Abstract
Cytogenetic abnormalities are important diagnostic and prognostic criteria for hematologic malignancies. Karyotyping and Fluorescent In Situ Hybridization, (FISH), are the conventional methods by which these abnormalities are detected. The sensitivity of these microscopy-based methods is limited by the abundance of the abnormal cells in the samples and therefore these analyses are commonly not applicable to minimal residual disease stages. A flow cytometry-based imaging approach was developed to detect chromosomal abnormalities following FISH in suspension (FISH-IS) which enables the automated analysis of several log-magnitude higher number of cells compared to the microscopy-based approaches. The present study demonstrates the applicability of FISH-IS for detecting numerical chromosome aberrations, establishes accuracy and sensitivity of detection compared to conventional FISH, and feasibility to study procured clinical samples of acute myeloid leukemia (AML). Male and female healthy donor peripheral blood mononuclear cells hybridized with combinations of chromosome enumeration probes (CEP) 8, X and Y served as models for disomy, monosomy, and trisomy. The sensitivity of detection of monosomies and trisomies amongst 20,000 analyzed cells was determined to be 1% with a high level of precision. A high correlation (R2=0.99) with conventional FISH analysis was found based on the parallel analysis of diagnostic samples procured from 10 AML patients with trisomy 8 (+8). Additionally, FISH-IS analysis of samples procured at the time of clinical remission demonstrated the presence of residual (+8) cells indicating that this approach may be used to detect minimal residual disease and associated chromosomal defects.
Key terms: FISH in suspension, acute myeloid leukemia, ImageStream, minimal residual disease
Introduction
Up to 60 percent of adults with de novo acute myeloid leukemia (AML) present with clonal chromosomal aberrations with 10–20% presenting with complex abnormal karyotypes defined as containing at least 3 chromosomal aberrations [1–7]. Based on multi-center studies, cytogenetic-based prognostication of treatment outcome for AML (favorable, intermediate and adverse) is now well-established (2–4) and the World Health Organization has adopted a re-classification of AML subtypes to include cytogenetic abnormalities independent of morphology and/or blast percentage [8].
Clinically, AML patients are declared to be in remission based primarily on morphological parameters (including bone marrow cellularity, maturation of all cell lineages, less than 5% blasts, etc) [9]. However, since the majority of these patients ultimately relapse, one can argue the adequacy of these remission criteria [10]. A multi-center study of AML cytogenetically evaluated samples at time of morphologic complete remission and found that conversion from aberrant karyotype at time of AML presentation to normal karyotype at time of morphologic remission was an important prognostic indicator [11]. This study and others [12] support the use of cytogenetic evaluation at remission as a criterion of complete remission in AML. Including cytogenetic analysis in defining complete remissions however, is compromised by the lack of sensitivity of traditional karyotyping and fluorescence in situ hybridization (FISH) techniques to detect chromosomal abnormalities during minimal residual disease stages. This lack of sensitivity is due to the relatively low number of cells, 20 for karyotyping, and 200–400 for FISH, that are evaluated. In case of structural chromosome rearrangements, PCR-based detection is possible but this will not be able to detect numerical abnormalities. Thus, during minimal (residual) disease stages, cytogenetic information on the numerically small, but potentially clinically significant aneuploid cell populations is commonly not available.
The ImageStream is a flow cytometry-based imaging platform that simultaneously captures multiple spectrally separated, but spatially correlated images from individual cells at high acquisition rates of up to 1000 cells/second. The ability to visualize and perform photometric/morphometric analysis of imagery from tens of thousands of cells thus combines quantitative image analysis with the statistical power of flow cytometry. One of the features that can be determined is the so-called “spot count/cell” which determines the number of “spots” that are defined by intensity and size parameters. This feature makes the ImageStream a potential candidate to enumerate cellular spots associated with FISH.
In order to adapt this technology for chromosome analysis, a method was established to perform FISH in suspension (FISH-IS). The current study determines the precision and sensitivity of FISH-IS to detect monosomies and trisomies by using controlled models derived from healthy donor peripheral blood. In addition, the sensitivity (compared to conventional FISH) and reproducibility of FISH-IS to detect numerical aberrations in samples procured from AML patients at diagnosis and remission is presented.
MATERIALS AND METHODS
All methods are described according to MIFlowCyt 1.0 guidelines. Any and all analysis files mentioned are available upon request.
Cells
Healthy donor peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll-Hypaque density centrifugation using Histopaque (Sigma-Aldrich, St. Louis, MO). Bone marrow samples from 10 AML patients, previously diagnosed by conventional cytogenetics as (+8), procured at time of diagnosis and at clinical remission, were obtained under protocols approved by the Scientific Review Committee and the Institutional Review Board at Roswell Park Cancer Institute (RPCI).
Conventional FISH hybridization
Diagnostic and remission bone marrow aspirate samples processed by the Cytogenetics Laboratory at -(RPCI) using standard techniques [13] were used for conventional FISH analysis. FISH was performed using a Chromosome Enumeration Probe (CEP) 8-SpectrumGreen (Cat #06J37-018, Abbott Molecular, Des Plaines, IL) according to manufacturer’s instructions. Slides were visualized and analyzed on a Nikon Microscope using the CytoVision Program (Applied Imaging, Inc.)
FISH-IS Hybridization
Following isolation of PBMCs by Ficoll-Hypaque density centrifugation, cells were fixed by adding freshly prepared Carnoy’s fixative (3:1 v/v methanol:glacial acetic acid) drop-wise to a loosened pellet of PBS-washed cells. The fixed cells were incubated for 10 minutes at room temperature followed by storage at −20°C for at least 4 hours and up to 3 months. For hybridization, fixed cells were washed twice in 1xPBS with 1% BSA, pelleted, and resuspended in 0.1% NP-40 and 2x Saline-Sodium Citrate (SSC) in PBS. From this, 1.5×106 cells/hybridization reaction were transferred to 1.5mL eppendorf tubes. Cells were pelleted by centrifugation and the supernatant carefully removed by pipetting. A solution of 28μL CEP hybridization buffer (supplied with CEP probe kit), 2μL of probe and 10μL of nuclease-free water was prepared for each hybridization reaction, and 40μL mix added to each cell pellet. Samples were then subjected to the following denaturing and hybridization steps: 5 min 80°C, 9h 42°C and an optional storage step of 4°C. Post hybridization, 200μL of 0.1% NP-40 in 2x SSC was added to each reaction mixture. Cells were then pelleted by centrifugation and resuspended in 200μL 0.3% NP-40 in 2x SSC pre-warmed to 73°C and incubated at 73°C for 2 min to degrade excess probe. 200μL of ice-cold fetal bovine serum (FBS) (Atlanta Biological, Lawrenceville, GA) was added to drop the temperature and to prevent clumping of cells. Cells were then pelleted and resuspended in 100μL ice-cold FBS and stored at 4°C before analysis on the ImageStream. An optional 50ng DAPI (Invitrogen, Grand Island, NY) is added to each sample to allow for visualization of the nucleus.
Models for aneuploidy
Healthy male donor PBMCs hybridized with CEPX (SpectrumGreen probe, Cat #05J10-033, Abbott Molecular, Des Plaines, IL) served as a model for monosomy. Healthy female donor PBMCs hybridized with CEPX or CEP8 served as a model for disomy whereas healthy male donor PBMCs simultaneously hybridized with CEPY (SpectrumGreen probe, Cat #05J10-034, Abbott Molecular, Des Plaines, IL) and CEP8 served as a model for trisomy. For experiments to determine the sensitivity of detection of aneuploid cells in a normal diploid cell population, male and female PBMCs were counted and mixed before hybridization according to the desired final ratios. The same cell mixtures were used to determine the sensitivity of detecting monosomies and trisomies by altering the hybridization probes from CEPX only (monosomy in male PBMC) to CEPY and CEP8 simultaneously (trisomy in male PBMC).
ImageStream acquisition
Data acquisition was performed with an ImageStream 100 (yrs 2008–2010) and, following an instrument upgrade, an ImageStreamX (yrs 2010–2012) cytometers (Amnis Corp, Seattle, WA). Relevant settings remained consistent between both instruments for all parameters measured. Images acquired include a brightfield image (Channel 1; 430–480nm), SpectrumGreen (Channel 2; 480–560nm) and, DAPI (Channel 7 ISX; 430–505nm). SpectrumGreen and DAPI were excited by 488nm and a 405nm lasers with outputs of 20mW and 5mW, respectively. All data were acquired in Extended Depth of Field (EDF) mode. EDF uses a combination of specialized optics and image processing algorithms to extend the in-focus range from 4 microns to 16 microns [14]. At least 20,000 cell events were acquired for each sample and IDEAS v4.0 software (Amnis) was used for data analysis.
ImageStream analysis
Following data acquisition, EDF-images were first deconvolved to obtain in-focus, spot-like images associated with the FISH hybridization [14]. From each cell, the brightfield, SpectrumGreen (500–560nm emission range) and, where applicable, DAPI (420–505 nm emission range) images were used for analysis. Hierarchical gating schemes, as detailed in the Results section, were employed to determine the spot count and total signal intensity of the cells of interest. Selected calculated image features from the IDEAS software were exported as FCS files into FCS EXPRESS (vs4 flow cytometry, DeNovo Software) for graphing single and dual parameter histograms.
RESULTS
Hierarchical gating strategy
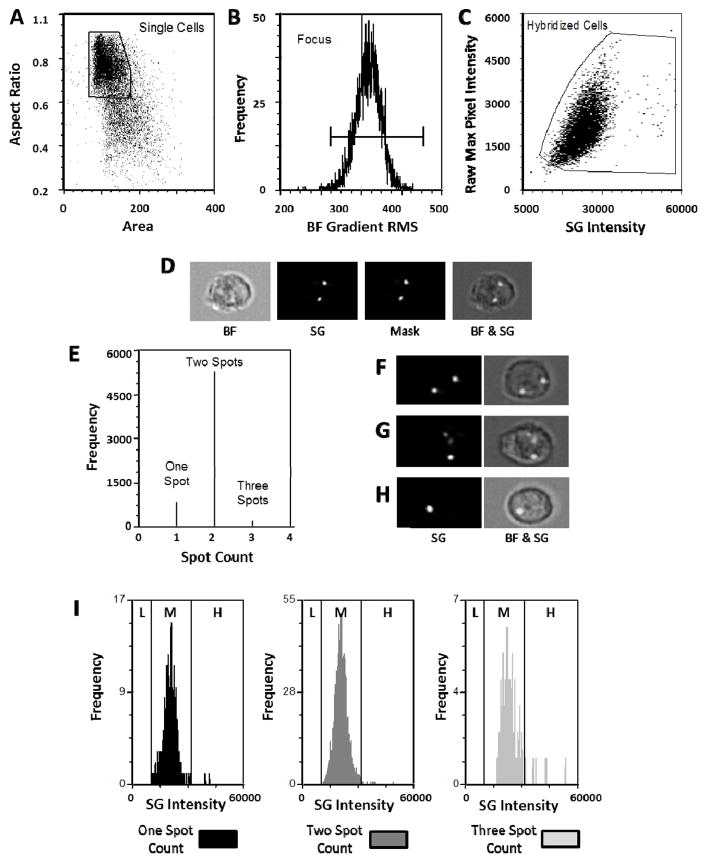
Using IDEAS, single cells are identified as having a high aspect ratio and a low area of associated brightfield images (Figure 1A). The focus quality of the images is then evaluated by visually inspecting cells according to the contrast parameter, Root Mean Square (RMS) of the pixel intensity gradient of the brightfield channel (Figure 1B). Due to the application of the EDF element close to 100% of the single cells are in focus. Of the single, in focus cells, events were identified as having adequate hybridization signal by plotting the raw maximum pixel intensity (a measure of the peak fluorescence in the cell, and therefore able to distinguish the spot-like distribution of the hybridization signal from more evenly distributed background signal) against the total cellular intensity in the appropriate fluorescence channel (Figure 1C). Using the fluorescence images (SpectrumGreen: SG) of the hybridized cells, a “spot mask” was created based on two user-input-dependent variables: the spot-to-background ratio and radius of the spots (Figure 1D). These masking parameters were visually optimized for each sample (optimization strategy of the masking parameters are provided in Suppl Figure 1) and a spot counting algorithm was then applied to assess the number of spots/cell. A representative example of a healthy donor hybridized with CEP8 is shown (Figure 1E). Since the images obtained are 2-dimensional projections of 3-dimensional (3-D) objects, overlap of 2 spots can occur depending on the rotation of a cell in the 3-D space with the potential for a discrepancy between spot count and ploidy. Furthermore, a hybridization signal of a disomy can be associated with more than 2 spots due to incomplete saturation of a hybridization spot or asynchronous replication of sequences following G2 separation. In order to correct for this, the measured intensity of hybridization signal for each of the populations was plotted as a single parameter histogram (Figure 1I) and evaluated for changes in intensity which corresponds to the amount of hybridized probes rather than the distribution (or spot count) of the hybridization signal. A medium (M) level of intensity range was determined for disomic cells (2n). Based on the 2n intensity range, a low intensity range (L) was assigned for aneuploid cells <2n (monosomies) and a high intensity (H) range for aneuploid cells >2n (trisomies). Those events that the software classified as one or three spots in Figure 1E are in the medium (2n) intensity range, so are actually disomies with a discrepant ploidy-spot count relationship for reasons explained above. Representative images of cells with spot counts of 2, 3, and 1 are shown in Figure 1F, G, and H respectively.
Figure 1. Hierarchical gating and analysis strategy to identify ploidy in a healthy female donor sample hybridized with a CEP8 probe.
Single cells are discriminated from debris and cell aggregates based on area and aspect ratio of the brightfield image (A). Of those cells, events which are in focus are selected on the basis of a high value of a contrast parameter (gradient RMS of the brightfield image) (B). Single, in focus cells that have a hybridization signal are then selected based on raw maximum pixel intensity of the SpectrumGreen image and the total cellular SpectrumGreen intensity (C). The SpectrumGreen images are segmented (masked) to identify hybridization spots by applying an algorithm that takes into account the signal to background ratio and the diameter of the spots. The corresponding brightfield (BF) and SpectrumGreen (SG) image of a representative cell, together with the associated spot-mask (Mask) and a collective overlay (BF & SG) of these images are shown in (D). A spot count feature is then applied (E) indicating that 12.4% cells contain 1 spot, 84.9% cells contain two spots, and 2.75% cells contain 3 spots. Panels F, G and H are examples of how a disomy can be associated with a 2 spot, 3 spot, and 1 spot count, respectively. Total fluorescence intensity distribution of each spot count category (1, 2, or 3) is also assessed (I) showing that most events fall within the medium (M) intensity range established for diploid (2n) cells. L=low intensity range (<2n); H=high intensity range (>2n). Note that the y-axes scales in fig 1I are normalized to better visualize the intensity distributions.
Controlled models of monosomies, disomies, and trisomies
In order to test the hierarchical gating strategy, representative models of aneuploidy were designed with predictable frequencies of numerical aberrations. Models of monosomy and trisomy were spiked into a disomy population prior to hybridization. Disomy populations were comprised of female healthy donor PBMCs hybridized with CEPX or CEP8 SpectrumGreen probes. Models for monosomy, and trisomy were male healthy donor PBMCs hybridized with CEPX only or simultaneously with CEP8 and CEPY, respectively. Male PBMCs were added to female PBMCs at ratios of 10%, 1% and 0.1%, for both models of monosomy and trisomy. A total of 10,000 cell events were collected for each sample and the results are summarized in Table 1.
Table 1.
Predictable models of aneuploidy in healthy donor PBMCs.
Aneuploidy was modeled by spiking male PBMCs into female PBMCs before hybridization with CEPX or CEPY + CEP8 resulting in monosomy or trisosmy for the male cells, and disomy for the female.
| Model | Probes Used | Expected | Results |
|---|---|---|---|
| 100% Female PBMCs | CEPX | 100% Disomy | 99.7% Disomy |
| 100% Male PBMCs | CEPX | 100% Monosomy | 100% Monosomy |
| 100% Male PBMCs | CEPY and CEP8 | 100% Trisomy | 99.5% Trisomy |
| 10% Male PBMCs | CEPX | 10.0% Monosomy | 9.3% Monosomy |
| 1.0% Male PBMCs | CEPX | 1.0% Monosomy | 1.0% Monosomy |
| 0.1% Male PBMCs | CEPX | 0.1% Monosomy | 0.5% Monosomy |
| 10% Male PBMCs | CEPY and CEP8 | 10.0% Trisomy | 9.4% Trisomy |
| 1.0% Male PBMCs | CEPY and CEP8 | 1.0% Trisomy | 1.2% Trisomy |
| 0.1% Male PBMCs | CEPY and CEP8 | 0.1% Trisomy | 0.5% Trisomy |
These data establish that the ImageStream approach is capable of accurately detecting numerical aberrations with a sensitivity level at least as low as 1 in 100 cells (1%). At levels below 1% the abnormal population is overestimated though the analysis allows for the manual inspection of the selected population. Post-analysis visual verification on the ‘enriched’ imagery can thus confirm the presence or absence of aneuploid populations. Figure 2 depicts images of visual verification of model trisomies at the 0.1% level.
Figure 2. Visual verification of trisomies from model 0.1% Trisomy sample.

The population identified as trisomies by FISH-IS analysis was manually examined and true trisomy events were identified. Cells in focus, shown by Brightfield image (BF, Left column); Spot images (2nd column); and nuclear staining shown (DAPI, 3rd column); with overlay on the right showing spots in the nucleus. DAPI signal was pseudo-colored red in analysis software to better display the presence of spots in the overlay.
Correlation between Spot Counts and fluorescence intensity
Monosomies have half of the genetic material for the affected chromosome than disomies, therefore it would be expected that when probed for an affected chromosome only 50% of the hybridization signal would be present. Likewise, trisomies, having an extra copy of the affected chromosome would be expected to display 150% of the hybridization signal compared to disomies. Figure 3A demonstrates that for the 10% monosomy/90% disomy model in which a single probe was used (CEPX) the results were in agreement with the expectations: intensity of monosomies = 50% of disomies. As indicated in Table 1, the low intensity population in the left graph of Figure 3A consists of 9.3% of the total events. For the 10% trisomy/90% disomy model for which 2 probes (CEP8 + CEPY) were used to create an artificial trisomy, aneuploidy was also detected in 9.4% of the total population but the intensity of the hybridization signal of the artificial trisomies was brighter than expected (Figure 3B, right graph). In the artificial trisomy model the greater than expected intensity of the cells is likely due to the larger hybridization signal of the CEPY probe compared to a single hybridization signal of a CEP8 probe. Figure 3C demonstrates that in a true trisomy sample (AML +8 sample) for which a single CEP8 probe is used, the intensity of the trisomy population is indeed 150% of that of the disomy population. This sample was determined to contain 90.5% trisomy by conventional FISH. The FISH-IS analysis of this same sample detected 89% trisomy. As expected, the intensity of the remaining 11% was within the medium (2n) intensity range, indicating the remaining events to be disomies. The presence of software-calculated ‘1 spot’ count in this sample is likely due to 3 overlapping spots of a trisomy, hence the high (>2n) signal intensity region, or 2 overlapping spots of a disomy (medium intensity range) (Figure 3C, left graph). The presence of 2 spots with high signal intensity range (>2n) is likely due to 2 of the 3 spots overlapping whereas the 2 spot count associated with a medium intensity range are true disomies (Figure 3C, center graph).
Figure 3. Correlation between spot count, ploidy and fluorescence intensity.
The analysis of male PBMCs mixed with female PBMCs at a 10:90 ratio following hybridization with CEPX (A) or CEPY+CEP8 (B) is shown. Low intensity (L) = <2n, Medium intensity (M) = 2n, and High intensity (H) = >2n; ranges are assigned according to the intensity distribution of diploid cells. In the 10% monosomy model (A) the fluorescence intensity of the male cells (1 X-chr) is 50% of that detected for the female cells (2 X-chr). In the artificial trisomy model (B) the intensity of the cells is greater than 150% of that of the disomies due to the larger hybridization signal of the CEPY probe compared to a single hybridization signal of a CEP8 probe. Figure 3C demonstrates that in a true trisomy sample (AML +8 sample) for which a single CEP8 probe is used, the intensity of the trisomy population is 150% of that of the disomy population. Fluorescence intensity distributions of A, B and C were normalized to position the disomy peaks at the same intensity level. Note that the y-axes scales are normalized to better visualize the intensity distributions.
CEP probes bind to chromosome-specific repetitive sequences in the centromeric regions of the chromosomes. It is noteworthy that the methodology is sufficiently sensitive to detect differences associated with relatively small differences in the size of hybridization target.
FISH-IS analysis versus conventional FISH analysis
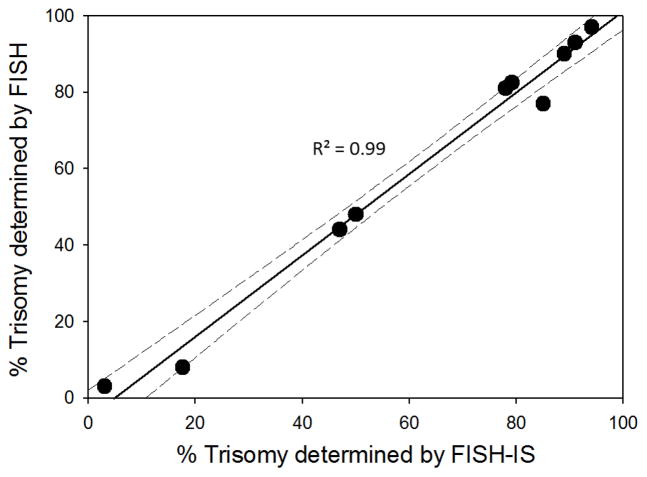
Cryopreserved diagnostic samples from ten AML patients with previously established +8 were evaluated by FISH-IS (Figure 4). The range of incidence of +8 varied from 3% to 97% (mean 62%). Throughout the entire range, a high correlation was observed between the original conventional FISH analysis results and the FISH-IS analysis (R2=0.99) but the intersect of the correlation line with the X-axis suggests greater sensitivity of FISH-IS in the lower detection range. At the time of diagnosis, bone marrow aspirates that were sent for cytogenetic analysis and cryopreservation were not necessarily pooled creating the possibility that the cryopreserved samples were not identical in terms of cellularity and heterogeneity compared to the sample that was cytogenetically analyzed at the time of diagnosis. Therefore, selected (n=3) samples that were obtained for FISH-IS analysis were also sent out for conventional FISH analysis. For those samples, original trisomy percentages determined at time of diagnosis of 11.5%, 82%, and 90.5%, were comparable to the repeat analysis of the corresponding cryopreserved samples with percentages of 8%, 97%, and 90%, respectively.
Figure 4. Correlation of conventional FISH and FISH-IS.
Ten cryopreserved diagnostic samples from patients previously established to be positive for (+8) were evaluated by both conventional FISH and FISH-IS. Graph shows percentage of trisomies in each sample by FISH-IS along the x axis, and conventional FISH along the y axis. Comparison of the two methods yields a correlation value of R2=0.99. First order linear regression of the data is shown by a solid black line and the corresponding 95% confidence interval is shown by the dashed lines.
Reproducibility of FISH-IS procedure
Of the 10 diagnostic patient samples processed, 5 had sample available with adequate cellularity to assess assay reproducibility (Figure 5). The five samples were thawed, fixed, and left to rest overnight at −20°C. On days one, four, and eight post-fixation, aliquots of each cell suspension were processed, hybridized, and run immediately on the ImageStreamX. The incidence of trisomies in the samples analyzed ranged from 3%–94% (median 79%). Results demonstrated that FISH-IS analysis of the same sample on three separate days is highly reproducible with standard deviations ranging from 0.3%–1.9%.
Figure 5. Reproducibility of trisomy identification.
Reproducibility was demonstrated by repeating the procedure on fixed patient samples from hybridization to final analysis on three separate days. On days one, four, and eight post-fixation, aliquots of each cell suspension were processed, hybridized, and run immediately on the ImageStreamX.
Identification of aneuploid cells during minimal residual disease stages
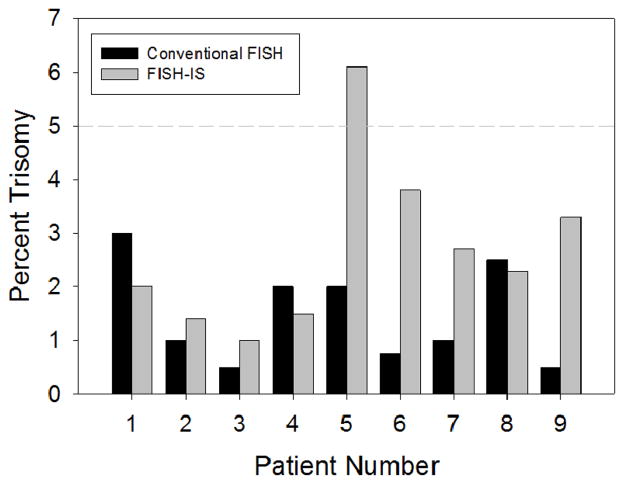
To evaluate the ability of the FISH-IS technique to detect aneuploidy during minimal residual disease, samples from nine (+8) patients, procured at time of clinical remission, were analyzed in parallel by conventional FISH and FISH-IS (Figure 6). The lower detection limit to be considered a positive sample by conventional FISH analysis is 10 positive cells out of 200 (5%). As expected, for all 9 samples the FISH analysis resulted in levels below this detection limit (range 0.5%–3%). The parallel FISH-IS analysis was very close to the FISH analysis in patients 1,2,3,4, and 8, but was considerably higher (range 2.7–6.6 fold) in patients 5, 6, 7, and 9, exceeding the 5% level in sample #5.
Figure 6. FISH-IS analysis of nine (+8) samples at clinical remission.
Samples procured at time of clinical remission from nine patients diagnosed with (+8), were analyzed in parallel by conventional FISH (black bars) and FISH-IS (grey bars). The 5% lower detection limit that has been established for conventional FISH is shown as a dashed line
Discussion
The correlation between flow cytometric detection of minimal residual disease (MRD) and clinical response in AML has become increasingly evident [10, 15, 16] and the importance of cytogenetic lesions and clinical response is well-established [2–4, 8]. Traditional flow cytometry is potentially an attractive technology to use in diseases involving karyotypic alterations due to the large number of events that can be collected in comparison to slide-based technologies. Combination of flow cytometric sorting and FISH analysis has been previously applied to overcome low target cell abundance that precludes FISH analysis of MRD [15, 17–21]. The present study demonstrates the applicability of ImageStream analysis to detect aneuploidy visualized by FISH-IS in relatively rare cell populations without sorting. The sensitivity and precision of this method was demonstrated by analyzing controlled and predictable models of monosomy and trisomy as well primary clinical samples. The sensitivity of this approach compares favorably to the conventional microscopy-based analysis of FISH, primarily due to the significantly increased numbers of cells that can be analyzed. Importantly, the data demonstrate that this method is applicable to the analysis of cryopreserved samples which would enable a retrospective study of aneuploidy present during MRD stages and quality of clinical response parameters such as remission duration.
A complicating issue of the FISH-IS spot count analysis compared to conventional FISH is the overlapping of hybridization spots due to spatial orientation of the cell in the two-dimensional imaging field which can lead to a discrepancy between aneuploidy and spot count. The data demonstrate that this can be corrected by taking into account the total fluorescence of a cell implying that the FISH-IS-associated fluorescence intensity is in fact the most discriminative parameter. However, particularly in case of rare events, the availability of images to visually verify aneuploidy in cells that were selected solely based on fluorescence intensity is an important advantage over the same approach if conventional flow cytometry would be used.
The sensitivity to detect differences in FISH-IS-associated fluorescence is high. This is not only demonstrated by the ability to detect differences in fluorescence between 1, 2, and 3 hybridization spots but also by the ability to detect small differences associated with the use of different probes with different sized hybridization regions. The latter is evident from the different fluorescence intensity ratios between disomies and trisomies when evaluating an artificial trisomy (male cells hybridized with CEP8 and Y, ratio >1.5) versus a true trisomy (AML cells hybridized with CEP8 only, ratio = 1.5). This sensitivity was also evident from the observation of inter-individual differences of hybridization-associated fluorescence using the same probe, likely associated with inter-individual differences in probe-specific hybridization regions [22]. While the intensity was found to vary between individuals, it was consistent for each person regardless as to whether samples were run on the same day or over a series of days (data not shown).
Identification of aneuploidy via FISH-IS was found to be accurate down to level of 1.0% when strictly using the analysis approach outlined in Figure 1. At levels lower than 1.0% the analysis appears to overestimate but in that case the gating strategy can primarily be used to narrow down a population of interest for subsequent visual inspection to arrive at a more accurate determination than achievable with the automated analysis. From a statistical standpoint, the improvement in sensitivity/accuracy of levels below the 1% threshold should be achievable by increasing the number of cells analyzed. We believe it is reasonable to assume that there is an inherent noise level associated with the FISH-IS labeling and subsequent ImageStream analysis and that no gating strategy will be perfect. An image, however, is very informative and the human interpretation of an image in many cases is a more efficient analysis than trying to apply iterative image analysis approaches that in the end have to uphold to the gold standard of a visual interpretation. The limitation of the visual interpretation is the number of images that can be analyzed. In the lower detection levels (less than 1%) we believe that the added value of the FISH-IS approach is to reduce the number of images to be visually inspected from several tens of thousands to, in most cases, less than 100 images which then can be efficiently visually checked for accuracy.
The data in Figs 4 and 6 suggest that the FISH-IS approach may be more sensitive than conventional FISH for detection of minimal residual disease however the statistical significance and clinical relevance of this will need to be confirmed in a larger study with clinical correlates that could include remission duration and comparing cytogenetic analysis at diagnosis and relapse. In this context it is noteworthy that patient #5 in Fig 6, relapsed with (+8) AML. Information on patients 6,7 and 9 was either not available or irrelevant due to the patients’ passing or receiving a bone marrow transplant.
The multispectral capabilities of the ImageStream offer the possibility of detecting additional fluorescent markers, including additional FISH probes of different colors or cell surface markers for immunophenotyping, and both these possibilities are currently being pursued. With regards to the detection of multiple FISH probes, it should be noted that multicolor FISH is commonly applied to detect structural aberrations for which alternative sensitive PCR-based detection methods are available) as opposed to numerical aberrations to which, in rare event cases, PCR analysis is not applicable. Additionally, the current data show that with the models used, in case of 2 spots, there is 12.4% (Figure 1E) chance for 2 spots to overlap due to orientation within the field of view. Based on this, the theoretical chance for overlap when 4 spots are present (2 spots each for 2 different colors), is greater than 70% (= 4!/(2!×2!) x M), where M = the chance that 2 spots overlap. Thus, in order to make FISH-IS applicable to multicolor hybridizations using fusion probes, it will be essential to develop a method that discriminates a fused signal due to spot overlap from one that is associated with a true structural chromosome change (such as a translocation). An alternative approach could be to use a break-apart hybridization probe under these circumstances in which case the translocation would be indicated by the detection of non-fused hybridization spots.
With regards to simultaneous FISH analysis and immunophenotyping, there are several publications that have successfully performed this combination with conventional slide-based FISH [23–29]. We are currently investigating whether a hybrid protocol between the FISH-IS and the published protocols can be established that would allow FISH-IS with simultaneous immunophenotyping.
Use of the current ImageStream FISH-IS protocol could potentially be extended to diagnose cytogenetic abnormalities that rely on a detectable quantitative difference of a hybridization signal. Examples are found not only in AML (for example (del 16)), but in other malignancies as well such as HER2 mutation in breast cancer. FISH-IS could also be utilized in syndromes associated with aneuploidy including Down syndrome (trisomy 21); Turner syndrome (monosomy X in females); and Klinefelter syndrome (disomy X in males). Modification of the protocol to incorporate use of fusion or break-apart probes, as mentioned above, would extend this technology’s use even further allowing measurement of, for example, bcr-abl fusion (Philadelphia chromosome) in CML; PML-RARα fusion in APL; and EML4-ALK fusion in non-small cell lung cancer.
In summary, the data presented herein demonstrate that aneuploidy can be detected by FISH-IS analysis with a high degree of sensitivity and accuracy. Because of the large sample size, this assay realizes cytogenetic analysis in rare event cases that was heretofore not easily accomplished and could have a utility in the monitoring of minimal residual disease.
Supplementary Material
A spot mask is dependent on two user-input-dependent variables: the so-called spot/background ratio and the spot radius. The effects of changing these variables on the spot masks are demonstrated on an image of a true disomy with a three spot count. The original brightfield (A) and SpectrumGreen (B) images are shown. A mask with a spot/background ratio set too low will pick up background noise (C). A mask with a spot/background ratio set too high will eliminate spots with weaker intensities (D). A mask with the radius set too high will merge individual spots in close proximity, incorrectly counting them as 1 spot (E). A mask set with the correct spot/background ratio and radius, accurately masking all spots is shown also shown (F).
Acknowledgments
The authors gratefully acknowledge the contributions of Drs Ibrahim Kebbewar (RPCI), Vidya Venkatachalam and Sherree Friend (Amnis Corp) to developing the applied ImageStream analysis algorithms.
Research supported by an award from the Roswell Park Alliance Foundation to HM and partial support by NIH 1S10RR022335-01 (PKW), NIH 4R33CA126667 (HM) and by the NCI Cancer Center Support Grant to the Roswell Park Cancer Institute (CA016056)
References
- 1.Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18(2):115–36. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 3.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83. [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 6.Farag SS, Archer KJ, Mrozek K, Ruppert AS, Carroll AJ, Vardiman JW, Pettenati MJ, Baer MR, Qumsiyeh MB, Koduru PR, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006;108(1):63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mrozek K. Cytogenetic, molecular genetic, and clinical characteristics of acute myeloid leukemia with a complex karyotype. Semin Oncol. 2008;35(4):365–77. doi: 10.1053/j.seminoncol.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR, Keating MJ, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8(5):813–9. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 10.DiNardo CD, Luger SM. Beyond morphology: minimal residual disease detection in acute myeloid leukemia. Curr Opin Hematol. 2012;19(2):82–8. doi: 10.1097/MOH.0b013e3283501325. [DOI] [PubMed] [Google Scholar]
- 11.Marcucci G, Mrozek K, Ruppert AS, Archer KJ, Pettenati MJ, Heerema NA, Carroll AJ, Koduru PR, Kolitz JE, Sterling LJ, et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22(12):2410–8. doi: 10.1200/JCO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Bacher U, Kern W, Schoch C, Schnittger S, Hiddemann W, Haferlach T. Evaluation of complete disease remission in acute myeloid leukemia: a prospective study based on cytomorphology, interphase fluorescence in situ hybridization, and immunophenotyping during follow-up in patients with acute myeloid leukemia. Cancer. 2006;106(4):839–47. doi: 10.1002/cncr.21665. [DOI] [PubMed] [Google Scholar]
- 13.Barch MJKT, Spurbeck JL. The AGT Cytogenetics Laboratory Manual. Philadelphia: Lippincott Raven; 1997. [Google Scholar]
- 14.Ortyn WE, Perry DJ, Venkatachalam V, Liang L, Hall BE, Frost K, Basiji DA. Extended depth of field imaging for high speed cell analysis. Cytometry A. 2007;71(4):215–31. doi: 10.1002/cyto.a.20370. [DOI] [PubMed] [Google Scholar]
- 15.Ossenkoppele GJ, van de Loosdrecht AA, Schuurhuis GJ. Review of the relevance of aberrant antigen expression by flow cytometry in myeloid neoplasms. Br J Haematol. 2011;153(4):421–36. doi: 10.1111/j.1365-2141.2011.08595.x. [DOI] [PubMed] [Google Scholar]
- 16.Paiva B, Gutierrez NC, Rosinol L, Vidriales MB, Montalban MA, Martinez-Lopez J, Mateos MV, Cibeira MT, Cordon L, Oriol A, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–91. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 17.Baines P, Austin S, Fisher J, Owen-Jones E, Lee-Jones L, Throp D, McKinley M, Hoy T, Mills K, Thompson P, et al. Increased circulating normal and BCR-ABL+Ve progenitor numbers in Philadelphia chromosome-positive acute myeloid leukaemia. Leuk Res. 2002;26(11):997–1005. doi: 10.1016/s0145-2126(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 18.Engel H, Drach J, Keyhani A, Jiang S, Van NT, Kimmel M, Sanchez-Williams G, Goodacre A, Andreeff M. Quantitation of minimal residual disease in acute myelogenous leukemia and myelodysplastic syndromes in complete remission by molecular cytogenetics of progenitor cells. Leukemia. 1999;13(4):568–77. doi: 10.1038/sj.leu.2401359. [DOI] [PubMed] [Google Scholar]
- 19.Gerber JM, Smith BD, Ngwang B, Zhang H, Vala MS, Morsberger L, Galkin S, Collector MI, Perkins B, Levis MJ, et al. A clinically relevant population of leukemic CD34(+)CD38(−) cells in acute myeloid leukemia. Blood. 2012;119(15):3571–7. doi: 10.1182/blood-2011-06-364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varella-Garcia M, Hogan CJ, Odom LF, Murata-Collins JL, Ai H, Chen L, Richkind K, Paskulin G, Andreeff M, Brizard A, et al. Minimal residual disease (MRD) in remission t(8;21) AML and in vivo differentiation detected by FISH and CD34+ cell sorting. Leukemia. 2001;15(9):1408–14. doi: 10.1038/sj.leu.2402219. [DOI] [PubMed] [Google Scholar]
- 21.White DL, Hutchins CJ, Turczynowicz S, Suttle J, Haylock DN, Hughes TP, Juttner CA, To LB. Detection of minimal residual disease in an AML patient with trisomy 8 using interphase fish. Pathology. 1997;29(3):289–93. doi: 10.1080/00313029700169115. [DOI] [PubMed] [Google Scholar]
- 22.Bayani J, Squire JA. Fluorescence in situ Hybridization (FISH) Curr Protoc Cell Biol. 2004;Chapter 22(Unit 22):4. doi: 10.1002/0471143030.cb2204s23. [DOI] [PubMed] [Google Scholar]
- 23.Batliwalla FM, Damle RN, Metz C, Chiorazzi N, Gregersen PK. Simultaneous flow cytometric analysis of cell surface markers and telomere length: analysis of human tonsilar B cells. J Immunol Methods. 2001;247(1–2):103–9. doi: 10.1016/s0022-1759(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson-Smith MA, Zheng YL, Carter NP. Simultaneous immunophenotyping and FISH on fetal cells from maternal blood. Ann N Y Acad Sci. 1994;731:73–9. doi: 10.1111/j.1749-6632.1994.tb55750.x. [DOI] [PubMed] [Google Scholar]
- 25.Hessel H, Mittermuller J, Zitzelsberger H, Weier HU, Bauchinger M. Combined immunophenotyping and FISH with sex chromosome-specific DNA probes for the detection of chimerism in epidermal Langerhans cells after sex-mismatched bone marrow transplantation. Histochem Cell Biol. 1996;106(5):481–5. doi: 10.1007/BF02473310. [DOI] [PubMed] [Google Scholar]
- 26.Korac P, Jones M, Dominis M, Kusec R, Mason DY, Banham AH, Ventura RA. Application of the FICTION technique for the simultaneous detection of immunophenotype and chromosomal abnormalities in routinely fixed, paraffin wax embedded bone marrow trephines. J Clin Pathol. 2005;58(12):1336–8. doi: 10.1136/jcp.2005.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Siebert R, Matthiesen P, Harder S, Theile M, Scherneck S, Schlegelberger B. Feasibility of simultaneous fluorescence immunophenotyping and fluorescence in situ hybridization study for the detection of estrogen receptor expression and deletions of the estrogen receptor gene in breast carcinoma cell lines. Virchows Arch. 2000;436(3):271–5. doi: 10.1007/s004280050040. [DOI] [PubMed] [Google Scholar]
- 28.Sargent RL, Cook JR, Aguilera NI, Surti U, Abbondanzo SL, Gollin SM, Swerdlow SH. Fluorescence immunophenotypic and interphase cytogenetic characterization of nodal lymphoplasmacytic lymphoma. Am J Surg Pathol. 2008;32(11):1643–53. doi: 10.1097/PAS.0b013e3181758806. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Subero JI, Chudoba I, Harder L, Gesk S, Grote W, Novo FJ, Calasanz MJ, Siebert R. Multicolor-FICTION: expanding the possibilities of combined morphologic, immunophenotypic, and genetic single cell analyses. Am J Pathol. 2002;161(2):413–20. doi: 10.1016/S0002-9440(10)64197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A spot mask is dependent on two user-input-dependent variables: the so-called spot/background ratio and the spot radius. The effects of changing these variables on the spot masks are demonstrated on an image of a true disomy with a three spot count. The original brightfield (A) and SpectrumGreen (B) images are shown. A mask with a spot/background ratio set too low will pick up background noise (C). A mask with a spot/background ratio set too high will eliminate spots with weaker intensities (D). A mask with the radius set too high will merge individual spots in close proximity, incorrectly counting them as 1 spot (E). A mask set with the correct spot/background ratio and radius, accurately masking all spots is shown also shown (F).