Abstract
Evidence indicates that astronauts experience significant bone loss during space mission. Recently, we used the NASA developed rotary cell culture system (RCCS) to simulate microgravity (μXg) conditions and demonstrated increased osteoclastogenesis in mouse bone marrow cultures. Autophagy is a cellular recycling process of nutrients. Therefore, we hypothesize that μXg control of autophagy modulates osteoclastogenesis. Real-time PCR analysis of total RNA isolated from mouse bone marrow derived non-adherent cells subjected to modeled μXg showed a significant increase in autophagic marker Atg5, LC3 and Atg16L mRNA expression compared to ground based control (Xg) cultures. Western blot analysis of total cell lysates identified an 8.0-fold and 7.0-fold increase in the Atg5 and LC3-II expression, respectively. Confocal microscopy demonstrated an increased autophagosome formation in μXg subjected RAW 264.7 preosteoclast cells. RT2 profiler PCR array screening for autophagy related genes identified that μXg upregulates intracellular signaling molecules associated with autophagy, autophagosome components and inflammatory cytokines/growth factors which coregulate autophagy in RAW 264.7 preosteoclast cells. Autophagy inhibitor, 3-methyladenine (3-MA) treatment of mouse bone marrow derived non-adherent mononuclear cells showed a significant decrease in μXg induced Atg5 and LC3 mRNA expression in the presence or absence of RANK ligand (RANKL) stimulation. Furthermore, RANKL treatment significantly increased (8-fold) p-CREB transcription factor levels under μXg as compared to Xg cultures and 3-MA inhibited RANKL increased p-CREB expression in these cells. Also, 3-MA suppresses μXg elevated osteoclast differentiation in mouse bone marrow cultures. Thus, our results suggest that μXg induced autophagy plays an important role in enhanced osteoclast differentiation and could be a potential therapeutic target to prevent bone loss in astronauts during space flight missions.
Keywords: Osteoclast, Autophagy, Microgravity, Rotary cell culture system (RCCS), NASA
Introduction
Space flight is a challenge for normal bone homeostasis in astronauts. Evidence is accumulating that unloading of the skeleton either due to space-flight or an altered gravitational environment results in a reduction of bone mineral density. Astronauts experience about 10–15% loss of bone mass in microgravity (μXg) [1,2] and the morphological changes resemble bones of osteoporotic patients [3,4]. In long-term space missions, astronauts can lose bone mass in the proximal femur in one month, as seen in postmenopausal women on earth in one year [5]. Therefore, high bone turnover in μXg conditions may lead to bone loss and fracture risk in astronauts. Although astronauts’ daily tasks include nutritional supplementation and regimented exercise for skeletal health, irreversible bone loss has serious implications for long-term inhabitants of the space station and space exploration.
The osteoclast (OCL) is the bone resorbing cell and M-CSF is required for proliferation and survival of OCL precursors. The TNF family member, receptor activator for nuclear factor κB ligand (RANKL), is critical for OCL precursor differentiation to form multinucleated OCL in the bone microenvironment. RANKL interaction with RANK receptor expressed on OCL progenitor cells results in activation of various signaling cascades during OCL differentiation and bone resorption [6]. In-flight studies conducted during the FOTON-3 mission revealed that OCLs and their precursors are direct targets for μXg and mechanical force could be responsible for modulating gene expression associated with OCL differentiation/activity [7]. Further, μXg is capable of stimulating OCL differentiation by regulating osteoblast secretion of RANKL and osteoprotegerin (OPG) [8]. Also, it has been shown that bone forming activity of osteoblast cells decreases under μXg conditions [9,10]. It has been reported that μXg reduces osteoblast life span and increases OCL activity which contributes to bone loss associated with weightlessness [11]. Furthermore, uncoupling of bone formation and resorption favors bone loss in cosmonauts during and after 180 days of space flight [12]. Similarly, intact limb bones of newts flown on board the biosatellite Cosmos-2229 revealed OCL activation and resorption on the endosteal surface [13]. It has been shown that skeletal unloading in mice diminishes bone quality in the tibia and fibula which leads to an increase in bone fracture risk [14]. Additionally, skeletal unloading in mice, bone mass is reduced due to elevated RANKL expression and osteoclastogenesis [15]. Also, RANKL expressed in osteocytes is responsible for bone loss associated with skeletal unloading in mice [16]. Similarly, it was demonstrated that isolated fetal mouse long bones under near weightlessness conditions show decreased mineralization and increased calcium release [9]. In addition, complicities of excess calcium mobilization from bones cause kidney stone formation.
Recently, we used the NASA developed ground based rotary cell culture system (RCCS) to simulate microgravity (μXg) conditions for mouse bone marrow cultures and showed increased osteoclastogenesis. We also determined the gene expression profiling during OCL differentiation of RAW 264.7 cells subjected to modeled μXg by Agilent microarray analysis. We thus identified that μXg significantly increases expression of critical molecules such as cytokines/growth factors, proteases and signaling proteins, which may enhance OCL differentiation/ function [17]. However, the mechanisms of μXg induced bone loss and the rational approaches to prevent fracture risk associated with prolonged weightlessness conditions are yet to be established.
Autophagy is a cellular, self-consumption process characterized by sequestration of bulk cytoplasm, long-lived proteins and organelle degradation that is critical for cellular homeostasis [18]. Autophagy has pleiotropic functions that are involved in cell survival, nutrient supply under starvation, defense against pathogens and antigen presentation [19]. In metabolic stress, endoplasmic reticulum (ER) stress, hypoxia and pathological conditions, autophagy is greatly increased, allowing the cell to degrade defected proteins and organelles to recycle macromolecular precursors, such as amino acids, fatty acids, and nucleotides [20]. Beclin-1 and autophagy proteins (Atgs) are involved in initiation and formation of autophagosome. It has been reported that autophagy proteins regulate the secretory lysosomes that are directed to fuse with the OCL ruffled border [21]. Similarly, interruption in the autophagic process has been shown to delocalize cathepsin K and reduce OCL bone resorption activity. Further, Rab7, which is required for OCL function, localizes to the ruffled border in an Atg5 dependent manner and participates in polarized secretion of lysosomal contents into the extracellular space by directing lysosomes to fuse with the plasma membrane [22]. Recently, it has been identified that autophagy is a pivotal regulator of hypoxia-induced OCL differentiation through the HIF-1α/ BNIP3 signaling pathway and that inhibition of oxidative stress decreased autophagy. Furthermore, autophagy inhibition by 3′-methyladenine (3-MA) LY294002, wortmannin or knock-down of Beclin-1/Atg 7 decreased OCL marker gene expression [23]. However, μXg control of autophagy and OCL differentiation is unknown.
Materials and methods
Modeled microgravity (μXg) and cell culture
The Rotating Wall Vessel Bioreactor (RWV) (Synthecon Inc., Texas) is a horizontally oriented rotary cell culture (RCC) system. A silicone membrane is located on the central axis of a rotation chamber to diffuse gases necessary for cell growth without creating turbulence. The solid-body rotation of the chamber causes a reduction of the medium’s shear stress [24]. The vessel was filled with α-minimum essential medium (MEM) free of bubbles and rotated at a constant speed of 16 rpm for 24 h to simulate microgravity (0.008 Xg), termed modeled microgravity (μXg). Ground based control or static gravity (1Xg) is termed Xg [25]. Bone marrow was flushed from long bones of 6–8 week-old mice (C57BL/6) using α-MEM. Cells were pelleted at 1500 rpm for 7 min at room temperature and plated in α-MEM with 10% fetal bovine serum (FBS) supplemented with M-CSF (10 ng/ml) and cultured overnight. Non-adherent mouse bone marrow cells (1.5 × 106/ml) or RAW 264.7 cells (1 × 104/ml) subjected to modeled μXg in RCCS for 24 h were cultured in a 24 well plate for 7 or 5 days, respectively, in the presence of M-CSF (10 ng/ml) and RANKL (75 ng/ml) (R&D Systems Inc., Minneapolis, MN). Cells were fixed with 2% glutaraldehyde in phosphate buffered saline (PBS) and stained for tartrate-resistant acid phosphatase (TRAP) activity using a histochemical kit (Sigma Chemical Co., St. Louis, MO). TRAP positive multinucleated cells (MNC) containing three or more nuclei were scored as osteoclasts (OCLs) under a microscope as described [26]. All procedures involving animal use were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Real-time RT-PCR
Total RNA was isolated from ground based control (Xg) and μXg subjected mouse bone marrow derived non-adherent cells treated with or without RANKL (75 ng/ml) and M-CSF (10 ng/ml) for 24 h using RNAzol reagent (Biotecx Labs, Houston, TX). The reverse transcription reaction was performed using iScript reverse transcriptase in a 20 μl reaction volume containing total RNA (2 μg), 4 μl amino acid buffer and nuclease-free water at 42 °C for 15 min followed by 99 °C for 5 min and 4 °C for 5 min. Real-time PCR was performed using IQ™ SYBR Green Supermix in an iCycler (iCycler iQ Single-color Real-Time PCR detection system; Bio-Rad, Hercules, CA). The forward and reverse primer sequences used to amplify the selected genes are listed as follows: Atg5 sense: 5′-CTG TCA AGT GCC TGC TGC T-3′, antisense: 5′-GTG AGC CTC AAC CGC ATC-3′; LC3 sense: 5′-CCA CCA AGA TCC CAG TGA TT-3′, antisense: 5′-CGC CGT CTG ATT ATC TTG ATG-3′; Atg16L sense: 5′-ACA TGA TGG TGC GTG GAA T-3′, antisense: 5′-TTG TCC TTC TGC TGC ATT TG-3′; and β-actin sense: 5′-CCA CAC CTT CCT ACA ATG AGC-3′, antisense: 5′-TAG AGG AAG ACG TAG GAC AG-3′. Thermal cycling parameters were 94 °C for 3 min, followed by 40 cycles of amplifications at 94 °C for 30 s, 60 °C for 1 min, 72 °C for 1 min, and 72 °C for 5 min as the final elongation step. Relative levels of mRNA expression were normalized in all analyzed samples with respect to levels of β-actin amplification as described [27].
RT2 profiler PCR array screening
RAW 264.7 cells (1 × 104 cells/ml) were subjected to modeled μXg or ground based control (Xg) cultures for 24 h and total RNA was isolated using RNAzol reagent. Reverse transcription reaction was done using poly-dT primer and Moloney murine leukemia virus reverse transcriptase as described above. Real-time PCR was performed using 2× RT qPCR Master Mix to screen the RT2 Profiler PCR Array System (PAMM-084A) in a 96-well plate to quantify expression levels of 84 autophagy related genes. Thermal cycling parameters were 95 °C for 10 min, followed by 40 cycles of amplifications at 95 °C for 15 s, 55 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min as the final elongation step. Relative levels of mRNA expression were normalized in all the samples with expression levels of housekeeping genes (GUSB, HPRT, HSP90AB1, GAPDH and β-actin) in triplicate studies, and data analysis was done using the Web portal.
Western blot analysis
Mouse bone marrow derived non-adherent cells cultured with α-MEM containing 10% FBS were subjected to μXg. Cells were stimulated with or without RANKL (75 ng/ml) and M-CSF (10 ng/ml) for 24 h and total cell lysates were prepared in a buffer containing 20 mM Tris–HCl at pH 7.4, 1% Triton X-100, 1 mM EDTA, 1.5 mM MgCl2, 10% glycerol, 150 mM NaCl, 0.1 mM Na3VO4 and 1× protease inhibitor cocktail. The protein content of the samples was measured using the BCA protein assay reagent (Pierce, Rockford, IL). Protein (100 μg) samples were then subjected to SDS-PAGE using 12% Tris–HCl gels and blot transferred on to a PVDF membrane, immunoblotted with antibody against Atg5, LC3-II, PLCγ2, p-CREB, CREB and β-actin. The bands were detected using the enhanced chemiluminescence detection system (Pierce, Rockford, IL) and band intensity was quantified by densitometric analysis using the NIH ImageJ Program.
Confocal microscopy
RAW 264.7 cells were exposed to μXg and normal gravity (Xg) conditions in parallel for 24 h and then cultured (1 × 103/well) in Lab-Tek 4-well chamber slides (Nunc Inc., Rochester, NY) with RANKL (75 ng/ml) and M-CSF (10 ng/ml) for 24 h. Cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, permeabilized with 0.1% Triton X-100 for 10 min and blocked for 1 h with PBS containing 2% horse serum at room temperature. Cells were incubated with LC3-II primary antibody for 3 h and treated with Alexa 488-conjugated anti-rabbit IgG in PBS containing 2% horse serum for 1 h at room temperature. Autophagosome formation was visualized by confocal microscopy (LSM 510; Carl Zeiss, Inc., Thornwood, NY).
Statistical analysis
Results are presented as mean ± SD for three independent experiments and compared by Student’s t-test. Values were considered significant at P < 0.05.
Results
Microgravity (μXg) induces autophagy in preosteoclast cells
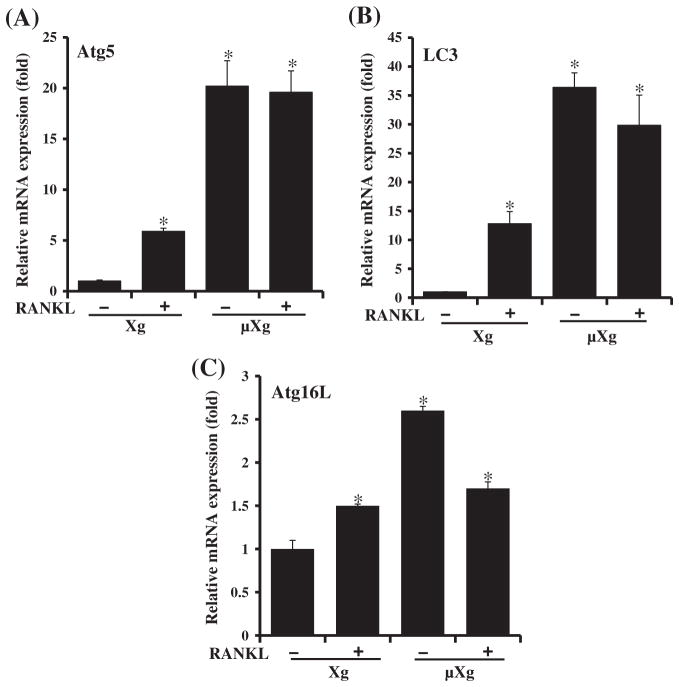
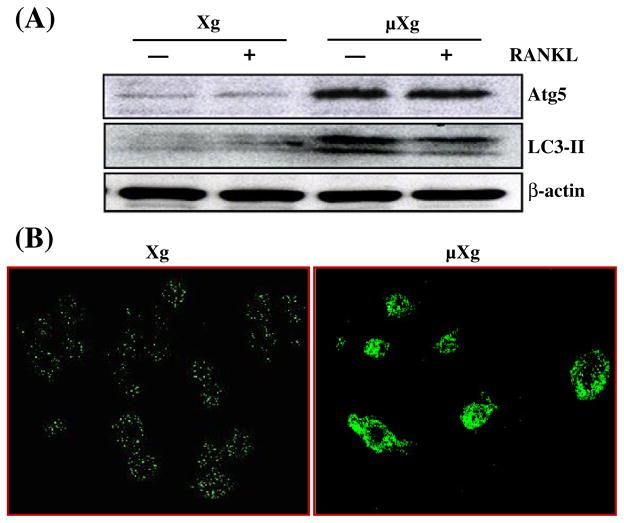
Previously, we showed that modeled μXg increases OCL formation in mouse bone marrow cultures [17]. However, the molecular mechanisms underlying μXg modulation of OCL differentiation are unclear. Recent studies implicated autophagy/autophagy proteins as playing an important role in enhanced osteoclastogenesis [21,23,28]. We therefore examined μXg modulation of autophagy markers in preosteoclast cells. Mouse bone marrow derived non-adherent cells were subjected to modeled μXg in RCCS for 24 h as described in the Materials and methods section. Real-time RT-PCR analysis of total RNA isolated from these cells demonstrated high levels of Atg5 (20-fold), LC3 (35-fold) and Atg16L (2.8-fold) mRNA expression without RANKL treatment when compared to ground based control (Xg) cultures without RANKL stimulation (Fig. 1). Western blot analysis of total cell lysates obtained from the μXg subjected preosteoclast cells without RANKL stimulation demonstrated an 8.0-fold and 7.0-fold increase in the Atg5 and LC3-II expression, respectively (Fig. 2A). Autophagy involves conversion of the cytoplasmic microtubule-associated protein 1 light chain 3 (LC3-I) into the membrane form of LC3-II during autophagosome formation [29,30]. Therefore, we performed confocal microscopy analysis for LC3-II expression in preosteoclast cells, and identified an abundant autophagosome formation in RAW 264.7 cells cultured under μXg in the absence of RANKL stimuli compared to ground based control (Xg) cultures (Fig. 2B).
Fig. 1.
Real-time RT-PCR analysis of autophagy marker Atg5, LC3 and Atg16L mRNA expression. Ground based control (Xg) and microgravity (μXg) subjected mouse bone marrow non-adherent cells were treated with or without RANKL (75 ng/ml) for 24 h. Total RNA isolated were subjected to real-time RT-PCR analysis using gene specific primers for (A) Atg5, (B) LC3 and (C) Atg16L. The mRNA expression was normalized with respect to GAPDH amplification. Each bar represents the mean ± SD of three independent experiments. *Significant (P < 0.05) difference when compared to ground based control without RANKL treatment.
Fig. 2.
μXg modulation of autophagosome formation. (A) Western blot analysis of Atg5 and LC3-II expression. Ground based control (Xg) and μXg subjected mouse bone marrow non-adherent cells were treated with or without RANKL (75 ng/ml) for 24 h. Total cell lysates were subjected to Western blot for Atg5 and LC3-II. β-actin expression served as control. (B) Autophagosome formation in preosteoclast cells. RAW 264.7 cells were cultured in Xg and μXg conditions for 24 h and autophagosomes were visualized by confocal microscopy analysis using anti-LC3-II antibody.
RT2 Profiler PCR array screening for μXg regulated autophagy related genes
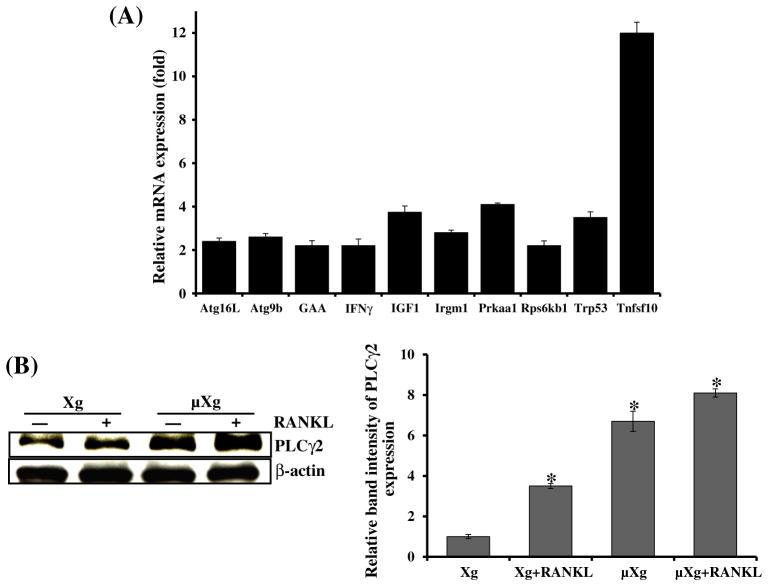
We next examined μXg regulation of autophagy related gene expression using the RT2 Profiler PCR array consisting of 84 different genes. We thus identified μXg upregulated mRNA expression of GAA, Trp53, Prkaa1, Rps6kb1, intracellular signaling molecules associated with autophagy, autophagosome components such as Atg16L, Atg9b, Irgm1, and inflammatory cytokines/growth factors such as Tnfsf10, IFN-γ and IGF1 which coregulate autophagy in preosteoclast cells (Fig. 3A). Calcium signaling has been shown to directly modulate autophagy [31,32]. Since PLCγ2 is involved in the regulation of intracellular Ca2+ level [33,34], we further examined μXg regulation of PLCγ2 expression in mouse bone marrow derived non-adherent cells. Western blot analysis of total cell lysates obtained from ground based control (Xg) and μXg subjected preosteoclast cells revealed that μXg significantly increased the PLCγ2 expression with or without RANKL stimulation compared to ground based control (Xg) cultures (Fig. 3B). β-actin expression served as control. These results indicate that μXg control of PLCγ2/calcium signaling modulates autophagy in preosteoclast cells.
Fig. 3.
(A) RT2 Profiler PCR array analysis for autophagy related gene expression. RAW 264.7 cells were cultured in Xg and μXg conditions for 24 h. Total RNA isolated from these cells was screened for autophagy related genes by real-time PCR in triplicate studies as described in the Materials and methods section. (B) PLCγ2 expression in mouse bone marrow derived non-adherent cells under μXg conditions. Cells were cultured in μXg for 24 h and stimulated with or without RANKL (75 ng/ml) for 24 h. Total cell lysates were subjected to western blot analysis using anti-PLCγ2 antibody. β-actin expression served as control. The band intensity was quantified by the National Institutes of Health ImageJ program, and PLCγ2 expression was normalized with β-actin expression in these cells. The values are expressed as mean ± SD for three independent experiments. *Significant (P < 0.05) difference when compared to ground based control without RANKL treatment.
Functional role of autophagy in μXg induced OCL differentiation
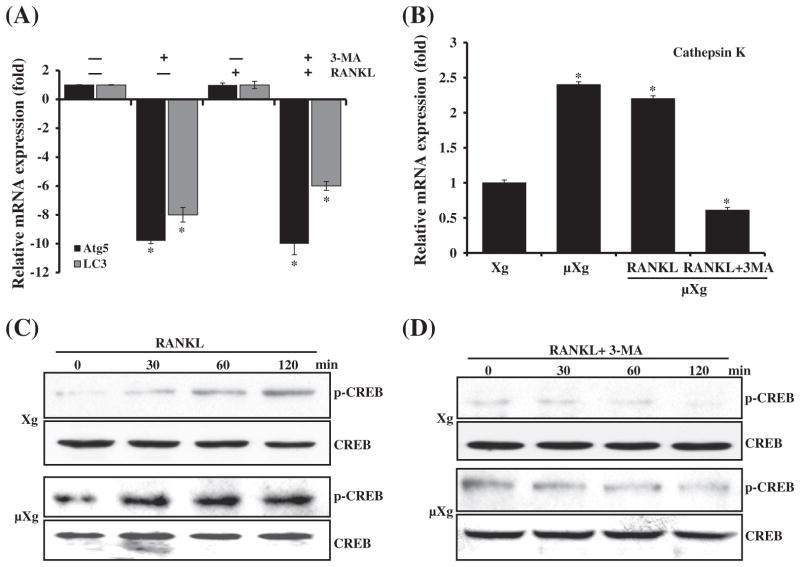
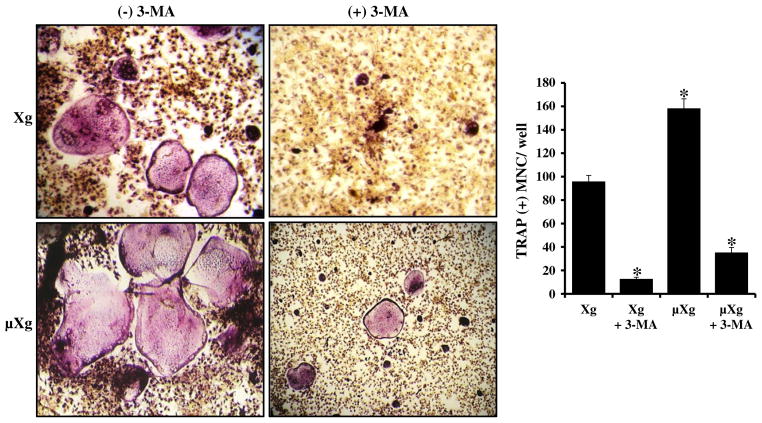
To determine the functional role of autophagy in OCL differentiation, mouse bone marrow derived non-adherent cells were subjected to μXg for 24 h and cultured with or without RANKL and autophagy inhibitor (3-MA). As shown in Fig. 4A, real-time RT-PCR analysis of total RNA isolated from these cells demonstrated that 3-MA significantly down-regulates μXg induced autophagosome markers, Atg5 and LC3 mRNA expression. We also identify that μXg upregulated cathepsin K mRNA expression compared to ground based control (Xg) conditions and 3-MA treatment (24 h) of preosteoclast cells inhibits μXg elevated cathepsin K mRNA expression (Fig. 4B). Furthermore, RANKL treatment significantly increased (8-fold) phosphorylation of cAMP response element (CRE)-binding protein (p-CREB), a critical transcription factor for osteoclast (OCL) differentiation under μXg as compared to Xg conditions. 3-MA markedly suppressed RANKL induced p-CREB expression under μXg and Xg conditions (Figs. 4C&D). Nevertheless, 3-MA treatment did not affect the viability of cells (data not shown). To determine the effect of autophagy on μXg enhanced OCL differentiation in vitro, mouse bone marrow derived non-adherent cells subjected to μXg or control Xg were stimulated with M-CSF, RANKL with or without 3-MA for 7 days to form multinucleated osteoclasts. We identified that 3-MA inhibits OCL differentiation in Xg conditions. Interestingly, 3-MA treatment resulted in suppression of μXg elevated OCL differentiation in mouse bone marrow cultures (Fig. 5).
Fig. 4.
(A) Autophagy inhibitor 3-methyladenine (3-MA) suppresses Atg5 and LC3 expression. Mouse non-adherent bone marrow cells were cultured in μXg for 24 h and treated with or without RANKL (75 ng/ml) and 3-MA (2 mM) for 24 h. Total RNA isolated were subjected to real-time RT-PCR analysis using gene specific primers for (A) Atg5 and LC3. (B) Cathepsin K gene expression. mRNA expression was normalized with respect to β-actin amplification. Each bar represents the mean ± SD of three independent experiments. *Significant (P < 0.05) difference when compared to ground based control without RANKL treatment. (C & D) RANKL stimulation and 3-MA inhibition of CREB activation under Xg and μXg conditions. Mouse non-adherent bone marrow cells were cultured in μXg for 24 h and treated with RANKL (75 ng/ml) in the presence and absence of 3-MA (2 mM) for different time points (0–120 min). Total cell lysate obtained from these samples was subjected to western blot analysis for p-CREB expression. Total CREB expression served as control.
Fig. 5.
Autophagy inhibitor (3-MA) inhibits μXg elevated OCL formation. Mouse bone marrow derived non-adherent cells were cultured with RANKL (75 ng/ml) and M-CSF (10 ng/ml) for 7 days in the presence and absence of 3-MA (2 mM) under Xg and μXg conditions. TRAP-positive multinucleated osteoclasts formed at the end of the culture period were scored. *Significant (P < 0.05) difference when compared to ground based control.
Discussion
Space flight alters normal bone homeostasis and causes increased bone resorption in astronauts. Studies conducted during space flight indicated that μXg directly regulates osteoclastogenesis [7]. In previous studies, we have shown that μXg simulation using a RCCS system increased osteoclastogenesis in bone marrow cultures [17]. This study further identified that μXg significantly increased autophagic marker Atg5 and LC3-II expression as well as autophagosome formation in preoteoclast cells. Therefore, since autophagy is a cellular recycling process of nutrients for survival and function it could enhance OCL formation. Recently, it has been shown that autophagy is activated by the pro-inflammatory cytokine TNF-α associated with inflammatory bone loss [35]. In this study, PCR array analysis identified that μXg upregulates inflammatory cytokines such as Tnfsf10 in preosteoclast cells. It is possible that μXg upregulation of inflammatory cytokines may elevate autophagic activity and osteoclast (OCL) formation. Autophagy proteins such as Atg5 have been shown to regulate the secretory component of osteoclastic bone resorption [21]. Our results have shown that autophagy inhibitor (3-MA) significantly decreased μXg induced cathepsin K expression in mouse bone marrow derived preosteoclast cells suggesting that autophagy inhibition may diminish OCL bone resorption activity under μXg conditions. p62/SQSTM1 interaction with autophagy-linked WDFY3 protein has been shown to play a functional role in osteoclasts [36]. Recently, it has been identified that aquaporin-9 knock-out mice attenuated bone loss and inhibited OCL formation in a hind-limb suspension mouse model [37]. Therefore, μXg may regulate autophagy in osteoclasts through complex molecular signaling mechanisms. Elevated Ca2+ oscillations modulate the CaMK–CREB pathway during OCL differentiation [17]. The CaMK–CREB pathway regulates the expression of OCL specific genes in cooperation with NFATc1 [38]. However, we previously found no significant change in the levels of NFATc1 expression, but rather increased levels of c-Jun, MITF, CREB transcription factors and cytosolic calcium levels in preosteoclast cells under μXg conditions [17]. It has been reported that calcium signaling directly stimulates autophagy [31,32] and PLCγ2 is involved in the regulation of intracellular Ca2+ level [33]. It has also been shown that space flight increases calcium release from bone [39]. Our findings that microgravity conditions increase PLCγ2 expression suggest that the PLCγ2/calcium signaling pathway modulates autophagy during OCL differentiation. CREB is activated by Ca2+/calmodulin-dependent kinase (CaMK) IV and is crucial for OCL differentiation and function [38,40]. We showed elevated levels of p-CREB in preosteoclast cells subjected to μXg compared to normal gravity (Xg) conditions [17]. Therefore, 3-MA inhibition of CREB activation under μXg conditions suggests that suppression of autophagy could negatively regulate gene expression essential for OCL activation. Accordingly, we have demonstrated that autophagy inhibitor (3-MA) inhibits μXg elevated OCL differentiation. Genetic and functional studies have implicated that autophagy is involved in bone cell function under normal and pathologic conditions [41]. In this study, we showed that 3-MA also inhibits OCL formation under Xg conditions which suggests that autophagy may play a role in bone loss associated with other skeletal disorders such as Paget’s disease. It has been shown that autophagic protein, Atg5 gene deletion protects against experimental postmenopausal osteoporosis [21]. Also, autophagy inhibitor treatment recapitulated deficiency of FIP200, an essential component of autophagy which resulted in osteopenia in mice [42]. More recently, it has been reported that suppression of autophagy in osteocytes contributes to the low bone mass associated with aging [43]. Long-term space flight is also challenged by other factors such as radiation. Irradiated mice subjected to mechanical unloading via hind-limb suspension demonstrated bone loss [44,45]. Irradiation has also been shown to induce autophagy in human bone marrow mesenchymal stem cells [46]. Thus, our results suggest that μXg induced autophagy enhances osteoclast differentiation and could be a potential therapeutic target to prevent bone loss in astronauts during space flight missions.
Acknowledgments
This work is supported by South Carolina EPSCoR Consortium grant (Dr. Reddy).
Footnotes
The authors declare no conflict of interests.
References
- 1.Carmeliet G, Bouillon R. The effect of microgravity on morphology and gene expression of osteoblasts in vitro. FASEB J. 1999;13(Suppl):S129–34. doi: 10.1096/fasebj.13.9001.s129. [DOI] [PubMed] [Google Scholar]
- 2.Sibonga JD. Spaceflight-induced bone loss: is there an osteoporosis risk? Curr Osteoporos Rep. 2013;11:92–8. doi: 10.1007/s11914-013-0136-5. [DOI] [PubMed] [Google Scholar]
- 3.Garber MA, McDowell DL, Hutton WC. Bone loss during simulated weightlessness: a biomechanical and mineralization study in the rat model. Aviat Space Environ Med. 2000;71:586–92. [PubMed] [Google Scholar]
- 4.Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–30. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh PR, Licata AA, Rice AJ. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravit Space Biol Bull. 2005;18:39–58. [PubMed] [Google Scholar]
- 6.Reddy SV. Regulatory mechanisms operative in osteoclasts. Crit Rev Eukaryot Gene Expr. 2004;14:255–70. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.20. [DOI] [PubMed] [Google Scholar]
- 7.Tamma R, Colaianni G, Camerino C, Di Benedetto A, Greco G, Strippoli M, et al. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 2009;23:2549–54. doi: 10.1096/fj.08-127951. [DOI] [PubMed] [Google Scholar]
- 8.Kanematsu M, Yoshimura K, Takaoki M, Sato A. Vector-averaged gravity regulates gene expression of receptor activator of NF-kappaB (RANK) ligand and osteoprotegerin in bone marrow stromal cells via cyclic AMP/protein kinase A pathway. Bone. 2002;30:553–8. doi: 10.1016/s8756-3282(02)00680-4. [DOI] [PubMed] [Google Scholar]
- 9.Van Loon JJ, Bervoets DJ, Burger EH, Dieudonne SC, Hagen JW, Semeins CM, et al. Decreased mineralization and increased calcium release in isolated fetal mouse long bones under near weightlessness. J Bone Miner Res. 1995;10:550–7. doi: 10.1002/jbmr.5650100407. [DOI] [PubMed] [Google Scholar]
- 10.Hughes-Fulford M, Lewis ML. Effects of microgravity on osteoblast growth activation. Exp Cell Res. 1996;224:103–9. doi: 10.1006/excr.1996.0116. [DOI] [PubMed] [Google Scholar]
- 11.Rucci N, Migliaccio S, Zani BM, Taranta A, Teti A. Characterization of the osteoblast-like cell phenotype under microgravity conditions in the NASA-approved Rotating Wall Vessel bioreactor (RWV) J Cell Biochem. 2002;85:167–79. [PubMed] [Google Scholar]
- 12.Caillot-Augusseau A, Lafage-Proust MH, Soler C, Pernod J, Dubois F, Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95) Clin Chem. 1998;44:578–85. [PubMed] [Google Scholar]
- 13.Berezovska OP, Rodionova NV, Grigoryan EN, Mitashov VI. Changes in the numbers of osteoclasts in newts under conditions of microgravity. Adv Space Res. 1998;21:1059–63. doi: 10.1016/s0273-1177(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 14.Ko CY, Seo DH, Kim HS. Deterioration of bone quality in the tibia and fibula in growing mice during skeletal unloading: gender-related differences. J Biomech Eng. 2011;133:111003. doi: 10.1115/1.4005350. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Liu W, Masuyama R, Fukuyama R, Ito M, Zhang Q, et al. Pyruvate dehydrogenase kinase 4 induces bone loss at unloading by promoting osteoclastogenesis. Bone. 2012;50:409–19. doi: 10.1016/j.bone.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambandam Y, Blanchard JJ, Daughtridge G, Kolb RJ, Shanmugarajan S, Pandruvada SN, et al. Microarray profile of gene expression during osteoclast differentiation in modelled microgravity. J Cell Biochem. 2010;111:1179–87. doi: 10.1002/jcb.22840. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 19.Fleming A, Noda T, Yoshimori T, Rubinsztein DC. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol. 2011;7:9–17. doi: 10.1038/nchembio.500. [DOI] [PubMed] [Google Scholar]
- 20.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–51. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 21.DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. 2011;21:966–74. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman A, Elazar Z. Autophagic factors cut to the bone. Dev Cell. 2011;21:808–10. doi: 10.1016/j.devcel.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Niu J, Kim H, Kolattukudy PE. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J Mol Cell Biol. 2011;3:360–8. doi: 10.1093/jmcb/mjr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–32. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JH, Long JP. Simulated microgravity impairs respiratory burst activity in human promyelocytic cells. In Vitro Cell Dev Biol Anim. 2001;37:209–15. doi: 10.1007/BF02577531. [DOI] [PubMed] [Google Scholar]
- 26.Shanmugarajan S, Irie K, Musselwhite C, Key LL, Jr, Ries WL, Reddy SV. Transgenic mice with OIP-1/hSca overexpression targeted to the osteoclast lineage develop an osteopetrosis bone phenotype. J Pathol. 2007;213:420–8. doi: 10.1002/path.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuvaraj S, Griffin AC, Sundaram K, Kirkwood KL, Norris JS, Reddy SV. A novel function of CXCL13 to stimulate RANK ligand expression in oral squamous cell carcinoma cells. Mol Cancer Res. 2009;7:1399–407. doi: 10.1158/1541-7786.MCR-08-0589. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia J, et al. Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1alpha/BNIP3 signaling pathway. J Cell Physiol. 2012;227:639–48. doi: 10.1002/jcp.22768. [DOI] [PubMed] [Google Scholar]
- 29.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Gao W, Ding WX, Stolz DB, Yin XM. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4:754–61. doi: 10.4161/auto.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson RL, van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, et al. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–41. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- 34.Patterson RL, van Rossum DB, Nikolaidis N, Gill DL, Snyder SH. Phospholipase C-gamma: diverse roles in receptor-mediated calcium signaling. Trends Biochem Sci. 2005;30:688–97. doi: 10.1016/j.tibs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Lin NY, Stefanica A, Distler JH. Autophagy: A key pathway of TNF-induced inflammatory bone loss. Autophagy. 2013:9. doi: 10.4161/auto.25467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hocking LJ, Mellis DJ, McCabe PS, Helfrich MH, Rogers MJ. Functional interaction between sequestosome-1/p62 and autophagy-linked FYVE-containing protein WDFY3 in human osteoclasts. Biochem Biophys Res Commun. 2010;402:543–8. doi: 10.1016/j.bbrc.2010.10.076. [DOI] [PubMed] [Google Scholar]
- 37.Bu G, Shuang F, Wu Y, Ren D, Hou S. AQP9: a novel target for bone loss induced by microgravity. Biochem Biophys Res Commun. 2012;419:774–8. doi: 10.1016/j.bbrc.2012.02.100. [DOI] [PubMed] [Google Scholar]
- 38.Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, et al. Regulation of osteoclast differentiation and function by the CaMK–CREB pathway. Nat Med. 2006;12:1410–6. doi: 10.1038/nm1515. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, McCoy T, Gazda D, Morgan JL, Heer M, Zwart SR. Space flight calcium: implications for astronaut health, spacecraft operations, and Earth. Nutrients. 2012;4:2047–68. doi: 10.3390/nu4122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderling TR, Stull JT. Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem Rev. 2001;101:2341–52. doi: 10.1021/cr0002386. [DOI] [PubMed] [Google Scholar]
- 41.Hocking LJ, Whitehouse C, Helfrich MH. Autophagy: a new player in skeletal maintenance? J Bone Miner Res. 2012;27:1439–47. doi: 10.1002/jbmr.1668. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, et al. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res. 2013;28:2414–30. doi: 10.1002/jbmr.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, et al. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288:17432–40. doi: 10.1074/jbc.M112.444190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willey JS, Lloyd SA, Nelson GA, Bateman TA. Ionizing Radiation and Bone Loss: Space Exploration and Clinical Therapy Applications. Clin Rev Bone Miner Metab. 2011;9:54–62. doi: 10.1007/s12018-011-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lloyd SA, Bandstra ER, Willey JS, Riffle SE, Tirado-Lee L, Nelson GA, et al. Effect of proton irradiation followed by hindlimb unloading on bone in mature mice: a model of long-duration spaceflight. Bone. 2012;51:756–64. doi: 10.1016/j.bone.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou J, Han ZP, Jing YY, Yang X, Zhang SS, Sun K, et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013;4:e844. doi: 10.1038/cddis.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]