Abstract
Cognitive decline is common in Parkinson's disease (PD), even in the early motor stage, and this non-motor feature impacts quality of life and prognosis tremendously. In this article, we discuss marker candidates for cognitive decline in PD from different angles, including functional and structural imaging techniques, biological fluid markers in cerebrospinal fluid, and blood genetic predictors, as well as gait as a surrogate marker of cognitive decline. Specifically, imaging-based markers of cognitive impairment in PD include cortical atrophy, reduced cortical metabolism, loss of cortical cholinergic and frontal dopaminergic function, as well as an increased cortical amyloid load. Reduced β-amyloid(1-42) in cerebrospinal fluid and lower plasma levels of epidermal growth factor are predictors for cognitive decline in PD. In addition, genetic variation in the apolipoprotein E (APOE), catechol-O-methyltransferase (COMT), microtubule-associated protein tau (MAPT), and glucocerebrosidase (GBA) genes may confer risk for cognitive impairment in PD; and gait disturbance may also indicate an increased risk for dementia. Other marker candidates have been proposed and are discussed. All of the current studies are hampered by gaps in our knowledge about the molecular causes of cognitive decline, which will have to be considered in future biomarker studies.
Keywords: Parkinson's disease, dementia, imaging, cerebrospinal fluid, blood, genetics, biomarker, gait
The point prevalence of dementia in patients with Parkinson's disease (PD) is 25%, and it rises to 80% in patients who live for ≥ 20 years with the disorder.1,2 Cognitive decline in PD worsens the patient's prognosis more than any other non-motor symptom. A prognostic marker delineating the probability for those patients at risk for cognitive decline would be of utmost importance. How can biomarkers help us to identify patients at risk for cognitive decline in PD? In this review, we summarize the current knowledge on imaging, cerebrospinal fluid (CSF), and blood proteins as well as genetic factors and the impairment of gait/postural instability as risk markers for cognitive decline in PD.
Imaging Dementia in Parkinson's Disease
Dementia in PD (PDD) is characterized both by a dysexecutive syndrome, which probably reflects striato-frontal dysfunction due to loss of dopaminergic projections, and also by the impairment of visuospatial capacities, attentional control, and short-term memory, probably due to loss of cholinergic function and local cortical Lewy body disease targeting the cingulate and posterior association areas.3 Microglial activation and incidental Alzheimer's disease (AD) or vascular pathology can also have an influence. Neuroimaging can help clarify the role of these factors in the development of PDD. Currently, we do not have an imaging marker for α-synuclein (αSyn) aggregation, which is the characteristic pathology of PD, although possible candidates include radiolabeled diphenylpyrazoles. Therefore, imaging has been used to detect the structural, metabolic, and pharmacological consequences of this pathology.
Structural Magnetic Resonance Imaging Studies
Voxel-based morphometry (VBM), a magnetic resonance imaging (MRI) technique that localizes signifi-cant changes in regional tissue volume or density between groups of individuals at a voxel level, detects significant posterior cortical atrophy in patients who have PD with mild cognitive decline (MCI).4 As the patients progress to develop frank dementia (PDD), the volume loss becomes more severe, targeting the hippocampus, the medial temporal lobe and parahippocampus, the cingulate gyrus, and frontal association areas.5 This gray matter loss correlates with motor disability and impairment of cognitive functions. VBM can also detect cortical volume changes in patients who have PD without dementia, and it has been reported that loss of frontal volume is correlated with poor performances on the Iowa Gambling Task and the facial expression recognition task.6
Diffusion tensor imaging measures the directionality (anisotropy) and magnitude (diffusivity) of water molecule flow in tissues. Damage to neuronal tracts results in reduced fractional anisotropy and increased mean diffusivity. Non-demented PD patients may show diffusion abnormalities in the posterior cingulate and hippocampus, even in the absence of volume loss.7 Levels of raised diffusivity correlate with cognitive performance on tests of memory, suggesting that diffusion tensor imaging could be used to predict the risk of later dementia in PD in the absence of overt atrophic changes.
Functional Imaging of Glucose Metabolism
18F-deoxyglucose (FDG)-positron emission tomography (PET) images show a consistent pattern of reduced regional cerebral glucose metabolism (rCMRGlc) in AD that begins in the posterior cingu-late, parietal, and temporal association regions and later spreads to the prefrontal cortex. A similar pattern is observed in patients with PD who develop later dementia, although the occipital cortex shows more severe hypometabolism, possibly explaining their greater tendency to develop hallucinosis.8 Yong and colleagues9 compared patterns of glucose metabolic reductions in patients with PD, in patients with PDD who had late dementia, and in patients who fulfilled consensus criteria for dementia with Lewy bodies (DLB). Compared with normal controls, patients with PDD and DLB had very similar patterns of hypometabolism targeting the parietal lobe, occipital lobe, temporal lobe, frontal lobe, and anterior cingulate. These two syndromes were only discriminated metabolically by a greater involvement of the cingulate in DLB. Compared with patients who had AD, there was a greater reduction in glucose metabolism in the anterior cingulate and occipital of patients who had DLB. Mild cognitive decline in PD is associated with a lesser degree of hypometabolism of the posterior parietal temporal cortex and cingulate. Non-demented PD patients have an abnormal profile of glucose metabolism, with relatively increased FDG-PET lentiform metabolism and with relatively reduced temporal, parietal, and frontal metabolism.10 This profile has been termed the PD-related profile, and its expression correlates with disease severity. Absolute values of cortical metabolism still may remain in the normal range in non-demented PD; however, in one series, one-third of PD patients individually exhibited either right or left posterior parietal rCMRGlc values that fell more than two standard deviations below the normal mean.11 The underlying cause of the temporoparietal dysfunction in non-demented PD patients could reflect either the presence of occult primary cortical Lewy bodies or Alzheimer pathology, or it may be secondary to a loss of cholinergic or monoaminergic input. Early metabolic reductions in visual association areas and the posterior cingulate cortex in PD appear to be markers of progression to later dementia. Of 23 patients with PD who underwent FDG-PET studies at baseline and again after 4 years of follow-up12 six patients (26%) developed dementia, and all of those PDD converters had significant occipital and posterior cingulate hypometabolism at baseline compared with controls.
Functional MRI can be used to assess functional connectivity changes in the brain. Blood oxygenation level-dependent (BOLD) MRI sequences are used to detect brain regions that, although anatomically separated, exhibit slow, synchronized oscillations of blood oxygenation in the resting state. Such resting state networks include the default mode network, which probably mediates attention. Compared with patients who have PD, those who have PDD exhibit significant alterations in resting connectivity between the frontal and parietal cortices.13
Functional Imaging of Dopamine Transporter
Iodine-123 (123I)-N-omega-fluoropropyl-2beta-car bomethoxy-3beta-(4-iodophenyl)nortropane (123I-FP-CIT) single photon emission computed tomography (SPECT) (DaTSCAN; GE Healthcare, Port Washington, NY), an in vivo marker of dopamine transporter binding, has been used to discriminate DLB from AD based on the detection of reduced putamen dopamine terminal function in the former. SPECT provided a sensitivity of 100% and a specificity of 100% for discriminating DLB from AD, whereas initial clinical impression provided a specificity of only 42% taking eventual autopsy findings as the standard of truth.14 Reduced caudate dopamine transporter uptake in the less affected hemisphere appears to provide a powerful approach for predicting cognitive decline in patients with de novo PD.15
Although striatal dopamine terminal dysfunction is a hallmark of DLB and PDD, loss of mesolimbic and mesocortical dopaminergic function is likely to be more relevant to PDD. This has been investigated with [18F]-L-dihydroxyphenylalanine (18F-dopa) PET, a marker of aromatic amino acid decarboxylase activity and dopa-mine storage capacity by the axonal terminal plexus. Using 18F-dopa PET and statistical parametric mapping, Ito and colleagues assessed changes in dopaminergic function in PD patients with and without later dementia who were matched for age, disease duration, and disease severity.16 Compared with the PD patients, the PDD patients had a 20% reduction in 18F-dopa uptake bilaterally in the ventral striata and the anterior cingulate. Klein and colleagues also observed extensive frontal dopamine loss in PDD and DLB compared with PD.17 These findings confirm that dementia in PD is associated in part with impaired mesolimbic, mesocortical, and ventral striatal dopaminergic function.18
Functional Imaging of the Cholinerigic System
The SPECT tracer 123I-iodobenzovesamicol (123IBVM), a marker of acetylcholine vesicle transport, detects cholinergic deficiency. Patients who have PD without dementia exhibit selectively reduced binding of 123I-BVM in parietal and occipital areas, whereas patients who have PDD and AD exhibit globally reduced cortical binding.18 Levels of acetyl cholinesterase (AChE) activity can be studied in PD with and without dementia using the selective PET substrates N-[11C]methylpiperidin-4-yl acetate (11C-MP4A) and 1-[11C]methylpiperidin-4-yl propionate (11C-PMP). Cortical 11C-MP4A uptake was reduced by 11% in PD but by 30% in PDD.19 No significant difference was noted in the level or extent of 11C-MP4A reductions in a comparison between PDD and DLB.17 Using 11C-PMP PET, a significant correlation was noted between levels of cortical AChE activity in a combined group of PD and PDD patients and their performances on a battery of attentional and executive tests.20 Interestingly, levels of cortical AChE deficiency did not appear to correlate with the degree of limb disability related to scores on the motor part of the Unified Parkinson's Disease Rating Scale (UPDRS) These imaging findings strongly validated the use of AChE inhibitors in PDD.
Visualization of Specific Pathologies
Radioligands are now available and some are licensed to image β-amyloid (Aβ) deposition in dementias. Aβ plaques and neurofibrillary tangles are the pathologic hallmarks of AD, and postmortem studies suggest that incidental Aβ deposition may occur a decade before the clinical symptoms of dementia. Because the prevalence of AD pathology is increased in PD, these PET ligands afford an opportunity to investigate the role of amyloid in PDD and DLB. 11C-PIB is a neutral thioflavin that, in AD brain slices, shows nanomolar affinity for neuritic Aβ plaques but low affinity toward amorphous, diffuse Aβ deposits; intracellular neurofibrillary tangles; and Lewy bodies.21
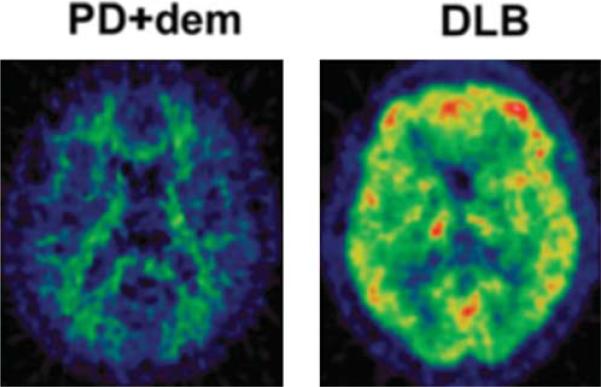
A majority of DLB patients show increased cortical 11C-PIB binding, which approaches the levels seen in AD.22,23 This suggests that, in fact, DLB is a dementia associated with both Lewy body pathology and amyloid pathology, explaining its aggressive nature. Patients with PDD, in contrast, have a reduced prevalence of amyloid plaques and lower levels of cortical 11C-PIB binding than patients with DLB (Fig. 1).22,24 This finding suggests that the dementia of PDD is more likely to be related to specific αSyn pathology rather than only dual pathology, in agreement with postmortem observations.25,26 However, others have suggested that the neuropathological correlate of PDD is a combination of different pathologies rather than the severity of any single pathology.27 Finally, it has been proposed that, in PDD and DLB, the presence of amyloid acts as a trigger for dementia but does not directly determine its nature.28 Incidental amyloid can be detected on occasion in older patients with PD,23 and it has been reported that non-demented PD patients who have raised cortical amyloid levels have a faster cognitive deterioration than patients who are amyloid-free at baseline.29
FIG. 1.
(11)C-labeled Pittsburgh Compound B (11C-PIB) positron emission tomography images are normal (left) in a patient who has Parkinson's disease with dementia (PD1dem) but show raised amyloid levels (right) in a patient who has dementia with Lewy bodies (DLB).
Similar to the manner in which 11C-PIB binding provides a marker for Aβ deposits, scientists hope to be able to visualize the distribution of αSyn in the brain with specific tracers, which currently are being developed. This endeavor is challenged by the lower abundance of αSyn in the brain and the need for ligands to pass a cell membrane to detect the intracellular aggregates. Possible candidates in trial are diphenylpyrazoles, which disaggregate αSyn in vitro and cross the blood-brain barrier. αSyn and tau protein radioligands like 18F-T807 will be of major importance in the future.30
Functional Imaging of Activated Microglial Activation
The activation of microglia is a nonspecific inflammatory response to brain injury. These activated cells are able to promote brain function by releasing growth factors, walling off necrotic tissue, and stripping and remodeling synapses.31 However, there is cumulative evidence suggesting that, in chronic neuro-degenerative diseases, activated microglia also may cause neuronal death and promote further neurode-generation through the release of a variety of cytokines and other neurotoxic factors. The isoquinoline [11C](R)-(1-[2-chlorophenyl]-N-methyl-N-[1-methylpropyl]-3-isoquinoine carboxamide (11C-[R]-PK11195 PET) is a selective, in vivo marker of activated microglia. The 18-kDa translocator protein (TSPO) is expressed on the mitochondrial membranes of activated (but not of resting) microglia and has a binding site for 11C-(R)-PK11195. Two studies have reported significant increases in 11C-(R)-PK11195 binding in both striatal and extrastriatal regions (mainly in the frontal and temporal cortices) in PD patients compared with normal controls.32,33 Since the publication of those studies, levels of striatal microglial activation have been correlated with disability rated according to the motor part of the UPDRS, whereas frontal microglial activation has been associated with impaired verbal fluency.34 Edison and colleagues35 have reported that PDD patients have more extensive microglia activation than nondemented PD patients, with significantly increased 11C-(R)-PK11195 binding observed in the anterior and posterior cingulate; striatum; and frontal, temporal, parietal, and occipital cortical regions compared with normal controls. In addition, in patients with PDD, cortical microglial activation is inversely correlated with Mini-Mental State Examination scores, suggesting that neuroinflammation may contribute to cognitive impairment in these patients.
Biological Fluids: CSF Biomarkers for Cognitive Impairment in PD
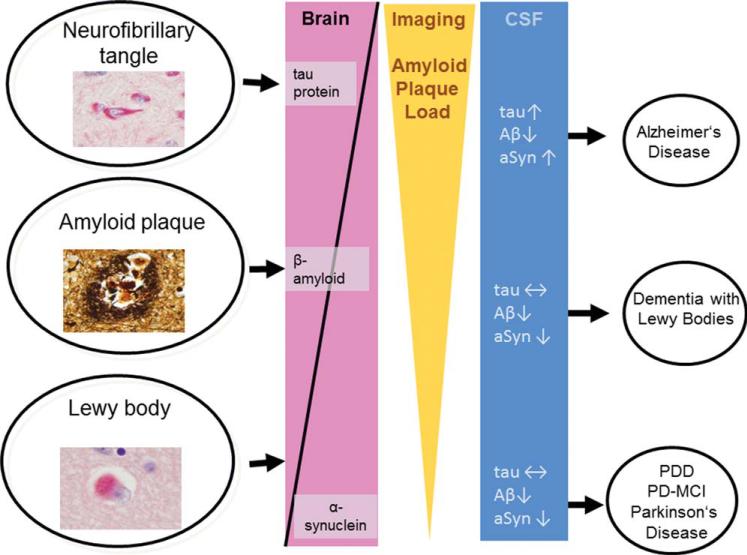
Eighty percent of CSF proteins are physiologically derived from the peripheral blood across the blood-CSF barrier. The remaining 20% of these proteins are synthesized within and released by resident cells of the CNS by active secretion, normal attrition, cell injury, or neural death.36 Because brain cell-derived proteins play an important role in the composition of CSF, it has been demonstrated that cellular and biochemical alterations of the brain metabolism will exhibit distinct alterations in the CSF proteome (Fig. 2).
FIG. 2.
Schematic summary (modified from Weinrich et al.136) of the most frequent and known pathomorphologic characteristics of Alzheimer's disease, dementia with Lewy bodies, and Parkinson's disease with dementia (PDD) shown with the respective patterns in cerebrospinal fluid (CSF) and with β-amyloid (Aβ) nuclear imaging. Shown are neurofibrillary tangle (top left) (immunohistochemistry reaction with the antibody AT-8 against hyper-phosphorylated tau protein), amyloid plaque (middle left) (Bielchowsky staining), and Lewy body (bottom left) (immunohistochemistry reaction with antibody LB-509 against α-synuclein [αSyn]). PD-MCI indicates Parkinson's disease with mild cognitive impairment.
Aβ peptides
Aβ peptides are important for neuronal information processing and are major constituents of amyloid plaques deposited in the brains of patients with, eg, AD and DLB.37,38 Decreased levels of the Aβ fragment 1-42 (Aβ1-42) in CSF are inversely correlated with in vivo amyloid imaging load, as in AD.39 However, in other neurodegenerative diseases, decreased Aβ1-42 levels also have been described as independent from the amyloid plaque load and, thus, are considered rather nonspecific.40 Amyloid plaques occur often in DLB, in which decreased Aβ1-42 levels in CSF also are observed; these levels reportedly were normal or slightly decreased in PD patients compared with controls.41 The CSF Aβ1-42 levels in PD were correlated with pattern recognition memory and scores on the Montreal Cognitive Assessment in the Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation-Parkinson's Disease (ICICLE-PD) study42 and tended to be lower with longer disease duration and cognitive decline.43,44 One study demonstrated a more pronounced decrease of CSF Aβ1-42 levels in patients with PD who had a more rapid cognitive decline using follow-up neuropsychological testing.45 Studies on longitudinal neuropsychological testing and CSF sampling are under way.
Other fragments, like Aβ1-40, Aβ1-38,46 and a post-translationally modified, oxidized form of Aβ1-40 that contains a-helical structures,47 have exhibited interesting patterns in PD, PDD, and DLB. This may point toward a specific pathophysiological metabolism, but it needs further clarification and study by an independent group.
Tau protein
The major constituent of neurofibrillary tangles is the natively unfolded phosphoprotein tau. In human diseases with filamentous deposits (eg, AD), tau protein becomes hyperphosphorylated, which is an early event that precedes filament assembly.48,49 Alternative splicing of exons of the tau gene (microtubule-associated protein tau [MAPT]) results in different isoforms. The imbalance of the tau isoforms with three (3R-tau) or four (4R-tau) microtubule-binding repeat domains is an important factor for the pathogenesis of neurodegeneration.50 Elevated CSF tau protein levels are increased in patients with AD20,21 but also can be observed in diseases with (rapid) neuronal cell loss independent from tau-related pathology.20,21 CSF tau protein, therefore, is not specific for tau-related disorders but, rather, is a marker for neuronal cell loss in general.51,52
Total tau protein levels can help to discriminate PD and PDD from AD53; however, compared with controls, the differences in CSF tau protein concentrations in PD and PDD are marginal.43 In DLB, CSF tau protein is lower compared with AD and higher compared with PD and PDD.54
Because hyperphosphorylation of tau protein promotes its aggregation into neurofibrillary tangles, the quantification of phosphorylated tau protein in CSF may serve as a specific marker for AD.55 In DLB, the neuropathological overlap with AD involves the phosphorylation of tau protein, but to a lesser extent.56,57 Some CSF studies have reported better specificity for the discrimination of PD/PDD from AD by using CSF tau protein phosphorylated at threonine 181 rather than total tau protein.58 A novel, sensitive assay for the quantification of 3R-tau and 4R-tau in CSF revealed a disease-specific pattern in PD and in other neurodegenerative diseases with dementia.59
α-Synuclein
Full-length αSyn, the major constituent of Lewy bodies, has been detected in biological fluids, including plasma, conditioned cell media, and CSF.60,61 There are discrepant findings by various investigators, who used several different platforms and standard operating procedures, as well as a tremendous overlap of values, but the majority of investigators demonstrated a reduction of CSF total αSyn in the synuclein-related disorders PD, DLB, and multiple system atrophy.62-64 A large multicenter study recently confirmed the decrease in CSF αSyn among patients who had early, drug-naive PD compared with healthy controls.65 Interestingly, patients who had PD with a tremor-dominant (TD) form had higher levels; whereas those who had a more severe, akinetic, rigid form (postural instability and gait disorder [PIGD]) had lower levels. Also, a subgroup of PD patients exhibited a CSF protein pattern similar to that observed in AD. Further follow-up investigations, including longitudinal sampling and cognitive testing, will provide insight into the robustness of this finding and help determine whether these CSF markers also may serve as markers of motor and/or cognitive progression.
In AD, CSF αSyn studies have demonstrated elevated levels,66 which may be the result of synergistic interactions between αSyn and Aα/tau as well as synaptic loss.67 Studies on post-translational modified forms of αSyn (eg, oligomers and phosphorylated αSyn) have been reported68,69 but need independent validation and analyses that include cognitive profiling.
Biological Fluids: Blood-Based Biomarkers for Cognitive Impairment in PD
Although CSF as a source of informative markers in PD is conceptually attractive because of the proximity to the brain, CSF proteins face significant barriers to widespread translation into clinical use as biomarkers. Specifically, CSF sampling requires relatively high invasiveness; CSF is prone to contamination by blood during sampling; and protein concentrations are low in CSF, leading to variability that is inherent in the measurement of low-abundance analytes. These considerations have led to efforts to identify markers in the blood, which is a much more easily assayed biofluid. Below, we discuss genetic risk factors and blood-based protein biomarkers for cognitive impairment in PD.
Epidermal growth factor
The level of plasma epidermal growth factor (EGF) was identified as a potential biomarker for cognitive impairment in PD after emerging from a screen of 102 plasma proteins.70 In the initial report, lower plasma EGF was correlated with poorer cognitive performance in a cross-sectional cohort of patients with PD, and it also predicted a higher rate of conversion to dementia in a subset of patients with PD that was followed longitudinally.70 An independent group of investigators subsequently reported the association of low EGF levels, this time measured in serum, with both poorer cognitive performance and future cognitive decline in early PD.71
Other marker candidates in blood, CSF, and other biofluids
Lower serum urate levels have been correlated with an increased risk for PD in multiple cohorts (reviewed by Cipriani et al.72), and at least one group also reported a potential correlation with an increased risk for domain-specific cognitive impairment in PD.73 In addition, higher plasma homocysteine levels reportedly were associated with depression and with poorer cognitive performance in PD.74 Other marker candidates, eg, serum heart-type fatty acid binding protein (HFABP), have been reported as potential diagnostic markers for Lewy body dementia in small cohorts.75 At this time, these blood-based markers, as well as other CSF marker candidates, need further validation in other cohorts. In addition, efforts to develop assays that are more robust and more sensitive, possibly based on other technical methods (eg, antibody-independent methods), are under way.
Increasing numbers of studies also have explored peripheral and more accessible body fluids as potential biomarker sources. In fact, after αSyn pathology was identified in submandibular salivary glands,76 a recent study identified αSyn in saliva as a possible biomarker.77
Genetic Risk Markers of Cognitive Impairment in PD
Apolipoprotein E
Genotype of the apolipoprotein E (APOE) gene is the most extensively studied potential genetic risk factor for cognitive impairment in PD. APOE has three common alleles, designated ∈2, ∈3, and ∈4. In non-PD populations, it is widely known that APOE genotype confers risk for cognitive impairment. Particularly in AD, the association of the e4 allele with the risk for dementia has been established for two decades (reviewed by the American College of Medical Genetics/American Society of Human Genetics Working Group on ApoE and Alzheimer Disease78).
In PD, several cross-sectional analyses have evaluated the association of the APOE genotype with cognitive impairment and dementia. Whereas several large cohorts demonstrated a significant enrichment of ∈4 allele carriers in PD patients with dementia (PDD) relative to PD patients without dementia,79,80 other studies either failed to find such an ∈4 allele effect81-83 or found an association of the ∈2 allele with PDD instead.84 A meta-analysis of these and other studies supported the enrichment of APOE ∈4 allele carriers in PDD versus PD without dementia,85 although the effect was modest (odds ratio, 1.74) compared with what was observed in AD. In addition, reconciliation of these conflicting data may lie in the findings of a longitudinal study, suggesting that PD ∈4 allele carriers have a faster rate of cognitive decline.86 Whether the modest effect of the APOE ∈4 allele in conferring an increased risk for dementia among PD patients is mediated through APOE effects on AD pathogenic pathways or through Lewy body disease-specific pathways remains to be determined.
Catechol O-methyltransferase gene
The catechol O-methyltransferase (COMT) gene encodes one of the main enzymes involved in the degradation of catecholamines, including dopamine. Within the coding region of the COMT gene, a common single nucleotide polymorphism (SNP) results in a methionine-to-valine change at amino acid position 158 (met158val). The valine (val) variant exhibits an overall increase in enzyme activity compared with the methionine (met) variant, which, in turn, may be responsible for alterations in cognitive performance. Whereas carriers of the COMT met allele reportedly demonstrated impaired performance in frontal executive function tasks,87 possibly dependent on an interaction with dopaminergic medication,88 other groups have not been able to replicate this association.86
MAPT and tau haplogroups
The MAPT gene encodes the microtubule-associated protein tau, a 16-exon gene on chromosome 17 that is found in two major haplogroups, termed H1 and H2. The H2 haplogroup represents an inversion of a 900-kb region containing MAPT as well as neighboring genes, is rarely found outside of individuals of European ancestry, and is believed to have arisen from a single founder. The H1 haplogroup is associated with an increased risk for a number of neurodegenerative disorders (reviewed by Pittman et al.89). An association of the MAPT H1 haplogroup with cognitive impairment and dementia in PD also has been reported,90 and other groups have subsequently replicated this association.91
Glucocerebrosidase
It has been demonstrated that heterozygous mutations in the glucocerebrosidase (GBA) gene are a major risk factor for PD.92 Homozygous mutations in glucocerebrosidase, an enzyme involved in the glycolipid metabolism, have long been known to cause the lysosomal storage disorder Gaucher's disease. Intriguingly, PD patients with GBA mutations may be more likely to develop cognitive impairment and dementia than PD patients without GBA mutations,92-94 although this link remains to be definitively established.
Numerous studies have evaluated the contribution of variants or mutations in APOE, COMT, MAPT, and GBA to the risk for cognitive impairment in PD. Although there are conflicting reports for all of these genes, the preponderance of the data suggests that a modestly increased risk for cognitive impairment is conferred by the APOE ∈4 allele, the COMT met variant, the MAPT H1 haplogroup, and mutations in GBA.
Gait as a Surrogate Marker for Cognitive Impairment
Gait is considered an important indicator of overall health, and poor gait performance is associated with greater mortality, morbidity, and fall risk.95 For the past decade, the notion that safe and effective gait is due solely to an intact motor system has given way to a more complex model that reflects the cognitive control of gait.96 Safe and independent negotiation of complex environments encountered in real-world settings requires the integration of external sensory information with neural networks involving cortical, subcortical, brainstem, and spinal cord structures.97,98 Some of these share common substrates with cognition.99 The link between cognition and gait has focused attention on gait measurement and what it can reveal about future cognitive states, particularly its role in identifying and predicting cognitive risk factors.
Association Between Gait and Cognition in Ageing
In cognitively normal, older adults, there is a robust association of gait speed and gait variability (for a review of gait characteristics, see Lord et al.100) with executive function, attention, and (to a lesser extent) memory and visuospatial function.101-104 The association becomes more emphatic under dual-task conditions, which sensitize the relationship between gait and cognition, particularly in MCI and dementia (for review, see Amboni et al.99). Although cross-sectional studies highlight the relationship between gait and cognition, longitudinal studies support the role of gait as a surrogate marker of cognitive decline. In community-dwelling, older adults, there is evidence that gait impairment both precedes105 and predicts cognitive decline,101,106 MCI, and different subtypes of dementia.106 Importantly, it seems that gait may be more sensitive than cognitive tests for detecting cognitive decline.101
Association Between Gait and Cognition in PD
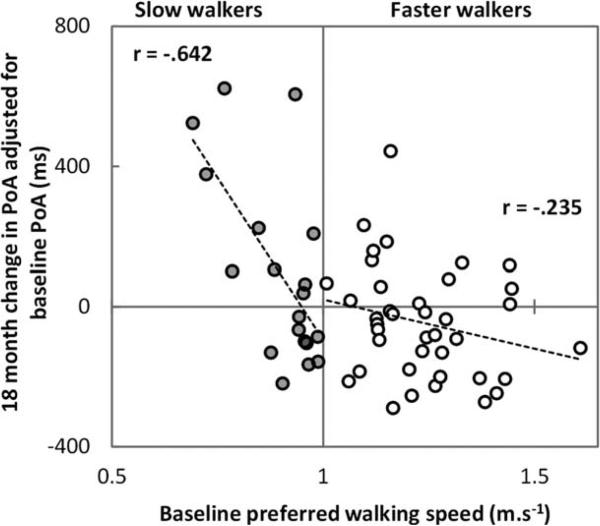
The evidence for gait as a surrogate marker for cognitive decline in movement disorders is limited to PD. Cognitive impairment presents early in PD,107 and progression to dementia in PD is complex and heterogeneous, with impairments in attention, executive and visuospatial domains, and memory.108-110 Early work demonstrated that a motor phenotype associated with disturbance of gait and posture (PIGD; categorized using the UPDRS) was a risk factor for cognitive impairment and subsequent dementia.111-113 Cognitive domains (executive and visuospatial function) were selectively associated with the PIGD phenotype,114 and this was evident even in early PD.115 Quantitative gait analysis is potentially a more sensitive tool than motor phenotype to predict cognitive risk. In PD, independent associations of executive function and attention with gait speed and variability116-119 are found; these associations strengthen under dual-task conditions in more advanced disease99 and support the potential for gait analysis as a cognitive marker. The relationship between different gait characteristics and MCI in PD has received less attention. However, a recent study found that gait was more significantly impaired in PD with MCI, and gait instability was specifically related to visuospatial impairment.120 Longitudinal studies are lacking, although one recent report shows promise in this respect, demonstrating that gait speed is a more sensitive predictor of decline in attention than baseline attention in early PD (Fig. 3).100 Relatively few studies have used a broad range of gait or cognitive outcomes. Therefore, the selective relationship and temporal course of gait and cognitive decline and predictive validity is yet to be determined. Studies are urgently needed to explore the predictive validity of gait as a marker of risk of cognitive decline and dementia.
FIG. 3.
Changes in the power of attention (PoA) over 18 months (a positive value means attention has worsened) since diagnosis are illustrated in 58 patients with idiopathic Parkinson's disease (mean age 6 standard deviation, 67.4 ± 10.6 years; 31 males, 21 females; Unified Parkinson's Disease Rating Scale motor part score [mean ± standard deviation], 10.6 ± 9.8; tested at peak dose of levodopa medication [controlling for baseline attention]). A slow walking speed at baseline predicted greater worsening of attention 18 months later, particularly for those who walked slowly (less than 1 ms−1) (from Lord et al., 2013100).
Relationship of Gait With Biomarkers of Cognitive Decline
Gait disturbance in PD shares neurochemical, pathological, structural, and genetic relationships with cognitive risk factors.108 It is believed that gait disturbance associated with the PIGD motor phenotype and dementia is underpinned by a common neuro-chemical deficit in cholinergic function.112 Recent studies indicate that comorbid cortical cholinergic denervation is a more robust indicator of reduced gait speed in PD than nigrostriatal denervation alone121; whereas, in early PD, cholinergic function (estimated using short-latency afferent inhibition) is independently associated with gait.122 Disturbed Aα metabolism, a pathological risk factor for dementia, is associated with the PIGD phenotype in PD with advanced disease and increased dementia risk123 and in early de novo PD.65,124 Brain imaging highlights shared structural correlates of gait and cognitive impairment.97,99,125 Finally, shared genetic determinants of gait and cognition have also been identified in older adults for COMT and APOE,126,127 although this is still to be determined in PD. Combined with evidence in older adults that gait changes may precede cognitive decline, these findings add validity to the role of gait as a surrogate marker of cognitive impairment. Longitudinal follow-up is required to explore the temporal relationship between these risk factors and their sensitivity and specificity.
Summary
Gait measures relate to different features of gait control in the same way that different cognitive tests relate to different aspects of cognitive function. The sensitivity of a range of gait characteristics to different cognitive functions is emerging, suggesting that a comprehensive approach is warranted.100 Adopting a more consistent, theoretical approach that captures a broad range of characteristics reduced to robust, independent gait domains will allow independent functions of gait to be explored with respect to cognitive function, which ultimately will be a more useful approach. This will enhance our understanding of the sensitivity and specificity of gait as a surrogate marker of cognitive impairment and dementia. Quantitative gait analysis is noninvasive and low-cost; moreover, the development of body-worn sensors is allowing measurement of gait to move from the laboratory to the clinic and home, increasing its utility. Further research to refine the role of gait as a surrogate marker for risk of cognitive impairment and dementia is required, and recommendations for future research are identified. Given the complexity of cognitive decline and dementia in movement disorders, gait may have an important place in a battery of marker candidates.
Other Marker Candidates for Cognitive Decline in PD
Other marker candidates in addition to those mentioned above have been proposed for cognitive decline in PD. In particular, oscillatory slowing in magnetoencephalography,128 short-latency afferent inhibition by conditioning motor-evoked potentials,129 lower mean frequency and higher variability in electroencephalogram (EEG),130 as well as low background rhythm frequency in quantitative EEG,131 the presence of rapid eye movement (REM) sleep behavior disorder (RBD) by polysomnogram,132 and pronounced hyposmia identified with the odor stick identification test133 and the self-administered University of Pennsylvania Smell Identification Test (UPSIT)134 either have been associated with or even have predicted cognitive decline in PD.
Conclusion
Optimal biomarkers reflect alterations of the underlying pathophysiology proximal to the disease. Here, we have summarized the current knowledge on imaging, CSF/blood, genetic, and clinical markers for cognitive decline in PD. All studies are hampered by too little knowledge about the underlying pathophysiological and molecular cause of cognitive decline in PD. It is still unclear how AD pathology, Lewy bodies, and other factors (eg, genetic variants, vascular changes) contribute to cognitive decline in PD135 and whether, eg, presynaptic αSyn aggregates and synapse rarefication, rather than Lewy bodies, promote cognitive decline.26 Due to these challenges, a single marker is unlikely to represent all specific and sensitive indicators to identify patients with PD who are at risk for cognitive decline/dementia (marker of trait), to indicate the manifestation of a dementia (marker of state), to signal the speed of the progression of cognitive decline (marker of rate), and to predict its further course (marker of fate). Although the imaging markers are helpful as markers of state and trait, genetic markers and gait evaluation can also be useful as markers of trait; and, in the future, biological fluid markers hopefully can be used as markers of trait, rate, and fate. Future biomarker candidates will rely on increasing knowledge of disease stratification (including disease endophenotyping) as well as an understanding of cognitive decline in PD at the patho-physiological and molecular levels.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. 2005;20:1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 3.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 4.Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord. 2011;26:289–296. doi: 10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- 5.Melzer TR, Watts R, MacAskill MR, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:188–194. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- 6.Ibarretxe-Bilbao N, Junque C, Tolosa E, et al. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson's disease. Eur J Neurosci. 2009;30:1162–1171. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- 7.Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78:1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- 8.Vander-Borght T, Minoshima S, Giordani B, et al. Cerebral metabolic differences in Parkinson's and Alzheimer's disease matched for dementia severity. J Nucl Med. 1997;38:797–802. [PubMed] [Google Scholar]
- 9.Yong SW, Yoon JK, An YS, Lee PH. A comparison of cerebral glucose metabolism in Parkinson's disease, Parkinson's disease dementia and dementia with Lewy bodies. Eur J Neurol. 2007;14:1357–1362. doi: 10.1111/j.1468-1331.2007.01977.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16 pt 2):1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu MTM, Taylor-Robinson SD, Chaudhuri KR, et al. Cortical dysfunction in non-demented Parkinson's disease patients: a combined 31Phosphorus MRS and 18FDG PET study. Brain. 2000;123:340–352. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- 12.Bohnen NI, Koeppe RA, Minoshima S, Giordani B, Albin RL, Frey KA, Kuhl DE. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J Nucl Med. 2011;52:848–855. doi: 10.2967/jnumed.111.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rektorova I, Krajcovicova L, Marecek R, Mikl M. Default mode network and extrastriate visual resting state network in patients with Parkinson's disease dementia. Neurodegener Dis. 2012;10(1-4):232–237. doi: 10.1159/000334765. [DOI] [PubMed] [Google Scholar]
- 14.Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT SPECT imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78:1176–1181. doi: 10.1136/jnnp.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnaldi D, Morbelli S, Morrone E, Campus C, Nobili F. Cognitive impairment in degenerative parkinsonisms: role of radionuclide brain imaging. Q J Nucl Med Mol Imaging. 2012;56:55–67. [PubMed] [Google Scholar]
- 16.Ito K, Nagano-Saito A, Kato T, et al. Striatal and extrastriatal dysfunction in Parkinson's disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain. 2002;125(pt 6):1358–1365. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- 17.Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- 18.Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- 19.Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–1722. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- 20.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. J Neurol. 2006;253:242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- 21.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 22.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 23.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jokinen P, Scheinin N, Aalto S, et al. [(11)C]PIB-, [(18)F]FDGPET and MRI imaging in patients with Parkinson's disease with and without dementia. Parkinsonism Relat Disord. 2010;16:666–670. doi: 10.1016/j.parkreldis.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol. 2005;58:773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 26.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134(pt 5):1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomperts SN, Locascio JJ, Marquie M, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27:965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomperts SN, Locascio JJ, Rentz D, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberling JL, Dave KD, Frasier MA. a-Synuclein imaging: a critical need for Parkinson's disease research. J Parkinsons Dis. 2013;3:565–567. doi: 10.3233/JPD-130247. [DOI] [PubMed] [Google Scholar]
- 31.Boche D, Perry VH, Nicoll JA. Activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 32.Ouchi Y, Yoshikawa E, Sekine Y, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 33.Gerhard A, Pavese N, Hotton G, et al. In vivo imaging of micro-glial activation with [(11)C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Simpson BS, Pavese N, Ramlackhansingh AF, Breen DP, Barker RA, Brooks DJ. Clinical correlates of brain inflammation in Parkinson's disease: a PET study [abstract]. Mov Disord. 2012;27(suppl 1):775. [Google Scholar]
- 35.Edison P, Ahmed I, Fan Z, et al. Microglia, amyloid, glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology. 2013;38:938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310:173–186. doi: 10.1016/s0009-8981(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 37.Glenner GG, Wong CW, Quaranta V, Eanes ED. The amyloid deposits in Alzheimer's disease: their nature and pathogenesis. Appl Pathol. 1984;2:357–369. [PubMed] [Google Scholar]
- 38.Jendroska K, Kashiwagi M, Sassoon J, Daniel SE. Amyloid beta-peptide and its relationship with dementia in Lewy body disease. J Neural Transm Suppl. 1997;51:137–144. doi: 10.1007/978-3-7091-6846-2_11. [DOI] [PubMed] [Google Scholar]
- 39.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 40.Mollenhauer B, Esselmann H, Roeber S, et al. Different CSF bamyloid processing in Alzheimer's and Creutzfeldt-Jakob disease. J Neural Transm. 2011;118:691–697. doi: 10.1007/s00702-010-0543-z. [DOI] [PubMed] [Google Scholar]
- 41.Kanemaru K, Kameda N, Yamanouchi H. Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies. Neurology. 2000;54:1875–1876. doi: 10.1212/wnl.54.9.1875. [DOI] [PubMed] [Google Scholar]
- 42.Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson's disease: the ICICLE-PD Study. Neurology. 2014;82:308–316. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mollenhauer B, Trenkwalder C, von Ahsen N, et al. Beta-amyloid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson's disease dementia. Dement Geriatr Cogn Disord. 2006;22:200–208. doi: 10.1159/000094871. [DOI] [PubMed] [Google Scholar]
- 44.Holmberg B, Johnels B, Blennow K, Rosengren L. Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson's disease and progressive supranuclear palsy. Mov Disord. 2003;18:186–190. doi: 10.1002/mds.10321. [DOI] [PubMed] [Google Scholar]
- 45.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid b1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian Park West study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 47.Bibl M, Mollenhauer B, Lewczuk P, et al. Validation of amyloid-beta peptides in CSF diagnosis of neurodegenerative dementias. Mol Psychiatry. 2007;12:671–680. doi: 10.1038/sj.mp.4001967. [DOI] [PubMed] [Google Scholar]
- 48.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 49.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 50.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 51.Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 52.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–1280. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 53.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 54.Mollenhauer B, Cepek L, Bibl M, et al. Tau protein, Abeta42 and S-100B protein in cerebrospinal fluid of patients with dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2005;19(2-3):164–170. doi: 10.1159/000083178. [DOI] [PubMed] [Google Scholar]
- 55.Hampel H, Goernitz A, Buerger K. Advances in the development of biomarkers for Alzheimer's disease: from CSF total tau and Abeta(1-42) proteins to phosphorylated tau protein. Brain Res Bull. 2003;61:243–253. doi: 10.1016/s0361-9230(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 56.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 57.Arima K, Hirai S, Sunohara N, et al. Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in Lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1999;843(1-2):53–61. doi: 10.1016/s0006-8993(99)01848-x. [DOI] [PubMed] [Google Scholar]
- 58.Parnetti L, Tiraboschi P, Lanari A, et al. Cerebrospinal fluid bio-markers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry. 2008;64:850–855. doi: 10.1016/j.biopsych.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Luk C, Compta Y, Magdalinou N, et al. Development and assessment of sensitive immuno-PCR assays for the quantification of cerebrospinal fluid three- and four-repeat tau isoforms in tauopathies. J Neurochem. 2012;123:396–405. doi: 10.1111/j.1471-4159.2012.07911.x. [DOI] [PubMed] [Google Scholar]
- 60.El-Agnaf OM, Salem SA, Paleologou KE, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 61.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid bio-markers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aerts MB, Esselink RA, Abdo WF, Bloem BR, Verbeek MM. CSF a-synuclein does not differentiate between parkinsonian disorders. Neurobiol Aging. 2012;33:430.e1–430.e3. doi: 10.1016/j.neurobiolaging.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebro-spinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med. 2010;4:683–699. doi: 10.2217/bmm.10.90. [DOI] [PubMed] [Google Scholar]
- 65.Kang JH, Irwin DJ, Chen-Plotkin A, et al. Association of cerebro-spinal fluid b-amyloid 1-42, T-tau, P-tau181, and a-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. doi: 10.1001/jamaneurol.2013.3861. [published online ahead of print 26 August 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF alpha-synuclein improves diagnostic and prognostic performance of CSF tau and Abeta in Alzheimer's disease. Acta Neuropathol. 2013;126:683–697. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larson ME, Sherman MA, Greimel S, et al. Soluble alpha-synuclein is a novel modulator of Alzheimer's disease pathophysiology. J Neurosci. 2012;32:10253–10266. doi: 10.1523/JNEUROSCI.0581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Tokuda T, Qureshi MM, Ardah MT, et al. Detection of elevated levels of a-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766–1772. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 69.El-Agnaf OM, Salem SA, Paleologou KE, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 70.Chen-Plotkin AS, Hu WT, Siderowf A, et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. 2011;69:655–663. doi: 10.1002/ana.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pellecchia MT, Santangelo G, Picillo M, et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson's disease patients. J Neurol. 2013;260:438–444. doi: 10.1007/s00415-012-6648-6. [DOI] [PubMed] [Google Scholar]
- 72.Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomark Med. 2010;4:701–712. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K. Uric acid associates with cognition in Parkinson's disease. Parkinsonism Relat Disord. 2008;14:576–578. doi: 10.1016/j.parkreldis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 74.O'Suilleabhain PE, Sung V, Hernandez C, Lacritz L, Dewey RB, Jr, Bottiglieri T, Diaz-Arrasyia R. Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations. Arch Neurol. 2004;61:865–868. doi: 10.1001/archneur.61.6.865. [DOI] [PubMed] [Google Scholar]
- 75.Mollenhauer B, Steinacker P, Bahn E, et al. Serum heart-type fatty acid-binding protein and cerebrospinal fluid tau: marker candidates for dementia with Lewy bodies. Neurodegener Dis. 2007;4:366–375. doi: 10.1159/000105157. [DOI] [PubMed] [Google Scholar]
- 76.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 77.Devic I, Hwang H, Edgar JS, et al. Salivary a-synuclein and DJ-1: potential biomarkers for Parkinson's disease [serial online]. Brain. 2011;134(pt 7):e178. doi: 10.1093/brain/awr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Statement on use of apolipoprotein E testing for Alzheimer disease. American College of Medical Genetics/American Society of Human Genetics Working Group on ApoE and Alzheimer Disease. JAMA. 1995;274:1627–1629. [PubMed] [Google Scholar]
- 79.Parsian A, Racette B, Goldsmith LJ, Perlmutter JS. Parkinson's disease and apolipoprotein E: possible association with dementia but not age at onset. Genomics. 2002;79:458–461. doi: 10.1006/geno.2002.6707. [DOI] [PubMed] [Google Scholar]
- 80.Pankratz N, Byder L, Halter C, et al. Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Mov Disord. 2006;21:45–49. doi: 10.1002/mds.20663. [DOI] [PubMed] [Google Scholar]
- 81.Ryu HG, Kwon OD. Apolipoprotein E epsilon 4 allele is not associated with age at onset or MMSE of Parkinson's disease in a Korean study. Parkinsonism Relat Disord. 2010;16:615–617. doi: 10.1016/j.parkreldis.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 82.Marder K, Maestre G, Cote L, et al. The apolipoprotein epsilon 4 allele in Parkinson's disease with and without dementia. Neurology. 1994;44:1330–1331. doi: 10.1212/wnl.44.7.1330. [DOI] [PubMed] [Google Scholar]
- 83.Ezquerra M, Campdelacreu J, Gaig C, et al. Lack of association of APOE and tau polymorphisms with dementia in Parkinson's disease. Neurosci Lett. 2008;448:20–23. doi: 10.1016/j.neulet.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 84.Harhangi BS, de Rijk MC, van Duijn CM, Van Broeckhoven C, Hofman A, Breteler MM. APOE and the risk of PD with or without dementia in a population-based study. Neurology. 2000;54:1272–1276. doi: 10.1212/wnl.54.6.1272. [DOI] [PubMed] [Google Scholar]
- 85.Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. J Neurol. 2009;256:493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 86.Morley JF, Xie SX, Hurtig HI, et al. Genetic influences on cognitive decline in Parkinson's disease. Mov Disord. 2012;27:512–518. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoogland J, de Bie RM, Williams-Gray CH, Muslimovic D, Schmand B, Post B. Catechol-O-methyltransferase val158met and cognitive function in Parkinson's disease. Mov Disord. 2010;25:2550–2554. doi: 10.1002/mds.23319. [DOI] [PubMed] [Google Scholar]
- 89.Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15(spec no 2):R188–R195. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- 90.Goris A, Williams-Gray CH, Clark GR, et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann Neurol. 2007;62:145–153. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- 91.Seto-Salvia N, Clarimon J, Pagonabarraga J, et al. Dementia risk in Parkinson disease: disentangling the role of MAPT haplotypes. Arch Neurol. 2011;68:359–364. doi: 10.1001/archneurol.2011.17. [DOI] [PubMed] [Google Scholar]
- 92.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chahine LM, Qiang J, Ashbridge E, et al. Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 2013;70:852–858. doi: 10.1001/jamaneurol.2013.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18):1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bohnen N, Jahn K. Imaging: what can it tell us about Parkinsonian gait? Mov Disord. 2013;28:1492–1500. doi: 10.1002/mds.25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28:1483–1491. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- 99.Amboni M, Braone P, Hausdorff J. Cognitive contributions to gait and falls: evidence and implications. Mov Disord. 2013;28:1520–1533. doi: 10.1002/mds.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. 2013;28:1534–1543. doi: 10.1002/mds.25545. [DOI] [PubMed] [Google Scholar]
- 101.Miekle MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 103.Verlinden VJ, van der Geest JN, Hofman A, Ikram MA. Cognition and gait show a distinct pattern of association in the general population. Alzheimers Dement; [published online ahead of print 9 July 2013]. [DOI] [PubMed] [Google Scholar]
- 104.Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, Callisaya ML. Cognitive function, gait and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:726–732. doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- 105.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127(pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 108.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, bio-markers and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 109.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 110.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 111.Burn DJ, Rowan EN, Minett T, et al. Extrapyramidal features in Parkinson's disease with and without dementia and dementia with Lewy bodies: a cross-sectional comparative study. Mov Disord. 2003;18:884–889. doi: 10.1002/mds.10455. [DOI] [PubMed] [Google Scholar]
- 112.Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77:585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 114.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- 115.Domellof M, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson's disease. Mov Disord. 2011;26:2183–2189. doi: 10.1002/mds.23814. [DOI] [PubMed] [Google Scholar]
- 116.Lord S, Baker K, Nieuwboer A, Burn D, Rochester L. Gait variability in Parkinson's disease: an indicator of non-dopaminergic contributors to gait dysfunction? J Neurol. 2011;258:566–572. doi: 10.1007/s00415-010-5789-8. [DOI] [PubMed] [Google Scholar]
- 117.Lord S, Rochester L, Hetherington V, Allcock LM, Burn D. Executive dysfunction and attention contribute to gait interference in “off” state Parkinson's disease. Gait Posture. 2010;31:169–174. doi: 10.1016/j.gaitpost.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 118.Rochester L, Nieuwboer A, Baker K, et al. Walking speed during single and dual tasks in Parkinson's disease: which characteristics are important? Mov Disord. 2008;23:2312–2318. doi: 10.1002/mds.22219. [DOI] [PubMed] [Google Scholar]
- 119.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 120.Amboni M, Barone P, Iuppariello L, et al. Gait patterns in Parkinsonian patients with or without mild cognitive impairment. Mov Disord. 2012;27:1536–1543. doi: 10.1002/mds.25165. [DOI] [PubMed] [Google Scholar]
- 121.Bohnen NI, Frey KA, Studenski S, et al. Gait speed in Parkinson's disease correlates with cholinergic degeneration. Neurology. 2013;81:1611–1616. doi: 10.1212/WNL.0b013e3182a9f558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, Burn DJ. Cholinergic dysfunction contributes to gait disturbance in early Parkinson's disease. Brain. 2012;135(pt 9):2779–2788. doi: 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muller ML, Frey KA, Petrou M, Kotagal V, Koeppe RA, Albin RL, Bohnen NI. b-Amyloid and postural instability and gait difficulty in Parkinson's disease at risk for dementia. Mov Disord. 2013;28:296–301. doi: 10.1002/mds.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alves G, Pedersen KF, Bloem BR, et al. Cerebrospinal fluid amyloid-beta and phenotypic heterogeneity in de novo Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013;84:537–543. doi: 10.1136/jnnp-2012-303808. [DOI] [PubMed] [Google Scholar]
- 125.Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31:523–531. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verghese J, Holtzer R, Wang C, Katz M, Barzilai N, Lipton R. Role of APOE genotype in gait decline and disability in aging. J Gerontol A Biol Sci Med Sci. 2013;68:1395–1401. doi: 10.1093/gerona/glt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olde Dubbelink KT, Hillebrand A, Twisk JW, et al. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology. 2014;82:263–270. doi: 10.1212/WNL.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 129.Yarnall AJ, Rochester L, Baker MR, et al. Short latency afferent inhibition: a biomarker for mild cognitive impairment in Parkinson's disease? Mov Disord. 2013;28:1285–1288. doi: 10.1002/mds.25360. [DOI] [PubMed] [Google Scholar]
- 130.Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease with dementia patients with a 2-year follow-up. Brain. 2008;131(pt 3):690–705. doi: 10.1093/brain/awm322. [DOI] [PubMed] [Google Scholar]
- 131.Klassen BT, Hentz JG, Shill HA, et al. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology. 2011;77:118–124. doi: 10.1212/WNL.0b013e318224af8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 133.Baba T, Kikuchi A, Hirayama K, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson's disease: a 3 year longitudinal study. Brain. 2012;135(pt 1):161–169. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- 134.Stephenson R, Houghton D, Sundarararjan S, et al. Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with Parkinson's disease. Mov Disord. 2010;25:2099–2104. doi: 10.1002/mds.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mollenhauer B, Schulz-Schaeffer W, Schlossmacher M. Synaptic alpha-synuclein pathology as the likely cause of Parkinson's disease dementia. Lancet Neurol. 2011;10:68–69. [Google Scholar]
- 136.Weinrich C, Wrede A, Mollenhauer B. [Analysis of cerebrospinal fluid proteins in the diagnosis of Parkinson's disease, Parkinson dementia and dementia with Lewy bodies.] Akt Neural. 2011;38:203–210. [Google Scholar]