Abstract
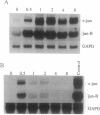
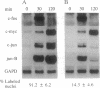
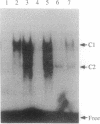
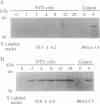
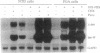
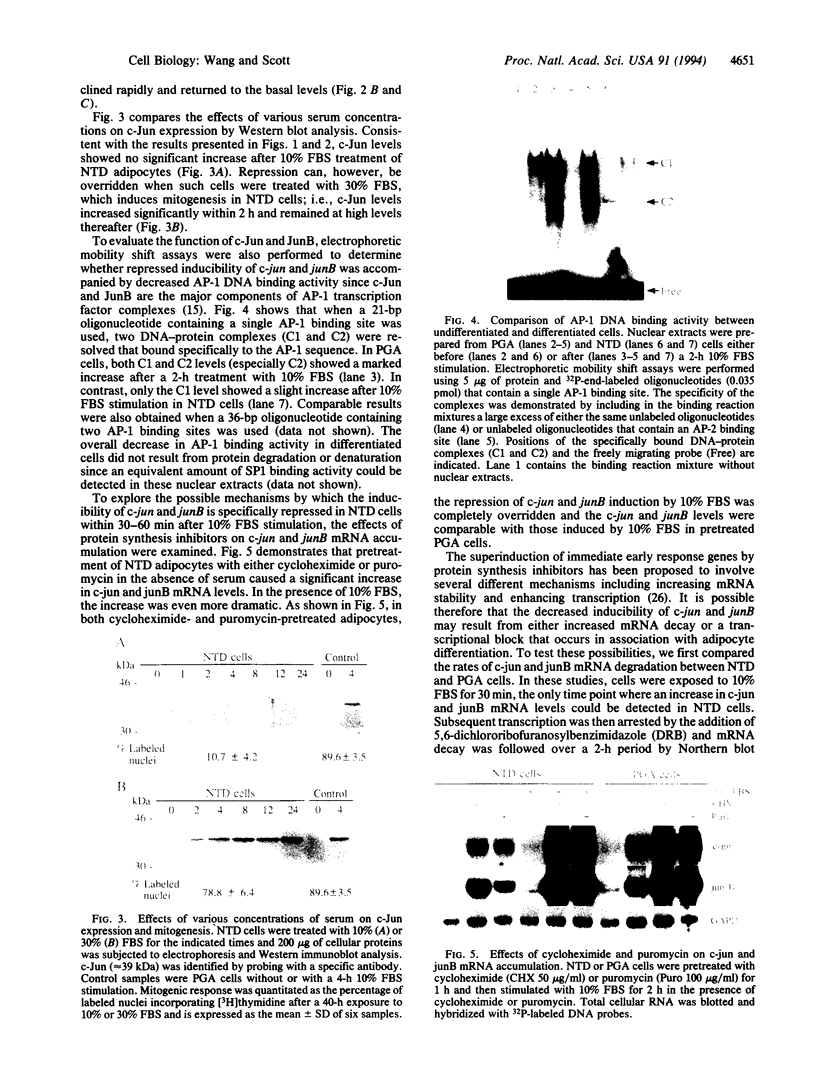
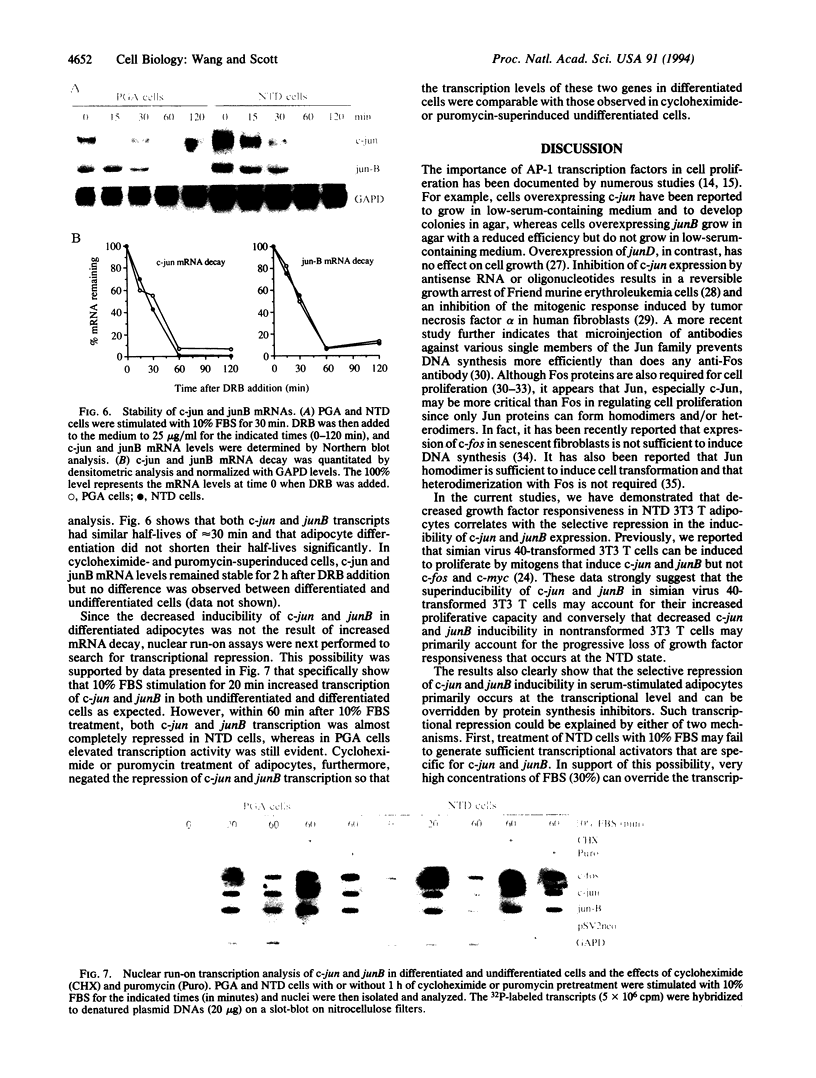
Nonterminally differentiated 3T3 T adipocytes are resistant to growth stimulation by 10% (vol/vol) fetal bovine serum even though they can be induced to proliferate with extremely high serum concentrations. We now report that in adipocytes 10% fetal bovine serum also fails to typically induce c-jun or junB. Rather, after 10% fetal bovine serum treatment, c-jun and junB expression is markedly repressed after a brief initial slight induction. Gel mobility shift studies confirm that AP-1 DNA binding activity is inhibited in adipocytes. Repression in c-jun and junB inducibility in adipocytes results from transcriptional mechanisms, can be reversed by treatment with protein synthesis inhibitors or higher serum concentrations, and does not affect c-fos or c-myc expression. These data suggest that adipocyte differentiation selectively and transcriptionally represses the inducibility of c-jun and junB so as to decrease the cell's ability to proliferate in response to 10% fetal bovine serum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Bengal E., Ransone L., Scharfmann R., Dwarki V. J., Tapscott S. J., Weintraub H., Verma I. M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992 Feb 7;68(3):507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Brach M. A., Gruss H. J., Sott C., Herrmann F. The mitogenic response to tumor necrosis factor alpha requires c-Jun/AP-1. Mol Cell Biol. 1993 Jul;13(7):4284–4290. doi: 10.1128/mcb.13.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., Spyrou G., La Vista N., Dangy J. P., Piu F., Yaniv M., Brun G. Overexpression of c-jun, junB, or junD affects cell growth differently. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8890–8894. doi: 10.1073/pnas.88.20.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Djian P., Phillips M., Green H. Suppression of SV40-promoted gene expression by differentiation of preadipose cells. Genes Dev. 1988 Oct;2(10):1251–1257. doi: 10.1101/gad.2.10.1251. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Mahadevan L. C. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992 Jul;11(7):2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estervig D. N., Minoo P., Tzen C. Y., Scott R. E. Inhibition of simian virus 40 T-antigen expression by cellular differentiation. J Virol. 1989 Jun;63(6):2718–2725. doi: 10.1128/jvi.63.6.2718-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S. O., Geddes T. J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992 Apr 17;256(5055):379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Hoerl B. J., Scott R. E. Nonterminally differentiated cells express decreased growth factor responsiveness. J Cell Physiol. 1989 Apr;139(1):68–75. doi: 10.1002/jcp.1041390111. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Sehgal A., Hadman M., Bos T. Heterodimerization with c-Fos is not required for cell transformation of chicken embryo fibroblasts by Jun. Cell Growth Differ. 1992 Dec;3(12):889–897. [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P., Sullivan W., Solomon L. R., Martin T. E., Toft D. O., Scott R. E. Loss of proliferative potential during terminal differentiation coincides with the decreased abundance of a subset of heterogeneous ribonuclear proteins. J Cell Biol. 1989 Nov;109(5):1937–1946. doi: 10.1083/jcb.109.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. D., Pignolo R. J., Cristofalo V. J. Insulin-like growth factor-I: specific binding to high and low affinity sites and mitogenic action throughout the life span of WI-38 cells. J Cell Physiol. 1987 Oct;133(1):135–143. doi: 10.1002/jcp.1041330117. [DOI] [PubMed] [Google Scholar]
- Phillips P. D., Pignolo R. J., Nishikura K., Cristofalo V. J. Renewed DNA synthesis in senescent WI-38 cells by expression of an inducible chimeric c-fos construct. J Cell Physiol. 1992 Apr;151(1):206–212. doi: 10.1002/jcp.1041510126. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Inducible binding of a factor to the c-fos enhancer. Cell. 1986 Dec 5;47(5):777–784. doi: 10.1016/0092-8674(86)90520-9. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K., Schiff J., Gilman M. Z. Transcription factor AP-1 activity is required for initiation of DNA synthesis and is lost during cellular aging. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):157–161. doi: 10.1073/pnas.89.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D. W., McCabe G., Feramisco J. R., Adler M. Expression of c-fos and AP-1 activity in senescent human fibroblasts is not sufficient for DNA synthesis. J Cell Biol. 1992 Dec;119(6):1405–1411. doi: 10.1083/jcb.119.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski H. B., Wheeler T. T., Young D. A. Gene expression during 3T3-L1 adipocyte differentiation. Characterization of initial responses to the inducing agents and changes during commitment to differentiation. J Biol Chem. 1992 Mar 5;267(7):4722–4731. [PubMed] [Google Scholar]
- Scott R. E., Estervig D. N., Tzen C. Y., Minoo P., Maercklein P. B., Hoerl B. J. Nonterminal differentiation represses the neoplastic phenotype in spontaneously and simian virus 40-transformed cells. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1652–1656. doi: 10.1073/pnas.86.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Hoerl B. J., Wille J. J., Jr, Florine D. L., Krawisz B. R., Yun K. Coupling of proadipocyte growth arrest and differentiation. II. A cell cycle model for the physiological control of cell proliferation. J Cell Biol. 1982 Aug;94(2):400–405. doi: 10.1083/jcb.94.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell C., Ptasznik A., Chang C. D., Swantek J., Cristofalo V. J., Baserga R. IGF-1 receptor levels and the proliferation of young and senescent human fibroblasts. Biochem Biophys Res Commun. 1993 Jul 15;194(1):259–265. doi: 10.1006/bbrc.1993.1813. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Sidhu R. S. Two-dimensional electrophoretic analyses of proteins synthesized during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1979 Nov 10;254(21):11111–11118. [PubMed] [Google Scholar]
- Smith M. J., Prochownik E. V. Inhibition of c-jun causes reversible proliferative arrest and withdrawal from the cell cycle. Blood. 1992 Apr 15;79(8):2107–2115. [PubMed] [Google Scholar]
- Subramaniam M., Schmidt L. J., Crutchfield C. E., 3rd, Getz M. J. Negative regulation of serum-responsive enhancer elements. Nature. 1989 Jul 6;340(6228):64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Trouche D., Grigoriev M., Lenormand J. L., Robin P., Leibovitch S. A., Sassone-Corsi P., Harel-Bellan A. Repression of c-fos promoter by MyoD on muscle cell differentiation. Nature. 1993 May 6;363(6424):79–82. doi: 10.1038/363079a0. [DOI] [PubMed] [Google Scholar]
- Wang H. L., Scott R. E. Insulin-induced mitogenesis associated with transformation by the SV40 large T antigen. J Cell Physiol. 1991 Apr;147(1):102–110. doi: 10.1002/jcp.1041470114. [DOI] [PubMed] [Google Scholar]
- Wang H., Scott R. E. Induction of c-jun independent of PKC, pertussis toxin-sensitive G protein, and polyamines in quiescent SV40-transformed 3T3 T cells. Exp Cell Res. 1992 Nov;203(1):47–55. doi: 10.1016/0014-4827(92)90038-a. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang J. Y., Johnson L. R., Scott R. E. Selective induction of c-jun and jun-B but not c-fos or c-myc during mitogenesis in SV40-transformed cells at the predifferentiation growth arrest state. Cell Growth Differ. 1991 Dec;2(12):645–652. [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Aproliferin--a human plasma protein that induces the irreversible loss of proliferative potential associated with terminal differentiation. Am J Pathol. 1986 Dec;125(3):546–554. [PMC free article] [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Defective control of terminal differentiation and its role in carcinogenesis in the 3T3 T proadipocyte stem cell line. Cancer Res. 1985 Jul;45(7):3339–3346. [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Polypeptide changes associated with loss of proliferative potential during the terminal event in differentiation. J Cell Biochem. 1987 Feb;33(2):137–150. doi: 10.1002/jcb.240330208. [DOI] [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Regulation of the terminal event in cellular differentiation: biological mechanisms of the loss of proliferative potential. J Cell Biol. 1986 May;102(5):1955–1964. doi: 10.1083/jcb.102.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. S., Samuels H. H. Cell- and sequence-specific binding of nuclear proteins to 5'-flanking DNA of the rat growth hormone gene. J Biol Chem. 1987 May 5;262(13):6313–6317. [PubMed] [Google Scholar]