Google Scholar]
- 85.Toh Y, Ohga T, Itoh S, et al. Treatment results of radical surgery and definitive chemoradiotherapy for patients with submucosal esophageal squamous cell cancinomas. Anticancer Res. 2006;26:2487–2492. [PubMed] [Google Scholar]
- 86.Adams R, Morgan M, Mukherjee S, et al. A prospective comparison of multidisciplinary treatment of esophageal cancer with curative intent in a UK cancer network. Eur J Surg Oncol. 2007;33:307–313. doi: 10.1016/j.ejso.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 87.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cell cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 88.Yamashita H, Nakagawa K, Yamada K, et al. A single institutional non-randomized retrospective comparison between definitive chemoradiotherapy and radical surgery in 82 Japanese patients with resectable esophageal squamous cell carcinoma. Dis Esophagus. 2008;21:430–436. doi: 10.1111/j.1442-2050.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 89.Ariga H, Nemoto K, Miyazaki S, et al. Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Rad Oncol Biol Phys. 2009;75:348–356. doi: 10.1016/j.ijrobp.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 90.Yamashita H, Okuma K, Seto Y, et al. A retrospective comparison of clinical outcomes and quality of life measures between definitive chemoradiation alone and radical surgery for clinical stage II–III esophageal carcinoma. J Surg Oncol. 2009;100:435–441. doi: 10.1002/jso.21361. [DOI] [PubMed] [Google Scholar]
- 91.Salek R, Tabrizi FV, Bezenjani SE, et al. Chemoradiotherapy alone as the standard treatment of epidermoid esophageal carcinoma. Oncology. 2011;81:214–219. doi: 10.1159/000333448. [DOI] [PubMed] [Google Scholar]
- 92.Morita M, Toh Y, Saeki H, et al. Chemoradiotherapy and surgical resection for cT4 esophageal cancer. Anticancer Res. 2012;32:3275–3282. [PubMed] [Google Scholar]
- 93.Motoori M, Yano M, Ishihara R, et al. Comparison between radical esophagectomy and definitive chemora-diotherapy in patients with clinical T1bN0M0 esophageal cancer. Ann Surg Oncol. 2012;19:2135–2141. doi: 10.1245/s10434-012-2231-8. [DOI] [PubMed] [Google Scholar]
- 94.Teoh AYB, Chiu PWY, Yeung WK, et al. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol. 2013;24:165–171. doi: 10.1093/annonc/mds206. [DOI] [PubMed] [Google Scholar]
- 95. [Accessed 6/30/2014]; http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=/portlets/contentViewer/show&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=committees/cancer/cancer_protocols/protocols_index.html&_pageLabel=cntvwr.
- 96. [Accessed 6/30/2014]; http://www.rcpath.org/publications-media/publications/datasets.
- 97.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon: World Health Organization; 2010. [Google Scholar]
- 98.Sobin L, Gospodarowicz ML, Wittekind CH. TNM Classification of Malignant Tumors. UICC, New York: John Wiley & Sons; 2010. [Google Scholar]
- 99.Chan DSY, Reid TD, Howell I, Lewis WG. Systematic review and meta-analysis of the influence of circumferential resection margin involvement on survival in patients with operable oesophageal cancer. Br J Surg. 2013;100:456–464. doi: 10.1002/bjs.9015. [DOI] [PubMed] [Google Scholar]
- 100.Lagarde SM, ten Kate FJ, de Boer DJ, et al. Extra-capsular lymph node involvement in node-positive patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Am J Surg Pathol. 2006;30:171–176. doi: 10.1097/01.pas.0000189182.92815.12. [DOI] [PubMed] [Google Scholar]
- 101.Peters CJ, Hardwick RH, Vowler SL, Fitzgerald RC. Generation and validation of a revised classification for oesophageal and junctional adenocarcinoma. Br J Surg. 2009;96:724–733. doi: 10.1002/bjs.6584. [DOI] [PubMed] [Google Scholar]
- 102.Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus. 2009;6:1–25. [Google Scholar]
- 103.Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol. 2013;3:262. doi: 10.3389/fonc.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 105.Ong CA, Shapiro J, Nason KS, et al. Three-gene immunohistochemical panel adds to clinical staging algorithms to predict prognosis for patients with esophageal adenocarcinoma. J Clin Oncol. 2013;31:1576–1582. doi: 10.1200/JCO.2012.45.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin DC, Du XL, Wang MR. Protein alterations in ESCC and clinical implications: a review. Dis Esophagus. 2009;22:9–20. doi: 10.1111/j.1442-2050.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 107.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 108.Deeter M, Dorer R, Kuppusamy MK, et al. Assessment of criteria and clinical significance of circumferential resection margins in esophageal cancer. Arch Surg. 2009;144:618–624. doi: 10.1001/archsurg.2009.115. [DOI] [PubMed] [Google Scholar]
- 109.Harvin JA, Lahat G, Correa AM, et al. Neoadjuvant chemoradiotherapy followed by surgery for esophageal adenocarcinoma: significance of microscopically positive circumferential radial margins. J Thorac Cardiovasc Surg. 2012;43:412–420. doi: 10.1016/j.jtcvs.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 110.Verhage RJ, Zandvoort HJ, ten Kate FJ, et al. How to define a positive circumferential resection margin in T3 adenocarcinoma of the esophagus. Am J Pathol. 2011;35:919–926. doi: 10.1097/PAS.0b013e31821a5692. [DOI] [PubMed] [Google Scholar]
- 111.O’Farrell NJ, Donohoe CL, Muldoon C, et al. Lack of independent significance of a close (<1 mm) circumferential resection margin involvement in esophageal and junctional cancer. Ann Surg Onc. 2013;20:2727–2733. doi: 10.1245/s10434-013-2899-4. [DOI] [PubMed] [Google Scholar]
- 112.DiMusto PD, Orringer MB. Transhiatal esophagectomy for distal and cardia cancers: implications of a positive gastric margin. Ann Thorac Surg. 2007;83:1993–1998. doi: 10.1016/j.athoracsur.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 113.Knott A, Pathak S, McGrath JS, et al. Consensus views on implementation and measurement of enhanced recovery after surgery in England: Delphi study. BMJ Open. 2012;2:e001878. doi: 10.1136/bmjopen-2012-001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gatenby P, Hainsworth A, Caygill C, et al. Projections for oesophageal cancer in England to 2033. Eur J Cancer Prev. 2011;20:283–286. doi: 10.1097/CEJ.0b013e32834572d2. [DOI] [PubMed] [Google Scholar]
- 115.Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg. 2013;30:70–78. doi: 10.1159/000350701. [DOI] [PubMed] [Google Scholar]
- 116.Low D, Kunz S, Schembre D, et al. Esophagectomy–it’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg. 2007;11:1395–402. doi: 10.1007/s11605-007-0265-1. [DOI] [PubMed] [Google Scholar]
- 117.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 118.Chadwick G, Groene O, Cromwell DA, et al. The National Oesophago-Gastric Cancer Audit: 2013 annual report. The NHS Information Centre; 2013. Cited Jan 8, 2013. https://catalogue.ic.nhs.uk/ [Google Scholar]
- 119.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 120.Blazeby JM, Brookes ST, Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut. 2001;49:227–230. doi: 10.1136/gut.49.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McLarty AJ, Deschamps C, Trastek VF, et al. Esophageal resection for cancer of the esophagus: long-term function and quality of life. Ann Thorac Surg. 1997;63:1568–1572. doi: 10.1016/s0003-4975(97)00125-2. [DOI] [PubMed] [Google Scholar]
- 122.Djarv T, Lagergren J, Blazeby JM, et al. Long-term health-related quality of life following surgery for oesophageal cancer. Brit J Surg. 2008;95:1121–1126. doi: 10.1002/bjs.6293. [DOI] [PubMed] [Google Scholar]
- 123.Gockel I, Gönner U, Domeyer M, et al. Long-term survivors of esophageal cancer: disease-specific quality of life, general health and complications. J Surg Oncol. 2009;102:516–522. doi: 10.1002/jso.21434. [DOI] [PubMed] [Google Scholar]
- 124.Zhang M, Wu QC, Li Q, et al. Comparison of the health-related quality of life in patients with narrow gastric tube and whole stomach reconstruction after oncologic esophagectomy: a prospective randomized study. Scand J Surg. 2013;102:77–82. doi: 10.1177/1457496913482234. [DOI] [PubMed] [Google Scholar]
- 125.De Boer AG, van Lanschot JJ, van Sandick JW, et al. Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. J Clin Oncol. 2004;22:4202–4208. doi: 10.1200/JCO.2004.11.102. [DOI] [PubMed] [Google Scholar]
- 126.Bennett JM, Wormald JCR, van Leuven M, Lewis MPN. Long term quality of life after oesophagectomy for cancer—comparison of cervical versus mediastinal anastomoses. Gastroenterol. 2013;144(Suppl 1):S–1062. [Google Scholar]