The binding of stromal interaction molecule 1 (STIM1) to ORAI calcium channels is critical for store-operated calcium entry (SOCE), a calcium influx pathway conserved in virtually all vertebrate organisms. Although many of the major steps of this process are well-understood, crucial details regarding the mechanism of STIM1 activation are unclear. A new study by Zhou et al. provides important new insights into the conformational dynamics of STIM1 activation.
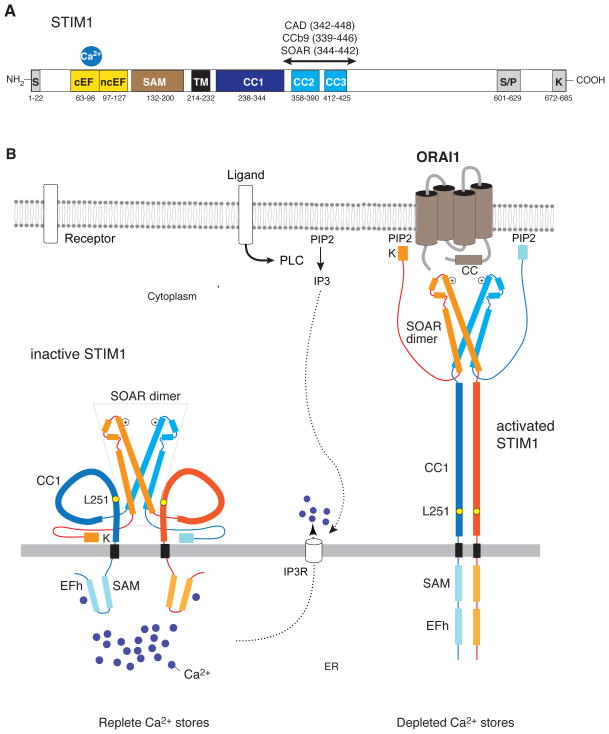
STIM1, and its homologue STIM2, are Ca2+ binding proteins located in the membrane of the ER 1,2. Their N and C termini are located in the ER lumen and cytoplasm, respectively, and these two domains function to sense the luminal ER Ca2+ concentration and activate ORAI calcium channels in the plasma membrane (PM) (Figure 1) 3,4. Stimulation of cells via receptors at their surface results in depletion of Ca2+ from the ER and the dissociation of Ca2+ from an EF-hand in the N terminus of STIM1 (STIM1NT) that is located adjacent to a sterile alpha motif (SAM). In cells with replete ER Ca2+ stores, the EF-SAM domain exists in a closed configuration 5. Upon Ca2+ release from the ER, hydrophobic regions in the EF-SAM domain are exposed, thereby allowing neighboring STIM1NT to dimerize 5,6. In fact, forced dimerization of STIM1NT using a rapalog system is sufficient to initiate STIM1 activation 7. Activated STIM1 translocates within the ER to ER-PM junctions where it accumulates into discrete puncta. Here, STIM1 binds to ORAI1, the pore-forming subunit of the Ca2+ release-activated Ca2+ (CRAC) channel 8, trapping ORAI1 channels into puncta, and enabling localized Ca2+ influx 9. STIM1 puncta formation and ORAI1 activation critically depend on STIM1 oligomerization, a process mediated by coiled-coil (CC) domains in STIM1CT 10,11. Three such domains, CC1–CC3, are present in STIM1CT and two of them, CC2 and CC3, are part of a ~ 110 amino acid domain, which is required for STIM1 oligomerization following store depletion. This domain is alternatively called CRAC activation domain (CAD) 12, STIM1-ORAI activating region (SOAR) 13 and Coiled-coil domain b9 (CCb9) 14, and its expression as a soluble protein is sufficient to activate CRAC channels 12–15. The recent crystal structure of the human SOAR domain reveals a V-shaped homodimer with the CC2 and CC3 domains forming a hair-pin motif. (Figure 1) 16. Collectively, these data suggest a model of STIM1 activation wherein in cells with replete Ca2+ stores, full-length STIM1 is partially dimerized via interactions of its cytoplasmic SOAR domain whereas its ER luminal N terminus exists in a folded and Ca2+-bound monomeric configuration 5.
Figure 1. Model of the structural dynamics underlying STIM1 activation and gating of ORAI channels.
Stimulation of cells by surface receptors results in the activation of phospholipase C, production of IP3 and IP3 receptor-mediated release of Ca2+ from ER Ca2+ stores. The resulting fall in [Ca2+]ER triggers store-operated Ca2+ entry (SOCE) via the activation of stromal interaction molecule 1 (STIM1) and opening of ORAI calcium channels. In resting cells with replete stores, Ca2+ ions bound to a canonical EF-hand in STIM1NT stabilize the binding of the EF-hand to a neighboring sterile alpha motif (SAM), preventing EF-SAM from engaging in protein-protein interactions (left) 5. Upon Ca2+ store depletion, the EF-SAM domain unfolds, thereby allowing neighboring EF-SAM domains to dimerize (right) 5,6. While the crystal structures of Ca2+-bound and Ca2+-free STIM1NT have been solved, the structure of STIM1CT is only partially known. STIM1CT contains 3 coiled-coil domains, CC1-CC3, two of which (CC2, CC3) are part of a critical STIM1-ORAI activating region (SOAR) 12–14. The crystal structure of the human SOAR reveals four α helices (α1–α4) arranged in a hairpin structure, with α1 and α4 corresponding roughly to CC2 and CC3, respectively 16. SOAR forms a homodimer with a V-like structure held together by hydrophobic interactions and hydrogen bonds at the base of the V-shaped dimer. It is likely that in cells with filled Ca2+ stores, STIM1 is partially dimerized via interactions of its cytoplasmic SOAR domain, whereas its ER luminal N terminus is a monomer. The study by Zhou et al. demonstrates that the CC1 domains in STIM1CT have a low α helical content and do not self-associate. Crosslinking of CC1 domains in vitro or their expected apposition following dimerization of STIM1NT in vivo stabilizes their α helices and facilitates their self-association. In addition, CC1-CC1 association weakens the binding of SOAR to CC1 and results in the unfolding of the STIM1CT. Together, these structural changes result in the elongation of STIM1CT, allowing it to bind to ORAI channels and anionic phospholipids in the plasma membrane and to activate SOCE. IP3, inositol 1,4,5-triphosphate; PIP2, phosphatidylionositol 4,5-bisphosphate; K, lysine-rich region.
Two overlapping questions regarding the mechanisms underlying STIM-mediated activation of ORAI channels arise from these findings. (1) How does dimerization of STIM1NT result in conformational changes in STIM1CT that enable activation of ORAI channels? (2) Why is STIM1 inactive in cells with replete Ca2+ stores although its SOAR domain appears to be already dimerized and has the potential, at least as a soluble protein, to activate ORAI channels? Mechanisms must be in place that restrain SOAR function in the context of full-length STIM1 in cells with replete Ca2+ stores?
Zhou et al. now use elegant biophysical approaches to elucidate the conformation alterations in STIM1CT to examine these questions. They show that dimerization of the membrane proximal CC1 domain of STIM1CT stabilizes the α-helical structure of CC1 and undocks SOAR from a binding site on CC1. This results in the exposure of a polybasic domain and an extended conformation of STIM1CT that likely allows it to bridge the gap between the ER and PM and to interact with ORAI channels and phospholipids in the PM (Figure 1).
In order to activate ORAI channels in the PM, STIM1CT has to bridge a distance of 10–25 nm, the estimated width of ER-PM junctions 17,18. Previous studies have suggested that a conformational change resulting in the unfolding of STIM1CT is required to achieve this feat 19,20. Using lanthanide-based resonance energy transfer (LRET), Zhou et al. analyzed the conformation of STIM1CT under conditions that mimic resting or activated forms of STIM1. They placed the Tb3+ LRET donor and a fluorescent acceptor at opposing ends of STIM1CT and analyzed energy transfer in wildtype STIM1CT and a L251S mutant that was previously shown to activate STIM1 function 20. Significant LRET is seen only in wildtype but not L251S mutant, which supports the conclusion that wildtype STIM1CT assumes a folded, inactive conformation in which the N and C terminal ends of STIM1CT are located close to each other (Figure 1). Mutation of L251 to serine abolishes this interaction, resulting in an elongated conformation of STIM1 that may be able to bridge the ER-PM junction gap. This interpretation is consistent with FRET studies using a shorter cytoplasmic STIM1 fragment (aa 233-474) which identified the STIM1 activating L251S mutation 20.
An important, unresolved question is how dimerization of the ER luminal STIM1NT after depletion of intracellular Ca2+ stores affects STIM1CT and enables activation of ORAI channels. This is an important issue because the cytoplasmic SOAR domain of STIM1 appears to be already dimerized 16 and therefore theoretically ready to bind to ORAI1 12–14. What mechanisms prevent this from happening in full-length STIM1 in non-stimulated cells? Zhou et al. use LRET measurements to demonstrate that the CC1 domains in soluble STIM1CT proteins are not closely associated since LRET was observed only when CC1 domains were forceably crosslinked. Interestingly, the study finds that soluble monomeric CC1 does not form a stable α-helix, whereas crosslinking of CC1 domains significantly increased their α-helical content, thereby further facilitating their dimerization. At first glance, the conclusion that the CC1 domains are not closely associated contrasts with an earlier finding that CC1 supports the resting self-association of full-length STIM1 11. However, in the model proposed by Zhou et al. apposition of CC1 domains in the context of full-length STIM1 is facilitated by the dimerization of the luminal EF-SAM domains following store depletion. Therefore, much like a zipper, the dimerization of STIM1NT would be predicted to alter the structure of cytoplasmic CC1 domains and trigger their dimerization. An important consequence of these changes is that the elongated α-helical CC1 dimer is likely to push the SOAR dimer towards the PM, bringing it into close proximity of ORAI channels.
What other effects does CC1 self-association have on the conformation and activation status of STIM1CT? Previous studies have shown that CC1 contains inhibitory domains that restrain STIM1 activation by interacting with residues in SOAR. For instance, an acidic domain (aa 302-322) in CC1 was reported to interact with a polybasic domain (aa 382-386) in SOAR, thereby keeping STIM1 in an inactive state and preventing ORAI1 activation 19. An inhibitory α helix (aa 310-337) in CC1 was identified by Yang et al. and shown to interact with residues at the base of the SOAR dimer 16. Deletion of the inhibitory α helix resulted in constitutive colocalization of STIM1 with ORAI1 and Ca2+ influx 16. Zhou et al. provide intriguing new data that support this model of CC1-mediated inhibition of SOAR function. Analyzing the interaction of monomeric and dimeric (i.e. crosslinked) CC1 with the isolated SOAR, they find that monomeric, but not dimeric, CC1 efficiently binds SOAR when immobilized on amylose resin. The conclusion that CC1 dimerization interferes with SOAR binding to CC1 is also strongly supported by findings in their study that disulfide crosslinking of CC1 abolishes LRET between donor/acceptor pairs placed at opposing ends of STIM1CT, arguing that CC1 dimerization changes the conformation of STIM1CT from a closed to open state.
The results of this study provide detailed mechanistic insights into the activation-induced conformational changes in STIM1CT and provide a consistent picture of how apposition of CC1 domains, triggered by depletion of ER Ca2+ stores and dimerization of the ER luminal STIM1NT, unfolds STIM1CT and releases inhibitory interactions between CC1 and SOAR that permit SOAR to bind to ORAI channels. Of course, these results also raise several fundamental questions about how CAD is exposed following STIM1CT extension, the nature of the tertiary structures of STIM1CT in the resting and active states, and whether there are alterations in SOAR itself during STIM1 activation. Further structural and function approaches will be necessary to resolve these questions.
References
- 1.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2012 Oct 5; doi: 10.1007/s00018-012-1175-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. The Journal of biological chemistry. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 7.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2006;103:16704–9. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell. 2010;21:1897–907. doi: 10.1091/mbc.E10-02-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muik M, et al. A Cytosolic Homomerization and a Modulatory Domain within STIM1 C Terminus Determine Coupling to ORAI1 Channels. J Biol Chem. 2009;284:8421–6. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5657–62. doi: 10.1073/pnas.1118947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–90. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 18.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korzeniowski MK, Manjarres IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3:ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muik M, et al. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J. 2011;30:1678–89. doi: 10.1038/emboj.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]