Abstract
Background
The influence of previous alcohol (ethanol) drinking experience on increasing the rate and amount of future ethanol consumption might be a genetically-regulated phenomenon critical to the development and maintenance of repeated excessive ethanol abuse. We have recently found evidence supporting this view, wherein inbred C57BL/6J (B6) mice develop progressive increases in the rate of binge-ethanol consumption over repeated Drinking-in-the-Dark (DID) ethanol access sessions (i.e. ‘front-loading’). The primary goal of the present study was to evaluate identical parameters in High Alcohol Preferring (HAP) mice to determine if similar temporal alterations in limited-access ethanol drinking develop in a population selected for high ethanol preference/intake under continuous (24hr) access conditions.
Methods
Using specialized volumetric drinking devices, HAP mice received 14 daily 2 hour DID ethanol or water access sessions. A subset of these mice was then given one day access to the opposite assigned fluid on day 15. Home cage locomotor activity was recorded concomitantly on each day of these studies. The possibility of behavioral/metabolic tolerance was evaluated on day 16 using experimenter administered ethanol.
Results
The amount of ethanol consumed within the first 15 minutes of access increased markedly over days. However, in contrast to previous observations in B6 mice, ethanol front-loading was also observed on day 15 in mice that only had previous DID experience with water. Furthermore, a decrease in the amount of water consumed within the first 15 minutes of access compared to animals given repeated water access was observed on day 15 in mice with 14 previous days of ethanol access.
Conclusions
These data further illustrate the complexity and importance of the temporal aspects of limited-access ethanol consumption, and suggest that previous procedural/fluid experience in HAP mice selectively alters the time course of ethanol and water consumption.
Keywords: Rate of alcohol/ethanol intake, high alcohol/ethanol preferring mice, genetic regulation of binge alcohol/ethanol consumption, Drinking in the Dark (DID), novelty of alcohol/ethanol access, alcohol/ethanol front-loading
There is increasing evidence that an organism’s genetic background has a great influence on its propensity to display binge ethanol-drinking behavior (Barkley-Levenson and Crabbe, 2013, Bauer and Ceballos, 2014, Chassin et al., 2002, Coon et al., 2014, Iancu et al., 2013, Rhodes et al., 2007, Watson et al., 2013). However, to date we know very little about how previous binge-drinking history, together with genetic background, might facilitate future binge-drinking behavior. Because binge-ethanol consumption leads to a myriad of detrimental acute and long-term health consequences including approximately half of the annual ethanol related deaths in the U.S. (Bouchery et al., 2011, Naimi et al., 2014), efforts to understand how repeated binge-ethanol consumption influences future binge-drinking in populations at risk for excessive ethanol intake are of utmost importance.
Increases in the rate of binge-like ethanol consumption in mice with genetic predisposition for excessive ethanol intake (C57BL/6J; B6) were recently described (Linsenbardt and Boehm, 2014). In these studies, mice given 15 consecutive days of limited access ethanol dramatically increased their rate of ethanol consumption over the course of the study without obvious asymptote. Water consuming control mice completely lacked this ‘front-loading’ behavior. Furthermore, when controls were given access to ethanol for a single session following repeated daily access to water, ethanol was consumed at a rate consistent with the first ethanol access session in repeated ethanol access groups. Together these results suggest that the observed increases in the rate of binge-ethanol intake in this genetically predisposed population were born out of previous binge-ethanol drinking experience.
However, as conclusions about the influence of genetic background/risk on ethanol drinking behavior drawn from one population of homozygous mice should be interpreted cautiously, the current experiments were conducted in a different population of high alcohol preferring mice, in part, to determine their generalizability.
High Alcohol Preferring (HAP) mice, bred for preference for ethanol over water under free-access conditions, were subjected to identical procedures as those recently described in B6 mice (Linsenbardt and Boehm, 2014) to determine 1) if similar temporal alterations in limited-access ethanol drinking (i.e. front-loading) develop in a population selected for high ethanol preference/intake under continuous (24hr) access conditions, and 2) if any such temporal alterations might persist when given access to one of these two solutions for the first time. Because the development of behavioral and metabolic tolerance has been shown to develop following 2 weeks of limited access binge-like ethanol intake in B6 mice (Linsenbardt et al., 2011), and HAP mice have been shown to develop sensitization to the locomotor stimulant effects of experimenter administered ethanol (Grahame et al., 2000), a final goal was to determine the extent to which repeated ethanol access/consumption might have led to metabolic and/or behavioral (locomotor) alterations in these studies.
Materials and Methods
Animals
Adult (postnatal day 70–90) male (N=40) and female (N=40) replicate 1 High Ethanol Preferring (HAP1) mice from the 45th generation of selection were bred on-site in the School of Science animal facility at Indiana University – Purdue University Indianapolis (IUPUI). For a detailed description of the production and response to selection in this and subsequent lines see Oberlin et al. (2011). All animals were acclimated to single housing in standard shoebox mouse cages and a 12 hour reverse light/dark cycle with lights off at 7:00 AM for at least a week prior to testing. Animals had ad lib access to food and water except during ethanol access sessions when only ethanol was available. All procedures were approved by the IUPUI School of Science Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academic Press, 2003).
Ethanol and Drug Solutions
Ethanol drinking and injection solutions (20% v/v) were made with 190 proof ethanol purchased from Pharmco, Inc (Brookfield, CT) and regular tap water or 0.9% physiological saline, respectively. Drinking solutions were made fresh every other day and stored in sealed fluid reservoirs connected to the Volumetric Drinking System (VDM) fluid dispensation system (Columbus Instruments Inc., Columbus, OH). Injectable solution was made immediately prior to use.
Drinking in the Dark (DID)
DID procedures used in these studies were recently described (Linsenbardt & Boehm, 2014). Briefly, three hours into the dark cycle each day, animals received access to an unsweetened 20% ethanol solution or tap water for 2 hours, by replacing water bottles with specialized volumetric sipper tubes (Columbus Instruments, Columbus, OH) containing the assigned fluid. The specialized volumetric drinking tubes recorded the total volume of fluid consumed in one minute epochs for each 2 hour DID session.
Home Cage Locomotor Apparatus
Home cage locomotion was monitored using a CI Multi-Device Interface (Columbus Instruments Inc., Columbus, OH) in conjunction with a Dell computer. The dimensions and other technical specifications of this apparatus have been previously described (Linsenbardt et al., 2011). Ambulatory activity was detected by the interruption of photocell beams positioned along the walls of each animal’s standard shoebox mouse cage. Data were collected in 1-min time intervals for a total of 2 hours during each DID session and translated into ambulatory counts using the provided software (version 1.4.0).
Blood Sampling
For the determination of blood ethanol concentrations (BECs), 50µl peri-orbital sinus blood samples were drawn following behavioral testing on days 15 and 16. Samples were centrifuged and plasma was withdrawn and stored at −20°C. BECs were then determined using an Analox Ethanol Analyzer (Analox Instruments, Lunenburg, MA).
Procedures
Alterations in the Time Course of Intake and Home Cage Locomotion in HAP 1 Mice
The procedures used in these studies were identical to a recently published report from our lab wherein male C57BL/6J mice were used (Linsenbardt & Boehm, 2014), in order to make direct comparisons possible between these two mouse genotypes/datasets and to facilitate our ongoing efforts of computer model generation/testing. Our experimental procedures are outlined in Table 1. Out of the 80 animals in this experiment, 40 were given access to ethanol and the remaining 40 were given access to water for 14 consecutive days using the DID procedures outlined above. On day 15, the type of fluid presented was switched in half of the mice; water consuming mice received ethanol (WE) and vice versa (EW). The other half continued to receive their previously assigned ethanol (EE) or water (WW) solution. Ten male and 10 female animals were included in each of these 4 subgroups. This allowed for a within and between subjects analysis of intake and home cage locomotor activity as a function of previous (14 day) fluid access history.
Table 1.
Procedures
| Days 1–14 | Day 15 | Day 16 | |
|---|---|---|---|
| (2-hour DID + HCL) | (2-hour DID + HCL) | (2-hour HCL) | |
| Male (N=40) | Ethanol (N=20) | Ethanol (EE; N=10) | Ethanol Inj. (2.0 g/kg; N=10) |
| Water (EW; N=10) | Ethanol Inj. (2.0 g/kg; N=9) | ||
| Water (N=20) | Water (WW; N=10) | Ethanol Inj. (2.0 g/kg; N=10) | |
| Ethanol (WE; N=10) | Ethanol Inj. (2.0 g/kg; N=10) | ||
| Female (N=40) | Ethanol (N=20) | Ethanol (EE; N=10) | Ethanol Inj. (2.0 g/kg; N=9) |
| Water (EW; N=10) | Ethanol Inj. (2.0 g/kg; N=10) | ||
| Water (N=20) | Water (WW; N=9) | Ethanol Inj. (2.0 g/kg; N=10) | |
| Ethanol (WE; N=10) | Ethanol Inj. (2.0 g/kg; N=10) | ||
Drinking-in-the-Dark (DID); Home-Cage Locomotion (HCL)
Three hours following the onset of the ‘lights off’ phase of the light cycle on the 16th day (24 hours following the previous day’s DID session), each animal was given a 2.0 g/kg ethanol injection (i.p.) and was immediately placed back into the home cage for 2 hours while locomotor activity was recorded. This was done primarily to evaluate possible alterations in ethanol metabolism, but also to assess how ethanol history might influence ethanol-induced home cage locomotion. Blood was sampled immediately after bottles were removed on day 15 and 2 hours following ethanol injection on day 16 for determination of BECs.
Statistics: Daily Mean Intake/Home Cage Locomotion and BECs
Fluid intake data from one female (WW) mouse was removed from analyses of day 15 due to a leaking sipper tube on that day. Mean total (2 hour) fluid intake and home cage locomotor activity of the ethanol and water consuming groups on days 1–14 for experiment 1 were first analyzed using a mixed 3-way analysis of variance (ANOVA) with day as the within subject’s factor and sex and fluid assignment group (ethanol or water) as the between groups factors. To determine if there were differences in mean intake on days 1–14 between day 15 assignment groups, fluid history subdivisions were analyzed separately such that the WW and WE groups were compared and the EE and EW groups were compared using ANOVA with intake on days 1–14 as the within subjects factor and the day 15 group assignment and sex as between subjects factors. Mean total fluid intake and home cage locomotion on day 15 were analyzed separately using a two-way ANOVA with repeated fluid assignment (days 1–14) and day 15 fluid assignment and sex as factors. Day 16 was analyzed identically to day 15, except as DID procedures were not performed, only 2 hour home cage locomotion was analyzed. Interactions with sex were not detected so all results and figures reported here are from subsequent analyses as reported above but collapsed on this factor.
Statistics: Correlations and Post-hoc Testing
Pearson’s correlations and linear regression were used to measure the relationship between several different variables of interest. Tukey and Dunnett’s post-hoc tests were performed when appropriate. Data were considered significant at p<0.05.
Statistics: Polynomial Regression Analysis
In order to better characterize the most prominent temporal alterations, a finite impulse response filter (known commonly as a central moving average) was calculated on 15 minute bin increments. Data was binned into 15 minute averages and ‘moved’ forward in time in 1 minute increments such that each subsequent bin included one additional minute into the future, and excluded one minute furthest in time. For example, bin 1 represents the mean of minutes 1–15 minutes, bin 2 represents the mean of minutes 2–16, and so on. Fifteen minute increments were chosen to allow for direct comparisons to previously published data in C57BL/6J mice (see Linsenbardt & Boehm, 2014), and because initial observations of intake data suggested that substantial changes occurred over DID sessions within the first 15 minutes of ethanol access. The results of this data smoothing were then subject to polynomial regression/curve fitting. Briefly, data were fit to 1st– 4th order polynomials according to fluid and/or treatment group as outlined above. The fit of each day’s data to one of the four polynomial equations were compared using an F-statistic to determine the equation that best described intake or locomotor activity data on a particular day.
Results
Days 1–14
General Linear Analysis
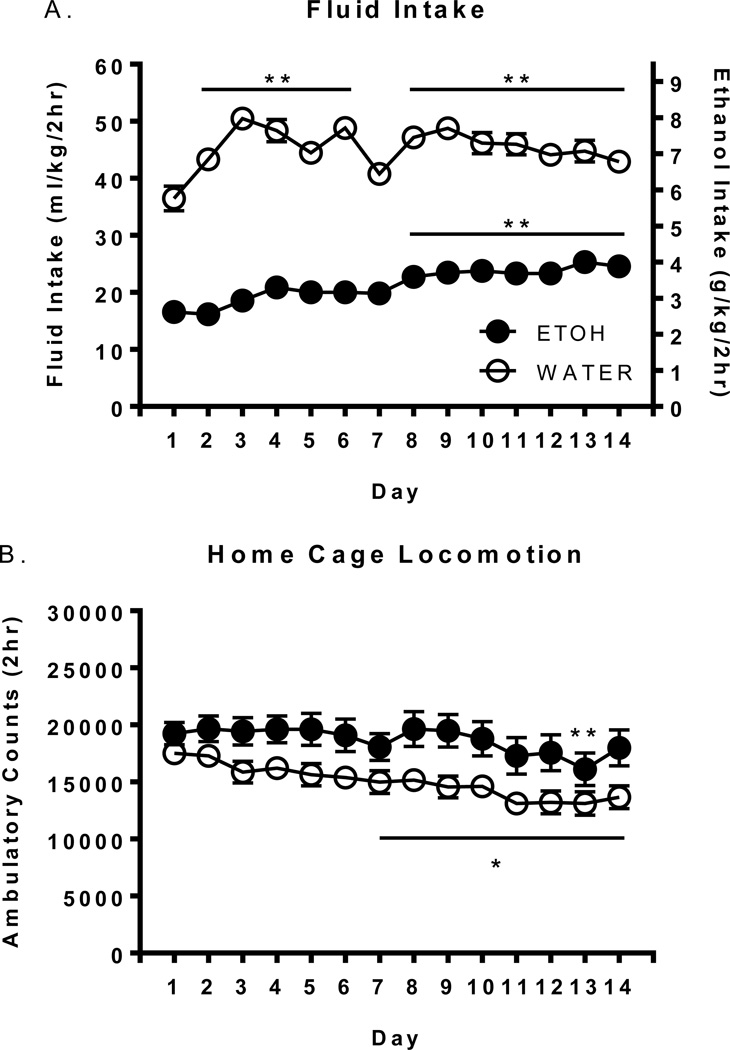
Total daily ethanol and water consumption on days 1–14 can be seen in Figure 1A. Analysis revealed significant main effects of fluid assignment [F(1, 76)=256.493 p<.0001] and day [F(13, 988)=10.67 p<.0001] with water assigned animals consuming more in volume by weight than ethanol, and overall fluid intake generally increasing over daily DID sessions.
Figure 1.
Total fluid intake and home cage locomotor activity on days 1–14. Mean daily fluid intake (A) and home cage locomotor activity (B) in ethanol and water consuming HAP mice over 14 consecutive DID sessions. The left y-axis refers to both ethanol and water groups, whereas the right y-axis refers to ethanol animals only. *’s indicate significant within-fluid-group differences vs day 1 (** p<.01, * p<.05).
Home cage locomotor activity on days 1–14 can be seen in Figure 1B. Analysis revealed significant main effects of fluid assignment [F(1, 76)=6.292 p<.05] and day [F(13, 988)=9.28 p<.0001] with ethanol assigned animals having significantly higher locomotor activity than water consuming animals and locomotor activity generally decreasing over days.
To determine within-fluid group increases/decreases in intake and locomotion over days, Dunnett’s post hoc tests were performed. Water intake was significantly higher on days 2–7 and 9–14 vs day 1 (p’s <0.01), while ethanol intake was significantly higher on days 8–14 vs day 1 (p’s <0.01). Locomotor activity in water consuming animals was significantly lower on days 7–14 vs day 1 (p’s <.05), while locomotor activity in ethanol consuming animals was significantly lower on day 13 only (p <.01).
There were no significant differences in water or ethanol consumption over the first 14 days as a function of day 15 fluid assignment; there were no significant day 15 fluid group assignment effects or interactions. In other words, ethanol (EE and EW) and water (WW and WE) groups had similar drinking histories prior to day 15 fluid access (data not shown). Furthermore, there were no significant differences in locomotor activity over the first 14 days as a function of day 15 fluid assignment; ethanol (EE and EW) and water (WW and WE) groups displayed similar locomotor activity prior to day 15 fluid access (data not shown). Because the two day 15 groups that received 14 days of ethanol access (EE and EW) had similar drinking histories/locomotion across these 14 days, as did day 15 groups that received 14 days of water access (WW and WE), all graphical representations of intake and home cage locomotor data in Figure 1 do not present groups broken down by day 15 fluid group assignment.
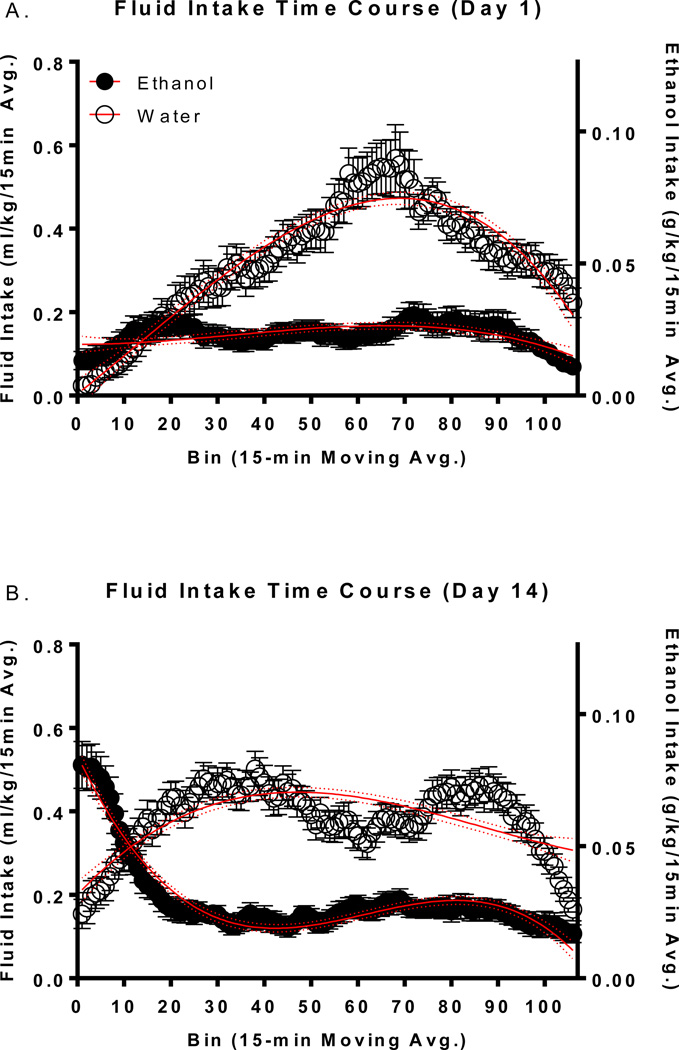
Polynomial Analysis
Central moving average representations for ethanol and water intake on days 1 and 14 can be seen in Figure 2. Central moving average values on days 1 and 14 were best fit to 3rd order polynomials. On both days 1 (Figure 2 A) and 14 (Figure 2 B) there were easily identifiable time course differences as a function of fluid assignment (i.e. the 95% confidence intervals of each of the fluids represented by the dotted lines were largely separated). Large differences in the time course of ethanol intake (i.e. ‘front-loading’) had developed by day 14 in HAP mice. There were no obvious differences in the temporal aspects of home cage locomotor activity so these data are not presented graphically.
Figure 2.
Time course of fluid intake over days 1–14. Time course of fluid intake on days 1 (A) and 14 (B). Data in A+B reflect 15 minute central moving averages. The solid red lines reflect the best fit line/polynomial line whereas the dotted red lines reflect the 95% confidence intervals of that line. The left y-axis refers to both ethanol and water groups, whereas the right y-axis refers to ethanol animals only.
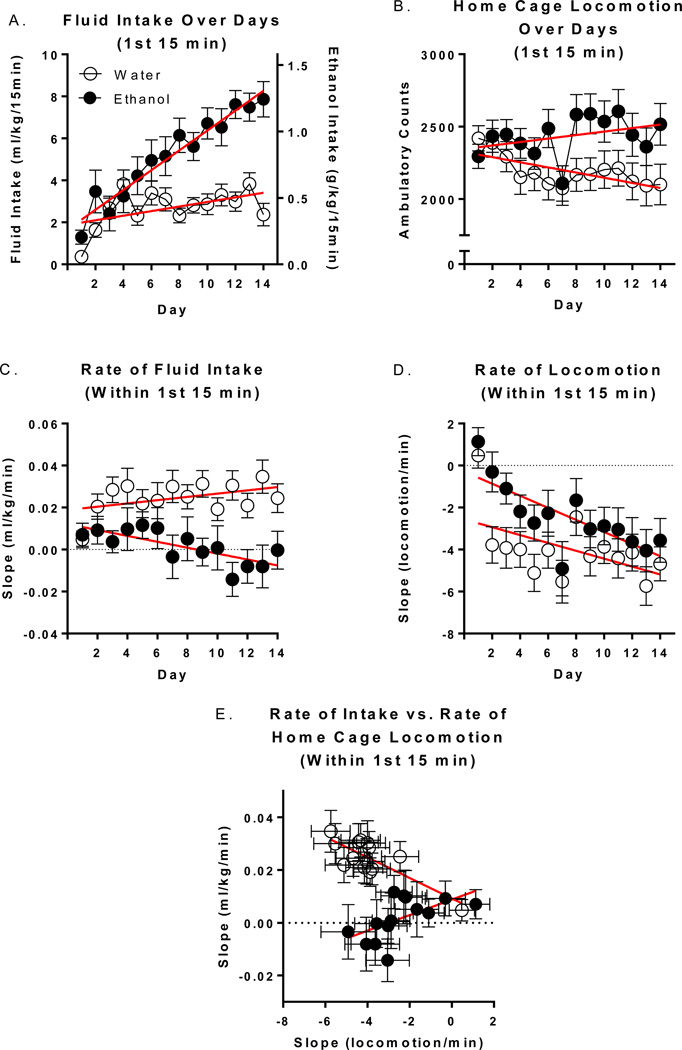
First 15 Minutes Analysis
To test the progressive nature of the above observed alterations, we analyzed the first 15 minutes of ethanol and water intake over all 14 days (Figure 3A). The results of this 2-way repeated measures ANOVA indicated significant main effects of fluid group [F(1, 78)=22.61 p<.0001], day [F(13, 1014)=10.44 p<.0001] and a fluid group*day interaction [F(13, 1014)=4.73 p<.0001] which post hoc tests confirmed was due to significant increases in ethanol intake compared to water intake. Significantly higher (within group) ethanol intake compared to day 1 was first detected on day 6 (p<.01) and continued through day 14 (p<.0001). Within-group increases in water consumption compared to day 1 occurred on days 4, 6, and 13 (p’s<.05). Both ethanol [R2=.13; p<.0001] and water [R2=.02; p<.001] intake increased linearly over days. However, the slopes of each fluid group were found to be significantly different [F=33.79; DFn/d=1/1116; p<.0001]. By way of comparison, 32% of total ethanol intake on day 14 was consumed during the first 15 minutes while less than 5% of total water intake was consumed during the first 15 minutes on this day.
Figure 3.
Fluid intake and home cage locomotion during the first 15 minutes of DID on days 1-14. Total amount of fluid consumed (A) and home cage locomotor activity (B) within the first 15 minutes of day 1–14. The rate (slope) of fluid intake (C) and home cage locomotor activity (D) that occurred within the first 15 minutes of days 1–14. (E) The relationship between the rate of intake and the rate of home cage locomotion that occurred within the first 15 minutes on days 1–14.
To evaluate if locomotor activity within the first 15 minutes changed over days, identical analyses to those above were conducted on home cage locomotion (Figure 3B). The results of this analysis indicated a significant main effect of day [F(13, 1014)=2.97 p<.001] and a fluid group*day interaction [F(13, 1014)=2.84 p<.001]. Locomotor activity within the first 15 minutes decreased linearly over days in the water group [R2=.01; p<.05].
To directly evaluate the rate of fluid intake within the first 15 minutes over days, the slope of intake over the first 15 one-minute bins for each day was assessed (Figure 3C). There was a significant main effect of fluid group only [F(1, 78)=30.18 p<.0001]. Regression analysis of these data over days indicated that the slope of fluid intake within the first 15 minutes decreased (i.e. rate increased) linearly over days in ethanol consuming animals [R2=.01; p<.05]. The slopes fit to these data over days of each fluid group’s regression line were found to be significantly different [F=9.18; DFn/d=1/1116; p<.01]. Identical analyses were then conducted for locomotor activity (Figure 3D). The results of this analysis indicated significant main effects of fluid group [F(1, 78)=4.80 p<.05] and day [F(13, 1014)=6.61 p<.0001]. The slope of locomotor activity within the first 15 minutes decreased (i.e rate increased) linearly over days in both ethanol [R2=.03; p<.0001] and water [R2=.02; p<.01] groups. These slopes were not significantly different.
An analysis of the relationship between the rate of intake and rate of home cage locomotion was then conducted. The rate of locomotion and the rate of ethanol intake were significantly positively associated [r=0.58; p<0.05], whereas the rate of locomotion and the rate of water intake were significantly negatively associated [r=−0.79; p<.001].
Day 15
General Linear Analysis
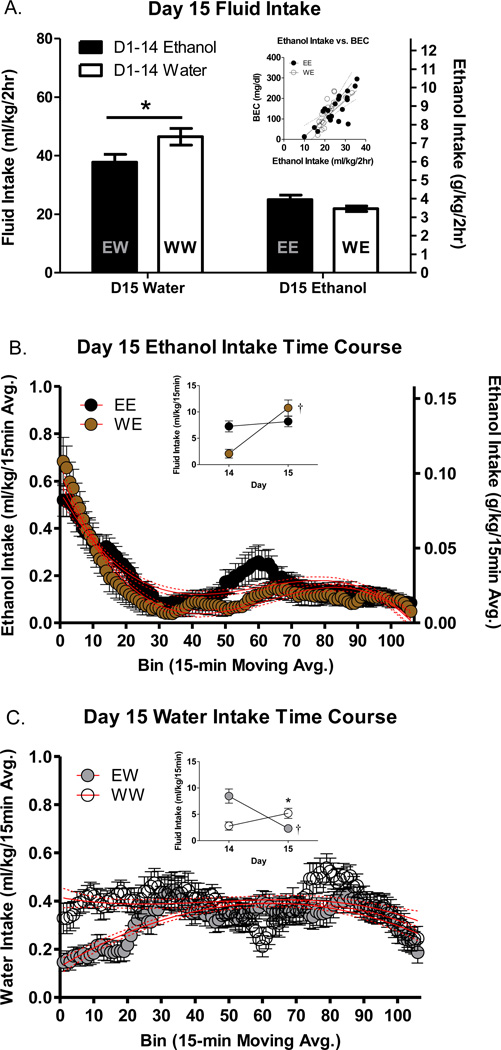
Total fluid consumption on day 15 can be seen in Figure 4A. Analysis of day 15 intake revealed a significant main effect of day 15 fluid assignment [F(1, 75)=76.67 p<.0001] with the groups consuming water drinking more fluid volume by weight than the groups consuming ethanol. There was also a significant D1–14 fluid assignment * D15 fluid assignment interaction [F(1, 75)=7.65 p<.01] driven in part by significantly higher water intake in the WW group compared to the EW group (p<.05). There were no differences in total ethanol intake between the EE and WE groups on day 15.
Figure 4.
Effect of 14 days of DID on Day 15 intake. (A) Two hour cumulative intake on day 15 in all groups. Inset reflects relationship between BEC and ethanol intake. The left y-axis refers to both ethanol and water groups, whereas the right axis refers to ethanol animals only (B) Time course of ethanol intake on day 15 in animals with 14 days of ethanol (EE) or water (WE) experience. (C) Time course of water intake on day 15 in animals with 14 days of water (WW) or ethanol (EW) experience. Insets in B+C reflect intake during the first 15 minutes of access on days 14 and 15. * indicates day 15 difference between groups assigned the same fluid on day 15 (*p<.05). †’s indicate within-group differences on day 15 compared to day 14 (†p<.05).
No significant differences were detected in mean home cage locomotion on day 15 (data not shown). However, BEC (mg/dl) was significantly positively associated with home cage locomotion on this day [R2=.21; N=40; p<.01].
There were no differences in mean day 15 BEC between the EE (146.44 ± 16.8 mg/dl) and WE (135.65 ± 14.56 mg/dl) groups. However, BECs were positively associated with ethanol intake in both the EE (R2=.63; N=20; p<.0001) and WE (R2=.67; N=20; p<.0001) groups (see Figure 4A inset).
Polynomial Analysis
Central moving average representations for ethanol and water intake on day 15 can be seen in Figure 4B+C. Central moving average values on day 15 were best fit to 3rd order polynomials in all but the EW group, where a 2nd order polynomial best fit the data. Surprisingly, the time course of ethanol intake was very similar between the EE and WE groups (Figure 4B), whereas the time course of water intake between the WW and EW groups differed early within the drinking session (Figure 4C).
To follow up on the observations of increased ethanol intake early in the DID session we then evaluated total fluid consumed within the first 15 minutes of access on days 14 and 15 (see Figure 4B+C insets). Day 15 fluid assignment groups were analyzed separately such that ethanol consuming animals were compared (EE vs. WE) and then water consuming animals were compared (WW vs. EW), to determine if/how the amount of intake in the first 15 minutes on day 15 had changed compared to the previous day.
Analysis of ethanol consuming groups (Figure 4B inset) revealed a significant main effect of day [F(1, 38)=28.15 p<.0001] and a significant day * D1–14 fluid assignment interaction [F(1, 38)=18.40 p=.0001]. Post-hoc testing indicated that WE animals consumed a similar amount of ethanol during the first 15 minutes compared to EE animals on day 15. This observation was due to a significant increase in ethanol fluid intake in the WE group (volume by weight) on day 15 compared to the previous days water consumption (p<.05).
Analysis of water consuming groups (Figure 4C inset) also revealed a significant main effect of day [F(1, 37)=4.67 p<.05] and a significant day * D1–14 fluid assignment interaction [F(1, 37)=24.19 p<.0001]. Post-hoc testing indicated that EW animals consumed less water during the first 15 minutes compared to WW animals on day 15. This observation was due to a significant decrease in water fluid intake in the EW group (volume by weight) on day 15 compared to the previous days ethanol consumption (p<.05). There were no obvious differences in the temporal aspects of home cage locomotor activity so these data are not presented graphically.
Day 16
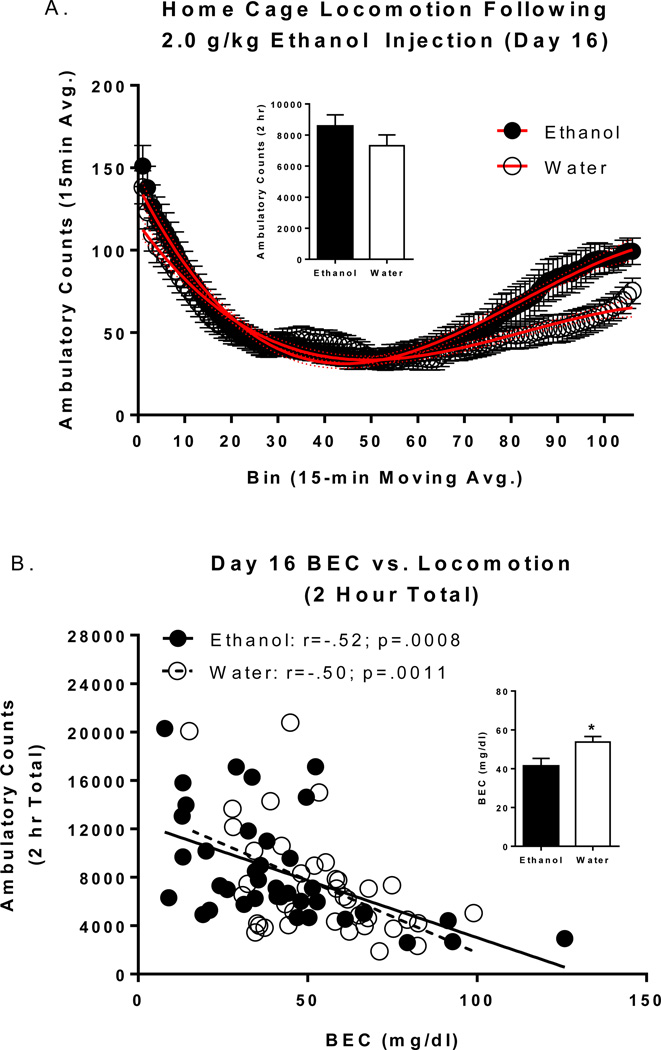
One male and one female mouse were removed from all analysis of day 16 due to incomplete/unsuccessful injections. There were no significant differences in mean locomotor activity in the home cages on day 16 as a function of repeated (D1–14; Figure 5A inset) or the previous day’s (D15) fluid assignment (data not shown). However, there was a significant main effect of D1–14 fluid assignment [F(1, 74)=6.47 p<.05; see Figure 5B inset] for BEC immediately following the session, with lower BECs in repeated ethanol consuming animals compared to repeated water consuming animals. Furthermore, BEC was significantly negatively correlated with total locomotor activity (Groups Combined: r=-.52; N=78; p<.0001; Ethanol only: r=-.52; N=38; p<.001; Water only: r=-.50; N=40; p=.001). Visualization of the time course analysis revealed that the most prominent differences in ethanol-induced home cage locomotor activity (Figure 5A) occurred towards the end of the 2 hour recording session. We evaluated the final 15 minute bin of locomotor activity to confirm these observations. Analysis of the final 15 minutes of home cage locomotion revealed a main effect of D1–14 fluid assignment [F(1, 74)=4.82p<.05] with higher activity in repeated ethanol consuming animals compared to repeated water consuming animals. Together these data suggest that mice with repeated ethanol access had developed some degree of metabolic tolerance to ethanol. However, additional dose-response and time course studies of BEC alterations are necessary to confirm the development of metabolic tolerance to ethanol.
Figure 5.
BECs and home cage locomotion following ethanol injections (2.0 g/kg) on day 16. (A) Time course of home cage locomotion immediately following ethanol injections in groups assigned to ethanol and water on days 1–14. Inset reflects mean 2hour home cage locomotion. (B) The relationship between BEC and 2 hour home cage locomotion. Inset reflects mean BECs. * indicates between group differences (p<.05).
Discussion
The results of these experiments provide additional evidence that mice with innate high 24 hour ethanol preference/intake rapidly develop ethanol front-loading when given ethanol access under limited conditions. Furthermore, and perhaps more importantly, previous DID fluid access and/or procedures differentially influenced the time course of intake of fluid solutions not yet experienced under DID procedures, suggesting that the relative novelty of the type of fluid offered and/or the DID procedures themselves led to an altered intake time course based on learned experience. These results, together with recent studies in the most commonly used strain of mouse in ethanol research (C57BL/6J or B6), emphasizes the complexity of genetic regulation of repeated binge-like ethanol intake using DID procedures, and suggest that learned associations with limited access procedures and/or the pharmacological effects of ethanol might drive similar patterns of intake in HAP and B6 mice, but for inherently different motivational outcomes.
Differences reported in the literature between B6 and HAP mice in ethanol-related behaviors support this view. HAP mice have been shown to consume more ethanol and achieve higher BECs than B6 mice under 24-hour 2 bottle choice conditions (Matson and Grahame, 2013), and have been found to be more sensitive to ethanol-induced locomotor stimulation (Grahame et al., 2000, Lessov et al., 2001, Melon and Boehm, 2011), conditioned taste aversion (Chester et al., 2003, Broadbent et al., 2002), and conditioned place preference (Grahame et al., 2001, Cunningham, 2014, Cunningham et al., 1992). Notably, with the exception of Matson & Grahame (2013), none of these studies were conducted contemporaneously or even in the same lab with identical procedures or apparatus. As such, these apparent population differences should be interpreted cautiously. Certainly, there are many possible motivational contributions to both the similarities and differences observed between HAP and B6 mice in this study and that of Linsenbardt & Boehm (2013). Thus, developing an understanding of factors that contribute to alterations in the timecourse of binge drinking in each of these and other unique high drinking mouse populations will bring us closer to understanding the causes and consequences of repeated excessive ethanol consumption.
Daily total cumulative intake and concomitant locomotor behavior over the course of 14 daily access sessions were fairly consistent within fluid assignment group. Although approximately twice the volume of fluid per unit of body weight was consumed in water assigned animals compared to ethanol assigned animals, both groups gradually increased intake over days. Interestingly however, and in contrast to our previous studies in B6 mice (Linsenbardt and Boehm, 2012, Linsenbardt and Boehm, 2014), ethanol consuming HAP mice consistently displayed greater locomotor activity relative to water consuming mice. Why ethanol drinking HAP mice in these studies displayed greater locomotor activity compared to water drinking mice over the course of many daily ethanol access sessions while ethanol drinking B6 mice quickly develop decreases in locomotor behavior over drinking days is not immediately clear. However, it is possible that ethanol-induced hypolocomotion was a prohibitive trait to further increases in 24-hour ethanol consumption after many generations of selection in this (HAP) population, and/or that the previously mentioned differences in ethanol-induced locomotor effects between these 2 mouse populations after experimenter administered ethanol (Grahame et al., 2000, Lessov et al., 2001, Melon and Boehm, 2011) generalizes to that of self-administered ethanol. In addition to these mouse population differences in ethanol consuming animals, there were also large differences between HAP and B6 mice in total home cage locomotor activity in water consuming animals. Water consuming HAP mice displayed approximately twice the level of home cage locomotor activity vs. water consuming B6 mice. The reason(s) for this dramatic difference are unknown, but innate activity differences, differences in response to DID procedures (experimenter entering and leaving room/bottle switching/etc.), or an interaction of these factors are all possibilities.
Alterations in the time course of fluid intake were observed in both ethanol and water consuming groups that resembled those observed previously reported in B6 mice. While more fluid was consumed earlier in the DID session over the course of the first 3–4 days in both fluid groups, (presumably due to habituation to the sipper tubes and general DID procedures), further increases in ethanol consumption (but not water) early in the DID sessions continued without obvious asymptote over subsequent days. By way of comparison, 6.57±0.85 ml/kg more ethanol was consumed within the first 15 minutes on day 14 compared to day 1, whereas only 2.01±0.60 ml/kg more water was consumed. There were other interesting behavior differences between water and ethanol consuming groups within the first 15 minutes of DID sessions that were observed. Most notably, the rate of intake within the first 15 minutes was significantly positively associated with the rate of home cage locomotion within this time frame in ethanol animals, whereas these measures were significantly negatively associated in water animals. That is, the more quickly ethanol was consumed, and the more slowly water was consumed, the more quickly locomotion decreased. However, significant alterations in the rate of intake and home cage locomotion in water animals were only evident when day 1 was included in the analyses; significance remained in all instances in ethanol animals when day 1 was excluded from the analyses. We believe inclusion of day 1 in these analyses is informative, and that these findings together might suggest that water animals adapted to the DID procedures with directionally opposite rates in fluid intake compared to ethanol animals, and that 1 day of DID was sufficient for the adaptation to manifest in the water group.
Perhaps the most interesting observations in fluid intake occurred on day 15, where half of each group of animals received the oppositely assigned fluid. There were no differences in total ethanol consumption in animals receiving ethanol for the first (WE) or 15th (EE) time. This was surprising given that ethanol intake increased gradually over days, with significantly greater intake vs. day 1 observed only on days 8–14 (Figure 1A). Furthermore, there were differences in total water consumed, with animals given access to water for the first time (EW) consuming significantly less water than those continuing to receive water (WW). Even more surprising, time course analysis of ethanol intake on day 15 revealed similar patterns of front-loading behavior between ethanol naïve and experienced groups, suggesting that this behavior in HAP mice is a trait regulated by previous experience with DID procedures and/or an alternative fluid. Similar patterns of ethanol intake were in contrast to differences observed in time course of water intake. Animals with previous ethanol access only (EW) consumed less water early within the DID session than did those with many days of previous water access (WW).
Day 15 time course effects were further evaluated by comparing the first 15 minutes of consumption on days 14 and 15 as a function of day 15 fluid assignment (Figure 4 insets). While there were no significant differences in fluid consumed within the first 15 minutes on day 14 and 15 in animals given access to the same fluid type (EE and WW), WE animals consumed significantly more (ethanol) solution compared to the previous days water consumption, and EW animals consumed significantly less (water) solution. Together these data suggest that providing novel (within DID procedures) solutions to HAP mice is sufficient to alter the time course of intake, in a bi-directional way perhaps based on the perceived rewarding value of the novel fluid based on previous day’s fluid experience. Future studies using similar methods but alternative reinforcing solutions such as sucrose or saccharine might further elucidate the motivational causes of these timecourse observations and should be explored.
To evaluate the consequences of repeated ethanol access on metabolic and/or behavioral tolerance, experimenter administered ethanol was given on day 16, 24 hours following day 15 fluid access. Significant differences in BEC were observed in animals given ethanol or water for 14 consecutive days (Figure 5B inset). Lower BECs in animals given access to ethanol and significant negative correlations between BEC and locomotor activity suggests that repeated ethanol consumption led to some degree of metabolic tolerance. That repeated binge-like ethanol intake might lead to metabolic tolerance in HAP mice is consistent with previous DID studies in B6 mice (Linsenbardt and Boehm, 2012, Linsenbardt et al., 2011) and provides additional support that increases in ethanol front-loading observed using this model may be associated with important physiological adaptations.
Conclusions
These studies provide additional evidence that mice with innate high 24 hour ethanol preference rapidly develop ethanol front-loading behavior when given ethanol access under limited-access conditions. Furthermore, that alterations in the time course of fluid consumption following daily repeated DID sessions in HAP mice was influenced by repeated DID experience, and/or the novelty of fluid type based on this experience, suggests that learned associations about the relative motivational properties of ethanol vs. water, interact with learned associations about the limited availability of this access, and dynamically regulate the time course of intake. Together these results emphasize the importance of monitoring preclinical behavioral measures with high temporal resolution, and highlight changes in behavior born out of previous experience that likely stem from both inherent/genetic and learned motivational properties.
Acknowledgments
This work was supported by NIAAA grant #’s AA015434 (SLB), AA016789 (SLB), AA07462 (DNL), and AA022268 (DNL).
References
- Barkley-levenson AM, Crabbe JC. High Drinking in the Dark Mice: A genetic model of drinking to intoxication. Alcohol. 2013 doi: 10.1016/j.alcohol.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Ceballos NA. Neural and genetic correlates of binge drinking among college women. Biol Psychol. 2014;97:43–48. doi: 10.1016/j.biopsycho.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol. 2002;70:67–78. [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, Grahame NJ. High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res. 2003;27:12–18. doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- Coon H, Piasecki TM, Cook EH, Dunn D, Mermelstein RJ, Weiss RB, Cannon DS. Association of the CHRNA4 Neuronal Nicotinic Receptor Subunit Gene with Frequency of Binge Drinking in Young Adults. Alcohol Clin Exp Res. 2014;38:930–937. doi: 10.1111/acer.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav Neurosci. 2014;128:430–445. doi: 10.1037/a0036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Chester JA, Rodd-henricks K, Li TK, Lumeng L. Alcohol place preference conditioning in high- and low-alcohol preferring selected lines of mice. Pharmacol Biochem Behav. 2001;68:805–814. doi: 10.1016/s0091-3057(01)00476-2. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Iancu OD, Oberbeck D, Darakjian P, Metten P, Mcweeney S, Crabbe JC, Hitzemann R. Selection for drinking in the dark alters brain gene coexpression networks. Alcohol Clin Exp Res. 2013;37:1295–1303. doi: 10.1111/acer.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berl) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Role of novelty and ethanol history in locomotor stimulation induced by binge-like ethanol intake. Alcohol Clin Exp Res. 2012;36:887–894. doi: 10.1111/j.1530-0277.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Alterations in the rate of binge ethanol consumption: implications for preclinical studies in mice. Addict Biol. 2013 doi: 10.1111/adb.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL., 2nd Alterations in the rate of binge ethanol consumption: implications for preclinical studies in mice. Addict Biol. 2014;19:812–825. doi: 10.1111/adb.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to ethanol's ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol. 2013;18:921–929. doi: 10.1111/j.1369-1600.2011.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Blanchette J, Nelson TF, Nguyen T, Oussayef N, Heeren TC, Gruenewald P, Mosher J, Xuan Z. A new scale of the U.S. alcohol policy environment and its relationship to binge drinking. Am J Prev Med. 2014;46:10–16. doi: 10.1016/j.amepre.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet. 2011;41:288–302. doi: 10.1007/s10519-010-9394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Watson NF, Buchwald D, Harden KP. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med. 2013;9:1333–1339. doi: 10.5664/jcsm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]