Abstract
Background
Trypanosomiasis is a neglected tropical disease with complex clinical manifestations, tedious diagnosis, and difficult treatments. The drugs available for the treatment of this endemic disease are old, expensive, and associated with other problems including safety and drug resistant parasites. Therefore, there is an urgent need for the development of new, effective, cheap, and safe drugs for its treatment. Plants are potentially rich sources of leads for new drugs against trypanosomiasis.
Vitex simplicifolia (Verbenaceae) is used traditionally for the treatment of tooth ache, edema, skin diseases, gout and trypanosomiasis in Nigeria. In a preliminary study, the methanol extract of Vitex simplicifolia was shown to exhibit a pronounced trypanocidal activity against T. b. rhodesiense.
The present study was undertaken to investigate the active component responsible for the acclaimed activity of the leaves of Vitex simplicifolia in the traditional treatment of trypanosomiasis in Nigeria and other African countries. Our investigations aim at assessing the plant as a new source of potential trypanocidal compounds.
Methods
The crude extracts were prepared from the dried leaves using methanol, successive extraction with hexane, dichloromethane, ethylacetate and butanol was also done. The ethylacetate fraction was further fractionated and compounds isolated using preparative chromatographic technique and their structures were elucidated by NMR, mass spectrometry and comparison with literature data. Trypanocidal activities and cytotoxicity, using rat skeletal myoblast (L6) cells were investigated and their selectivity indices were determined.
Results
The chromatographic separations of the methanol extracts gave rise to seven compounds. The isolated compounds 2, 3, 6 and 7 exhibited promising trypanocidal activity with IC50 values ranging from 4.7-12.3 μg/ml and cytotoxicity in the range of 1.58- 46.20 μg/ml. Compound 6, however, showed the most selective trypanocidal activity with a selectivity index of 9.8. This is the first report of trypanocidal activity of flavonoids from this plant genus.
Conclusions
The isolated compounds from Vitex simplicifolia exhibited noteworthy trypanocidal activities and hence may provide a source of new antitrypanosomal agents. These results also support the traditional use of Vitex simplicifolia in the treatment of trypanosomiasis. This is the first report of trypanocidal effect of flavonoids from this plant genus.
Keywords: Vitex simplicifolia, Verbenaceae, Flavonoids, Trypanosoma brucei rhodesiense
Background
Human African trypanosomiasis also known as African sleeping sickness is caused by the protozoan parasites Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense in countries of sub-Saharan Africa [1]. World Health Organization (WHO) categorized it among the neglected tropical diseases which threatens primarily rural populations and is fatal unless treated. The use of the currently available drugs for the treatment of sleeping sickness has been limited owing to their numerous side effects, difficulty in administration and a certain loss of efficacy [2]. There is a great need for new safe chemotherapeutic agents preferably with oral application. In the drug pipeline managed by the Drugs for Neglected Diseases initiative (DNDi) two molecules are in clinical trial phases, fexinidazole and SCYX-7158 [3,4]. Drug discovery efforts are also directed towards natural products as an alternative source to synthetic compounds. Studies have revealed that many plant species are potential sources of novel trypanocidal compounds [5-9].
Vitex simplicifolia (Verbenaceae) is a sprawling shrub that can grow as tall as 1.5 m in height [10]. The leaves are strongly aromatic, intensifying when crushed and measure 2–6.5 cm in length and 1–4.5 cm in width [11]. The leaves and the edible fruits of V. simplicifolia are used in traditional medicine for the treatment of malaria, skin diseases, toothache, edema, gout and dermatitis [12]. In southern China, V. simplicifolia is used for the treatment of various pain disorders, such as stomach ache, hernia ache, dysmenorrhea, arthralgia, and piles [13]. Over the years, folk medicine has remained a veritable platform for researchers for sourcing lead compounds for the development of potent therapeutic agents. Several trypanocidal molecules have been isolated from plants with undefined side effects [14,15]. Flavonoids are one of the largest and most abundant classes of secondary metabolites in plants and mainly found in fruits and vegetables. Many flavonoids of plant origin have shown to possess a variety of medicinal properties [16-18]. Previous studies have demonstrated some structure activity relationships among flavonoid derivatives and their trypanocidal and /or antileishmanial activities [19-23]. So far, no similar flavonoids or its derivatives have been reported to possess trypanocidal activity against African trypanosomes. In this study, we report for the first time the trypanocidal activity of some methylated flavonoid derivatives from Vitex simplicifolia.
Methods
General experimental procedures
Optical rotations were determined on a Perkin-Elmer-241 MC polarimeter. 1D and 2D NMR spectra were recorded on an Avance DMX 600 NMR spectrometer. Chemical shifts were referenced to the residual solvent peak at δH 7.26 (CDCl3) and 2.05 (acetone-d6) for 1H, and δC 77.0 (CDCl3) and 29.92 (acetone-d6) for 13C, respectively. Mass spectra were measured with a LCMS HP1100 Agilent Finnigan LCQ Deca XP Thermoquest and high-resolution electrospray ionization mass spectroscopy (HRESIMS) were recorded with an UHR-TOF maXis 4G (Bruker Daltonics, Bremen) mass spectrometer. HPLC analysis was performed with a Dionex P580 system coupled to a photodiode array detector (UVD340S); routine detection was at 235, 254, 280, and 340 nm. The separation column (125 × 4 mm) was prefilled with Eurosphere-10 C18 (Knauer, Germany), and the following gradient was used (MeOH, 0.1% HCOOH in H2O): 0–5 min (10% MeOH); 5–35 min (10-100% MeOH); 35–45 min (100% MeOH).
Semi-preparative HPLC was performed using a Merck Hitachi HPLC System (UV detector L-7400; Pump L-7100; Eurosphere-100 C18, 300 × 8 mm, Knauer, Germany). Column chromatography was performed on Silica gel 60 M (230–3400 mesh ASTM, Macherey-Nagel GmbH & Co. KG, Dueren, Germany) and Sephadex LH-20 (Sigma). TLC was carried out on precoated silica gel plates (silica gel 60 F-254, Merck KGaA, Darmstadt, Germany) for monitoring of fractions. Detection was performed at 254 and 366 nm.
Plant material
The leaves of Vitex simplicifolia were collected between March and April 2012 from Nsukka, Enugu State, Nigeria. The plant material was authenticated by Mr. Alfred Ozioko of the Centre for Ethnomedicine and Drugs Development, a subsidiary of Bioresources Development and Conservation Program (BCDP), Nsukka, Enugu State. The voucher specimens were deposited at the herbarium of the Department of Pharmacognosy, University of Nigeria, Nsukka. The leaves were cleaned, dried under room temperature and pulverized.
Extraction and isolation procedures
About 600 g of the powdered leaves of V. simplicifolia was extracted with 2.5 l of methanol for 24 hours with constant stirring using a magnetic stirrer. The methanol extract (45 g) was dispersed in water and successively extracted with hexane, dichloromethane (DCM), ethylacetate and butanol. The chromatographic separations of the DCM fraction (428.8 mg), using Vacuum Liquid Chromatography (VLC) 22 x 4 cm on silica gel (230–400 mesh) with gradient of n-hexane: ethylacetate led to 10 VLC fractions A-J. Fraction E (72.2 mg) eluted with 50% n-hexane: ethylacetate was subjected to semi-preparative HPLC (Merck, Hitachi L-7100) using a Eurosphere 100–10 C18 column (300 × 8 mm, i.d.) with the following gradient (MeOH:H2O): 0 mins, 50% MeOH; 0 mins, 50% MeOH; 4 mins, 60% MeOH; 5 mins, 65% MeOH; 12 mins, 85% MeOH; 14 mins 90% MeOH; 15 mins, 100% MeOH; 16 mins, 100% MeOH to obtain compounds 1 (8.0 mg), 2 (5.8 mg), 3 (1.8 mg), 4 (1.9 mg), 5 (3.0 mg), 6 (1.8 mg) and 7 (2.0 mg).
Determination of in vitro trypanocidal activity and cytotoxicity
Minimum essential medium (50 μl) supplemented according to a standard method [24], with 2-mercaptoethanol and 15% heat inactivated horse serum was added to each well of a 96-well microtiter plate. Serial three-fold compound dilutions were prepared covering a range from 90 to 0.123 μg/ml. Then 104 bloodstream forms of Trypanosoma brucei rhodesiense STIB 900 (a clone of a population isolated in 1982 from a patient in Tanzania) in 50 μl culture medium were added to each well and the plate incubated at 37°C under a 5% CO2 atmosphere for 72 h. Ten microlitres of Alamar Blue (12.5 mg resazurin dissolved in 100 ml distilled water) were then added to each well and incubation continued for a further 2–4 h. The plate was then read in a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and emission wavelength of 588 nm [25]. Fluorescence development was measured and expressed as percentage of the control. Data were transferred into the graphic programme Softmax Pro (Molecular Devices) which calculated IC50 values. Melarsoprol was used as standard drug.
Cytotoxicity was determined using a rat skeletal myoblast cell line (L6 cells). The culture medium was RPMI 1640 supplemented with L-glutamine 2 mM, HEPES 5.95 g/l, NaHCO3 2 g/l and 10% foetal bovine serum. Podophyllotoxin (Sigma-Aldrich) was used as the reference drug. The assay was performed following the antitrypanosomal assay protocol as described above. The IC50 values were calculated from the sigmoidal growth inhibition curves using Softmax Pro software (Molecular Devices Corp.). Tests were done in three independent experiments in duplicate.
Results and discussion
In a previous preliminary study, the methanol extract of Vitex simplicifolia was shown to exhibit a moderate trypanocidal activity against T. b. rhodesiense with an IC50 value of 14.2 μg/ml [26]. The methanol extract was therefore subjected to further fractionation and purification to identify the active components leading to the isolation of seven flavonoid derivatives (1–7).
The structures of the isolated flavonoid derivatives in Figure 1 were elucidated by a combination of 1D and 2D NMR and mass spectral analyses and comparison of the data with those reported in the literature as 2-(5′-methoxyphenyl)-3,4′,5,7,8-trihydroxychroman-4-one (1), 2-(5′-methoxyphenyl) 4′,5,7-trihydroxy-3-methoxychromen-4-one (2), Penduletin (3), 2-(4′-hydroxyphenyl)-5-hydroxy 3,7- dimethoxy chromen-4-one (4), 2-(4-hydroxyphenyl)-3,5,7-trihydroxy chromen-4-one (5), Artemetin (6) and 2-(3′,4′-dimethoxyphenyl)-7-hydroxychromen-4-one (7). All the seven compounds in Figure 1 were isolated from Vitex simplicifolia for the first time. Compounds 3 and 6 have been isolated from a related Vitex species Vitex trifolia, while the others were isolated from the genus Vitex for the first time.
Figure 1.
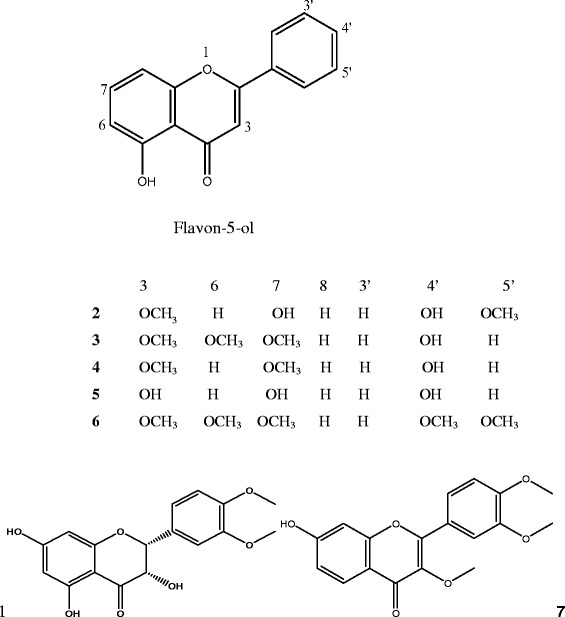
Chemical structures of the seven flavonoid derivatives isolated from Vitex simplicifolia (1–7).
All isolated compounds were subjected to in vitro assays assessing the trypanocidal and cytotoxic activities. The results are shown in Table 1.
Table 1.
In vitro trypanocidal activity and cytotoxicity of flavonoids from Vitex simplicifolia against T.b rhodesiense and L6 cells respectively
| Compound | T.b.rhodesiense | L6 Cells | SI |
|---|---|---|---|
| IC 50 μg/ml | IC 50 μg/ml | ||
| 1 | 10.2 | 100 | 9.8 |
| 2 | 12.3 | 6.64 | 0.5 |
| 3 | 13.8 | 14.0 | 1.0 |
| 4 | 19.4 | 28.2 | 1.4 |
| 5 | 23.7 | 100 | 4.2 |
| 6 | 4.7 | 46.2 | 9.8 |
| 7 | 10.8 | 1.58 | 0.2 |
| Melarsoprol | 0.002 | ||
| Podophyllotoxin | 0.005 |
SI: selectivity index (IC50 of L6 cells/ IC50 of T.b.rhodesiense).
Four of the isolated compounds 2, 3, 6 and 7 exhibited moderate trypanocidal activities with IC50 values ranging from 4.7-13.8 μg/ml, however, selectivity versus mammalian L6 cells was completely missing for 2, 3, and 7. Compound 6 showed the most promising and selective trypanocidal activity (IC50 = 4.7 μg/ml) with a selectivity index of 9.8. A close look at the structures of the isolated compounds reveals some structural features that can be correlated with the activities. The trypanocidal activity appears to increase with increase in the methylation of the hydroxyl groups. A plausible explanation for this observation is the corresponding increase in lipophilicity, which increases the permeability of the compounds across the parasite’s membranes. This is in line with other investigations which stated that substitution at C-4” especially with methoxyl group seems relevant for antiplasmodial activity [20]. Similar studies inferred that methylation at C-4” of the flavonols potentiates the trypanocidal activities [18]. Another report stated that association of methoxyl group at C-7 with hydroxyl group at C-4” is responsible for the trypanocidal activities of some flavonoids [27]. It might as well imply that hydroxyl at C-7 associates with the methoxyl group at C-4”. In this study, it can be inferred that the presence of methoxyl group at C-3, which is common to all the active compounds in this case, could be attributed to their activities. Furthermore, the absence of an OH group on the ring B which is the case with the most active compound could be another reason for its activity. According to a similar finding, the effect of the methoxyl groups on the flavonoid rings in relation to our findings needs further classifications [19].
Conclusions
In conclusion, the flavonoids isolated from V. simplicifolia showed low to moderate trypanocidal activity in vitro with compound 6 as the most active of the isolated molecules with a promising trypanocidal activity and some selectivity. A similar study shows compounds isolated from plants with IC50s from 1.6 to 19.4 μM and selectivity indices between 0.5 and 6.5 [14]. To the best of our knowledge this is the first report on trypanocidal activity of this class of compounds from this plant genus. Future optimization of these compounds through structural alteration may lead to molecules with improved trypanocidal activity and selectivity.
Acknowledgements
The Authors wish to acknowledge the grant from the Federal Ministry of Education and Research (BMBF), Germany, awarded to PP and AD which helped in actualizing this work.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NJN isolated, identified and prepared the manuscript; FBC contributed in the interpretation of the spectra and also in the proofreading of the manuscript. DL elucidated the structures of the compounds. AD is part of the grant that supported this work. PP made available the laboratory, including equipments, consumables and supervised isolation of these compounds. RB was responsible for the bioassays and contributed in the writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Ngozi Nwodo, Email: nwodong@hotmail.com.
Festus Okoye, Email: basdenc@yahoo.com.
Daowan Lai, Email: laidaowan123@gmail.com.
Abdesammed Debbab, Email: Abdessamad.debbab@uni-duesseldorf.de.
Marcel Kaiser, Email: marcel.kaiser@unibas.ch.
Reto Brun, Email: reto.brun@unibas.ch.
Peter Proksch, Email: proksch@uni-duesseldorf.de.
References
- 1.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010;375(9709):148–59. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- 2.Burri C, Chappuis F, Brun R. Human African trypanosomiasis. In: Farrar J, Hotez PJ, Junghans T, Kang G, Lalloo D, White N, editors. Manson’s tropical diseases. 23. Edinburgh: Saunders/Elsevier; 2014. pp. 606–21. [Google Scholar]
- 3.Brun R, Don R, Jacobs RT, Wang MZ, Barrett MP. Development of novel drugs for human African trypanosomiasis. Future Microbiol. 2011;6:677–91. doi: 10.2217/fmb.11.44. [DOI] [PubMed] [Google Scholar]
- 4.Drugs for Neglected Diseases initiative. DNDi R&D Projects - 2013 Outlook. http://www.dndi.org/diseases-projects/portfolio.html (accessed 22 May 2014).
- 5.Jones AJ, Grkovic T, Sykes ML, Avery VM. Trypanocidal activity of marine natural products. Marine Drugs. 2013;11(10):4058–82. doi: 10.3390/md11104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asuzu IU, Chineme CN. Effects of Morinda lucida leaf extract on Trypanosoma brucei brucei infection in mice. J Ethnopharm. 1990;30:307–13. doi: 10.1016/0378-8741(90)90109-7. [DOI] [PubMed] [Google Scholar]
- 7.Nwodo NJ, Brun R, Osadebe PO. In vitro and in vivo evaluation of antitrypanosomal activity of fractions of Holarrhena africana. J Ethnopharmacol. 2007;113:556–9. doi: 10.1016/j.jep.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Ngure RM, Ongeri B, Karori SM, Wachira W, Maathai RG, Kabugu JK, et al. Anti-trypanosomal effects of Azadirachta indica (neem) extract on Trypanosoma brucei rhodesiense infected mice. Eastern J Med. 2009;14:2–9. [Google Scholar]
- 9.Nwodo NJ, Agbo MO. Antitrypanosomal effects of methanolic extracts of Nauclea diderrichii (Merr) and Spathodea campanulata stem bark. J Pharm Allied Sci. 2010;7(5):1219–27. [Google Scholar]
- 10.Munir AA. A taxonomic revision of the genus Vitex L (Verbenaceae) in Australia. J Alelaide Botanic Garden. 1987;10(1):31–79. [Google Scholar]
- 11.Keay RWJ: The trees of Nigeria. 1964, 20:340–345.
- 12.Liang F, Zhou X, Cao L. Investigation report on Vitex trifolia L.var. Simplicifolia cham. Medicinal plant resources in china. Medicinal Plant. 2012;3(1):16–9. [Google Scholar]
- 13.Zhoug S, QIU GD, LIU YB, et al. Pharmacological activity comparism of Vitex trifolia L var simplicifolia cham, Fructus viticis, Vitex negundo chastetree fruit. Pharmacol Clin Chinese Materia Medica. 1996;12(1):37. [Google Scholar]
- 14.Julianti T, Yoshie Y, Zimmermann S, Kaiser M, Hamburger M, Adams M. Antitrypanosomal sesquiterpene lactones from Saussurea costus. Fitoterapia. 2011;82:955–9. doi: 10.1016/j.fitote.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann F, Sporer F, Tahrani A, Wink M. Antitrypanosomal properties of Panax ginseng C. A. Meyer: New possibilities for a remarkable traditional drug. Phytother Res. 2013;27:86–98. doi: 10.1002/ptr.4692. [DOI] [PubMed] [Google Scholar]
- 16.Tim Cushnie TP, Lamb AJ. Review antimicrobial activity of flavonoids. Int J Antimicrobial Agents. 2005;26(5):343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joe ES, Eunjung L, Sang GS, Jihoon L, Jong EK, Jiyoung K, et al. Eupatilin, a major flavonoid of artemisia, attenuates aortic smooth muscle cell proliferation and migration by inhibiting PI3K, MKK3/6, and MKK4 activities. Planta Med. 2013;79:1009–16. doi: 10.1055/s-0033-1350621. [DOI] [PubMed] [Google Scholar]
- 18.Taleb-Contini SH, Salvador MJ, Balanco JMF, Albuquerque S, de Oliveira DCR. Antiprotozoal effect of crude extracts and flavonoids isolated from Chromolaena hirsuta (asteraceae) Phytotherapy Res. 2004;18(3):250–4. doi: 10.1002/ptr.1431. [DOI] [PubMed] [Google Scholar]
- 19.Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, et al. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, In vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother. 2006;50(4):1352–64. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weniger B, Vonthron-Se’ne´cheau C, Kaiser M, Brun R, Anton R. Comparative antiplasmodial, leishmanicidal and antitrypanosomal activities of several biflavonoids. Phytomedicine. 2006;13:176–80. doi: 10.1016/j.phymed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Jorda˜o CO, Vichnewski W, de Souza GE P, Albuquerque S, Lopes JLC. Trypanocidal activity of chemical constituents of Lychnophora salicifolia Mart. Phytother Res. 2004;18:332–4. doi: 10.1002/ptr.1366. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka T, Inokuchi T, Fujioka S, Kimura Y. Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. J Pharm Pharmcol. 2007;59:1307–12. doi: 10.1211/jpp.59.9.0016. [DOI] [PubMed] [Google Scholar]
- 23.Keun MY, Kun HS, Hyeun WC, Sam SK, Hyun PK. Vitexicarpin, a flavonoid from the fruits of Vitex rotundifolia, inhibits mouse lymphocyte proliferation and growth of cell lines In vitro. Planta Med. 1998;64:546–50. doi: 10.1055/s-2006-957511. [DOI] [PubMed] [Google Scholar]
- 24.Baltz T, Baltz D, Giroud C, Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985;4:1273–7. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Räz B, Iten M, Grether-Bühler Y, Kaminsky R, Brun R. The Alamar blue® assay to determine drug sensitivity of African trypanosomes (T. b. Rhodesiense and T.b. Gambiense) in vitro. Acta Trop. 1997;68:139–47. doi: 10.1016/S0001-706X(97)00079-X. [DOI] [PubMed] [Google Scholar]
- 26.Nwodo NJ, Agbo MO, Brun R. In vitro and In vivo antitrypanosomal studies of the leaf extract of Vitex simplicifolia. Afri J Pharma Res Dev. 2012;4(1):35–40. [Google Scholar]
- 27.Grecco SS, Reimão JQ, Tempone AG, Sartorelli P, Rodrigo LOR, Cunha ROR, et al. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae) Exp Parasitol. 2012;130:141–5. doi: 10.1016/j.exppara.2011.11.002. [DOI] [PubMed] [Google Scholar]


