Abstract
Background
Beneficial effects of angiotensin converting enzyme (ACE) inhibitors seem to be mediated by mechanisms that are partly independent of blood pressure lowering. The present study evaluates effects of an ACE-inhibitor (i.e. fosinopril) intervention on novel cardiovascular risk factors.
Methods
Data are from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN), a double-blind, crossover, randomized, placebo-controlled trial enrolling subjects aged ≥55 years and older with high cardiovascular disease risk profile. Biomarkers of hemostasis (i.e. plasminogen activator inhibitor-1 [PAI-1], D-dimer), inflammation (i.e. C-reactive protein [CRP], interleukin-6 [IL-6]), and endothelial function (i.e. endothelin-1, vascular cell adhesion molecule-1 [VCAM-1]) were measured at the baseline, at the mid-term, and at end of follow-up (after one year) clinic visits. Paired t-test analyses (after Sidak’s adjustment, p value<0.009) were performed to compare biomarkers modifications after fosinopril/placebo interventions.
Results
Mean age of the sample (n=290, women 43.4%) was 66.0 years old. No significant differences were reported for CRP, IL-6, PAI-1, VCAM-1, and endothelin-1 levels in the comparisons between fosinopril and placebo interventions. D-Dimer was the only biomarker showing a significant difference between fosinopril intervention (median 0.32 [interquartile range, IQR 0.22–0.52] µg/mL) and placebo (median 0.29 [IQR 0.20–0.47] µg/mL, p=0.007) when analyses were restricted to participants with higher compliance to treatment and receiving the maximum ACE-inhibitor dosage.
Conclusions
ACE-inhibition does not significantly modify major biomarkers of inflammation, hemostasis, and endothelial function. Further studies should confirm the possible effect of ACE-inhibitors on the fibrinolysis pathway.
Keywords: ACE-inhibition, inflammation, clinical trial, coagulation/thrombosis, endothelium, clinical science, antihypertensive drug
INTRODUCTION
Several therapies, including aspirin, lipid lowering agents, beta-blockers, Angiotensin converting enzyme (ACE) inhibitors, calcium channel blockers, diuretics, and other antihypertensive drugs, are available for the prevention of the complications of atherosclerotic disease. The knowledge of the mechanisms by which these drugs may prevent cardiovascular events is important for targeting treatments according to the individual risk profile. In addition to the established cardiovascular risk factors, such as hypertension and dyslipidemia, novel risk factors are being identified, including markers of inflammation, impairment of fibrinolysis, and endothelial dysfunction which may be used for targeting therapies.
ACE-inhibitors have been shown to prevent major cardiovascular outcomes in patients with heart failure(1;2), diabetes(3–6), hypertension(3–5;7), and high cardiovascular risk profile(8). Many beneficial effects of ACE inhibitors seem to be mediated by mechanisms that are independent of blood pressure lowering. In fact, in the high-risk patients enrolled in the Heart Outcomes Prevention Evaluation (HOPE) trial, most of the benefits of ramipril compared to placebo appeared unrelated to blood pressure modifications(8). Moreover, other clinical trials have suggested that ACE inhibitors may be superior to other drugs for the prevention of cardiovascular events, despite minimal differences in achieved blood pressure levels(4;5).
There is currently very limited knowledge of the therapeutic mechanisms potentially underlying the benefits from ACE inhibition. It has been suggested that ACE inhibitors may favorably modify markers of hemostasis (such as plasminogen activator inhibitor-1 [PAI-1] and the coagulation activation marker D-dimer), inflammation (such as C-reactive protein [CRP] and interleukin-6 [IL-6]) and endothelial function (such as endothelin-1 and vascular cell adhesion molecule-1 [VCAM-1]), all of which are associated with atherosclerosis and a higher cardiovascular risk(9–11).
The impairment of the hemostatic mechanism plays an important role in the development of the atherosclerotic disease. In patients with type 2 diabetes and hypertension, ACE inhibitors have been shown to significantly decrease PAI-1 concentrations(12), but evidence is extremely sparse and results are inconclusive(13;14). A hypothetical anti-inflammatory effect of ACE inhibitors has also been evaluated in several studies, but with discordant results(15–18). Similarly, though some reports have suggested that the beneficial effects of ACE inhibitors might stem from improvement in endothelial function(19), but the available evidence cannot be considered definitive(20). The main concerns limiting interpretation of the few clinical trials available examining the effects of ACE-inhibition on novel cardiovascular risk biomarkers in humans are small sample sizes, short follow-up periods, and/or adoption of animal models.
In this paper, we present the principal results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN; ClinicalTrials.gov identifier: NCT0051389)(21). This double-blind, crossover, randomized, placebo-controlled trial was specifically designed to assess the biological mechanisms by which ACE-inhibition may improve clinical outcomes in persons aged 55 years and older who have a high cardiovascular disease risk profile. The aim of the present study is to evaluate effects of an ACE-inhibitor (fosinopril) intervention on a wide spectrum of novel cardiovascular risk factors.
METHODS
The TRAIN study design
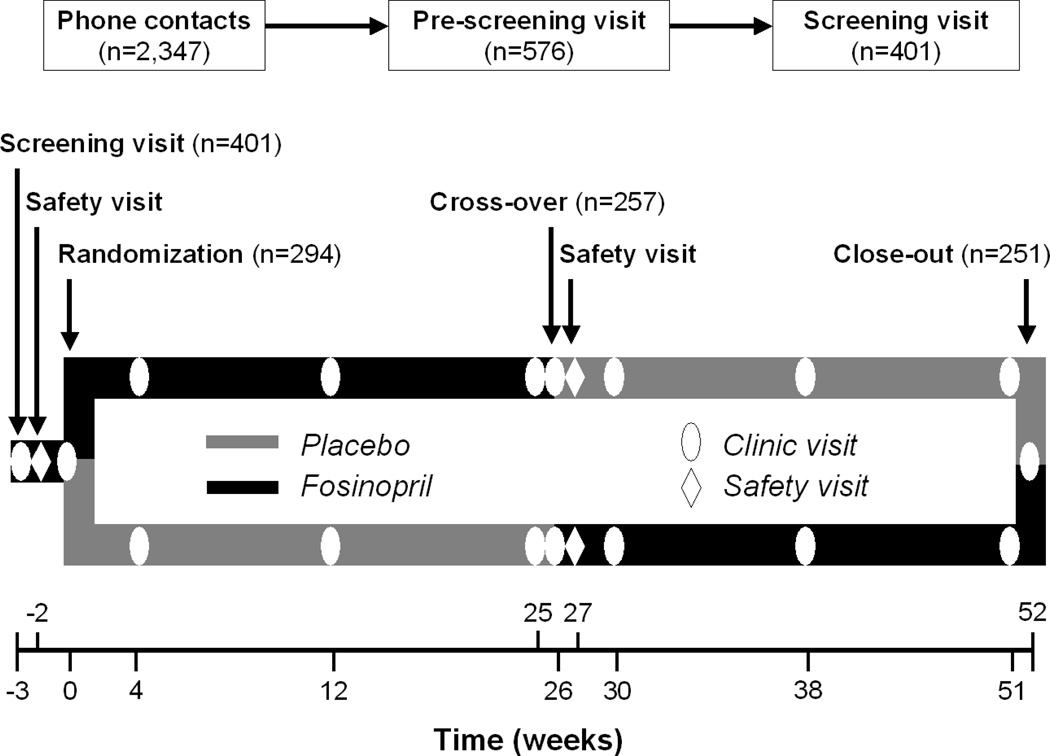
The TRAIN study design is presented in Figure 1. In a crossover design each participant was randomized to a sequence of treatments. Advantages of this design include the ability to control for all known and unknown confounding variables with implicit matching of data, improved recruitment because every participant receives the intervention, and a substantially higher statistical power compared to the independent parallel groups design. The 6-month follow-up of active treatment ensured the maximization of the effects on biological markers, but not the induction of permanent changes in the progression of the disease. For participants randomized to active intervention during the first, 6-month follow-up of placebo period was intended to ensure complete wash-out of the drug effects on biological markers. Previous studies demonstrated that the explored biological changes are fully reversible after 6 months of wash-out(12;22;23), and that a short-term (less than 1 year) treatment with ACE-inhibitor is unlikely to cause definitive changes in disease progression(8).
Figure 1.
Design of the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors.
Study participants
To be included in the TRAIN study, participants had to present with at least one of the following indicators of high cardiovascular risk: 1) coronary heart disease; 2) peripheral vascular disease; 3) history of stroke (>6 months); 4) diabetes along with at least one other cardiovascular risk factor (hypertension, total cholesterol >200 mg/dl, HDL cholesterol <40 mg/dl for men or <50 mg/dl for women, triglycerides ≥150 mg/dl, current cigarette smoking, obesity [body mass index, BMI ≥30 kg/m2, or waist >102 cm for men and >88 cm for women], known microalbuminuria, any evidence of previous vascular disease). Exclusion criteria for the TRAIN study were: 1) current use of or known hypersensitivity to ACE inhibitors; 2) diagnosis of specific cardiovascular conditions (including previous myocardial infarction, ejection fraction <40%, hemodynamically significant primary valvular or outflow tract obstruction, constrictive pericarditis, complex congenital heart disease, syncopal episodes likely to be due to life-threatening arrhythmias, planned cardiac surgery or angioplasty within three months, uncontrolled hypertension, cor pulmonale, planned cardiac surgery or angioplasty within three months); 3) conditions affecting results of or safe participation in the trial (significant renal disease [i.e. renal artery stenosis; creatine clearance <0.6 mL/s or serum creatinine ≥2.26 mg/dL; overt nephropathy; serum potassium >5.5 mEq/L], life-threatening illness, recent surgical procedure (<6 months), simultaneous enrollment in another experimental drug trial); 4) compliance issues (plans to leave the area in the next three months, substance abuse, compliance <80% during the pre-randomization phase). All the inclusion and exclusion criteria were based on medical history, medical records review, physical examination, and laboratory data of participants.
All the participants signed an informed consent for the study at the screening visit. The Wake Forest University School of Medicine Institutional Review Board approved the study protocol.
Organization of the study
Participants were recruited from the communities of Winston Salem, NC and Greensboro, NC through several recruitment strategies. Participants were first screened by a phone interview for eligibility (n=2,347). A clinical pre-screening visit, aimed at reviewing medical history and records of potential participants, was arranged for those (n=576) who successfully met the study inclusion and exclusion criteria as checked during the phone interview. Subjects who successfully completed the pre-screening visit (n=401) were entered in a screening and single-blind one-week run-in phase, in which the tolerability of ACE-inhibitor and compliance were evaluated.
Participants who successfully completed all the preliminary phase interviews and visits (n=294) were then randomized to placebo or active intervention (treatment with fosinopril) of the study during the baseline clinical visit. After 3 visits in a 6-month follow-up, the cross-over occurred. After further 6 months and 3 mid-term clinical visits, participants attended close-out visits. A special safety visit was also performed one week after the cross-over. According to the adopted double sampling method, one week before the 6-month visit (cross-over visit), an additional blood draw was performed for assays of biomarkers. The drug titration and follow-up procedures of the first phase were repeated in the following 6 months of the trial.
The present analyses are conducted on a sample of 290 participants, after exclusion of 4 participants in which none of the biomarkers of interest was assessed. Participants with the biomarker measurements at all time points (baseline, 6-month, and 12-month clinic visits) were 243 for CRP, 246 for IL-6, 247 for PAI-1, 238 for D-Dimer, 236 for VCAM-1, and 236 for endothelin-1. No significant sociodemographic, clinical, and biological differences were present between participants considered in the present analyses and those excluded because of missing values.
Intervention
At randomization, patients were assigned to receive fosinopril versus matching placebo at a dose of 20 mg daily for one week, then 40 mg daily. If the dosage was not tolerated because of hypotension, cough or other problems, the participants were given the lowest tolerated dose. The rationale for use of high dose fosinopril was the attempt to maximize potential effects on the biomarkers of interest, despite minimal differences in blood pressure control seen with higher doses (about 2–3 mmHg)(12;17). Fosinopril was chosen over other ACE-inhibitors because of the more favorable pharmacokinetic profile (i.e. high trough-to-peak ratio, both renal and hepatic elimination)(24;25), low incidence of adverse events(26;27), and overall evidence suggesting comparable efficacy of different ACE-inhibitors(28–30). Fosinopril is a phosphonate-containing ACE inhibitor, characterized by a partial extra-renal excretion. It is administered as a prodrug, and then rapidly converted by the liver and the gastrointestinal mucosa to the active form (i.e. fosinoprilat). The active form half-life is about 12 hours. Fosinopril was donated by Bristol-Myers Squibb (New York, NY).
Study drug was supplied at an interim 3-month visit. Before each visit, investigators assessed the participant’s adherence by counting the study pills left. Serum creatinine and potassium were monitored throughout the study. Moreover, participants were asked to report to investigators any suspected adverse event they might have experienced during the follow-up. The possible onset of adverse events was also specifically assessed by investigators at each clinic visit. All medical records connected to a serious adverse event were reviewed by study investigators.
Cardiovascular risk biomarkers
To minimize circadian rhythm and other fluctuations in biomarkers levels, all participants underwent a blood draw by venipuncture in the morning after ≥6 hours of fasting at screening and at each clinical visit. Specimens were cold processed within one hour, and then they were aliquoted into cryovials, frozen and stored at −80°C until analyses.
Serum levels of IL-6 were assessed with a high-sensitivity monoclonal antibody-based Enzyme Linked ImmunoSorbent Assay (ELISA; R&D Systems, Minneapolis, MN). Sensitivity was <0.10 pg/ml, with an expected detection range of 0.15–10.0 pg/mL, and a coefficient of variation (CV) of 11.6%. Serum levels of CRP were measured by an ELISA developed in-house based on purified CRP and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA)(31). The CRP assay had a sensitivity of 0.08 µg/mL, with a standard reference range of between 0.5 and 2.5 mg/L. The mean CV for CRP across assay runs was 4.2%(32). Serum levels of PAI-1 antigen were measured by a two-site ELISA(33). The analytical CV for this assay was 3.5%. This assay present is not sensitive to PAI-1 in complex with TPA, but only to free PAI-1 (both latent and active). Serum levels of VCAM-1 were measured by commercially available ELISA (R&D Systems). VCAM-1 interassay CV was6.6%. Endothelin-1 in extracted EDTA plasma was measured by a solid-phase ELISA (R&D Systems). The lower detection level is 1.0 pg/mL, and the analytical CV was 4.9%. D-Dimer was measured using an immuno-turbidimetric assay (STA Liatest D-DI, Diagnostica Stago, Asnieres, France) on the Sta-R coagulation analyzer, with an analytical CV of 18.6%.
In a subsample of 200 randomly selected participants, Angiotensin-II levels were measured by radioimmunoassay (RIA) to obtain a reference measure showing the biological effectiveness of the intervention. The RIA kit (ALPCO Diagnostics, Salem, NH) had an intra-assay and an inter-assay precision of 8.3% and 11.5%, respectively. The assessment of eight of these participants was not completed at all the required time points of interest due to insufficient specimen and/or technical issues.
Sample population characteristics
Sociodemographic (age, gender, race, current smoking) and self-reported clinical conditions (diabetes, angina, myocardial infarction, cancer, stroke, peripheral artery disease, lung disease) were collected at the baseline assessment. BMI was calculated as body mass (in kilograms) divided by squared height (in meter2). Body mass was measured by Delphi (Hologic Inc., Bedford, MA) dual-energy X-ray absorptiometry (DXA) scan. Height was self-reported by participants. Blood pressure (in mmHg) was calculated as the average of three measures taken in standardized sitting position.
Sample size analysis
The TRAIN study was designed to yield a minimum of 90% statistical power to detect a reduction in the levels of four biomarkers (i.e. PAI-1, IL-6, CRP, and VCAM-1) by at least 25% of the standard deviation of difference in the response of matched pairs (paired effect size 0.25). The detectable reduction in PAI-1 and IL-6 is smaller than the actual reduction observed in the Fosinopril versus Amlodipine Comparative Treatments Study (FACTS) after only 4 weeks (paired effect size 0.26 and 0.32, respectively)(12).
The effect size in the TRAIN study was expected to be larger because 1) the longer duration of treatment (6 months vs. 4 weeks), and 2) the advantage of the double sampling method. According to previous evidence on the predictive value of IL-6(34), PAI-1(35;36), and CRP(34;37) for cardiovascular events, differences smaller than those detectable in TRAIN are unlikely to be clinically significant.
To meet these goals, sample size analysis indicated the need to randomize a total of 290 participants. A 12% loss-to-follow-up-rate (i.e. n=25) was taken into account when calculating this estimate, and included participants who could not be tracked, refused further blood sampling during the follow-up, missed follow-up visits, or had insufficient blood samples. The sample size projections were based on paired t-test analyses. Two-sided tests based on alpha level of 0.0125 were planned to account for the multiple comparisons related to the four primary biomarkers.
Statistical analysis
The mean values of biomarker levels were calculated for mid-term and end of follow-up visits (visits 6 and 7, and 11 and 12, respectively). If a value among the two measured at each visit was missing, the other existing value was used. The effect of fosinopril was evaluated on biomarker levels was measured at visits 6 and 7 for those who received the medication during the first part of the trial, and at visits 11 and 12 for those who received fosinopril in the second part of the trial. The opposite was done for the placebo. Paired t-test analyses were performed to compare biomarker levels between fosinopril and placebo intervention. Non-normally distributed variables were normalized by log-transformation before comparison, and results expressed as medians (interquartile ranges). Because all comparisons are done by matched pairs, no adjustment is needed for baseline factors, such as age, gender, or other potential confounders. A value of p<0.009 was considered as statistically significant, according to Sidak’s adjustment to account for the multiple comparisons perfomed in the present analyses and considering six biomarkers of interest.
RESULTS
The main characteristics of the TRAIN sample population (n=290) are shown in Table 1. The median study participants’ compliance to the treatment throughout the study was 92.1% (interquartile range 49.8%–98.5%). Most of the participants (89.8%) received the maximum dosage of the study medication (fosinopril 40 mg).
Table 1.
Main characteristics of the TRAIN study population (n=290).
| Value (mean ± SD, or %) |
|
|---|---|
| Age (years) | 66.0 ± 7.4 |
| Gender (Women) | 43.4 |
| Race | |
| White | 74.1 |
| African American | 23.8 |
| Asian | 0.7 |
| Other | 1.3 |
| Current smoker | 11.4 |
| Body Mass Index (kg/m2) | 29.0 ± 4.7 |
| Diabetes | 22.1 |
| Angina | 8.3 |
| Cancer | 14.8 |
| Stroke | 7.9 |
| Peripheral artery disease | 3.8 |
| Lung disease | 10.7 |
| C-Reactive Protein (mg/L)* | 2.46 (1.03–4.99) |
| Interleukin-6 (pg/mL)* | 2.94 (2.30–4.23) |
| Plasminogen Activator Inhibitor-1 (ng/mL)* | 31.67 (16.53–53.52) |
| D-Dimer (µg/mL)* | 0.28 (0.20–0.46) |
| Vascular cell adhesion molecule-1 (ng/mL)* | 1047.1 (819.4–1244.7) |
| Endothelin (pg/mL)* | 1.50 (1.23–1.85) |
| Angiotensin-II (pg/mL)* | 11.00 (7.50–17.02) |
| Systolic blood pressure (mmHg) | 137.4 ± 15.3 |
| Diastolic blood pressure (mmHg) | 80.8 ± 10.3 |
Expressed as median (interquartile range).
Blood pressure and angiotensin-II
The mean blood pressure at the baseline visit was 137.4 (SD 15.3)/80.8 (SD 10.3) mmHg. Significant reductions were found after fosinopril intervention compared to placebo in both systolic (128.5 [SD 14.4] mmHg vs. 133.4 [SD 13.5] mmHg; p<0.001) and diastolic (77.6 [SD 8.7] mmHg vs. 80.6 [SD 9.1] mmHg; p<0.001) blood pressure values. Significantly lower levels of angiotensin-II (n=192) were reported after fosinopril treatment compared to placebo (median value [interquartile range]: 11.10 [7.10–17.90] pg/mL vs. 12.50 [7.70–19.40] pg/mL, respectively; p<0.001; Table 2).
Table 2.
Cardiovascular risk biomarkers and ACE-inhibition.
| N | Fosinopril | Placebo | p | |
|---|---|---|---|---|
| Angiotensin-II (pg/mL) | 192 | 11.10 (7.10–17.90) | 12.50 (7.70–19.40) | <0.001 |
| C-Reactive protein (mg/L) | 243 | 2.40 (1.03–5.07) | 2.34 (1.17–4.89) | 0.62 |
| Interleukin-6 (pg/mL) | 246 | 2.37 (1.72–3.85) | 2.54 (1.75–3.80) | 0.89 |
| Plasminogen activator inhibitor-1 (ng/mL) | 247 | 25.95 (16.50–40.96) | 25.77 (16.42–45.60) | 0.70 |
| D-Dimer (µg/mL) | 238 | 0.32 (0.22–0.53) | 0.29 (0.20–0.50) | 0.02 |
| Vascular cell adhesion molecule-1 (ng/mL) | 236 | 1057.0 (832.2–1331.7) | 1054.5 (830.7–1319.5) | 0.98 |
| Endothelin-1 (pg/mL) | 236 | 1.52 (1.21–1.84) | 1.49 (1.26–1.85) | 0.62 |
Values are expressed as median (interquartile range) and p values calculated using log-transformed values and t-test analyses.
Cardiovascular risk biomarkers
The comparisons between biomarker levels after treatment with fosinopril and placebo are reported in Table 2. No significant difference was reported for CRP, IL-6, PAI-1, VCAM-1, endothelin-1 levels in the comparisons between fosinopril and placebo. Levels of D-dimer showed a borderline, not-statistically significant modification (p=0.02) after treatment with fosinopril (0.32 [0.22–0.53] µg/mL) compared to placebo (0.29 [0.20–0.50] µ/mL).
Further analyses were performed restricted to participants who were at least 80% compliant to treatment and received the maximum ACE-inhibitor dosage (i.e. fosinopril 40 mg). Participants included in these secondary analyses did not significantly differ for sociodemographic and clinical characteristics from those excluded because less compliant to treatment and/or receving a lower fosinopril dosage. No significant effect of fosinopril intervention compared to placebo was found for CRP (n=186; 2.43 [1.04–5.21] mg/L versus 2.56 [1.84–3.68] mg/L, respectively; p=0.86), IL-6 (n=187; 2.38 [1.77–3.94] pg/mL versus 2.60 [1.84–3.68] pg/mL, respectively; p=0.72), PAI-1 (n=188; 25.44 [15.96–39.62] ng/mL versus 24.67 [15.67–47.30], respectively; p=0.54), endothelin-1 (n=180; 1.51 [1.20–1.84] pg/mL versus 1.48 [1.26–1.89] pg/mL, respectively; p=0.43), and VCAM-1 (n=180; 1062.1 [827.4–1321.9] ng/mL versus 1042.3 [817.6–1266.8] ng/mL, respectively; p=0.88). D-Dimer was the only biomarker showing a statistically significant difference between fosinopril intervention and placebo (n=183; 0.32 [0.22–0.52] µg/mL versus 0.29 [0.20–0.47] µg/mL, respectively; p=0.007). To explore the directional consistency of D-dimer modifications, restricted analyses were performed in participants randomized to fosinopril during the first 6 months of the trial, so to determine whether D-dimer changes due to ACE-inhibition were transient. D-dimer concentrations tended to increase during the first 6 months of intervention (n=119; baseline 0.28 [0.19–0.44] µg/mL; 6-month visit 0.31 [0.22–0.48] µg/mL) and decrease when placebo was administered during the following 6 months (12-month visit 0.28 [0.18–0.49] µg/mL). The directional consistency of these changes was also confirmed among the most compliant participants receiving the highest dosage of fosinopril (n=92).
All the presented analyses considered as reference concentrations those measured at the end of the six months placebo administration (i.e. 6-month clinic visit for participants randomized to placebo during the first phase of the trial, and 12-month clinic visit for participants randomized to placebo during the second phase of the trial). Consistent results were reported if biomarkers concentrations measured at the baseline clinic visit (i.e. randomization time-point) were considered as reference (placebo) values in all the previously described analyses.
Adverse events during the TRAIN study
A total of 461 adverse events were reported throughout the study by 191 participants. The most commonly reported adverse events were cough (66 participants, 14.5%), dizziness (34 participants, 7.4%), cold (33 participants, 7.2%) diarrhea (8 participants, 1.8%), headache (8 participants, 1.8%), fatigue (7 participants, 1.5%), and significant increase of creatinine levels (7 participants, 1.5%). Thirty-four serious adverse events were reported by 22 participants during the entire study. Only two of these events were likely due to the study medication (a definite drug-related hospitalization for angioedema, and a probable drug-related hospitalization for dizziness). No significant differences in biomarkers concentrations were found between participants who developed vs. those who did not developed adverse events to fosinopril (all p values >0.3).
DISCUSSION
To our knowledge, the TRAIN study is the first randomized controlled trial with 1) a large sample size to reach the adequate statistical power needed to detect significant changes in biomarkers levels after ACE-inhibition, and 2) a relatively long follow-up (6 months) to maximize the biological effects of the medication. Contrary to our hypothesis, ACE-inhibition was not associated with significant reductions in most of the novel cardiovascular risk factors, including markers of inflammation (IL-6, CRP), hemostasis (PAI-1), and endothelial function (VCAM-1, endothelin-1). A marginal increase in D-dimer levels was associated with the intervention. Consistent results were reported when analyses were restricted to the most compliant participants receiving the maximum fosinopril dosage.
Our findings seem not to support the hypothesis that inflammatory-, and endothelial function-related pathways substantially justify the beneficial effects of ACE-inhibitors on cardiovascular risk, above and beyond their action on blood pressure and neurohormonal profile. Thus, alternative hypotheses to explain the beneficial effects of ACE-inhibitors on cardiovascular risk are needed.
Unexpectedly, we found a statistically significant (about 10%) increase in D-dimer concentrations after fosinopril treatment among most compliant participants receiving the highest dosage of medication. Secondary (but underpowered) analyses conducted among participants initially randomized to the first phase of fosinopril intervention may support this finding. In fact, D-dimer concentrations tended to increase during the initial six months of ACE-inihibiton, and to subsequently return to the baseline levels after the following six months of placebo administration. Given the lack of information on the topic (and the consequent impossibility of comparing results with previous evidence), we can not draw definitive conclusions and exclude that this finding might be due to a type I error. In fact, the analytical method for the D-dimer measurement was limited by a relatively high coefficient of variation. Moreover, despite our main analyses were adjusted according to the Sidak’s method to take into account the multiple-hypothesis testing, the additional models performed in the restricted samples of participants might have increased the risk of false positive findings. In this context, it is also noteworthy that D-dimer concentrations were not considered as one of the primary outcomes when originally designing the TRAIN study. Therefore, our sample size might have been insufficient to adequately explore the effect of ACE-inhibition on this biomarker levels. Nevertheless, some support for this result can be found in few available preclinical studies on atherosclerotic animal models showing higher levels of this breakdown product of fibrin in the presence of ACE-inhibitor or angiotensin type II receptor antagonist treatment(38;39). It has been suggested that increased levels of D-dimer in subjects with atherosclerosis may reflect increased rather than inhibited fibrinolysis(40;41). There is some evidence that atherosclerosis may be attributed, at least in part, to an impaired fibrinolytic balance(42). ACE-inhibition may promote a pro-fibrinolytic mechanism rebalancing the fibrinolysis-coagulation mechanism, possibly through an increase in bradykinin levels(43). In this scenario, the increased levels of D-dimer may represent the degradation of preexisting fibrin(40). Interestingly, it has been demonstrated that submaximal physical exercise may lead to small, but significant increase of D-dimer levels in patients with peripheral atherosclerosis(40). However, further studies are needed to confirm our results, and verify whether ACE-inhibitors may indeed positively act on the fibrinolytic pathway.
The reduction of blood pressure we found following the fosinopril treatment (~5/3 mmHg) is consistent with previous studies(12). The effectiveness of our intervention is also confirmed by the significant reduction in levels of plasma angiotensin-II, a potent vasoconstrictor and the basis of the blood pressure effects of ACE-inhibitors.
Some study limitations need to be mentioned. In our study, we have not a precise estimate the activation status of the Renin-Angiotensin-Aldosterone system. If the Renin-Angiotensin-Aldosterone system was not sufficiently activated, the expected effects of the ACE-inhibitor intervention might have not been detectable or biologically significant. In this context, the relatively limited sample size of the TRAIN study did not allowed stratified analyses according to different baseline concentrations of angiotensin-II. Future studies should specifically explore whether different risk profiles based on the activation of the Renin-Angiotensin-Aldosterone system may differently respond to ACE-inhibition intervention in varying the studied biomarkers concentrations. Our findings showed that the ACE inhibitor effect on the angiotensin II levels was relatively modest. It is possible that the so-called “ACE escape” phenomenon took place in our sample. This only partially understood mechanism is probably associated with alternate synthesis pathways for angiotensin II. For example, specific enzymes (e.g. cathepsin G, elastase) may directly convert angiotensinogen to angiotensin II, or facilitate an alternative conversion of angiotensin I to angiotensin II (e.g. chymases, cathepsin G). The possible existence of this mechanism may be responsible for an underestimation of the role played by the Renin-Angiotensin-Aldosterone System on the studied biomarkers and pathways. Future studies may explore a more efficient blockage of the Renin-Angiotensin-Aldosterone System by, for example, simultaneously acting on the ACE and the angiotensin receptors. Our findings might be specifically related to the ACE-inhibitor molecule adopted in the TRAIN study (i.e. fosinopril). We cannot exclude that different results might be found with the use of other ACE-inhibitors. However, there is an overall agreement suggesting the efficacy of individual ACE-inhibitors to be equivalent(28–30). Our results were obtained from a high cardiovascular risk profile sample population, and may not be appliable to subjects with a lower risk profile. Finally, our results are based on the evaluation of several markers representing different biological pathways (i.e. inflammation, hemostasis, fibrinolysis, and endothelial function). Given the complexity (and the possible overlappings) of these mechanisms, the evaluation of single biomarkers may not be sufficient to extend our results to the entire pathways. Therefore, our results should not be considered as conclusive. Further studies are needed to confirm and extend our findings by evaluating additional markers part of the studied biological pathways.
Perspectives
Our study demonstrates that ACE-inhibition with fosinopril does not significantly modify major biomarkers of inflammation, hemostasis, and endothelial function. Further studies are needed to 1) explore additional mechanistic pathways (e.g. metabolic functioning and oxidative damage), and 2) confirm our results suggesting a possible direct role played by ACE-inhibitors on D-dimer concentrations.
Acknowledgments
SOURCES OF FUNDING
The TRAIN study is National Institute of Health-funded project (NIH grant R01-HL68901). The TRAIN study was also (partially) supported by the University of Florida Claude D. Pepper Older Americans Independence Center (NIH grant 1P30-AG028740), Wake Forest University Claude D. Pepper Older Americans Independence Center (NIH grant 5P30-AG021332), and the Wake Forest University General Clinical Research Center (NIH grant M01-RR07122).
Footnotes
Clinical Trial Registration Information: The TRAIN study is registered at the ClinicalTrials.gov website (http://clinicaltrials.gov/ct2/show/NCT00051389). The identification number is NCT0051389.
DISCLOSURES
None of the Authors had significant conflict of interest to disclose for the present work. Fosinopril was donated by Bristol-Myers Squibb (New York, NY).
REFERENCES
- 1.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 2.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med. 1992;327(10):685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 3.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. Br Med J. 1998;317(7160):713–720. [PMC free article] [PubMed] [Google Scholar]
- 4.Tatti P, Pahor M, Byington RP, DiMauro P, Guarisco R, Strollo G, et al. Outcome results of the Fosinopril versus Amlodipine Cardiovascular Events randomized Trial in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. doi: 10.2337/diacare.21.4.597. [DOI] [PubMed] [Google Scholar]
- 5.Estacio RO, Jeffers BW, Hiatt WR, Biggi SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular events in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–652. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 6.Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Furberg CD. Therapeutic benefits of ACE inhibitors and other antihypertensive drugs in patients with type 2 diabetes. Diabetes Care. 2000;23:888–892. doi: 10.2337/diacare.23.7.888. [DOI] [PubMed] [Google Scholar]
- 7.Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomized trial. Lancet. 1999;353:611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 8.The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 9.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(11):2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 10.Koenig W. Haemostatic risk factors for cardiovascular diseases. Eur Heart J. 1998;19(Suppl C):C39–C43. [PubMed] [Google Scholar]
- 11.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252(4):283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 12.Pahor M, Franse LV, Deitcher SR, Cushman WC, Johnson KC, Shorr RI, et al. Fosinopril versus Amlodipine Comparative Treatments Study: a randomized trial to assess effects on plasminogen activator inhibitor-1. Circulation. 2002;105:457–461. doi: 10.1161/hc0402.102929. [DOI] [PubMed] [Google Scholar]
- 13.Paterna S, Di Garbo V, Avellone G, Di Pasquale P, Cacia A, Tuttolomondo A, et al. Effects of losartan and delapril on the fibrinolytic system in patients with mild to moderate hypertension. Clin Drug Investig. 2003;23(11):717–724. doi: 10.2165/00044011-200323110-00004. [DOI] [PubMed] [Google Scholar]
- 14.Trifiletti A, Barbera N, Scamardi R, Bagnato L, Pizzoleo MA, Nevoso A, et al. Effects of medium-term antihypertensive therapy on haemostatic parameters in patients with essential hypertension. Haemostasis. 1997;27(1):35–38. doi: 10.1159/000217431. [DOI] [PubMed] [Google Scholar]
- 15.Tsikouris JP, Suarez JA, Simoni JS, Ziska M, Meyerrose GE. Exploring the effects of ACE inhibitor tissue penetration on vascular inflammation following acute myocardial infarction. Coron Art Dis. 2004;15(4):211–217. [PubMed] [Google Scholar]
- 16.White M, Lepage S, Lavoie J, De Denus S, Leblanc MH, Gossard D, et al. Effects of combined candesartan and ACE inhibitors on BNP, markers of inflammation and oxidative stress, and glucose regulation in patients with symptomatic heart failure. J Card Fail. 2007;13(2):86–94. doi: 10.1016/j.cardfail.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gullestad L, Aukrust P, Ueland T, Espevik T, Yee G, Vagelos R, et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol. 1999;34(7):2061–2067. doi: 10.1016/s0735-1097(99)00495-7. [DOI] [PubMed] [Google Scholar]
- 18.Sheth T, Parker T, Block A, Hall C, Adam A, Pfeffer MA, et al. Comparison of the effects of omapatrilat and lisinopril on circulating neurohormones and cytokines in patients with chronic heart failure. Am J Cardiol. 2002;90(5):496–500. doi: 10.1016/s0002-9149(02)02521-3. [DOI] [PubMed] [Google Scholar]
- 19.Hlubocka Z, Umnerova V, Heller S, Peleska J, Jindra A, Jachymova M, et al. Circulating intercellular cell adhesion molecule-1, endothelin-1 and von Willebrand factor-markers of endothelial dysfunction in uncomplicated essential hypertension: the effect of treatment with ACE inhibitors. J Hum Hypertens. 2002;16(8):557–562. doi: 10.1038/sj.jhh.1001403. [DOI] [PubMed] [Google Scholar]
- 20.Schalkwijk CG, Smulders RA, Lambert J, Donker AJM, Stehouwer CDA. ACE-inhibition modulates some endothelial functions in healthy subjects and in normotensive type I diabetic patients. Eur J Clin Invest. 2000;30:853–860. doi: 10.1046/j.1365-2362.2000.00721.x. [DOI] [PubMed] [Google Scholar]
- 21.Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BWJH, Lenchik L, et al. Sarcopenia, obesity and inflammation - Results from the TRAIN study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 22.Koh KK, Mincemoyer R, Bui MN, Csako G, Pucino F, Guetta V, et al. Effects of hormone-replacement therapy on fibrinolysis in postmenopausal women. N Engl J Med. 1997;336(10):683–690. doi: 10.1056/NEJM199703063361002. [DOI] [PubMed] [Google Scholar]
- 23.Cushman M, Costantino JP, Tracy RP, Song K, Buckley L, Roberts JD, et al. Tamoxifen and cardiac risk factors in healthy women: suggestion of an anti-inflammatory effect. Arterioscler Thromb Vasc Biol. 2001;21(2):255–261. doi: 10.1161/01.atv.21.2.255. [DOI] [PubMed] [Google Scholar]
- 24.Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000;57(Suppl 1):S3–S7. doi: 10.1093/ajhp/57.suppl_1.S3. [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum R, Zucchelli P, Caspi A, Nouriel H, Paz R, Sclarovsky S, et al. Comparison of the pharmacokinetics of fosinoprilat with enalaprilat and lisinopril in patients with congestive heart failure and chronic renal insufficiency. Br J Clin Pharmacol. 2000;49(1):23–31. doi: 10.1046/j.1365-2125.2000.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansson L, Forslund T, Hoglund C, Istad H, Lederballe-Pedersen O, Kristinsson A, et al. Fosinopril versus enalapril in the treatment of hypertension: a double-blind study in 195 patients. J Cardiovasc Pharmacol. 1996;28(1):1–5. doi: 10.1097/00005344-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Wagstaff AJ, Davis R, McTavish D. Fosinopril: a reappraisal of its pharmacology and therapeutic efficacy in essential hypertension. Drugs. 1996;51(5):777–791. doi: 10.2165/00003495-199651050-00006. [DOI] [PubMed] [Google Scholar]
- 28.Pahor M, Psaty B, Alderman MH, Williamson JD, Applegate WB, Cavazzini C, et al. The health outcomes associated with calcium antagonists compared with other first-line antihypertensive therapies: a meta-analysis of randomized controlled trials. Lancet. 2000;356:1949–1954. doi: 10.1016/S0140-6736(00)03306-7. [DOI] [PubMed] [Google Scholar]
- 29.Neal B, MacMahon S, Chapman N Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000;356(9246):1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 30.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 31.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological implications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 32.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 33.Declerck PJ, Alessi MC, Verstreken M, Kruithof EK, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor 1 in biological fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988;71(1):220–225. [PubMed] [Google Scholar]
- 34.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 35.Juhan-Vague I, Pyke SD, Alessi MC, Jespersen J, Haverkate F, Thompson SG. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation. 1996;94(9):2057–2063. doi: 10.1161/01.cir.94.9.2057. [DOI] [PubMed] [Google Scholar]
- 36.Held C, Hjemdahl P, Rehnqvist N, Wallen NH, Bjorkander I, Eriksson SV, et al. Fibrinolytic variables and cardiovascular prognosis in patients with stable angina pectoris treated with verapamil or metoprolol. Results from the Angina Prognosis study in Stockholm. Circulation. 1997;95(10):2380–2386. doi: 10.1161/01.cir.95.10.2380. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 38.Oubina MP, De Las Heras N, Cediel E, Sanz-Rosa D, Aragoncillo P, Diaz C, et al. Synergistic effect of angiotensin-converting enzyme (ACE) and 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibition on inflammatory markers in atherosclerotic rabbits. Clin Sci (Lond) 2003;105:655–662. doi: 10.1042/CS20030127. [DOI] [PubMed] [Google Scholar]
- 39.Oubina MP, De Las Heras N, Vasquez-Perez S, Cediel E, Sanz-Rosa D, Ruilope LM, et al. Valsartan improves fibrinolytic balance in atherosclerotic rabbits. J Hypertens. 2002;20:303–310. doi: 10.1097/00004872-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Mustonen P, Lepantalo M, Lassila R. Physical exertion induces thrombin formation and fibrin degradation in patients with peripheral atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:244–249. doi: 10.1161/01.atv.18.2.244. [DOI] [PubMed] [Google Scholar]
- 41.van der Bom JG, Bots ML, Haverkate F, Meyer P, Hofman A, Grobbee DE, et al. Fibrinolytic activity in peripheral atherosclerosis in the elderly. Thromb Haemost. 1999;81(2):275–280. [PubMed] [Google Scholar]
- 42.Fogari R, Zoppi A. Antihypertensive drugs and fibrinolytic function. Am J Hypertens. 2006;19(12):1293–1299. doi: 10.1016/j.amjhyper.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 43.Cruden NL, Newby DE. Clots, kinins and coronaries. Atherosclerosis. 2005;183(2):189–198. doi: 10.1016/j.atherosclerosis.2005.05.038. [DOI] [PubMed] [Google Scholar]