Abstract
Background
Prefrontal cortex (PFC) dysfunction is believed to contribute to the transition from controlled substance use to abuse. Because astrocytes have been suggested to play a key role in the development and maintenance of drug-seeking behaviors, we sought to determine if PFC astrocytes are affected by ethanol self-administration.
Methods
Ethanol consumption was modeled in rats by three self-administration paradigms where ethanol was made concurrently available with water in the home cage either continuously (CEA) or intermittently (IEA). In the third paradigm, ethanol was only available in the operant chamber (OEA). To avoid the potential confound of acute ethanol effects, all rats were sacrificed either 24 h or 3 wks abstinence. In all groups, the effect of ethanol consumption on PFC astrocytes was measured using unbiased stereological counting of cells expressing the astrocyte marker glial fibrillary acidic protein (GFAP). GFAP immunoreactivity commonly changes in response to pharmacological insult or injury.
Results
GFAP-positive astrocyte number increased in the prelimbic and anterior cingulate cortex regions of the PFC after IEA. No change was found in the infralimbic or orbitofrontal cortex after IEA. After 3 weeks abstinence, there was a reduction of astrocytes in the prelimbic and orbitofrontal cortex of the CEA cohort as well as a reduction in the orbitofrontal cortex of the OEA cohort.
Conclusion
These findings demonstrate that discrete PFC subregions contain GFAP-positive astrocyte populations that respond differentially to distinct ethanol consumption paradigms. A better understanding of how specific astrocyte populations uniquely adapt to ethanol consumption could provide insight for targeted therapeutic interventions.
Keywords: Astrocyte, Glial Fibrillary Acidic Protein, Self-administration, Stereology, Prefrontal Cortex
Introduction
There is considerable interest in identifying enduring adaptations that occur during ethanol consumption and abstinence because these adaptations may contribute to ethanol-seeking behavior (Spanagel, 2009). Chronic ethanol consumption has long been associated with structural and functional abnormalities within specific brain regions, including in the mesocorticolimbic circuit (Bossert et al., 2013). The mesocorticolimbic circuit is associated with drug-seeking behavior across many drug classes, including ethanol (Spanagel, 2009; Buhler and Mann, 2011; George et al., 2012; Koob, 2013). The prefrontal cortex (PFC) is a region that operates within the mesocorticolimbic circuit to exert executive control over drug-seeking behavior. The PFC can be said to encompass several regions of the anterior frontal lobe including the anterior cingulate, orbital, medial, and lateral cortices. Specific PFC subregions parse highly processed information in order to guide goal-directed behaviors, thoughts, and emotions (Goldman-Rakic, 1996; Miller et al., 2002).
Here, we sought to better understand the cytoarchitecture of the rat PFC following voluntary ethanol self-administration. Most of the literature has focused on adaptations affecting neurons. However, it is now understood that astrocytes play an essential role in neurotransmission and can directly modulate neuronal plasticity by shaping both excitatory and inhibitory synaptic integration, synchrony, and synaptogenesis (Araque et al., 2014). Importantly, astrocytes are affected by ethanol ex vivo (Adermark and Lovinger, 2006) and in vivo. For example, the number of astrocytes increased in rat cortical regions following either repeated ethanol gavage (Udomuksorn et al., 2011) or consumption of an alcohol-containing diet (Dalcik et al., 2009). In contrast, fewer astrocytes were found in the prelimbic region of the medial PFC of rats genetically inbred to prefer ethanol over water (Miguel-Hidalgo, 2005). Likewise, postmortem analysis of ethanol-dependent human brains revealed that astrocyte number was decreased in the dorsolateral PF (Miguel-Hidalgo et al., 2002) and orbitofrontal cortex (Miguel-Hidalgo et al., 2006). However, it is unknown what, if any, astrocyte responses occur in the PFC following voluntary ethanol consumption by standard outbred rats.
To begin addressing this unknown, the PFC astrocyte population was examined after abstinence from three commonly used ethanol consumption paradigms: intermittent ethanol access (IEA), continuous ethanol access (CEA), and operant ethanol access (OEA). Multiple paradigms were used since no single model can recapitulate the complexity of alcohol use disorders. Because the IEA paradigm is increasingly employed due to escalating ethanol consumption and inflexible ethanol-seeking behavior, a non-abstinent IEA cohort was also included for comparison (Simms et al., 2008; Hopf et al., 2010). In all models, our primary dependent measure was the number of astrocytes that were expressing glial fibrillary acidic protein (GFAP). GFAP is a well-established marker for astrocytes, and GFAP expression levels are sensitive to pharmacological insult or injury (Sofroniew and Vinters, 2010), including ethanol exposure (Miguel-Hidalgo et al., 2006; Miguel-Hidalgo et al., 2002; Udomuksorn et al., 2011; Miguel-Hidalgo, 2005). Thus, analysis of GFAP-expressing cell number was chosen as our primary dependent measure in order to aid integration of these findings with earlier literature. Four major regions of the rat PFC were analyzed: the prelimbic and infralimbic regions of the medial cortex, the anterior cingulate, and the orbitofrontal cortex. These regions are implicated in ethanol-related behaviors and exhibit rudimentary functional parallels to the primate PFC (Uylings et al., 2003; Seamans et al., 2008). While the rat lacks the anatomical equivalent of the primate dorsolateral PFC, the rat medial PFC is thought to be a rough functional approximation of both the primate medial and lateral PFC (Uylings et al., 2003; Seamans et al., 2008).
Material and methods
Animals
Male Han Wistar rats (weighing 225–250 g, approximately 8 weeks old at start of experimentation) were housed in a climate-controlled vivarium (lights on: 7 am–7 pm). The same investigators conducted all studies between 10am and 7pm, except during overnight training sessions. Experiments adhered to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local institutional Animal Care and Use committee. Rats were handled daily throughout experimentation.
Behavioral apparatus
Home cage drinking (the IEA and CEA cohorts) was carried out in standard polycarbonate cages (46.5 cm × 24.5 cm × 20.5 cm) with two drinking spouts (separated by 6cm, 10cm above the cage floor). Cages were located in the vivarium, and exhaust fans provided background noise. Operant training proceeded in standard 29 cm × 25 cm × 29 cm chambers (Coulbourn Instruments, Whitehall, PA) in which a 100 μL liquid dipper was located between two retractable levers located 7.5 cm above the floor. Cue lights were located 8.5 cm above each lever. Chambers were housed in sound-attenuating cubicles.
Blood Ethanol Levels
Blood ethanol concentrations were determined, as previously described (Bowers et al., 2008; Bull et al., 2014) Briefly, lateral rostral tail vein blood was collected under light isoflurane anesthesia either immediately after the last operant session or 30 min into the last IEA or CEA session in a room adjacent to the vivarium. Blood was spun (19,000 RCF, 4°C), serum decanted, and stored at −80°C until use. Serum was shaken under heat (70 °C, 10 min) and 1 mL headspace subjected to isothermal chromatography (50 °C, 30 m BAC-1 capillary, 40 ml/min He carrier). Ethanol was detected by flame ionization (200 °C, H2/Air) on an Agilent 6980 with a 0.01 mM detection limit. The integrated area under the curve for each sample was normalized to a 1-propanol (0.1mg/mL) external standard and compared to known EtOH standards in order to calculate mg%. Samples were run in triplicate.
Ethanol consumption paradigms
Three paradigms of ethanol consumption (20%, v/v) were studied. (1) The most common rodent model of regular human ethanol consumption employs co-exposure to two bottles in the home cage where one of these bottles contains ethanol, and the other contains water (Sanchis-Segura and Spanagel, 2006). Rodents have uninterrupted access to both bottles until, if desired, abstinence is imposed. We refer to this paradigm as Continuous Ethanol Access (CEA). (2) The Intermittent Access to Ethanol paradigm (IEA), which was originally developed in the 1970s (Wise, 1973), has recently regained attention in part because this paradigm appears to capture specific aspects of inflexible behavior that is exhibited by human alcoholics (Hopf et al., 2010; Simms et al., 2008). IEA rats had intermittent, 24h home cage ethanol access starting at 7 pm every Monday, Wednesday, and Friday. Thus, no ethanol was available Tuesday, Thursday, Saturday, or Sunday. Water was available at all times, but bottle position was alternated with each ethanol presentation. Rats were allowed to self-administer ethanol on either the IEA or CEA paradigm for 10 weeks before a 3 week period of abstinence was imposed. (3) The third paradigm was Operant Ethanol Access (OEA) in which 3 lever depressions on the ethanol-paired lever were required in order to obtain an ethanol-filled dipper cup, which was available for 4 seconds. The OEA paradigm models the reinforcing effects of ethanol (Sanchis-Segura and Spanagel, 2006). OEA rats were allowed to self-administer ethanol for 8 weeks and then were placed into 3 weeks abstinence. Increased ethanol-seeking behavior has been observed during abstinence in heavy social drinkers (Burish et al., 1981), non-human primates (Weerts et al., 2006), and rodents (Sinclair and Senter, 1968). To avoid the potential confound of acute ethanol effects, IEA rats were perfused 24 h after the last drinking session. The other cohorts were perfused after 3 wks abstinence. All rats were perfused at the same age. GFAP-expressing astrocytes in the PFC were quantified either before or after 3 wks forced abstinence. Figure 1A illustrates experimental timelines.
Figure 1. Ethanol consumption and abstinence altered the number of cells expressing Glial Fibrillary Acidic Protein (GFAP) in the prelimbic cortex.
A) Experimental timelines. i) During the 10 weeks of Intermittent Ethanol Access (IEA), rats had 24 hour access to ethanol and water (EtOH+water) every Monday, Wednesday, and Friday (M, W, F). On Tuesday, Thursday, Saturday, and Sunday (T, Th, Sa, Su) there was access to water only. ii) Continuous Ethanol Access (CEA) rats were allowed access to both ethanol and water 24 hours a day for 10 weeks. iii) Rats in the Operant Ethanol Access (OEA) paradigm consumed ethanol during 1 hour daily sessions for 8 weeks on a fixed ratio 3 schedule of reinforcement. Some rats in the IEA cohort and all in the CEA and OEA cohorts underwent 3 weeks of forced abstinence in the home cage. B) The region of prelimbic cortex that was analyzed spanned approximately from +3.70 mm to +2.20 mm relative to Bregma (Paxinos and Watson, 1998). C) A significant one-way ANOVA (F(4,25)=7.26, p<0.001) followed by a Dunnett’s post-hoc indicated a transient increase in the number of GFAP-expressing astrocytes after IEA as well as a decrease after 3 weeks abstinence from CEA. D) The decrease in astrocytes after CEA followed by abstinence was reflected in a decrease in astrocyte density as well (F(4,25)=5.85, p<0.01). E) There was a transient increase in region volume after IEA F(4,25)=2.83, p<0.05). F) The number of Nissl-stained cell bodies not expressing GFAP was unchanged in all treatment groups (F(4,25)=2.12, p>0.05). G) Representative micrographs of analyzed sections. Bar=50 μm. Data represent mean±SEM, *p<0.05, n=6. Naive: age and handling matched controls; IEA: Intermittent Ethanol Access; CEA: Continuous Ethanol Access; OEA: Operant Ethanol Access; abst: abstinence.
Operant training
Rats in the OEA cohort acquired operant responding via use of a sucrose fade, as we have previously reported (Bull et al., 2014; Bowers et al., 2008). Briefly, rats were trained to respond for ethanol (20% v/v) in decreasing concentrations of sucrose (10%-0% g/v) over a period of approximately 2 weeks. After that time, water and food sated rats self-administered 20% ethanol in tap water on a fixed ratio 3 reinforcement schedule (FR3). After FR3 completion, a 100 μl dipper cup containing the reinforcer was elevated. The dipper cup and stimulus light above the ethanol-paired lever were illuminated and a tone was activated for 4 seconds. If the ethanol-containing dipper cup was not licked more than 4 times within the first 2 seconds, the cup fell and a null response was recorded. Null responses were not included in g/kg calculations.
Tissue preparation
Rats underwent transcardial perfusion (300mL cold PBS followed by 300 mL 4% paraformaldehyde) under pentobarbital anesthesia, brains were postfixed (2% paraformaldehyde, 4°C, 15 hours), cryoprotected (20% followed by 30% sucrose, 4°C), rapidly frozen, and embedded in OCT (Tissue-Tek, Torrance, CA). Forty μm thick coronal sections were serially collected through the forebrain using a cryostat (Leica, Buffalo Grove, IL). Sections were stored in cryoprotection buffer (25% glycerin, 25% ethylene glycol in 46 mM NaH2PO4, 154 mM Na2HPO4, −20°C) until use, generally within one month.
Immunohistochemistry
Free-floating sections from all treatment groups were processed in parallel. Sections were rinsed with PBS (3 × 15 minutes, room temperature), blocked (0.02% TritonX-100, 1% normal goat serum, PBS, 1 hour, room temperature), and incubated with primary GFAP antisera in blocking buffer (1:15,000, polyclonal, Dako, Glostrup, Denmark, 4°C, 15 hours). Sections were washed (3 × 15 minutes, room temperature), incubated in biotinylated secondary, avidin-biotin peroxidase anti-peroxidase complex (Vectastain Elite ABC, Vector Laboratories, Burlingame, CA) assembled, and sections developed in 3, 3′-diaminobenzidine (Peroxidase Substrate Kit, Vector Laboratories). After mounting onto slides, sections were rehydrated (steps of 95%, 70%, 50% ethanol), briefly Nissl counter stained (1.5 minutes cresyl violet, Sigma, St. Louis, MO), differentiated (1% glacial acetic acid/ 70% ethanol), dehydrated (ethanol steps: 50%, 70%, 95%, 100%), cleared (xylene, Fisher, Pittsburg, PA), and coverslipped (Cytoseal, Richard-Allan Scientific, Kalamazoo, MI).
Stereological quantification
Unbiased quantitative stereology was used to avoid sampling bias and artifacts that arise from incorrectly identifying unique cells across sections and/ or by neglecting potential changes in region volume (the reference space). A brightfield microscope (BH-2, Olympus, Center Valley, PA), CCD camera (TXD13C, Baumer Optronic, Southington, CT), and motorized stage (Prior Scientific, Rockland, MA) were interfaced to a computerized stereology system (Stereologer, SRC, Boca Ratan, FL) running on a Macintosh computer (10.6.8, Cupertino, CA). A single, treatment-blinded investigator conducted the analysis. A preliminary study indicated that a systematic-random sampling of every sixth section through the structure of interest generated a coefficient of error < 10%. The coefficient of error describes accuracy of the stereological estimate (Schmitz and Hof, 2005). This sampling yielded 5–6 sections for the prelimbic and infralimbic cortex [AP: 2.20–3.70 mm or 2.20–3.20 mm from Bregma (Paxinos and Watson, 1998), respectively]. Additionally, 6–7 sections were sampled for the anterior cingulate and orbitofrontal cortex [AP: 1.60-0.48 mm or 2.20–3.30 mm from Bregma (Paxinos and Watson, 1998), respectively]. The reference space (the brain region of study) was outlined by tracing the contours of the region of interest in each of these serial sections using a low power objective (4x, Olympus), motorized stage, and Stereologer. Section thickness was measured for each sample. No section was less than 10 μm. The reference space volume for each brain region under study was estimated with the Cavalieri principle (Mouton, 2002; Mouton, 2014), where a systematic array of points with a known area was superimposed on the surface of each section in the series. The number of points falling within the traced area was then used along with section thickness to estimate volume. Simultaneously, cells falling within the outlined region of interest were counted at high magnification (63x, 1.4NA, oil-immersion, PlanApo, Zeiss, Oberkochen, DE), using the optical fractionator that applied the disector principle and unbiased counting rules (West et al., 1991; Mouton, 2002). In brief, the optical disector is a virtual cube that is randomly placed at regular intervals within a tissue section by Stereologer’s optical fractionator algorithm. Thus, the disector ensures that samples are taken in all three dimensions with equal probability (Sterio, 1984). Only the cell bodies falling within the optical disector cube or that were touching any of the three “inclusion” sides were counted. Cell bodies falling outside of the optical dissector cube or touching any of the three “exclusion” lines were not counted. Two types of cells were counted. 1) GFAP-expressing cell bodies encompassing Nissl-stained nuclei and 2) GFAP-negative Nissl-stained nuclei. At least 200 GFAP-positive astrocytes and GFAP-negative Nissl-stained bodies were counted across approximately 100–200 disectors per subject and region. Packing density was calculated as the total number of cells counted in the reference space divided by the volume of the reference space, which is the product of disector number and the total disector volume (Courchesne et al., 2011).”
Statistics
Data were analyzed by a one-way ANOVA with treatment as the factor. Significant main effects were followed by a Dunnett’s post-hoc test (Prism, GraphPad, La Jolla, CA). p<0.05 was considered significant.
Results
Ethanol Consumption
The average ethanol consumption during the last 10 days of the paradigm averaged 5.53±0.41 g/kg/24 h, 5.35±0.34 g/kg/24 h, and 0.85±0.08 g/kg/1 h for the IEA, CEA, and OEA cohorts, respectively. Blood ethanol concentrations collected on the last drinking day were 53.43±8.22 mg%, 50.43±8.2 mg%, and 4.2±0.3 mg% for the IEA, CEA, and OEA cohorts, respectively. Operant responding for the OEA cohort can be found in Supplementary Figure S1. While OEA rats consumed significantly less ethanol than either the CEA or IEA cohorts, the high level of responding on the ethanol-paired lever compared to the inactive lever in food- and water-sated rats suggests that the pharmacological properties of ethanol rather than purely caloric value was likely predominantly responsible for maintaining responding. In support of this conclusion, others have shown that rats bred for high ethanol preference will operantly self-administer ethanol directly into the ventral tegmental area, thereby bypassing systemic influences (Gatto et al., 1994; Rodd-Henricks et al., 2000).
Prelimbic cortex
GFAP-expressing astrocytes and non-GFAP expressing cells were analyzed in the prelimbic region of the rat medial PFC following each of the drinking paradigms outlined above and illustrated in Figure 1A. Shading in Figure 1B represents the approximate location of the first and last sections that were included in the region studied via systematic-random sampling. A one-way ANOVA revealed a main effect of treatment on astrocyte numbers (Figure 1C). Post-hoc comparisons indicated that the number of astrocytes expressing GFAP increased by almost one third (31%) in the IEA cohort prior to abstinence compared to age and handling-matched naive rats. No change was seen in a similarly trained IEA cohort after 3 weeks abstinence, suggesting that this effect returned to baseline after prolonged abstinence. In contrast, post-hoc analysis indicated that the number of GFAP-expressing astrocytes in the CEA cohort decreased by 30% after 3 weeks abstinence when compared to naive. Decreased numbers were mirrored by a similar decrease (31%) in the density of GFAP-expressing astrocytes in the CEA cohort after 3 weeks abstinence when compared to naive (Figure 1D). No change was observed in GFAP-expressing astrocyte density for the IEA cohort despite increased astrocyte number, which likely stems from a 16% increase in region volume when compared to naive (Figure 1E). Interestingly, 3 weeks of abstinence from IEA resulted in a normalization of both GFAP-expressing astrocyte numbers as well as volume (Figure 1C and 1E; p>0.05 comparing naive vs. IEA abst). Future studies will be required to determine the precise timeline of these effects. No changes were found in the number of cells that were not expressing GFAP in any of these cohorts (Figure 1F). No differences were observed in any of these measures for the OEA cohort compared to naive. Representative immunohistochemistry is illustrated in Figure 1G.
Infralimbic cortex
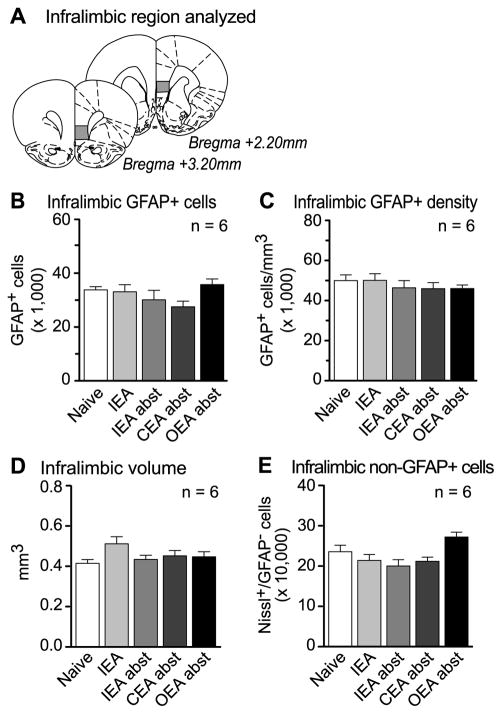
GFAP-expressing astrocytes and non-GFAP expressing cells were analyzed in the infralimbic region of the rat medial PFC following each of the drinking paradigms outlined above. Figure 2A shows the approximate locations of the first and last sections that were included in the systematic-random sampling of the infralimbic cortex. In contrast to the neighboring prelimbic cortex, no changes were observed in the infralimbic cortex following any of the drinking paradigms employed. Specifically, no main effect of treatment was found with regards to the number of GFAP-expressing astrocytes (Figure 2B), astrocyte density (Figure 2C), volume (Figure 2D), or GFAP-negative Nissl-stained cell bodies (Figure 2E).
Figure 2. No infralimbic cortex changes were observed.
A) The region of infralimbic cortex that was analyzed spanned approximately +3.20 mm to +2.20 mm, relative to Bregma (Paxinos and Watson, 1998). B) The number of GFAP-expressing astrocytes was unchanged in the infralimbic cortex following any treatment studied (F(4,25)=1.81, p>0.05). C) Similarly, no change was found in the density of GFAP-expressing cells (F(4,25)=0.54, p>0.05), D) volume (F(4,25)=1.97, p>0.05), or the number of Nissl-stained cell bodies not expressing GFAP (F(4,25)=4.11, p>0.05). Data represent mean±SEM, n=6. Naive: age and handling matched controls; IEA: Intermittent Ethanol Access; CEA: Continuous Ethanol Access; OEA: Operant Ethanol Access: abst: abstinence.
Anterior cingulate cortex
GFAP-expressing astrocytes and non-GFAP expressing cells were analyzed in the rat anterior cingulate cortex following each of the drinking paradigms outlined above. Figure 3A shows the approximate locations of the first and last sections included in the systematic-random sampling of the anterior cingulate cortex. A one-way ANOVA revealed a significant main effect of treatment on the number of GFAP-expressing astrocytes (Figure 3B). A post-hoc analysis indicated that there was a 29% increase in the number of GFAP-expressing astrocytes in the IEA cohort compared to naive, which returned to baseline after 3 weeks abstinence. This response was similar to that observed in the prelimbic cortex (Figure 1C). However, in contrast to the prelimbic cortex, no changes were observed in the number of GFAP-expressing astrocytes in the anterior cingulate of the CEA cohort. A decrease was also observed in the density of GFAP-expressing astrocytes in the OEA cohort (Figure 3C). Neither the overall number of GFAP-expressing cells (Figure 3B) nor region volume (Figure 3D) changed in the OEA cohort. Similar to both regions of the medial PFC studied (the prelimbic and infralimbic cortices), no changes were seen in the number of Nissl-stained cells that were not expressing GFAP (Figure 3E).
Figure 3. The number of cells expressing Glial Fibrillary Acidic Protein (GFAP) increased in the anterior cingulate after Intermittent Ethanol Access (IEA).
A) The region of the anterior cingulate cortex analyzed spanned approximately +1.70 mm to +0.48 mm relative to Bregma (Paxinos and Watson, 1998). B) A one-way ANOVA revealed a significant main effect of treatment on the number of GFAP-expressing astrocytes (F(4,25)=5.76, p<0.01). A Dunnett’s post-hoc analysis revealed an increase in the number of GFAP-expressing astrocytes in the IEA cohort compared to naive, but this was no longer seen after 3 weeks abstinence. C) Decreased density of GFAP-expressing astrocytes was observed in the Operant Ethanol Access (OEA) cohort (F(4,24)=5.51, p<0.01). D) No change was observed in region volume (F(4,24)=1.10, p>0.05) or E) the number of Nissl-stained cells that were not expressing GFAP (F(4,24)=2.03, p>0.05). Data represent mean±SEM, *p<0.05, n=6. Naive: age and handling matched controls; IEA: Intermittent Ethanol Access; CEA: Continuous Ethanol Access; OEA: Operant Ethanol Access; abst; abstinence.
Orbitofrontal Cortex
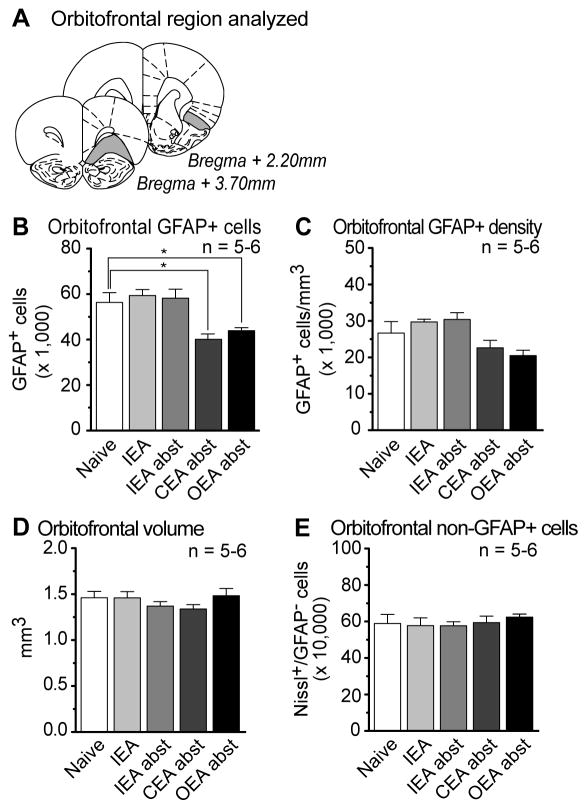
GFAP-expressing astrocytes and non-GFAP expressing cells were analyzed in the rat orbitofrontal cortex following each of the drinking paradigms outlined above. Figure 4A shows the approximate locations of the first and last sections that were included in the systematic-random sampling of the orbitofrontal cortex. A one-way ANOVA indicated a significant main effect of treatment on the number of GFAP-positive astrocytes in the orbitofrontal cortex (Figure 4B). In contrast to the prelimbic cortex, a post-hoc analysis did not show any differences in astrocyte number for the IEA cohort compared to controls. However, similar to the prelimbic cortex, there was a 28% decrease in astrocyte numbers in the CEA cohort after abstinence when compare to naive, and a 22% decrease in the OEA cohort as well. Future experiments will be required to determine the precise timeline of these effects. No changes were observed in the density of GFAP-expressing astrocytes (Figure 4C), orbitofrontal cortex volume (Figure 4D), or the number of non-GFAP expressing cells that were stained with Nissl (Figure 4E). For the orbitofrontal cortex only, one data point in both the CEA and OEA cohorts was excluded from analysis due to falling greater than two standard deviations away from the mean.
Figure 4. The number of cells expressing Glial Fibrillary Acidic Protein (GFAP) changed in the obitofrontal cortex after daily, but not intermittent ethanol consumption.
A) The region of the anterior cingulate cortex spanned approximately +3.70 mm to +2.20 mm relative to Bregma (Paxinos and Watson, 1998). B) A one-way ANOVA indicated a significant main effect of treatment on the number of GFAP-positive astrocytes in the orbitofrontal cortex (F(4,23)=7.26, p<0.001). A Dunnett’s post hoc analysis demonstrated a significant decrease in the Continuous Ethanol Access (CEA) and Operant Ethanol Access (OEA) cohorts. No effects were found on the C) density of GFAP-expressing astrocytes (F(4,23)=4.76, p>0.05), D) region volume (F(4,23)=0.71, p>0.05) or the number of Nissl-stained cells not expressing GFAP (F(4,23)=0.11, p>0.05). Data represent mean±SEM, *p<0.05, n=5–6. Naive: age and handling matched controls; IEA: Intermittent Ethanol Access; CEA: Continuous Ethanol Access; OEA: Operant Ethanol Access; abst; abstinence.
Discussion
Numerous studies have identified that dysfunction of specific PFC subregions including the medial, anterior cingulate, and orbitofrontal cortices can promote drug- or ethanol-seeking behavior (Bossert et al., 2013; Lasseter et al., 2010). This is the first study of GFAP-expressing astrocytes in the PFC following voluntary ethanol consumption by standard outbred rats. Broadly speaking, we observed that different ethanol self-administration paradigms mediated unique changes in the number of GFAP-expressing astrocytes within discrete PFC subregions.
In the IEA cohort, a transient increase in the number of GFAP-expressing astrocytes was observed in both the medial prelimbic cortex and anterior cingulate that returned to baseline after 3 weeks abstinence. This study also identified decreased GFAP-expressing astrocytes in discrete PFC subregions. Specifically, we observed a decrease in the prelimbic cortex after 3 weeks abstinence from CEA as well as a decrease in the orbitofrontal cortex after 3 weeks abstinence from either CEA or OEA. These findings are noteworthy because activity of the anterior cingulate (Myrick et al., 2004) and medial PF (Grusser et al., 2004) has been correlated with subjective ratings of craving that are made by ethanol-dependent subjects. Moreover, activation of these regions appeared to be most pronounced in subjects that had relapsed within 3 months of abstinence (Grusser et al., 2004). The differential impact of ethanol consumption on the prelimbic versus infralimbic medial PFC is also interesting, given that the prelimbic and infralimbic cortices have been suggested to have contrasting functions (Bossert et al., 2013; Lasseter et al., 2010). For example, the prelimbic cortex is thought to be involved in the planning and execution of drug-seeking behaviors. The prelimbic cortex mainly innervates the nucleus accumbens core, and this projection is critical for many forms of drug-seeking behavior (Bossert et al., 2013; Lasseter et al., 2010). In contrast, the infralimbic cortex mainly innervates the nucleus accumbens shell, and the infralimbic cortex appears to be uniquely involved in promoting drug-seeking behavior. More specifically, the infralimbic cortex can promote cocaine-seeking behavior following forced abstinence yet it also appears to inhibit cocaine seeking after extinction training (Bossert et al., 2013; Lasseter et al., 2010). The current study only examined GFAP-expressing astrocytes after forced abstinence. Given these findings with cocaine, a future study examining the response of PFC astrocytes after extinction training may be merited.
Additional experiments in rodent models of ethanol consumption also further highlight the importance of the medial PFC. For example, lower numbers of GFAP-positive astrocytes have been observed in the prelimbic cortex of rats genetically bred for their high preference for ethanol over water (Miguel-Hidalgo, 2005), and infusion of gliotoxins or a gap junction blocker into the rat prelimbic cortex increased preference for ethanol (Miguel-Hidalgo et al., 2008). Similarly, temporary inactivation of the prelimbic, but not the infralimbic cortex increased the context-induced self-administration of alcoholic beer by previously abstinent rats (Willcocks and McNally, 2013). While these prelimbic cortex findings are discordant with the psychostimulant literature, where prelimbic inactivation decreased drug-seeking behavior (Bossert et al., 2013; Lasseter et al., 2010), these earlier ethanol data are nonetheless consistent with the function of the prelimbic cortex in initiating previously learned behaviors in order to receive reinforcement (Corbit and Balleine, 2003). Regardless, given the broad understanding that the prelimbic and anterior cingulate cortices support drug-seeking behavior after an abstinence period (Bossert et al., 2013; Myrick et al., 2004), the decrease that we observed in the number and/or density of GFAP-expressing astrocytes in these regions after forced abstinence is especially intriguing.
Similar to the medial PFC and anterior cingulate, activity of the orbitofrontal cortex has also been significantly correlated with subjective ratings of craving made by ethanol-dependent subjects, but not controls (Myrick et al., 2004). The rat orbitofrontal cortex is a rough approximation of the primate, with the notable exception that the primate orbitofrontal cortex integrates information about the abstract and affective value of expected outcomes (Wallis, 2012). In contrast, the rat orbitofrontal cortex does not appear to integrate this information, but instead encodes specifics about behavioral responses leading to outcomes (Wallis, 2012). In humans, the orbitofrontal cortex is thought to encode compulsive drives (Volkow and Fowler, 2000). Similarly, the rodent orbitofrontal cortex is thought to encode compulsive ethanol consumption (Vengeliene et al., 2009). Given that compulsions can be defined as a highly repetitive behavior, it is interesting that we only observed orbitofrontal GFAP-positive astrocyte changes in the continuous access and operant cohorts that had self-administered ethanol over several contiguous days as opposed to the intermittent ethanol access cohort.
Despite the changes in the number of GFAP-expressing astrocytes within some PFC subregions, no change was detected in the number of cells that were not expressing GFAP. However, it is possible that the Nissl stain did not detect some changes that may have occurred within specific cell populations. For example, it is known that neuronal loss occurs in the orbitofrontal cortex of rats following repeated ethanol consumption (Collins et al., 1996). Regardless, the observed alterations in GFAP-expressing cells could have profound impact on neural plasticity since astrocytes can actively modulate neuronal activity (Araque et al., 2014). A fruitful future line of investigation could include examining if astrocyte stimulation, trophic factors and/or transplants could abate some effects of ethanol.
While the current study was not designed to elucidate if altered numbers of GFAP-expressing astrocytes could impact ethanol-seeking behavior, the literature suggests that astrocytes can dramatically affect drug-seeking behavior through the regulation of extracellular glutamate. For example, facilitation of glutamate efflux in the nucleus accumbens via activation of astrocytic glutamate antiporters has been shown to reduce cocaine-seeking behavior (Baker et al., 2003). Because astrocytes are the primary mechanism of clearing excess glutamate from the synaptic cleft, a long-lasting reduction in the number of astrocytes after ethanol consumption could disrupt glutamate homeostasis. This could be important given that reduced astrocytic glutamate uptake in the PFC leads to an anhedonic state (John et al., 2012), and anhedonic-like symptoms are observed in ethanol-consuming rats (Briones and Woods, 2013; Koob, 2003). Lastly, stimulation of nucleus accumbens core astrocytes reduced the motivation to self-administer ethanol after abstinence and increased reward perception (Bull et al., 2014). Lastly, future studies could build upon the present work by including paradigm-specific controls rather than a generic age- and handling-matched ethanol naive cohort. In other words, one may consider including sucrose or polycose drinking controls for each paradigm. Additionally, one may wish to rigorously control ethanol levels between cohorts in order to test the hypothesis that orbitofrontal astrocyte plasticity occurs beyond a specific blood ethanol concentration threshold.
In conclusion, we found that GFAP-expressing astrocyte numbers within discrete PFC subregions uniquely adapt to specific ethanol self-administration paradigms. These findings add to a growing understanding that specific paradigms of alcohol consumption can lead to unique repertoires of astrocytic adaptations. A better understanding of these adaptations could provide insight for targeted therapeutic interventions.
Supplementary Material
Acknowledgments
This work was supported by the AMBRF/ Foundation for Alcohol Research, NIAAA/P30-AA019372, NIH/NIAAA P50 AA022537, and NIH/NCATS UL1 TR000058.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Adermark L, Lovinger DM. Ethanol effects on electrophysiological properties of astrocytes in striatal brain slices. Neuropharmacology. 2006;51:1099–108. doi: 10.1016/j.neuropharm.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–39. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–49. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, Bonci A, Diamond I. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci U S A. 2008;105:12533–38. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–34. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–93. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Bull C, Freitas KC, Zou S, Poland RS, Syed WA, Urban DJ, Minter SC, Shelton KL, Hauser KF, Negus SS, Knapp PE, Bowers MS. Rat Nucleus Accumbens Core Astrocytes Modulate Reward and the Motivation to Self-Administer Ethanol after Abstinence. Neuropsychopharmacology. 2014;39:2835–45. doi: 10.1038/npp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J Stud Alcohol. 1981;42:1013–20. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–92. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–57. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–10. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Dalcik H, Yardimoglu M, Filiz S, Gonca S, Dalcik C, Erden BF. Chronic ethanol-induced glial fibrillary acidic protein (GFAP) immunoreactivity: an immunocytochemical observation in various regions of adult rat brain. Int J Neurosci. 2009;119:1303–18. doi: 10.1080/00207450802333672. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–64. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–61. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–53. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–73. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CS, Smith KL, Van’t Veer A, Gompf HS, Jr, Carlezon WA, Cohen BM, Ongur D, Bechtholt-Gompf AJ. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology. 2012;37:2467–75. doi: 10.1038/npp.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–17. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo J, Shoyama Y, Wanzo V. Infusion of gliotoxins or a gap junction blocker in the prelimbic cortex increases alcohol preference in Wistar rats. J Psychopharmacol. 2008 doi: 10.1177/0269881108091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biol Psychiatry. 2002;52:1121–33. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clin Exp Res. 2005;29:766–72. doi: 10.1097/01.alc.0000164378.92680.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–55. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Freedman DJ, Wallis JD. The prefrontal cortex: categories, concepts and cognition. Philos Trans R Soc Lond B Biol Sci. 2002;357:1123–36. doi: 10.1098/rstb.2002.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. 1. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- Mouton PR. Neurostereology: Unbiased Stereology Of Neural Systems. 1. Wiley-Blackwell; 2014. [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li TK. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000;24:8–16. [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–31. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–62. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–67. [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Udomuksorn W, Mukem S, Kumarnsit E, Vongvatcharanon S, Vongvatcharanon U. Effects of alcohol administration during adulthood on parvalbumin and glial fibrillary acidic protein immunoreactivity in the rat cerebral cortex. Acta Histochem. 2011;113:283–89. doi: 10.1016/j.acthis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addict Biol. 2009;14:384–96. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–36. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–68. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–10. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.