Abstract
Purpose of review
This review summarizes recent findings on the regulation of vascular tone by the nuclear receptor transcription factor, peroxisome proliferator-activated receptor (PPAR) γ. Much of the recent work utilize genetic tools to interrogate the significance of PPARγ in endothelial and smooth muscle cells and novel PPARγ target genes have been identified.
Recent findings
Endothelial PPARγ prevents inflammation and oxidative stress, while promoting vasodilation by controlling the regulation of NADPH oxidase, catalase and superoxide dismutase gene expression. Moreover, the protective functions of endothelial PPARγ appear more prominent during disease conditions. Novel findings also suggest a role for endothelial PPARγ as a mediator of whole body metabolism. In smooth muscle cells, PPARγ regulates vascular tone by targeting genes involved with contraction and relaxation signaling cascades, some of which via transcriptional activation, and some through novel mechanisms regulating protein turnover. Furthermore, aberrant changes in renin-angiotensin system components and exacerbated responses to angiotensin II-induced vascular dysfunction are observed when PPARγ function is lost in smooth muscle cells.
Summary
With these recent advances based partially on lessons from patients with PPARγ mutants, we conclude that vascular PPARγ is protective and plays an important role in regulation of vascular tone.
Keywords: PPARγ, endothelial cells, smooth muscle cells, vasculature, vessel tone
Introduction
The purpose of this review is to highlight mechanisms by which peroxisome proliferator-activated receptor (PPAR) γ regulates vascular tone. Vascular tone is a key determinant of systemic vascular resistance and local tissue perfusion. It is dependent on the function of the endothelium, smooth muscle cells and to some extent on the perivascular adipose tissue (PVAT). As vascular cell type-specific functions of PPARγ have just started to emerge, the authors will discuss key findings from previous studies that provide insights into the mechanisms of PPARγ action. We will focus the review on the function of PPARγ in endothelial and smooth muscle cells, particularly mechanisms uncovered by the use of genetically engineered mice as models to overcome the limitations of systemic thiazolidinedione (TZD) administration. Overall, numerous reports consistently support the concept that vascular PPARγ is protective.
PPARγ and Mechanism of Actions
PPARγ is a nuclear hormone receptor transcription factor. Two human PPARγ isoforms have been identified, PPARγ1 and PPARγ2, which differ by a 30-amino acid extension at the N terminus. PPARγ2 (the longer form) is highly expressed in adipose tissue, where its function is a prerequisite for adipogenesis [1]. PPARγ1 is ubiquitously expressed at a low level in other tissues where it exerts cell-specific functions. PPARγ in macrophages regulates immune defense through the regulation of a distinct set of genes, with little overlap with PPARγ-target genes in adipocytes [2].
PPARγ regulates gene expression by forming a heterodimeric complex with the retinoid X receptor (RXR) at the PPAR response elements (PPRE). Without ligand, the transcription of PPARγ target genes is repressed due to the interaction of PPARγ with co-repressor complexes. Upon ligand binding, PPARγ undergoes a conformational change in the ligand binding domain and promotes gene expression through recruitment of a co-activator complex [1, 3].
Clues to PPARγ-Regulated Blood Pressure and Vascular Function
The evidence that implicates PPARγ in the regulation of cardiovascular homeostasis comes primarily from the observations that: 1) TZD, potent PPARγ agonists, lower blood pressure and alleviate cardiovascular diseases [4] and 2) patients with some PPARγ mutations develop severe early-onset hypertension [5]. However, the molecular mechanism and the site of action for PPARγ-mediated blood pressure regulation requires further investigation.
TZDs are high affinity synthetic ligands of PPARγ, previously used to treat patients with type 2 diabetes. The effectiveness of the TZD class in regulating glycemic control occurs through multiple mechanisms including augmented β-cell function and enhanced insulin sensitivity in skeletal muscle, adipocyte and liver [6**]. Induction of adiponectin, fibroblast growth factor family (FGF) 1 and FGF21 by TZD were reported to be important mediators for the insulin sensitizing effect of a TZD [3, 7-9]. In combination, the activation of these mechanisms by TZD provides a long lasting glycemic control compared to sulfonylureas and metformin [6*]. However, reports of serious adverse events including weight gain, congestive heart failure, fluid retention and osteoporosis have limited the clinical usefulness of TZD. The controversy was sparked in 2007 by a meta-analysis which reported the association of rosiglitazone with increased risk of myocardial infarction and cardiovascular deaths [10]. Despite the limitations of meta-analysis, this study raised serious public concerns and the prescription of rosiglitazone was subsequently restricted. As of last year, the FDA recommended that this restriction be removed after an independent re-adjudication showed no cardiovascular harms associated with the use of rosiglitazone [11**]. Although the results were not significantly different, fewer deaths from a cardiovascular cause were observed in patients received rosiglitazone compared to those treated with metformin and sulfonylurea. Another member of the TZD class, pioglitazone has been shown to reduce cardiovascular outcomes in diabetic patients [11**]. It has been speculated that the improvement of lipid profile by pioglitazone might account for these differences [6**]. However, it is important to note that safety concerns reporting increased risk of bladder cancer with pioglitazone have also been raised [12**].
Interestingly, multiple studies in human and animal models have shown that TZDs cause a modest decrease in both diastolic and systolic blood pressure, an effect that is not observed with other insulin sensitizer drugs [4]. It is interesting to note that TZD-mediated blood pressure lowering occurs despite edema and plasma volume expansion, implicating robust non-renal actions of PPARγ to lower blood pressure. Consistent with this is the observation that patients with some PPARγ mutations exhibit severe early onset hypertension [5, 13**, 14, 15]. This suggests the plausible hypothesis that there is a direct non-renal action of PPARγ, perhaps in the vasculature. That the same mutations also cause lipodystrophy, dyslipidemia, insulin resistance and type 2 diabetes argues either that the effects of the mutations are pleiotropic, that is, they target multiple tissues, or the hypertension might be a secondary consequence from metabolic changes. A knock-in mouse model carrying the mouse equivalent to one of the PPARγ mutations (P465L) originally identified in patients, exhibited hypertension and cerebral arterial dysfunction despite normal insulin sensitivity, thus arguing against the latter [16]. This is further supported by studies where PPARγ activity was manipulated by deletion or over-expression of PPARγ mutants in a vascular cell type-specific manner which resulted in blood vessel dysfunction, independent of metabolic abnormalities [17-21**]. Moreover, transfection of vascular cells in vitro with two different mutants in PPARγ (R165T and L339X), which were reported to cause severe hypertension in patients, recapitulated a robust induction of the renin-angiotensin system (RAS) and increased inflammation, and phenocopied what occurred in cells isolated from patients [13**]. Taken together, these studies reinforce the concept of the direct actions of PPARγ in vascular cells.
Roles of PPARγ in Endothelium
Many studies have shown that endothelial PPARγ has anti-inflammatory and anti-oxidant actions, while promoting vasodilatation (Figure 1). TZD treatment of cultured vascular endothelial cells increases nitric oxide (NO) production via post-translational modification of eNOS [22] and preserves NO bioavailability through suppressing NADPH oxidase expression [23]. Activation of PPARγ in the vasculature counteracts endothelin-1-induced constriction by induction of endothelin receptor type B expression in endothelial layers [24]. It was reported that endothelial PPARγ is required for the blood pressure lowering effect of TZD [25, 26]. Endothelial-specific disruption of a conditional allele of PPARγ (PPARγflox) with Tie2-promoter driven Cre-recombinase (Tie2Cre) resulted in mild hypertension and endothelial dysfunction that was associated with reduced NO production, increased reactive oxygen species, and enhanced NFκB activity [27]. In contrast, another study with Tie2Cre-mediated PPARγ disruption reported no change in systemic blood pressure at baseline but disrupted diurnal variations of blood pressure and heart rate [28]. Femoral arterial reactivity to phenylephrine, angiotensin II and KCI were significantly increased in Tie2Cre/PPARγflox mice [26]. Other phenotypes outside the vasculature were reported in studies using Tie2Cre, most likely because the promoter is active in cells other than endothelium [29, 30]. For this reason, other investigators have utilized mice with vascular endothelial-cadherin (cdh5)-Cre recombinase-driven PPARγ deletion. These mice showed aggravated ischemia-induced blood-brain barrier disruption due to cerebrovascular permeability, with no change in systemic blood pressure [31**]. Mechanistically, Kruppel-like factor (KLF)-11 has been identified as a PPARγ gene target and was reported to function as a PPARγ co-regulator in cerebral vascular endothelial cells. Pioglitazone-mediated vascular protection following middle cerebral artery occlusion was significantly lost in KLF11 null mice [31**].
Figure 1. Role of PPARγ in Vascular Endothelial Cells.
In endothelial cells, PPARγ modulates target gene expression involved with oxidative stress, inflammation, cell survival and fatty acid transporters. Endothelial PPARγ promotes vasodilation through enhanced nitric oxide (NO) production, increased NO bioavailability and decreased reactive oxygen species (ROS). Activation of PPARγ in the vasculature counteracts endothelin-1-induced vasoconstriction by induction of ETBR expression. PPARγ ameliorates inflammation in endothelial cells, perhaps through trans-repression of NFκB. It has been shown that KLF11 is necessary for pioglitazone-mediated vascular protection following middle cerebral artery occlusion. KLF11 is a direct target of PPARγ and also functions as a PPARγ co-regulator in cerebral vascular endothelial cells. Other evidence also suggests that PPARγ in endothelial cells is important for metabolic balance and lipid accumulation in the periphery, possibly by regulating the fatty acid transporters, Fatty acid translocase (CD36), Fatty acid binding protein 4 (aP2) and retinol binding protein (RBP)-7. Retinoid X receptor (RXR); Cu/Zn superoxide dismutase (SOD); Endothelin receptor type B (ETBR); Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); Kruppel-like factor (KLF)-11.
Emerging evidence highlights the prominent role of endothelial PPARγ during stress conditions. Transgenic mice expressing a dominant negative PPARγ mutant (V290M or P467L) specifically in endothelium exhibited reduced vasodilation to acetylcholine in basilar artery and aorta after prolonged high fat diet treatment [20] or during dyslipidemia induced by disruption of Apolipoprotein E, but not at baseline [32**]. This impairment was restored by superoxide scavenger, suggesting increased oxidative stress caused by the loss of PPARγ function. A significant increase in transcription of pro-oxidant genes such as p22phox, Noxo2, and NoxA2, concomitant with a reduction of mRNA of anti-oxidant catalase and Cu/Zn SOD was observed in vascular endothelium from these mice [20]. This is consistent with catalase and Cu/Zn SOD being PPARγ target genes [33, 34]. It is entirely possible that endogenous PPARγ ligands, perhaps derived from free fatty acids, induce upregulation of these genes during high fat feeding in normal mice, and this provides a protective mechanism. The downregulation of catalase and Cu/Zn SOD transcripts in the transgenic mice is potentially a direct consequence of the failure of endogenous PPARγ ligands to increase the transcriptional activity of the V290M and P467L mutants of PPARγ which are located in the ligand binding domain [15].
Endothelial dysfunction is often superimposed with obesity and type 2 diabetes. A novel role for endothelial cells in energy storage through fatty acid release in and out of the peripheral tissues has started to emerge [35]. Indeed, classic PPARγ transcriptional targets involved with fatty acid metabolism such as CD36, aP2 and RBP7 were shown to be expressed in endothelial cells [18]. Activation of PPARγ in microvascular endothelial cells markedly increased expression of aP2 and CD36 [36*]. Down-regulation of these fatty acid transporters mRNA in endothelium from endothelial-specific PPARγ knockout mice was associated with dyslipidemia after high fat overload [18,36*]. Unexpectedly, these mice manifested reduced white adipose tissue mass and improved insulin sensitivity following high fat diet, effects which persisted even after reconstitution of hematopoietic cells in the endothelial-specific PPARγ knockout, suggesting that endothelial PPARγ is critical for metabolic balance and lipid accumulation in periphery [18]. Despite improving insulin sensitivity, impaired vasorelaxation and hypertension following high fat diet was observed [18]. These findings suggest that endothelial PPARγ might regulate vascular function by targeting a set of genes distinct from those responsible for metabolism. This study also enforces the hypothesis that endothelial PPARγ is required for vascular protection during fatty acid overload.
Roles of PPARγ in Smooth Muscle
The net signaling in smooth muscle initiated by neurohumoral factors as well as intrinsic properties of the vessels determines the tone of the vasculature (Figure 2). Specific expression of dominant negative mutant PPARγ (P467L) in smooth muscle cells of transgenic mice (termed S-P467L) resulted in hypertension, vascular dysfunction, cerebral arteriole remodeling, tachycardia and baroreflex dysfunction [19, 37**]. Aberrant vascular reactivity is observed in both conduit and resistance arteries, emphasizing the critical role of PPARγ in regulation of the blood vessel tone. In aorta and cerebral arteries, interference of PPARγ contributed to loss of responsiveness to NO-dependent vasorelaxation [38, 39**]. Strikingly, the hypercontractile phenotype in response to receptor-dependent agonists is apparent in these transgenic mice. Inhibition of Rho kinase not only blunted the augmented constriction, but it also restored the impaired NO responsiveness in aorta and cerebral arteries from these transgenic mice [38, 39**], which is consistent with aberrantly increased Rho kinase activity. Mechanistically, mutant PPARγ led to a defect in RhoA degradation caused by a reduced expression of Cullin-3, a component of an E3-ubiquitin ligase complex-mediating proteasomal degradation of different substrates including RhoA. In smooth muscle cells, down-regulated Cullin-3 expression significantly increased RhoA [38]. Pre-incubation of isolated aorta from a normal mouse with a pan-Cullin inhibitor resulted in augmented vasoconstriction that was dependent on Rho kinase, resembling the phenotype observed in S-P467L aorta. RhoBTB1, a potential Cullin-3 adapter was identified as a transcriptional target of PPARγ and its expression was dramatically decreased by P467L mutant PPARγ [38]. It remains unclear if loss of this specific adapter contributes to suppression of Cullin-3 activity and reduced RhoA degradation.
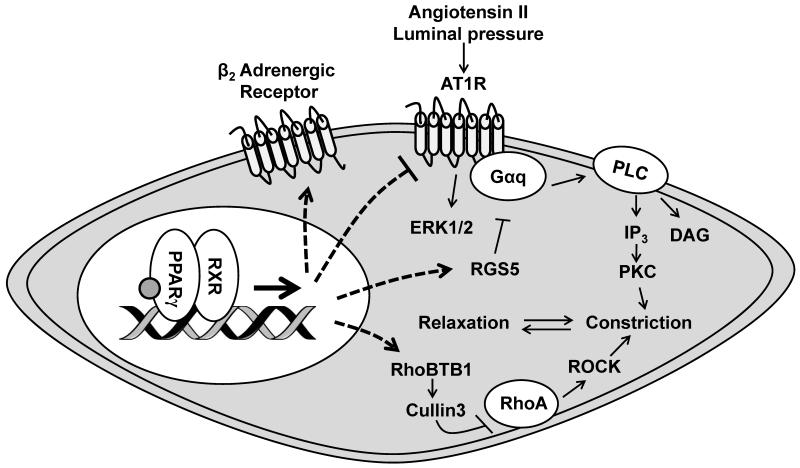
Figure 2. Role of PPARγ in Vascular Smooth Muscle Cells.
PPARγ in smooth muscle cells regulates vascular tone via transcriptional activation, and some through novel mechanisms regulating protein turnover (RhoBTB1/Cullin 3). Downregulation of RhoBTB1/Cullin 3 in the blood vessel caused by smooth muscle PPARγ mutant is associated with increased RhoA/Rho kinase activity, leading to increased contraction. Smooth muscle PPARγ is also important for normal myogenic tone via regulating Regulator of G-protein signaling (RGS)-5 transcript. Furthermore, exacerbated responses to angiotensin II either by up-regulated Angiotensin II receptor, type 1 (AT1R) or decreased RGS5 are observed when PPARγ function is lost in smooth muscle cells and that potentially contributes to increased oxidative stress and vascular hypertrophy. In addition to AT1R, other evidence from newly identified PPARγ mutants indicates that PPARγ is critical in regulating renin and angiotensinogen expression. Transcription of β2 adrenergic receptor has also been reported to be regulated by smooth muscle PPARγ, thereby promoting vasodilation. Retinoid X receptor (RXR); Rho-related BTB domain-containing protein (Rho BTB)-1; Ras homolog family member A (RhoA); Rho-associated protein kinase (ROCK); Inositol trisphosphate (IP3); Diacylglycerol (DAG); Phospholipase C (PLC); Protein kinase C (PKC); Mitogen-activated protein kinase (ERK1/2).
The role of PPARγ in regulation of myogenic tone has also been reported. Myogenic tone is an intrinsic property of smooth muscle in resistance arterial beds. This mechanism allows precise control of local blood flow despite the fluctuation of systemic blood pressure. Inhibition of PPARγ function mediated by dominant negative expression in smooth muscle (S-P467L) resulted in a marked increase in myogenic constriction in small mesenteric arteries. Augmented basal tone is attributable to a robust downregulation of the regulator of G protein signaling 5 (RGS5) mRNA and subsequent increase in protein kinase C activity. Loss of RGS5 transcript in S-P467L mesenteric arteries is also associated with a selective increase in angiotensin II-induced vasoconstriction and ERK1/2 activation [40, 41*], consistent with findings from RGS5 null mice [42*]. Several lines of investigation reveal that RGS5 is a novel PPARγ target. For example, PPARγ bound to a PPRE in the RGS5 locus and TZD increased RGS5 transcript, an effect that was completely lost in resistance vessels with dominant negative PPARγ expression [40].
Down-regulation of PPARγ expression or activity has often been correlated with increased AT1 receptor (AT1R) expression, and a recent study implicated hypoxia-inducible factor (HIF)-1α [43*]. Loss of HIF1α in smooth muscle resulted in suppressed PPARγ expression and subsequent upregulation of AT1R; and smooth muscle-specific HIF1α knockout mice exhibited augmented vasoconstriction to angiotensin-II and increased systolic blood pressure. Recent clinical evidence also highlights the interaction between PPARγ and the renin-angiotensin system (RAS). Patients with mutations in the DNA binding domain (R165T) or ligand binding domain (L339X, causing a truncation) of PPARγ exhibited lipodystrophy, insulin resistance and severe hypertension. It is surprising to note that in addition to AT1R, other RAS components including renin and angiotensinogen were markedly increased in fibroblasts or blood mononuclear cells isolated from these patients [13**]. The authors reported that the effect of the mutations was reproduced in transfected vascular smooth muscle cells, which lead them to conclude the induction of the RAS was due to a direct effect of loss of PPARγ function. AT1R antagonist reportedly improved blood pressure control in these patients [13**], but blood vessel function was not studied. It is notable that AT1R has been recently shown to act as a mechanosensor [44*]. Although the mechanism whereby reduced PPARγ activity leads to increased AT1R remains unclear, it is possible that increased AT1R in these patients may have contributed to increased myogenic tone, vascular dysfunction, and oxidative stress.
Mice with lifelong PPARγ deficiency specifically in smooth muscle (SM22Cre/PPARγflox) exhibited slightly increased systemic blood pressure and impaired circadian variations of mean arterial pressure [28]. Another study with inducible smooth muscle specific PPARγ inactivation in adult mice using smooth muscle myosin heavy chain promoter reported no difference in baseline blood pressure before or after angiotensin-II infusion compared to control mice [21**]. Interestingly, the mesenteric arteries from these mice exhibited exacerbated angiotensin-II-induced endothelial dysfunction, vascular remodeling and inflammation [21**]. These findings are consistent with the hypothesis that smooth muscle PPARγ is protective against hypertension and vascular dysfunction. In contrast to these studies, another line of smooth muscle PPARγ deficient mice generated by crossing PPARγflox mice with knock-in mice expressing Cre-recombinase in the endogenous SM22 gene locus manifested decreased blood pressure and enhanced vasodilation to β-adrenergic receptor [17]. The surprising and inconsistent results were potentially reconciled when the same author later reported that these mice completely lack perivascular adipose tissue (PVAT) resulting from the transient activation of SM22 promoter in PVAT during development [45]. Whereas it is entirely possible that loss of PVAT might contribute to discrepant results compared with other studies, it also underscores the potential importance of PVAT in regulating vascular tone.
New Surprises
The progressive work on the function of PPARγ in the vasculature consistently support the notion that PPARγ is required to maintain homeostasis of the blood vessel (Figures 1 and 2). Emerging evidence has recently revealed that the complexity of PPARγ extends far beyond the classic view as a regulator of gene transcription. For example, PPARγ was reported to act as E3 ligase that promotes ubiquitination of p65, a subunit of NFκB, through a direct PPARγ-p65 interaction to regulate inflammatory responses [46]. PPARγs activity as an E3 ligase for p65 would be functionally analogous, but mechanistically distinct from its better characterized trans-repression of inflammatory gene transcription [47]. Other studies demonstrated that the activity of PPARγ can be regulated by post-translational modifications. This has become a very provocative topic of discussion and investigation. With relevance to anti-inflammatory activities of PPARγ, ligand dependent-sumoylation of PPARγ inhibits pro-inflammatory gene targets of NFκB or AP1 through a trans-repression mechanism [48]. The transcriptional activity of PPARγ can also be modulated by phosphorylation and acetylation. Phosphorylation of PPARγ at Ser273 selectively decreases expression of a subset of genes involved with insulin sensitivity [49]. Deacetylation of PPARγ at Lys268 and Lys293 is involved with browning of white adipose tissue, a phenomenon that promotes enhanced metabolism [50]. Thus, PPARγ activity may not only be impaired by mutation, but also under conditions which promote its post-translation modification. Accordingly, it is entirely possible that the mediators often correlated with vascular diseases and hypertension such as oxidative stress, angiotensin II and free fatty acids are able to modify and impair PPARγ activity via these mechanisms which further promotes vascular dysfunction.
Conclusion
Understanding the complexity of PPARγ function, particularly in specific tissues or cell types will help develop new classes of drugs that can either target PPARγ or the physiological pathways it regulates more selectively. With new data suggesting that activation of PPARγ in the brain and bone [12**] can contributes to weight gain and bone loss, respectively suggests the need for a new therapy that provides less accessibility to these tissues. Although it would be technically challenging to chemically activate PPARγ selectively in the vasculature it may be possible to selectively activate or inhibit some of the final common pathways regulated by PPARγ in endothelium or smooth muscle.
Key points.
PPARγ, a nuclear receptor plays an important role in the vasculature but the mechanisms remain under investigation.
Recent studies from genetically modified mouse models have emphasized the specific functions of PPARγ in endothelial and smooth muscle cells, where it provides protective mechanism against cardiovascular diseases.
Endothelium PPARγ modulates target genes expression involved with oxidative stress, inflammation and fatty acid transporters.
PPARγ regulates genes in signaling cascades related to constriction and relaxation in smooth muscle.
PPARγ may control the expression and activity of the renin-angiotensin in smooth muscle cells.
Acknowledgements
The authors would like to thank Dr. Henry L. Keen for his comments and assistance for the preparation of this manuscript.
Financial support and sponsorship: This work was supported through research grants from the NIH to CDS (HL048058, HL061446, HL062984, HL084207). The authors also gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Conflicts of interest: Supported by research grants from the NIH and research and salary support from the Roy J. Carver Trust.
References
- 1.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends in endocrinology and metabolism: TEM. 2014;25(6):293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefterova MI, Steger DJ, Zhuo D, et al. Cell-specific determinants of peroxisome proliferator-activated receptor gamma function in adipocytes and macrophages. Molecular and cellular biology. 2010;30(9):2078–89. doi: 10.1128/MCB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadian M, Suh JM, Hah N, et al. PPARgamma signaling and metabolism: the good, the bad and the future. Nature medicine. 2013;19(5):557–66. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giles TD, Sander GE. Effects of thiazolidinediones on blood pressure. Current hypertension reports. 2007;9(4):332–7. doi: 10.1007/s11906-007-0060-0. [DOI] [PubMed] [Google Scholar]
- 5.Sigmund CD. A clinical link between peroxisome proliferator-activated receptor gamma and the renin-angiotensin system. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(4):676–8. doi: 10.1161/ATVBAHA.112.301125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Eldor R, DeFronzo RA, Abdul-Ghani M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes care. 2013;36(Suppl 2):S162–74. doi: 10.2337/dcS13-2003. Summarizes evidence on the pleiotropic effects of TZD on glycemic control and insulin sensitivity and highlights the long lasting glycemic control of TZD over other anti-diabetes drugs.
- 7.Dutchak PA, Katafuchi T, Bookout AL, et al. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148(3):556–67. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Scherer PE. The PPARgamma-FGF1 axis: an unexpected mediator of adipose tissue homeostasis. Cell research. 2012;22(10):1416–8. doi: 10.1038/cr.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonker JW, Suh JM, Atkins AR, et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485(7398):391–4. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356(24):2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- **11.Press announcements—FDA requires removal of certain restrictions on the diabetes drug Avandia [press release] 2013. FDA removed the restriction for rosiglitazone use in patients with type 2 diabetes after the re-adjudicated of results of the RECORD trial showed no significant increased risk in heart attack or death in patients treated with rosiglitazone.
- **12.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the Promise of Insulin Sensitization in Type 2 Diabetes. Cell metabolism. 2014 doi: 10.1016/j.cmet.2014.08.005. Risk-benefit analysis of TZD based on clinical data and basic sciences and novel mechanisms regulating PPARγ activity and potential new therapeutics targeting PPARγ with less side effect.
- **13.Auclair M, Vigouroux C, Boccara F, et al. Peroxisome proliferator-activated receptor-gamma mutations responsible for lipodystrophy with severe hypertension activate the cellular renin-angiotensin system. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(4):829–38. doi: 10.1161/ATVBAHA.112.300962. Identified two new PPARγ mutations in patients with lipodystrophy, insulin resistance and severe hypertension associated with a cellular increase in renin-angiotensin system, oxidative stress and inflammation.
- 14.Agostini M, Schoenmakers E, Mitchell C, et al. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell metabolism. 2006;4(4):303–11. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 16.Beyer AM, Baumbach GL, Halabi CM, et al. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51(4):867–71. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Villacorta L, Zhang J, et al. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119(16):2161–9. doi: 10.1161/CIRCULATIONAHA.108.815803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda T, Brown JD, Orasanu G, et al. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. The Journal of clinical investigation. 2009;119(1):110–24. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halabi CM, Beyer AM, de Lange WJ, et al. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell metabolism. 2008;7(3):215–26. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beyer AM, de Lange WJ, Halabi CM, et al. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circulation research. 2008;103(6):654–61. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Marchesi C, Rehman A, Rautureau Y, et al. Protective role of vascular smooth muscle cell PPARgamma in angiotensin II-induced vascular disease. Cardiovascular research. 2013;97(3):562–70. doi: 10.1093/cvr/cvs362. Despite unaltered systemic blood pressure, loss of smooth muscle PPARγ exacerbated angiotensin II-induced small mesenteric arteries remodeling, endothelial dysfunction and vascular inflammation.
- 22.Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(9):1810–6. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 23.Hwang J, Kleinhenz DJ, Lassegue B, et al. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. American journal of physiology Cell physiology. 2005;288(4):C899–905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 24.Tian J, Wong WT, Tian XY, et al. Rosiglitazone attenuates endothelin-1-induced vasoconstriction by upregulating endothelial expression of endothelin B receptor. Hypertension. 2010;56(1):129–35. doi: 10.1161/HYPERTENSIONAHA.110.150375. [DOI] [PubMed] [Google Scholar]
- 25.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. American journal of hypertension. 2005;18(4 Pt 1):549–56. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Wang N, Symons JD, Zhang H, et al. Distinct functions of vascular endothelial and smooth muscle PPARgamma in regulation of blood pressure and vascular tone. Toxicologic pathology. 2009;37(1):21–7. doi: 10.1177/0192623308328545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinhenz JM, Kleinhenz DJ, You S, et al. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. American journal of physiology Heart and circulatory physiology. 2009;297(5):H1647–54. doi: 10.1152/ajpheart.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang N, Yang G, Jia Z, et al. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell metabolism. 2008;8(6):482–91. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Y, Saghatelian A, Chong LW, et al. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes & development. 2007;21(15):1895–908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nature medicine. 2007;13(12):1496–503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- **31.Yin KJ, Fan Y, Hamblin M, et al. KLF11 mediates PPARgamma cerebrovascular protection in ischaemic stroke. Brain: a journal of neurology. 2013;136(Pt 4):1274–87. doi: 10.1093/brain/awt002. Selective disruption of PPARγ in vascular endothelial cells led to increased cerebrovascular permeability and brain damage during ischemia. KLF11 was identified as a new PPARγ co-regulator in endothelial cells required for TZD-mediated cerebrovascular protection during ischemic stroke.
- **32.Pelham CJ, Keen HL, Lentz SR, Sigmund CD. Dominant negative PPARgamma promotes atherosclerosis, vascular dysfunction, and hypertension through distinct effects in endothelium and vascular muscle. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304(9):R690–701. doi: 10.1152/ajpregu.00607.2012. Endothelial dysfunction and hypertension were unmasked when loss of apolipoprotein E was compounded with the presence of mutant PPARγ in endothelial cells.
- 33.Girnun GD, Domann FE, Moore SA, Robbins ME. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Molecular endocrinology. 2002;16(12):2793–801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- 34.Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234(1):87–91. doi: 10.1016/s0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 35.Mehrotra D, Wu J, Papangeli I, Chun HJ. Endothelium as a gatekeeper of fatty acid transport. Trends in endocrinology and metabolism: TEM. 2014;25(2):99–106. doi: 10.1016/j.tem.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Goto K, Iso T, Hanaoka H, et al. Peroxisome proliferator-activated receptor-gamma in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. Journal of the American Heart Association. 2013;2(1):e004861. doi: 10.1161/JAHA.112.004861. Activation of PPARγ facilitated fatty acid uptake in microvessel endothelial cells through induction of classic target genes, aP2 and CD36.
- **37.Borges GR, Morgan DA, Ketsawatsomkron P, et al. Interference with peroxisome proliferator-activated receptor-gamma in vascular smooth muscle causes baroreflex impairment and autonomic dysfunction. Hypertension. 2014;64(3):590–6. doi: 10.1161/HYPERTENSIONAHA.114.03553. Altered carotid arterial remodeling and neurovascular signaling caused by compromised smooth muscle PPARγ activity links vascular dysfunction to impaired baroreflex.
- 38.Pelham CJ, Ketsawatsomkron P, Groh S, et al. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARgamma and RhoA/Rho-kinase. Cell metabolism. 2012;16(4):462–72. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.De Silva TM, Modrick ML, Ketsawatsomkron P, et al. Role of Peroxisome Proliferator-Activated Receptor-gamma in Vascular Muscle in the Cerebral Circulation. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03935. Emphasizes the critical role of PPARγ in brain circulation.
- 40.Ketsawatsomkron P, Lorca RA, Keen HL, et al. PPARgamma regulates resistance vessel tone through a mechanism involving RGS5-mediated control of protein kinase C and BKCa channel activity. Circulation research. 2012;111(11):1446–58. doi: 10.1161/CIRCRESAHA.112.271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Carrillo-Sepulveda MA, Keen HL, Davis DR, et al. Role of vascular smooth muscle PPARgamma in regulating AT1 receptor signaling and angiotensin II-dependent hypertension. PloS one. 2014;9(8):e103786. doi: 10.1371/journal.pone.0103786. Expression of PPARγ mutant in smooth muscle cells resulted in increase angiotensin-II signaling.
- *42.Holobotovskyy V, Manzur M, Tare M, et al. Regulator of G-protein signaling 5 controls blood pressure homeostasis and vessel wall remodeling. Circulation research. 2013;112(5):781–91. doi: 10.1161/CIRCRESAHA.111.300142. RGS5 plays a critical role in blood pressure regulation and vascular homeostasis through angiotensin II signaling with PKC, MAPK and Rho kinase as downstream effectors.
- *43.Huang Y, Di Lorenzo A, Jiang W, et al. Hypoxia-inducible factor-1alpha in vascular smooth muscle regulates blood pressure homeostasis through a peroxisome proliferator-activated receptor-gamma-angiotensin II receptor type 1 axis. Hypertension. 2013;62(3):634–40. doi: 10.1161/HYPERTENSIONAHA.111.00160. HIF1α deficiency increased AT1 receptor in smooth muscle and that correlated with reduced PPARγ expression.
- *44.Schleifenbaum J, Kassmann M, Szijarto IA, et al. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circulation research. 2014;115(2):263–72. doi: 10.1161/CIRCRESAHA.115.302882. Provides evidence that identified AT1R as a mechano-sensor during myogenic constriction.
- 45.Chang L, Villacorta L, Li R, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126(9):1067–78. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou Y, Moreau F, Chadee K. PPARgamma is an E3 ligase that induces the degradation of NFkappaB/p65. Nature communications. 2012;3:1300. doi: 10.1038/ncomms2270. [DOI] [PubMed] [Google Scholar]
- 47.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature reviews Immunology. 2010;10(5):365–76. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 49.Choi JH, Banks AS, Estall JL, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466(7305):451–6. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiang L, Wang L, Kon N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150(3):620–32. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]