Abstract
The Tor1/2p signal transduction pathway regulates nitrogen catabolite repression (NCR)-sensitive (GAP1, GAT1, DAL5) and retrograde (CIT2, DLD3, IDH1/2) gene expression by controlling intracellular localization of the transcription activators, Gln3p and Gat1p, and Rtg1p and Rtg3p, respectively. The accepted pathway for this regulation is NH3 or excess nitrogen ⫞ Mks1p ⫞ Ure2p ⫞ Gln3p → DAL5, and rapamycin or limiting nitrogen ⫞ Torp → Tap42 ⫞ Mks1p → Rtg1/3p → CIT2, respectively. In current models, Mks1p positively regulates both Gln3p (and DAL5 expression) and Rtg1/3p (and CIT2 expression). Here, in contrast, we show the following. (i) Mks1p is a strong negative regulator of CIT2 expression and does not effect NCR-sensitive expression of DAL5 or GAP1. (ii) Retrograde carbon and NCR-sensitive nitrogen metabolism are not linked by the quality of the nitrogen source, i.e. its ability to elicit NCR, but by the product of its catabolism, i.e. glutamate or ammonia. (iii) In some instances, we can dissociate rapamycin-induced CIT2 expression from Mks1p function, i.e. rapamycin does not suppress Mks1p-mediated down-regulation of CIT2 expression. These findings suggest that currently accepted models of Tor1/2p signal transduction pathway regulation require revision.
The GATA family of transcription factors are conserved from yeast to man. The Saccharomyces cerevisiae family consists of at least eight members, each possessing a homologous zinc-finger motif, CX2CN17–20CX2C, which binds to a sequence containing GATA at its core. Two of the best-studied GATA factors, Gln3p and Gat1p, activate transcription of many genes including those whose expression is Nitrogen Catabolite Repression (NCR)1-sensitive (1–4, 6, 7, 32).
Yeast cells face environments of widely varying nitrogen sources and, like most microorganisms, transport, accumulate, and utilize good nitrogen sources in preference to poor ones. NCR is the physiological mechanism for achieving this selectivity and is the preeminent control exerted over nitrogen catabolic gene expression in yeast. In excess nitrogen (e.g. glutamine, ammonia), transcription of genes encoding proteins needed to transport and degrade poor sources (e.g. proline, allantoin) is low, i.e. “repressed.” On the other hand, when nitrogen is limiting, transcription of these genes increases, i.e. is “derepressed” (1, 2).
Recently, our understanding of NCR-sensitive expression has greatly increased. Ure2p negatively regulates the ability of Gln3p and Gat1p to function (8, 9). When cells are grown in excess nitrogen, Gln3p and Gat1p are not bound to their target promoter sequences thus making these GATAs available, in some instances, to serve as surrogate TATA elements (10). This behavior correlates with green fluorescent protein-Gln3p and green fluorescent protein-Gat1p being nuclear when NCR-sensitive expression is high and cytoplasmic when low (10).
A major breakthrough in our understanding of the mechanism of NCR derives from reports that rapamycin, an immunosuppressant macroclide, which inhibits the Tor1/2 proteins, induces NCR-sensitive expression (11–14). Tor1, a non-essential protein, facilitates translational initiation and G1 to Sphase progression (15). Tor2p is essential and functions as does Tor1p but additionally participates in actin cytoskeleton reorganization and other functions as well (16–18). In cells growing in rich medium, Gln3p (and in some laboratories Ure2p as well) is hyperphosphorylated, forms a complex with Ure2p, and is localized to the cytoplasm (11–14). Inhibition of Torp activity with rapamycin or by limiting nitrogen results in dephosphorylation of Gln3p, decreased Ure2p-Gln3p complex formation, and nuclear localization of Gln3p (11–14).
Mks1p, a recent addition to the NCR regulatory network, has been identified three different ways. MKS1 (multicopy compensator of a kinase suppression) was first isolated as a gene whose products function downstream of protein kinase A (19). Second, MKS1 was found to be identical to LYS80, at first thought to encode a repressor of the lysine biosynthetic genes (20) but later found to down-regulate the α—ketoglutarate pool and several tricarboxylic acid cycle enzyme activities (21). The last identification of MKS1 was as a high copy suppressor of the NCR-sensitivity of ureidosuccinate (USA) uptake (22). USA, the first unique intermediate in uracil biosynthesis, can fulfill the auxotrophic requirement generated by a ura2 mutation with proline, but not ammonia, as a nitrogen source (8, 9). Nitrogen source restriction derives from USA permease (encoded by NCR-sensitive DAL5) production being repressed by ammonia (8, 9, 23). A ura2 transformant, containing approximately the 3′ half of MKS1 on a high copy vector, can use USA to cover the ura2 auxotrophy even with ammonia as the nitrogen source (22). Because ure2 is epistatic to mks1, which in turn is epistatic to rtg2, the regulatory circuit, NH3 → Rtg2p ⫞ Mks1p ⫞ Ure2p ⫞ Gln3p → DAL5, was proposed (22, 24). Mks1p is also required for conversion of Ure2p to its prion form [Ure3p] (25).
More recently, genomic analyses led Shamji et al. to agree that Mks1p is a positive regulator of rapamycin-induced, Gln3p-dependent gene expression and a positive regulator of Rtg1/3p-dependent retrograde gene expression (7). The retrograde regulon consists of genes, including CIT2, IDH1/2, and DLD3, whose expression is stimulated by damage to mitochondria and reduced in cells grown with glutamate as the sole nitrogen source (26, 27). Komeili et al. also reported that retrograde gene expression is rapamycin-induced and correlates with the quality of the nitrogen source provided, being lowest with preferred nitrogen sources thereby linking carbon and nitrogen regulation (28). They also, in agreement with Sekito et al., demonstrated that Rtg1/3p intracellular localization correlates with the level of retrograde expression, being nuclear when it is high and cytoplasmic when low (27).
Several observations, however, raise questions about our current understanding of the role of Mks1p in Tor1/2p signal transduction and NCR-sensitive and retrograde gene expression. (i) Mks1p, a proposed negative regulator of Ure2p, doesn’t play a role in Ure2p dephosphorylation, i.e. Ure2p dephosphorylation occurs normally in an mks1 mutant (7). (ii) Tap42p is phosphorylated by Tor1/2p (29), and a mutant allele, tap42–11, has been isolated in which Tap42 has lost its ability to respond to rapamycin addition. As a result, rapamycin-induction of processes downstream of Tap42p, e.g. Gln3p-dephosphorylation, are lost in the mutant as well (11). Surprisingly, however, rapamycin-induced Ure2p dephosphorylation occurs normally in the tap42–11 mutant (7). (iii) In most genetic backgrounds, glutamine and ammonia are more strongly repressive nitrogen sources than urea or glutamate (30). Yet in the measurements of Komeili et al., CIT2 expression occurs at the same high level with ammonia and urea as the nitrogen source and is severely reduced when glutamine or glutamate is provided instead (28). In other words, CIT2 expression doesn’t correlate with the quality of nitrogen source.
Paradoxical observations such as these prompted us to reinvestigate the relation of nitrogen quality to retrograde gene expression and the role of Mks1p as a positive regulator of Rtg1/3p-dependent transcription and negative regulator of Ure2p. Here we show that: (i) retrograde gene expression is controlled not by the quality of the nitrogen source, but the product to which it is degraded, (ii) Mks1p is not a positive but a strong negative regulator of retrograde gene expression, and (iii) Mks1p does not significantly or directly influence NCR-sensitive, GATA-mediated gene expression.
MATERIALS AND METHODS
Strains and Media
We used strains M970 (MATalys5/MATαlys2), YHE677 (MATa/MATα mks1Δ::G418/mks1Δ::G418 ura3/+), YHE815 (MATα ura2 leu2Δ::hisG trp1Δ::hisG (cross 83, spore 2a)), and YHE823–1b (MATα ura2 leu2Δ::hisG trp1Δ::hisG mks1Δ::G418 (cross 83, spore 1b)). Strains were grown at 30 °C to mid-log phase (A600 nm = 0.50–0.55) in Wickerham’s minimal medium (31) containing 2% glucose and the indicated nitrogen sources. Rapamycin (Sigma) (stock solution, 1 mg/ml in 10% Tween 20 plus 90% ethanol) was added to cultures, where indicated, to yield a final concentration of 0.3 μg/ml. Wickerham’s solid medium containing 2% glucose and 0.1% of either l-glutamic acid, l-glutamine, urea, ammonium sulfate, or asparagine, 0.2% l-proline, or 0.2% allantoin was used to evaluate growth. Plates were incubated at 30 °C for 3 days.
RNA Isolation and Northern Blot Analyses
Total RNA preparation and Northern blot analyses were performed as described (10) with the exception that the breaking buffer contained 0.5 m Tris base and diethoxydiformate was not added and 9 μg of total RNA were loaded per lane. Radiolabeled PCR products, using primers CIT2 (5′-TCAGGGAA-CAATATCAACAC-3′, 5′-CTGTTCTAATAGAACATCGC-3′); IDH1 (5′-CTGGTAACAATCAAGGTTCA-3′, 5′-CTTCTTTATGATCTGTTTGC-3′); IDH2 (5′-GAACCTGAATTACCTTGGTAG-3′, 5′-GACAAAGATAGGGCTAACATC-3′); DLD3 (5′-GTGCCAAAATGCTCCTCAAT-3′, 5′-TCTTCTGGAGCTTGAGAGTT-3′); GAT1 (5′-GTTCTGTTCATCGCATGTGCA-3′, 5′-GTATTATTGGCGATGCTGGGA-3′); GAP1 (5′-TGCCCAAACTCCATTGAAGC-3′, 5′-AATCTCCCACGGGGAATACA-3′); DAL5 (5′-AGATTTCCACTAGTTCAGCGG-3′, 5′-CCTACCAATTCAACAGCACCT-3′); MKS1 (5′-ACCCCAGAGCGATTGAATTT-3′, 5′-TCTTCATCATCTTCAGCG-3′); LYS1 (5′-ATGGCTGCCGTCACATTACAT-3′, 5′-TTGGCAGCAAAGAAGGCAA-3′); and H3 (5′-AAGCAAACAGCAAGAAAGTC3-′, 5′-CCTTCTTTTGGATAGTGACA3-′) were used as probes.
β-Galactosidase Assays
Strains, transformed with DAL5-lacZ pRR29, were grown in 2% glucose yeast nitrogen base medium with 0.1% proline or glutamine as the nitrogen source. Uracil (20 mg/liter), leucine (120 mg/liter), and tryptophan (20 mg/liter) were added to provide for auxotrophic requirements. β-galactosidase assays were performed as described (5).
RESULTS
Mks1p-regulated Retrograde Gene Expression Does Not Correlate with the Quality of the Nitrogen Source but the Product of Its Degradation
Komeili et al. concluded that rapamycin signaling links nitrogen quality to the activity and nuclear localization of retrograde transcription factors Rtg1/3p (31). Urea or ammonia as the nitrogen source elicited high retrograde gene expression, whereas glutamate and glutamine yielded low expression (28). However, for most strains, urea and glutamate are poorer nitrogen sources than ammonia and glutamine (30), arguing against the quality of the nitrogen source being the important determinant. There is, however, a physiologically significant correlation in the data of Komeili et al. It is the product of the degraded nitrogen source. Retrograde gene expression was high in both nitrogen sources that yielded ammonia as the degradative product and low in those yielding glutamate (28). To test this hypothesis, we measured gene expression in cells provided with a spectrum of nitrogen sources ranging from poor to good, some being degraded to ammonia and others to glutamate (Fig. 1). Expression of GAP1 and GAT1 was used as the control to monitor NCR, i.e. quality of the nitrogen source, while CIT2 and DLD3 expression were the reporters for retrograde gene expression.
Fig. 1. Effects of various nitrogen sources on NCR-sensitive and retrograde gene expression.
Wild type (M970) cells were grown in Wickerham’s minimal medium containing 0.2% l-proline (PRO), 0.2% allantoin (ALL), or 0.1% l-glutamic acid (GLU), urea, ammonium sulfate (+NH4), or l-glutamine (GLN) as the nitrogen source. Northern blots were hybridized with radiolabeled CIT2 (A), DLD3 (B) GAP1 (C), or GAT1 (D) probes. Histone (H3) was used as a control to assess loading and transfer.
Proline and allantoin, both poor nitrogen sources, support high level GAP1 expression (Fig. 1C, lanes A and B). Glutamate and urea behave similarly, i.e. support high GAP1 expression (lanes C and D). Ammonia and glutamine, in contrast, are good nitrogen sources for this strain and strongly repress GAP1 expression (lanes E and F). The NCR-sensitive GAT1 expression profile is similar to that of GAP1 (Fig. 1D). CIT2 and DLD3 expression profiles differ from those of GAP1 and GAT1, being higher with allantoin, urea, and ammonia than with proline or glutamate (Fig. 1, A and B). Allantoin and urea are degraded to ammonia, while proline is degraded to glutamate. The results with ammonia and glutamine are similarly correlated but more complex. Ammonia elicits less CIT2 expression than urea but more than glutamine or glutamate (Fig. 1A, lanes C–F). Ammonia is transported and assimilated faster than urea, which accounts for ammonia being repressive, while urea is not. Ammonia and urea assimilation occurs predominantly through the formation of glutamate, hence intracellular glutamate is higher with ammonia as the nitrogen source than with urea. Therefore, the CIT2 results are not surprising because CIT2 and DLD3 expression is highly repressed by glutamate (26–28). By this reasoning, glutamine assimilation, which yields equimolar amounts of glutamate and ammonia, should support less CIT2 and DLD3 expression than ammonia alone even though ammonia and glutamine are equally repressive nitrogen sources, and that is what occurs (compare lanes E and F). These data suggest the degradative product rather than ability to elicit NCR controls CIT2 and DLD3 expression.
Mks1p Is a Negative Regulator of Retrograde Gene Expression
A substantial regulatory network has developed around Mks1p and its relation to NCR-sensitive gene expression (7, 22, 24). Schreiber’s laboratory, using genomic analyses, reported that Mks1p transmits a signal from Tap42p to Rtg1/3p via the pathway rapamycin ⫞ Torp → Tap42 ⫞ Mks1p → Rtg1/3p → retrograde (CIT2) gene expression. In their model, Mks1p positively regulates Rtg1/3p (7). In light of data in Fig. 1, we reinvestigated Rtg1/3p-dependent expression in wild type and mks1Δ strains, measuring CIT2 expression in cells provided with proline, ammonia, or glutamine as the nitrogen source (Fig. 2A, lanes A and G, C and I, E and K). CIT2 is expressed at undetectable levels in wild type cells with proline and low levels with ammonia or glutamine as the nitrogen source (lanes A, C, and E). With all three nitrogen sources, CIT2 expression increases dramatically in the mks1Δ (lanes G, I, and K). Therefore, in contrast to the conclusions of Schreiber and co-workers (7), we find that Mks1p is a strong negative regulator of CIT2 expression. To ensure that the observations with CIT2 are not gene-specific but reflect retrograde expression per se, we performed similar experiments with DLD3, IDH1, and IDH2, all genes used in earlier analyses by Komeili et al. and Shamji et al. (Refs. 28 and 7, respectively). Expression of all three genes is low to undetectable in wild type proline-grown cells and greatly increases in the mks1Δ (Figs. 3C and 4, A and B, lanes A and G). Mks1p negative regulation of retrograde gene expression is not restricted to the use of proline as a nitrogen source. It also occurs with both ammonia and glutamine (Figs. 2–4, lanes C, E, I, and K). Changes between wild type and mks1Δ were less dramatic because of the greater basal level of retrograde gene expression with these nitrogen sources.
Fig. 2. Effects of mks1Δ and rapamycin on CIT2 and GAP1, or lysine on LYS1 expression.
Northern blot analyses of RNA from Wild type (W.T., M970) and mks1Δ (YHE677) strains grown in minimal medium with either 0.1% l-proline (PRO), 0.5% ammonium sulfate (+NH4), or 0.1% l-glutamine (GLN) as the sole nitrogen source with (+RAP) or without rapamycin treatment. Blots were hybridized with radiolabeled CIT2 (A) or GAP1 (B) probes. When lysine (66 mg/liter) was added to the medium, histidine (20 mg/liter) was also added to prevent basic amino acid imbalance.
Fig. 3. Effects of mks1Δ and rapamycin on DAL5, GAT1, and DLD3 expression.
Experiments were performed as in Fig. 2. Northern blots were hybridized with radiolabeled DAL5 (A), GAT1 (B), or DLD3 (C) probes.
Fig. 4. Effects of mks1Δ and rapamycin on IDH1, IDH2, and MKS1 expression.
Experiments were performed as in Fig. 2. Northern blots were hybridized with radiolabeled IDH1 (A), IDH2 (B), or MKS1 (C) probes. Histone (H3) was used as control to assess loadings.
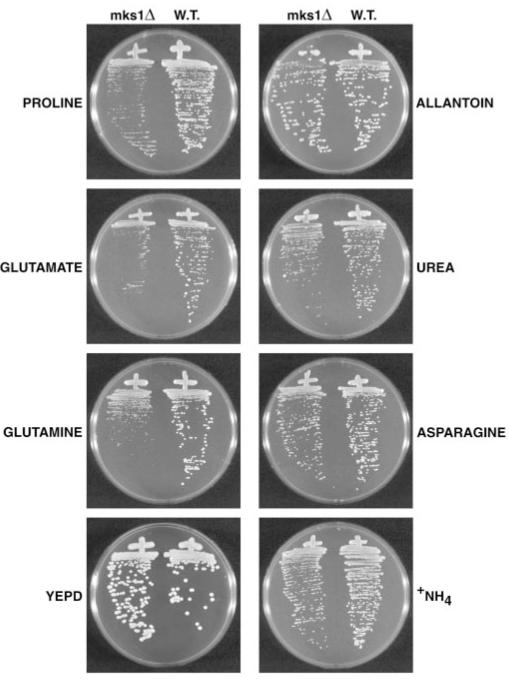
To determine whether failure to down-regulate retrograde gene expression adversely affects the cell, we grew wild type and mks1Δ strains on minimal glucose medium with a range of nitrogen sources. There was little if any effect of deleting MKS1 when the nitrogen source, irrespective of its quality, provided is degraded to ammonia (Fig. 5, right panel, allantoin, urea, asparagine). However, mks1Δ cells grow more slowly than wild type when the nitrogen source is degraded to glutamate (Fig. 5, left panels), arguing that excess retrograde gene expression is detrimental in the presence of glutamate.
Fig. 5. Growth of wild type (W. T., M970) and mks1Δ (YHE677) strains on various nitrogen sources.
W.T., cells were streaked onto yeast extract/peptone/dextrose or Wickerham’s minimal medium plates containing 0.2% l-proline, 0.2% allantoin, or 0.1% l-glutamic acid, urea, l-glutamine, l-asparagine, or ammonium sulfate as the sole nitrogen source. Plates were incubated at 30 °C for 3 days.
NCR-sensitive Gene Expression Is Not Affected in a mks1Δ
Significant differences between our views of Mks1p and those of Shamji et al., Edskes et al., and Pierce et al. prompted us to further query the conclusion that Mks1p is a negative regulator of Ure2p (7, 22, 24). If Mks1p is a negative regulator of Ure2p, it would be a positive regulator of Gln3p, Gat1p, and NCR-sensitive gene expression (22). Therefore, we compared the NCR sensitivity of GAP1 expression in wild type and mks1Δ strains. GAP1 expression is equally NCR-sensitive in the two strains; we see no evidence of the Mks1p-dependent GAP1 expression predicted by existing models (Fig. 2B, compare lanes A, C, and E with G, I, and K). To ensure that the observations were not GAP1-specific but reflected NCR-sensitive expression per se, we additionally assayed DAL5 and GAT1 expression and β-galactosidase production supported by a DAL5-lacZ fusion plasmid. The DAL5 expression profile is similar to that of GAP1, i.e. there is no demonstrable Mks1p dependence of expression or alteration of NCR sensitivity in the mks1Δ relative to wild type. The GAT1 expression profile differs little from that of DAL5, except that its NCR sensitivity is greater in the mks1Δ than wild type (Fig. 3B). NCR sensitivity is similarly unaltered when measured with a β-galactosidase assay. Wild type (YHE815) produced 28,276 and 949 units in proline versus glutamine medium, respectively, compared with 8,951 and 210 for mks1Δ YHE823–1b. These data argue against Mks1p having a role in NCR-sensitive gene expression.
A possible explanation of our failure to observe differences in NCR-sensitive gene expression between wild type and mks1Δ strains might have been due to previously unnoticed nitrogen-dependent modulation of MKS1 expression or a defect in the mks1Δ construction. Therefore, we measured MKS1 expression in wild type and mutant cells. It is affected by neither nitrogen source nor rapamycin-treatment of wild type cells (Fig. 4C, lanes A–F). MKS1 mRNA could not be detected in the mks1Δ (Fig. 4C, lanes G–L); the same was true when the autoradiogram was heavily overexposed or the blot analyzed with a phosphorimaging device (data not shown).
To rectify Mks1p data with that derived from lys80 mutants of Feller et al., we compared LYS1 expression in wild type and mks1Δ strains. Ramos et al. and Feller et al. report saccharopine dehydrogenase (encoded by LYS1) activity is 3–5-fold higher in a lys80 mutant than wild type growing in minimal ammonia + lysine medium (20, 21). We confirm their enzymatic data; LYS1 expression increases in an mks1Δ strain relative to wild type (Fig. 2C).
Rapamycin Induction of CIT2 Is Independent of Mks1p Regulation
According to the Schreiber model, rapamycin ⫞ Torp → Tap42 ⫞ Mks1p → Rtg1/3p → retrograde gene expression (7). That model posits Mks1p to be a positive regulator of Rtg1/3p, connecting retrograde transcription to the upper portions of the Torp pathway. Our evidence demonstrating Mks1p negatively, rather than positively, regulates CIT2 expression calls this model into question and prompted us to query the extent to which Mks1p mediates Tor1/2p control of CIT2 expression. We assayed retrograde and NCR-sensitive gene expression in wild type and mks1Δ strains treated with rapamycin. Higher concentrations of rapamycin than used by some investigators do not induce CIT2 expression in the wild type with proline as the nitrogen source even though they strongly induce DAL5 expression as previously reported by other laboratories (7, 12, 14) (Fig. 2, lanes A and B). Rapamycin does induce CIT2 expression with ammonia or glutamine as the nitrogen source (Fig. 2, lanes C–F), but in neither case does deletion of MKS1 affect expression (Fig. 2, lanes I–J). In contrast, deletion of MKS1 dramatically increases CIT2 expression in proline-grown cells even though rapamycin cannot. DLD3, IDH1, and IDH2 expression responded similarly, but here rapamycin, in contrast to the Schreiber model, was slightly inhibitory in the mks1Δ strain regardless of the nitrogen source (Figs. 3C and 4). At least with proline as the nitrogen source, the effects of rapamycin treatment and MKS1 deletion are clearly separable and suggest that Mks1p is unlikely to be a direct participant in rapamycin-induced CIT2 expression.
DISCUSSION
Four observations suggest that revisions are needed in the current model of Tor1/2p signal transduction, retrograde, and NCR-sensitive gene regulation. We find that: (i) Mks1p does not directly control NCR-sensitive gene expression, (ii) Mks1p is a strong negative regulator of retrograde gene expression, (iii) nitrogen catabolic and carbon retrograde gene expression are not linked by good versus poor nitrogen sources but by the product of nitrogen source degradation, i.e. compounds degraded to ammonia do not down-regulate retrograde expression, whereas those degraded to glutamate do, and (iv) Mks1p-mediated repression of retrograde gene expression is dissociable from that exerted by the Tor1/2p-mediated pathway.
Retrograde control regulates the amount of α-ketoglutarate available for ammonia assimilation into glutamate as manifested by down-regulation of retrograde gene expression with glutamate as the nitrogen source. Mks1p mediates this down-regulation as evidenced by the dramatic phenotype of mks1 mutants. While glutamate is the predominant regulator, ammonia may also play a role because CIT2 expression is greater with glutamine (which is degraded to ammonia and glutamate) than glutamate as the nitrogen source. Whether a direct link exists between Tor1/2p and Mks1p is questionable because the effects of rapamycin can be completely dissociated from Mks1p function with proline as the nitrogen source. The question cannot be answered for ammonia or glutamine because although CIT2 expression increases upon rapamycin treatment in wild type, it is already high in untreated mks1Δ cells and in some cases decreases on treatment with rapamycin. Mks1p affects NCR-sensitive expression only indirectly via α-ketoglutarate production and hence the rate of ammonia assimilation. To us, the function of Mks1p remains unknown. If Mks1p is a direct participant in control of CIT2 expression, it most likely does so by controlling nuclear access of Rtg1/3p, probably by regulating the phosphorylation state of these transcription factors. Consistent with this suggestion, Pierce et al. have shown that: (i) Mks1p is localized to the cytoplasm and (ii) an mks1 mutation is epistatic to one at rtg2; hence Mks1p functions between Rtg2p and the transcription factors Rtg1/3p that are required for activation of CIT2 expression (24). We have confirmed the conclusions of Pierce et al. concerning the mks1-rtg2 epistasis relationships using Northern blot analyses of GAP1, DAL5, and CIT2 gene expression (data not shown).
Differences between our conclusions and accepted models of NCR-sensitive and retrograde expression prompt an attempt to rectify the observations. Mks1p was concluded to be a positive regulator of retrograde expression from transcriptome analyses in which the ratios of CIT2 and DLD3 expression in treated versus untreated wild type cells (+7.1 and +6.4, respectively) decreased (+1.0 and −1.2, respectively) in mks1Δ cells (7). However, ratios can be ambiguous. The ratio of CIT2 expression in Fig. 2A, lanes D and J is close to one but much greater if lanes C and D are compared. This appears to concur with the Shamji et al. conclusion (7) even though the opposite is true when all of the data can be analyzed.
Komeili et al. report that the quality of a nitrogen source determines whether it will stimulate or inhibit retrograde gene expression, i.e. “preferred” nitrogen sources (glutamine and glutamate) down-regulate it (28). In contrast, we conclude that the significant feature is not nitrogen source quality but the product of degradation. Differences in interpretation derive from the S288c strain background they used and how one classifies various nitrogen sources. Ammonia is not a highly repressive nitrogen source in an S288c genetic background. Although the precise reason for this diminished repression has not been directly identified, it is an idiosyncrasy of this specific nitrogen source because other sources, e.g. asparagine or glutamine, elicit similarly repressive responses in both S288c and Σ1278b. Based on the data Komeili et al. observed, ammonia was not classified as a good nitrogen source (28). It was rather grouped with urea, a nitrogen source widely reported to elicit little NCR. This grouping may have diverted attention away from a correlation involving the end product of degradation, thereby leading them to conclude that nitrogen source quality was the important determinant. According to the conclusions of Komeili et al., both proline and allantoin should support high CIT2 expression because both are poor nitrogen sources. This expectation, however, isn’t supported experimentally (Fig. 1).
The third difference concerns whether Mks1p transmits the Tor1/2p signal of excess nitrogen to the retrograde genes. Here, the conclusion depends upon the data considered. mks1Δ, but not rapamycin-treatment, increases CIT2 expression in proline medium. With ammonia or glutamine, rapamycin induces CIT2 expression in the wild type. This increase cannot be concluded to occur in the mks1Δ, however, because CIT2 expression is already high and in some cases decreases upon treating mutant cells with rapamycin (Fig. 2). At least three interpretations immediately come to mind: (i) Mks1p and rapamycin belong to different regulatory pathways as far as CIT2 expression is concerned, (ii) Mks1p belongs to the Tor1/2p pathway and the differences are quantitative, or (iii) additional, unknown factors exist that function differentially, one set when Tor1/2p are active and another when they inactive, e.g. with proline as nitrogen source.
The last, and by far most challenging, observations to rectify derive from the USA uptake experiments (22). There is no doubt that overexpression of MKS1 suppresses NCR-sensitivity of DAL5 expression (22), but this can occur in two different ways: (i) Mks1p can negatively regulate Ure2p function, the prevailing view or (ii) Mks1p can diminish the ability of ammonia to bring about NCR. We suggest the latter best accounts for all of the data. We show that Mks1p is a strong negative regulator of CIT2 expression, which extends the earlier report that α-ketoglutarate increases in lys80/mks1 mutants along with citrate synthase, aconitase, and isocitrate dehydrogenase activities (21). We suggest that overproduction of Mks1p excessively down-regulates CIT2 expression and hence citrate synthase production needed to synthesize α-ketoglutarate. The resulting limitation of α-ketoglutarate decreases the rate of ammonia assimilation to glutamate thereby decreasing its ability to elicit NCR. This permits sufficient DAL5/UREP expression to meet the requirements of the selection scheme. This explanation also accounts for the recent report that DAL5-lacZ expression increases in an rtg2Δ (24). Because Rtg2p is required for nuclear localization of the retrograde transcriptional activators, Rtg1/3p, its loss would result in diminished CIT2 expression just as occurs with overexpression of MKS1 (26–28).
The most difficult incongruity to explain is the heterologous DAL5-lacZ expression data obtained by Edskes et al., Pierce et al., and in our laboratory (Ref. 22 and 24, respectively). The former researchers find that DAL5-lacZ expression decreases 70-fold in an mks1Δ relative to wild type cells growing in proline medium, whereas we see only a small (2.5-fold) decrease, which is not substantiated by our Northern blot data. Further, the NCR sensitivity of the β-galactosidase production we measure is not significantly different in wild type and mks1Δ cells. These differences may be an example of heterologous gene expression failing to mirror steady state mRNA data.
Acknowledgments
We thank Dr. Reed Wickner for very generously providing strains, Tim Higgins for preparing the artwork, and the University of Tennessee Yeast Group for suggestions to improve the manuscript.
Footnotes
The abbreviations used are: NCR, nitrogen catabolite repression; USA, ureidosuccinate.
This work was supported by National Institutes of Health Grant GM-35642.
REFERENCES
- 1.Hoffman-Bang J. Mol. Biotechnol. 1999;12:35–73. doi: 10.1385/MB:12:1:35. [DOI] [PubMed] [Google Scholar]
- 2.ter Schure EG, van Riel NA, Verrips CT. FEMS Microbiol. Rev. 2000;24:67–83. doi: 10.1111/j.1574-6976.2000.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Magasanik B. In: The Molecular and Cellular Biology of the Yeast Saccharomyces cerevisiae. Jones E, Pringle J, Broach J, editors. Cold Spring Harbor Press; Cold Spring Harbor, N Y: 1992. pp. 283–318. [Google Scholar]
- 4.Cooper TG. In: Mycota. Marzluf G III, Bambrl R, editors. Springer Verlag; Berlin: 1996. pp. 139–169. [Google Scholar]
- 5.Rai R, Genbauffe FS, Sumrada RA, Cooper TG. Mol. Cell. Biol. 1989;9:602–608. doi: 10.1128/mcb.9.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox KH, Pinchak AB, Cooper TG. Yeast. 1999;15:703–713. doi: 10.1002/(SICI)1097-0061(19990615)15:8<703::AID-YEA413>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Shamji AF, Kuruvilla FG, Schreiber SL. Cur. Biol. 2000;10:1574–1581. doi: 10.1016/s0960-9822(00)00866-6. [DOI] [PubMed] [Google Scholar]
- 8.Drillien R, Lacroute F. J. Bacteriol. 1972;109:203–208. doi: 10.1128/jb.109.1.203-208.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drillen RM, Aigle M, Lacroute F. Biochem. Biophys. Res. Commun. 1973;53:367–372. doi: 10.1016/0006-291x(73)90671-2. [DOI] [PubMed] [Google Scholar]
- 10.Cox KH, Rai R, Distler M, Daugherty JR, Coffman JA, Cooper TG. J. Biol. Chem. 2000;275:17611–17618. doi: 10.1074/jbc.M001648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck T, Hall MN. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 12.Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardwick JS, Kuruvilla FG, Tong JF, Shamji AF, Schreiber SL. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram PG, Choi JH, Carvalho J, Ai W, Zeng C, Chan T-F, Zheng XFS. J. Biol. Chem. 2000;275:35727–35733. doi: 10.1074/jbc.M004235200. [DOI] [PubMed] [Google Scholar]
- 15.Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. Mol. Biol. Cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennis PB, Fumagalli S, Thomas G. Cur. Opin. Genet. Develop. 1999;9:49–54. doi: 10.1016/s0959-437x(99)80007-0. [DOI] [PubMed] [Google Scholar]
- 17.Schmelzle T, Hall MN. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 18.Rohde J, Heitman J, Cardenas ME. J. Biol. Chem. 2001;276:9583–9586. doi: 10.1074/jbc.R000034200. [DOI] [PubMed] [Google Scholar]
- 19.Matsuura A, Anraku Y. Mol. Gen. Genet. 1993;238:6–16. doi: 10.1007/BF00279524. [DOI] [PubMed] [Google Scholar]
- 20.Ramos F, Wiame J-M. Mol. Gen. Genet. 1985;200:291–294. doi: 10.1007/BF00425438. [DOI] [PubMed] [Google Scholar]
- 21.Feller A, Ramos F, Pierard A, Dujbois E. Yeast. 1997;13:1337–1346. doi: 10.1002/(SICI)1097-0061(199711)13:14<1337::AID-YEA186>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Edskes HK, Hanover JA, Wickner RB. Genet. 1999;153:585–594. doi: 10.1093/genetics/153.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turoscy V, Cooper TG. J. Bacteriol. 1987;169:2598–2600. doi: 10.1128/jb.169.6.2598-2600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce MM, Maddelein M-L, Roberts T, Wickner R. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13213–13218. doi: 10.1073/pnas.181486098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edskes HK, Wickner RB. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6625–6629. doi: 10.1073/pnas.120168697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhegchang L, Butow RA. Mol. Cell. Biol. 1999;19:6720–6728. doi: 10.1128/mcb.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekito T, Thornton J, Butow RA. Mol. Biol. Cell. 2000;11:2103–2115. doi: 10.1091/mbc.11.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komeili A, Wedaman KP, O’Shea EK, Powers T. J. Cell Biol. 2000;151:863–878. doi: 10.1083/jcb.151.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Broach JR. EMBO J. 1999;18:2782–2792. doi: 10.1093/emboj/18.10.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper TG. In: The Molecular Biology of the Yeast Saccharomyces cerevisiae: Metabolism and Gene Expression. Strathern JN, Jones EW, Broach J, editors. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1982. pp. 39–99. [Google Scholar]
- 31.Wickerham LJ. J. Bacteriol. 1946;52:293–301. doi: 10.1128/JB.52.3.293-301.1946. [DOI] [PubMed] [Google Scholar]
- 32.Cooper TG. FEMS Microbiol. Rev. 2002;737:1–16. doi: 10.1111/j.1574-6976.2002.tb00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]