Abstract
Purpose
This study aimed to evaluate the clinical significance of Ki-67 and p53 expressions in patients with pancreatic head cancer.
Methods
Between May 2008 and April 2013, immunohistochemical staining for Ki-67 and p53 was performed in 34 patients with pancreatic head cancer (ductal adenocarcinoma). All 34 patients underwent pancreaticoduodenectomy at Chonnam National University Hwasun Hospital, Hwasun, Korea. Clinical and histopathological characteristics were analyzed, relative to p53 expression.
Results
Thirty (88.2%) and twenty-one (61.7%) of the 34 pancreatic head cancers exhibited positive expression of Ki-67 and p53, respectively. Patients expressing Ki-67 and p53 experienced more frequent tumor recurrences within 1 year after surgical resection (P = 0.003 and P = 0.030, respectively). However, no correlation was detected between Ki-67 and p53 expression. Ki-67 expression was correlated with pathological grade, lymph node metasatsis, and clinical stage (P < 0.05). Importantly, Ki-67 was the independent predictive factor for postoperative recurrence within 1 year in both univariable and multivariable analyses (odds ratio, 27.219; 95% confidence interval, 1.403-528.135; P = 0.029).
Conclusion
The expression of Ki-67 and p53 are significantly related to early postoperative recurrence within 1 year after surgical resection in pancreatic head cancer. Especially, Ki-67 was the independent predictive factor for postoperative recurrence within 1 year. Therefore, immunohistochemical staining for Ki-67 and p53 may be applied as a predictive marker for early postoperative recurrence in pancreatic head cancer.
Keywords: Ki-67 antigen, Tumor suppressor protein p53, Pancreatic ductal carcinoma, Immunohistochemistry, Risk factors
INTRODUCTION
Pancreatic ductal adenocarcinoma is the fifth leading cause of cancer deaths in Europe and the United Stated, with an estimated 5-year overall survival of less than 5% [1,2]. Pancreatic cancer arises from precursor lesions called pancreatic intraepithelial neoplasia, which are characterized by the sequential accumulation of alterations in the KRAS oncogene and loss of the CDKN2A, TP53, and/or SMAD4 tumor suppressors in many cases [3]. Although we know the frequencies of such mutations in pancreatic cancer, their specific functions during the development of pancreatic cancer remain unclear. But molecular research has indicated the close relationship between the dysfunction of apoptosis-related genes and the incidence of pancreatic cancer.
Ki-67 protein (also known as MKI67) is a cellular marker for proliferation [4]. It is strictly associated with cell proliferation. During interphase, the Ki-67 antigen can be exclusively detected within the cell nucleus, whereas in mitosis most of the protein is relocated to the surface of the chromosomes. Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent from resting cells (G0). Importantly, expression of Ki-67 reflects tumor proliferation rates and correlates with initiation, progression, metastasis and prognosis of many tumors [5,6,7]. Although Ki-67 is broadly used as a proliferation marker, the physiologic function of Ki-67 still remains unclear.
p53 protein can induce cell apoptosis to prevent the mutated DNA passage to the next generation in cases of failed DNA repair. Due to the loss of cell supervision of p53 protein after p53 gene mutation, cells are susceptible to entry of S phase with injured DNA and genetic instability is the source of gene mutation and chromosomal aberration, leading to malignant cell change and tumor formation [8]. p53 tumor suppressor gene is frequently mutated in human pancreatic cancer (37%-76%), predominantly through messense mutation [9,10,11,12]. An earlier study showed that the mutation of p53 may have a connection with the more malignant biologic behavior of pancreatic cancer [8,13,14]. However, Some reports showed that p53 was not an independent prognostic marker for survival of patients with pancreatic cancer [10,11,12,15].
In the present study, we investigated the expression patterns of Ki67 and p53 in pancreatic head cancer and determined its association with patient clinicopathological parameters. Moreover, we analyzed the relationship between Ki-67 expression, p53 expression, postoperative recurrence and patient survival after surgical resection.
METHODS
Patients
Two hundred fifty-five patients underwent pancreaticoduodenectomy at Chonnam National University Hwasun Hospital, Hwasun, Korea, between May 2008 and April 2013. Among these, one hundred thirty-four patients had pancreatic head cancer. All the patients proved to have pancreatic head cancer (ductal adenocarcinoma) on histological examination of surgical specimens. Eighteen patients were excluded due to R1 resection (4 patients) and pre- or postoperative chemotherapy (14 patients). Eighty-two patients did not receive Ki-67 and p53 immunohistochemical staining and were excluded. Therefore, thirty-four patients with pancreatic head cancer were enrolled in this study.
Clinicopathological parameters collected included age, sex, tumor size, lymph node metastasis, TNM staging, differentiation of the tumor, lympohovascular invasion, perineural invasion and preoperative level of serum CA 19-9. The clinical staging of all tumors was completed in accordance with the 7th American Joint Committee on Cancer (AJCC) TNM staging system [16].
Immunohistochemistry
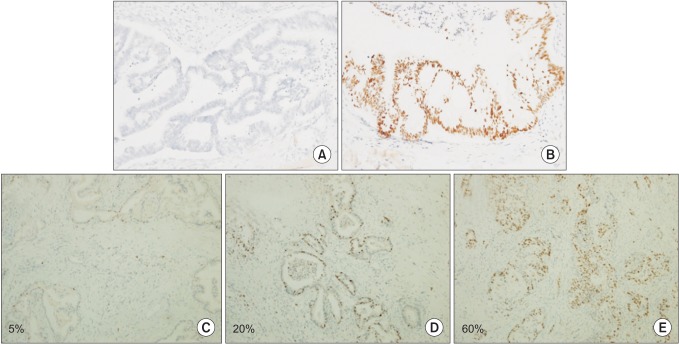
Immunohistochemical staining was performed using the ventastain avidin-biotin complex technique. Formalin-fixed, paraffin-embedded blocks of tumor tissues and adjacent normal mucosa were sliced into 1-µm-thick sections, mounted in silene-coated slides. After melting paraffin at 65℃ for 30 minutes, the sections were deparaffinized and rehydrated. Endogenous peroxidase activity was eliminated by incubation with H2O2 in methanol for 10 minutes, sections were immersed in citrate buffer (pH 6.0) in a microwave-resistant container. After washing with citrate buffer, the slides were incubated for 1 hour at room temperature with the primary antibody for Ki-67 (MIB-1, Dako, Glostrup, Denmark) and p53 (1:500; R&D systems Inc., Minneapolis, MN, USA). After further washing, the slides were incubated for 10 minutes at room temperature with the secondary antibody (antimouse IgG, DAKO), washed and then incubated with avidin-biotin peroxidase for 10 minutes. Peroxidase was detected by adding diaminobenezidine tetrahydrocholoride. All slides were counterstained with haematoxylin dehydrated and mounted with Permount. Immunoreactivity for p53 was seen as brown, fine to coarse granular staining on the cytoplasmic membrane. The signal intensity of p53 was categorized into two grades: 0, negative (Fig. 1A); 1, positive (Fig. 1B). Ki-67 staining appears as brownish-yellow granules in the nucleus. The signal intensity of Ki-67 was categorized into four grades: 0, negative; 1, weak, ≤10% (Fig. 1C); 2, moderate, 10%-50% (Fig. 1D); and 3, strong, ≥50% (Fig. 1E).
Fig. 1. Representative immunohistochemical staining of p53 and Ki-67 in pancreatic head cancer. Negative expression for p53 (A, ×200) and positive expression for p53 (B, ×200). Positive staining suggests a mutant p53 gene with accumulation of mutant p53 proteins. Weak expression (C, ×100), moderate expression (D, ×100), and strong expression for Ki-67 (E, ×100).
Outcome measures
The following operative variables were collected for each patient: demographics; tumor histology, size of pancreatic head cancers; operative detail; most recent follow-up data; disease-free status (e.g., recurrence vs. nonrecurrence); and date of recurrence. Survival status (alive vs. dead) was determined by review of the medical records as well as through the use of Jeonnam regional cancer center death index. Disease-free survival was calculated from the date of operation to the date of recurrence or last follow-up.
The cumulative overall and recurrence-free survival rates were analyzed on the basis of Ki67 and p53 expression in pancreatic head cancer tissues. The clinicopathological variables were analyzed to determine the predictive factors for postoperative recurrence after surgical resection.
Statistical analysis
Summary statistics were reported using mean or median values where appropriate. Student t-test and Mann-Whitney U test were used for mean comparison of continuous variables and for ordinal data, respectively, whereas chi-squre test and Fisher exact test were used to compare frequencies of categorical variables between groups. Survival rates were calculated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Predictors for postoperative recurrence were determined by univariable and multivariable analyses using Cox proportional hazard model. Significance was defined as P ≤ 0.05. All statistical analyses were performed using the statistical package SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) and STATA 12.1 (StataCorp LP., College Station, TX, USA).
RESULTS
Ki-67 and p53 expression in pancreatic head cancer
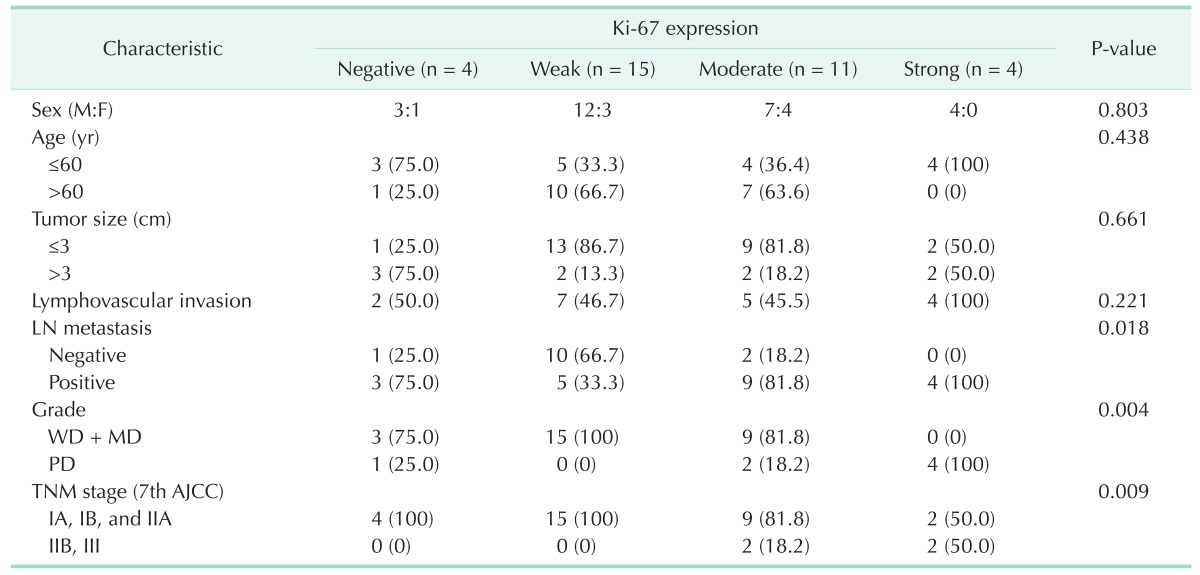
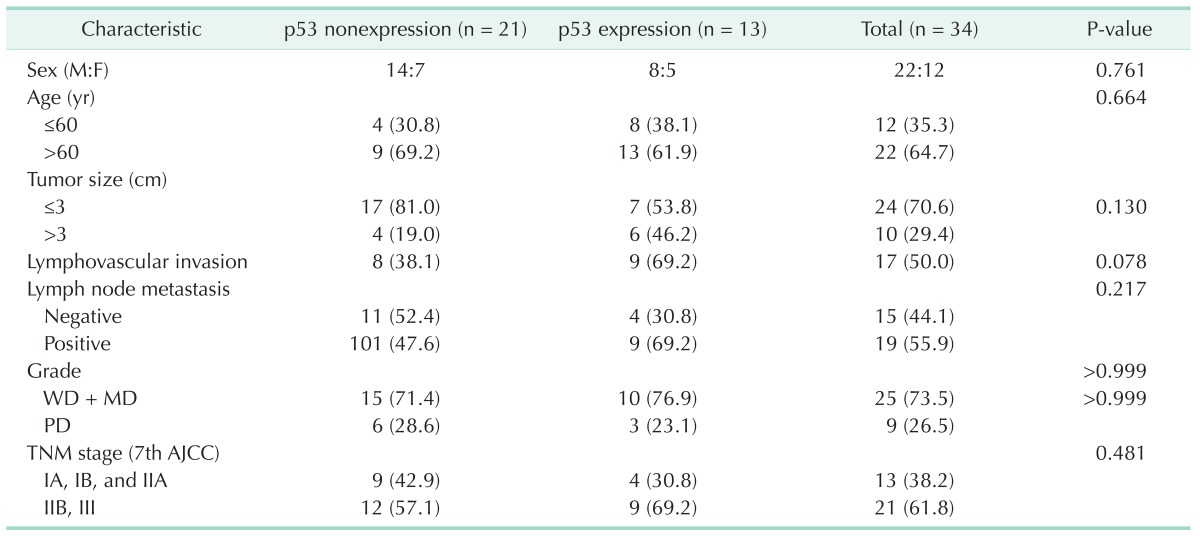
The demographic and clinicopathological characteristics of these patients are listed in Tables 1 and 2. There were 22 males (64.7%) and 12 females (35.3%), with the mean age of 63.0 ± 10.5 years. The average tumor diameter was 2.5 cm (1.2-5.8 cm). According to AJCC cancer stage system, 7th ed. [16], 13 patients (38.2%) had stage IA, IB, IIA disease, and 21 patients (61.8%) had stage IIB and III. In total, 17 patients (50.0%) displayed lymphovascular invasion, 23 patients (67.6%) displayed perineural invasion on microscopic examinations. Lymph node metastasis was positive in 19 patients (55.9%). Differentiation of the tumor was well or moderately differentiated in 25 patients (73.5%) and poorly differentiated in 9 patients (26.5%).
Table 1. Clinicopathological characteristics of patients with pancreatic head cancer.
Values are presented as number (%).
LN, lymph node; AJCC, American Joint Committee on Cancer; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated.
Table 2. Correlation between Ki-67 and p53 expression in pancreatic head cancer.
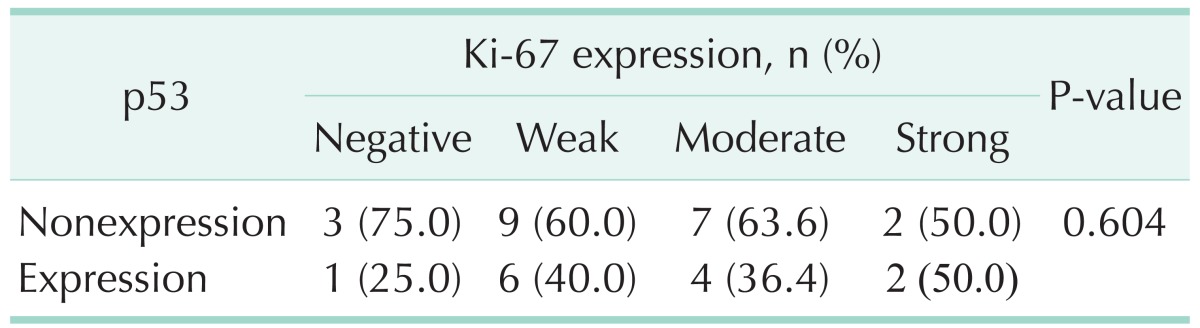
Ki-67 was detected in nearly all the tumor samples (30/34, 88.2%). Ki-67 expression was correlated with pathological grade (P = 0.004), lymph node metasatsis (P = 0.018), and clinical stage (P = 0.009); neither was associated with gender, age, tumor size and serum CA19-9 levels (Table 1). Interestingly, there was no correlation between Ki-67 and p53 expression in pancreatic cancer tissues (P = 0.604) (Table 2). p53 was expressed in 61.8% (21 of 34) of pancreatic head cancer. p53 expression did not significantly differ in any clinicopathological factors (Table 3).
Table 3. Clinicopathological characteristics of patients with and without p53 expression.
Values are presented as number (%).
WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; AJCC, American Joint Committee on Cancer.
Ki-67 and p53 expression and postoperative recurrence of pancreatic head cancer
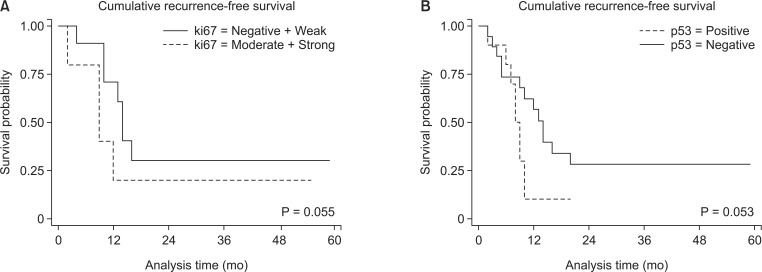
During the follow-up with a mean period of 19.7 ± 14.4 months, the overall recurrence-free survival rates were not different between patients with Ki-67 and p53 expression (P = 0.055 and P = 0.053, respectively) (Fig. 2). However, differences in the recurrence rates were significantly evident within 1 year after surgical resection. The recurrence rates in pancreatic head cancer patients with Ki-67 expression were 0% (nonexpression group), 46.7% (weak), 81.8% (moderate), and 100% (strong) at 1 year (P = 0.001). The recurrence rates with and without p53 expression were 84.6% and 42.8% at 1 year (P = 0.030).
Fig. 2. Cumulative recurrence rates after surgical resection according to the degree of Ki-67 (A) and p53 expression (B) in pancreatic head cancer tissue. Overall recurrence-free survival rates were not different between patients with Ki-67 and p53 expression (P = 0.055 and P = 0.053, respectively). However, the recurrence-rate within 1 year after surgical resection was significantly lower in pancreatic head cancer patients expressing Ki-67 (P = 0.003) and p53 (42.8% vs. 84.6%, P = 0.030).
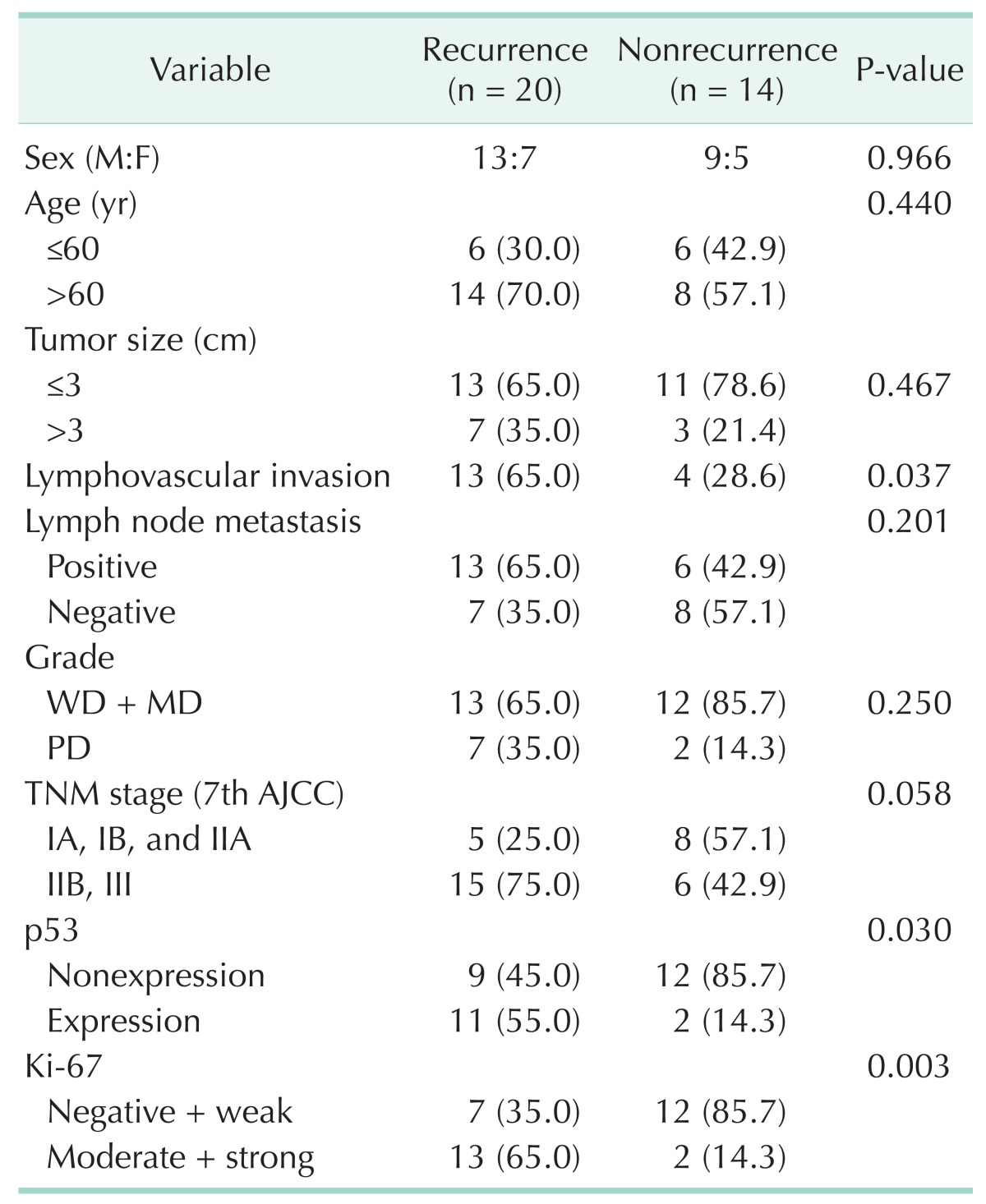
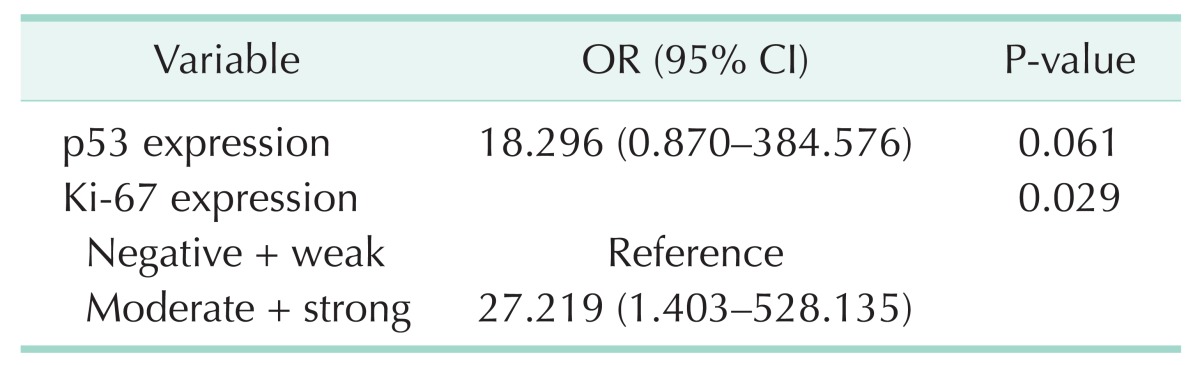
Univariable analysis found Ki-67 expression, p53 expression, and lymphovascular invasion as the predictive factors for recurrence within 1 year after pancreaticoduodenectomy (Table 4). In the multivariable analyses, we chose gender, Ki-67 expression, p53 expression, lymphovascular invasion, pathological grade, and clinical stage as variables and Ki-67 expression was the only independent predictive factor for tumor recurrence within 1 year after surgical resection (odds ratio, 27.219; 95% confidence interval, 1.403-528.135; P = 0.029) (Table 5).
Table 4. Univariable analysis of predictive factors for early postoperative recurrence within 1 year after pancreaticoduodenectomy.
Values are presented as number (%) unless otherwise indicated.
WD, well-differentiated; MD, moderately-differentiated; PD, poorly differentiated; AJCC, American Joint Committee on Cancer.
Table 5. Multivariable analysis of predictive factors of early postoperative recurrence within 1 year after pancreaticoduodenectomy.
OR, odds ratio; CI, confidence interval.
Ki-67 and p53 expression and cumulative overall survival of pancreatic head cancer
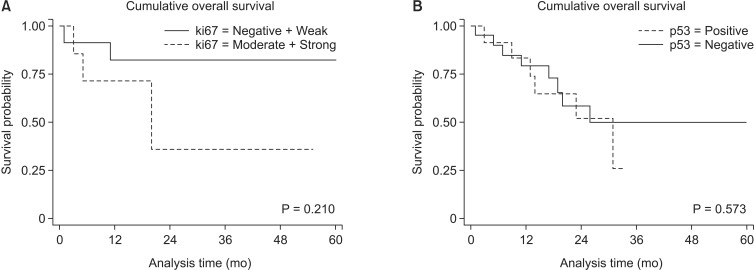
Consequently, the cumulative overall survival rates were not different between patients with Ki-67 and p53 expression (P = 0.210 and P = 0.573, respectively) (Fig. 3).
Fig. 3. Cumulative survival rates after surgical resection according to the degree of Ki-67 (A) and p53 expression (B). Cumulative overall survival rates were not different between patients with Ki-67 and p53 expression (P = 0.210 and P = 0.573, respectively).
DISCUSSION
Ki-67 is a nuclear protein expressed in proliferating cells [4]. A recent review of Ki-67 shows mixed results regarding its prognostic value in pancreatic cancer. Stanton et al. [17] concluded that Ki-67 did not appear to correlate with survival and was not a useful prognostic marker in pancreatic cancer. On the other hand, others reported that Ki-67 expression was correlated with pathological grade, lymph node metastasis, and clinical stage [18]
Notably, our study showed the overall recurrence-free survival rates were not different between patients with and without Ki-67 expression (P = 0.055) (Fig. 3A). However, Ki-67 expression was closely related to tumor recurrence within 1 year after pancreaticoduodenectomy, indicative of the more aggressive behavior of these tumors. Ki-67 tended to increase with the decreasing degree of differentiation and was correlated with lymph node metastasis and clinical stages. These findings suggest that Ki-67 expression promotes pancreatic cancer [18]. Consistent with this finding, univariable and multivariable analysis revealed that Ki-67 expression is an independent predictive factor for early postoperative recurrence within 1 year after surgical resection. Usually, early recurrence after surgical resection is due to pre-existing intra-abdominal spread or the incomplete removal of tumors, while late recurrence is mostly due to nonsynchronous, multifocal tumorigenesis. In our experiment, only one patient experienced tumor recurrence along the resection margin; therefore incomplete resection did not contribute to the early recurrence. These suggested that pancreatic cancer cells expressing Ki-67 may reflect tumors of an aggressive nature. Whereas Ki-67 did not appear to correlate with survival, Ki-67 may be a useful prognostic marker in pancreatic cancer.
The tumor suppressor gene p53 is critical to normal cellular function, and its amino acid sequence is highly conserved among many species. Following DNA damage, p53 protein levels increase because of posttranslational changes in protein stability. The p53 response to DNA damage leads to both cell cycle arrest and apoptosis [19]. Mutation of the p53 gene are frequent genetic abnormalities in various human malignancies, including pancreatic adenocarcinoma [20].
The p53 expression in pancreatic cancer is reported to be 37%-76% [9,10,11,12]. In our study, it was 61.8%. The correlation between p53 expression and TNM stage, differentiation of the tumor and the prognosis is still controversial. Some studies have reported association between p53 gene and the cell cycle, and have discussed the role of p53 in the carcinogenesis of pancreatic cancer [12,21,22]. However, researchers have proposed various results in the association between p53 mutation and clinicopathologic properties and survival rate. Many studies report that there is no association between p53 mutation and clinicopathologic properties [10,11,12], while Yokoyama et al. [23] reported an association between p53 mutation and clinical stages. In this study, p53 expression had no association with any clinicopathological factors.
Nakamori et al. [20] reported that mutations of the p53 gene might play an important role in cancer aggressiveness and could be a clinically useful predictor of prognosis in patients with pancreatic adenocarcinioma. And Sato et al. [24] reported p53 protein expression may be a beneficial prognostic factor for patients with pancreatic cancer. In our study, p53 expression was predictive factor for tumor recurrence within 1 year after surgical resection. However, p53 expression is not an independent predictive factor in the multivariable analysis and the cumulative overall survival rates were not different between patients with and without p53 expression.
This study has the limitation of any retrospective study and limited cases. For example, lymph node metastasis and tumor size are known as an independent prognostic factor for pancreatic cancer [16,25]. In our study, patients with lymph node metastasis and tumors more than 3 cm had a higher recurrence-rate within 1 year after surgical resection than patients without these characteristics. However, this result was not statistically significant. We thought this may have resulted from limited cases. In addition, this study is confined to Ki-67 and p53 expression and doesn't consider the compounding effect of molecular markers such as MDM2, p21Waf1, and SMAD4. Therefore, the accumulation of more, larger groups of patients and further examinations (more rigorous research design) will be necessary to draw any definitive conclusion.
In summary, Ki-67 and p53 is frequently expressed in pancreatic cancer. Our study indicates that the expression of Ki-67 and p53 is closely related to postoperative recurrences within 1 year after surgical resection. Especially, Ki-67 was the independent predictive factor for postoperative recurrence within 1 year. This study suggests that immunohistochemical staining for Ki-67 and p53 may be applied as a predictive marker for early postoperative recurrence after surgical resection in pancreatic head cancer.
ACKNOWLEDGEMENTS
This work was supported by clinical research grant from Pusan National University Hospital 2014.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Jensen OM, Esteve J, Moller H, Renard H. Cancer in the European Community and its member states. Eur J Cancer. 1990;26:1167–1256. doi: 10.1016/0277-5379(90)90278-2. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 4.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006;48:674–682. doi: 10.1111/j.1365-2559.2006.02402.x. [DOI] [PubMed] [Google Scholar]
- 6.Gentile V, Vicini P, Giacomelli L, Cardillo MR, Pierangeli A, Degener AM. Detection of human papillomavirus DNA, p53 and ki67 expression in penile carcinomas. Int J Immunopathol Pharmacol. 2006;19:209–215. [PubMed] [Google Scholar]
- 7.Lee CS. Differences in cell proliferation and prognostic significance of proliferating cell nuclear antigen and Ki-67 antigen immunoreactivity in in situ and invasive carcinomas of the extrahepatic biliary tract. Cancer. 1996;78:1881–1887. doi: 10.1002/(sici)1097-0142(19961101)78:9<1881::aid-cncr6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Dong M, Dong Q, Zhang H, Zhou J, Tian Y, Dong Y. Expression of Gadd45a and p53 proteins in human pancreatic cancer: potential effects on clinical outcomes. J Surg Oncol. 2007;95:332–336. doi: 10.1002/jso.20684. [DOI] [PubMed] [Google Scholar]
- 9.Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, et al. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993;142:1534–1543. [PMC free article] [PubMed] [Google Scholar]
- 10.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 11.Kawesha A, Ghaneh P, Andren-Sandberg A, Ograed D, Skar R, Dawiskiba S, et al. K-ras oncogene subtype mutations are associated with survival but not expression of p53, p16(INK4A), p21(WAF-1), cyclin D1, erbB-2 and erbB-3 in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2000;89:469–474. doi: 10.1002/1097-0215(20001120)89:6<469::aid-ijc1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, et al. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994;118:150–154. [PubMed] [Google Scholar]
- 13.Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol. 2005;11:2162–2165. doi: 10.3748/wjg.v11.i14.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bold RJ, Hess KR, Pearson AS, Grau AM, Sinicrope FA, Jennings M, et al. Prognostic factors in resectable pancreatic cancer: p53 and bcl-2. J Gastrointest Surg. 1999;3:263–277. doi: 10.1016/s1091-255x(99)80068-7. [DOI] [PubMed] [Google Scholar]
- 15.Salek C, Minarikova P, Benesova L, Nosek V, Strnad R, Zavoral M, et al. Mutation status of K-ras, p53 and allelic losses at 9p and 18q are not prognostic markers in patients with pancreatic cancer. Anticancer Res. 2009;29:1803–1810. [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 17.Stanton KJ, Sidner RA, Miller GA, Cummings OW, Schmidt CM, Howard TJ, et al. Analysis of Ki-67 antigen expression, DNA proliferative fraction, and survival in resected cancer of the pancreas. Am J Surg. 2003;186:486–492. doi: 10.1016/j.amjsurg.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Hu HY, Liu H, Zhang JW, Hu K, Lin Y. Clinical significance of Smac and Ki-67 expression in pancreatic cancer. Hepatogastroenterology. 2012;59:2640–2643. doi: 10.5754/hge12071. [DOI] [PubMed] [Google Scholar]
- 19.Shimamura A, Fisher DE. p53 in life and death. Clin Cancer Res. 1996;2:435–440. [PubMed] [Google Scholar]
- 20.Nakamori S, Yashima K, Murakami Y, Ishikawa O, Ohigashi H, Imaoka S, et al. Association of p53 gene mutations with shor t survival in pancreat ic adenocarcinoma. Jpn J Cancer Res. 1995;86:174–181. doi: 10.1111/j.1349-7006.1995.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch HT, Brand RE, Lynch JF, Fusaro RM, Kern SE. Hereditary factors in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2002;9:12–31. doi: 10.1007/s005340200001. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri BA, Huang L, Berger D, Chang H, Klein-Szanto AJ, Goodrow T, et al. Molecular pathology of primary and metastatic ductal pancreatic lesions: analyses of mutations and expression of the p53, mdm-2, and p21/WAF-1 genes in sporadic and familial lesions. Cancer. 1997;79:700–716. [PubMed] [Google Scholar]
- 23.Yokoyama M, Yamanaka Y, Friess H, Buchler M, Korc M. p53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994;14(6B):2477–2483. [PubMed] [Google Scholar]
- 24.Sato Y, Nio Y, Song MM, Sumi S, Hirahara N, Minari Y, et al. p53 protein expression as prognostic factor in human pancreatic cancer. Anticancer Res. 1997;17(4A):2779–2788. [PubMed] [Google Scholar]
- 25.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–296. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]