Abstract
Background
Diabetes mellitus is a common and serious disorder. A search of the literature reveals no comprehensive quantitative assessment of the association between insulin use and incidence of diabetic macular edema. Therefore, we performed a meta-analysis of observational studies to evaluate the effect of insulin use on the risk of developing macular edema.
Material/Methods
Comparative studies published until May 2014 were searched through a comprehensive search of the Medline, Embase, and the Cochrane Library electronic databases. A systematic review and quantitative analysis of comparative studies reporting the effect of insulin use on the incidence of macular edema was performed. All analyses were performed using the Review Manager (RevMan) v.5 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
A total of 202 905 individuals were included in the present meta-analysis. In a random-effects meta-analysis, the use of insulin was found to be associated with increased risk of macular edema (RR, 3.416; 95% CI, 2.417–4.829; I2, 86.6%). Analysis that just included high-quality studies showed that insulin use increased the risk of macular edema (RR, 2.728; 95% CI, 1.881–3.955; I2=77.7%). In cohort studies (RR, 4.509; 95% CI, 3.100–6.559; I2, 77.7%) but not in case-control studies (RR, 1.455; 95% CI, 0.520 to 4.066; I2, 95.9%), increased incidence of macular edema was observed.
Conclusions
The results of this meta-analysis of observational studies demonstrate that insulin use is a risk factor for diabetic macular edema. However, available data are still sparse, and in-depth analyses of the assessed associations in the context of additional longitudinal studies are highly desirable to enable more precise estimates and a better understanding of the role of insulin use in incidence of diabetic macular edema.
MeSH Keywords: Case-Control Studies, Cohort Studies, Insulins, Insulins, Macular Edema, Meta-Analysis
Background
Diabetes mellitus is a common and serious disorder. Mostly because of chronic complications associated with the condition, diabetes mellitus accounts for over $100 billion in annual health care expenditures in the U.S. alone [1]. By 2030, an estimated 350 million people will have diabetes worldwide [2]. Diabetic retinopathy is the most important ocular complication in patients with diabetes mellitus and previous epidemiology studies have reported that the prevalence rate of diabetic retinopathy ranges between 6% and 18.4% [3]. Some patients with diabetic retinopathy develop macular edema [4,5]. Macular edema is one of the major causes of vision loss in individuals with diabetes and its development depends, in part, on the breakdown of the blood-retinal barrier. Diabetic macular edema is the major cause of vision loss associated with diabetic retinopathy. Worldwide, there are approximately 93 million people with diabetic retinopathy, 17 million with proliferative diabetic retinopathy, and 21 million with diabetic macular edema. The overall prevalence of DME is 6.81% (6.74–6.89) for DME in people with diabetes worldwide [7], accounting for 12% of new cases of blindness annually [8]. According to studies of the natural history of DME, 24% of eyes with DME will lose at least 3 lines of vision within 3 years.
The prevalence of diabetic macular edema depends on the type and duration of diabetes. In patients with type I diabetes, DME occurs in the first 5 years following diagnosis of diabetes, with the prevalence gradually increasing to 40% over 30 years. Around 5% of type II diabetes patients had diabetic macular edema when diabetes was diagnosed, gradually increasing to 30% within 25–30 years [6]. Several systemic risk factors have been identified in population-based epidemiological studies. In patients <age 30, independent risk factors for diabetic macular edema included duration of diabetes, proteinuria, sex, history of cardiovascular disease, use of diuretics, and elevated HbA1C [7]. In patients >30 years old, the incidence of diabetic macular edema is associated with longer duration of diabetes, elevated systolic blood pressure, and elevated glycosylated hemoglobin [8]. Proteinuria was positively associated with insulin dependence but not in those that were not using insulin. The prevalence of diabetic macular edema was also significantly associated with high serum cholesterol levels in patients with type I diabetes [9]. A sharp reduction (from 2.3% and 0.9%) in the prevalence of diabetic macular edema was noted in a Wisconsin population with better blood glucose control over 2 decades, confirming that chronic hyperglycemia is a critical factor in the pathogenesis of diabetic macular edema [10]. A cross-sectional study enrolling patients with type 2 diabetes who agreed to undergo blood sampling showed that exogenous insulin therapy is a independent risk factor for macular edema (p<0.05; odds ratio=3.8) [11]. Several observational studies were conducted to investigate the association between insulin use and macular edema incidence; however, the results were inconsistent [12–14]. To the best of our knowledge, there has been no comprehensive quantitative assessment of the association between insulin use and diabetic macular edema incidence. Therefore, we performed a meta-analysis of observational studies to evaluate the effect of insulin consumption on the risk of developing macular edema.
Material and Methods
Study identification
This meta-analysis was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [15], and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [16]. A literature search was carried out using PubMed (1966 to May 2014), Embase (1947 to May 2014), and Cochrane Library Central database (1967 to May 2014). There were no restrictions on origin or languages. Search terms included: “insulin”, “antihyperglycemic” in combination with “macular edema” or “diabetic retinopathy”. The reference lists of each comparative study included in this meta-analysis and previous reviews were manually examined to identify additional relevant studies.
Study selection
Two reviewers independently selected eligible case-control and cohort studies that investigated insulin use and macular edema risk. Disagreement between the 2 reviewers was settled by discussing with the third reviewer. Inclusion criteria were: (i) used a case-control or cohort study design; (ii) evaluated the association between insulin use and macular edema risk; (iii) presented odds ratio (OR), relative risk (RR), or hazard ratio (HR) estimates with 95% confidence interval (CI). When there were multiple publications from the same population, only data from the most recent report were included in the meta-analysis and the remaining were excluded. Studies reporting different measures of RR (e.g., risk ratio, rate ratio, hazard ratio, and odds ratio) were included in the meta-analysis. In practice, these measures of effect yield a similar estimate of RR.
Data extraction
The following data were collected by 2 reviewers independently using a purpose-designed form: name of first author, publication date, country of the population studied, study design, study period, number of cancer cases and subjects, sex, the study-specific adjusted ORs, RRs, or HRs with their 95% CIs for the insulin use and risk of macular edema, and confounding factors for matching or adjustments.
Methodological quality assessment
We used the Newcastle-Ottawa scale to assess the methodologic quality of cohort and case-control studies [17]. The Newcastle-Ottawa scale contains 8 items in 3 categories: selection (4 items, 1 star each), comparability (1 item, up to 2 stars), and exposure/outcome (3 items, 1 star each). A “star” presents a “high-quality” choice of individual study. Hence, the full score was 9 stars, and a high-quality study was defined as a study with ≥6 awarded stars.
Data synthesis and analysis
Heterogeneity was assessed using the Cochran Q and I2 statistics. For the Q statistic, a P value<0.10 was considered statistically significant for heterogeneity; for the I2 statistic, heterogeneity was interpreted as absent (I2: 0–25%), low (I2: 25.1–50%), moderate (I2: 50.1–75%), or high (I2: 75.1–100%) [18]. Subgroup analyses were carried out according to: (i) study quality, (ii) study design (cohort versus case-control studies), (iii) geographic location (Europe vs. Asia vs. North America), and (iv) number of adjustment factors (n ≥5 vs. n ≤6). Pooled RR estimates and corresponding 95% CIs were calculated using the inverse variance method. Considering that this is a meta-analysis based on observational studies, regardless of whether heterogeneity was significant (I2 ≥50%), the summary estimate based on the random-effects model (DerSimonian-Laird method) was reported, which assumes that the studies included in the meta-analysis had varying effect sizes. We carried out sensitivity analyses by excluding 1 study at a time to explore whether the results were strongly influenced by a specific study. Publication bias was assessed using Begg and Mazumdar adjusted rank correlation test and the Egger regression asymmetry test. All analyses were performed using Stata version 11.0 software (StataCorp, College Station, TX).
Results
Identification and selection of studies
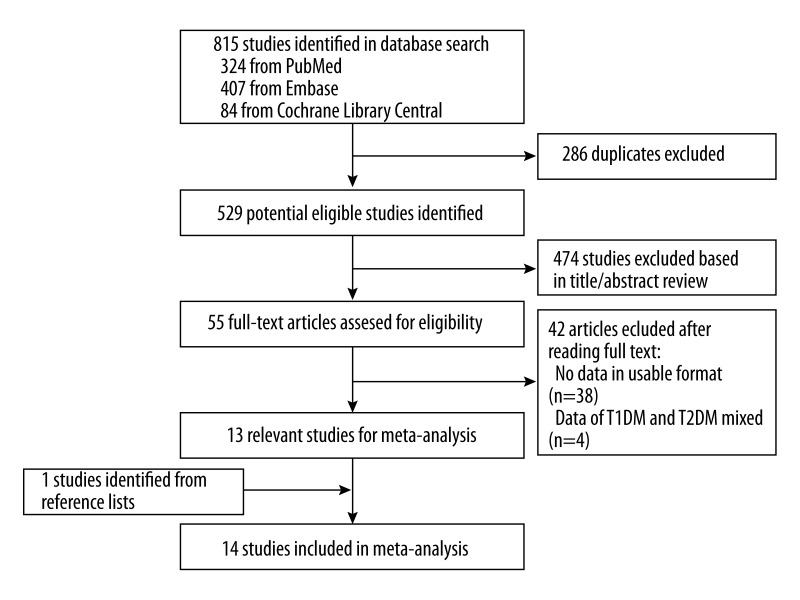
The initial 815 articles (324 from PubMed, 407 from Embase and 84 from Cochrane Library Central) were identified. After 286 duplicates and 474 unrelated articles were excluded, 55 full-text articles were assessed for eligibility. From these 55 articles, we excluded 4 articles that did not report the incidence of macular edema and 38 articles that did not report the data in usable format. One reference was included from reviewing the reference lists of the related articles. A total of 14 studies were included in this study [12–14, 19–29]. Figure 1 shows the flow of search results.
Figure 1.
Flow chart of the literature search. The literature search was conducted in Medline, EMBASE, and Cochrane Library. The reference lists of the relevant studies were also reviewed.
Study characteristics and quality
A total of 202 905 individuals were included in the present current meta-analysis. The characteristics of these included studies were shown in Table 1. Among the 14 included studies, 3 studies were case-control studies and 11 were cohort studies. Geographic distribution of all included studies was 6 in the Americas, 6 in Europe and 2 in Asia. The duration of all the studies differed – the longest was about 20 years and the shortest was less than 1 year.
Table 1.
Study characteristics of included studies.
| Name | Country | Study duration | Follow-up | case/control | Hospital/population | DM type | Adjusted factors |
|---|---|---|---|---|---|---|---|
| Henricsson M. | Sweden | 1997 | 3.1±1.3 Y | 2414 | Hospital based Cohort study | 2 | Age, smoking, antihypertensive treatment |
| Bertram B. | German | 1997 | <1 Y | 496 | Hospital based Cohort study | 2 | Age, treatment |
| Klein R. | USA | 1979–1980 | 10 Y | 891 | Population based Cohort study | 2 | Age, sex, age of diagnosis, smoking history, aspirin use, cardiovascular disease |
| Leske M.C. | Barbados | 2003 | 4 | 410 | Population based Cohort study | 2 | Age of onset, systolic blood pressure, treatment with insulin, and oral medication |
| Aroca P.R. | Spain | 2000–2004 | 4 | 93 | Hospital based case-control study | 2 | Age, sex, duration of DM |
| Romero-Aroca P. | Spain | 2004.1–2004.6 | 11 M | 123 | Hospital based Cohort study | 2 | Age, sex, duration of DM, arterial hypertension |
| Lee S.J. | Korea | 2002.9–2004.3 | < 1 Y | 496 | Population based Cohort study | 2 | Age, sex, BMI, hypertension |
| Hirai F.E. | USA | 1980–1982 | 20 Y | 2366 | Population based Cohort study | 1,2 | Age, sex, BMI, HbAlc, CVD history, hypertension |
| Shen L.Q. | USA | 2002.5.1–2003.5.31 | 2.8 Y | 282 | Population based Cohort study | 2 | Age, sex, race, duration of DM, HbA1c, blood pressure, use of antihypertensive drugs, pedal edema |
| Liu L.Y. | China | 2001–2005 | 32 M | 1974 | Hospital based Case-control study | 1,2 | Age, sex |
| Fong D.S. | USA | 2002–2006 | 1 Y | 143257 | Population based Cohort study | 2 | age and HgA1c, and excludes patients without drug benefit, no eye exam and HgA1c 7.0 |
| Motola D. | USA | 2005.1–2008.10 | 4 Y | 49589 | Population based Case-control study | 2 | NA |
| Idris I. | UK | 2000.1.1–2009.11.30 | 10 Y | 103368 | Population based Cohort study | 2 | Age, sex, BMI, blood pressure, HbA1c, HDL, LDL |
| Bertelsen G. | Norway | 2007.10–2008.11 | 1 Y | 514 | Population based Cohort study | 2 | Sex, blood pressure, BMI, cholesterol, somking |
Thirteen studies among all the included studies provided adjusted RR/OR value and the adjusted factors (e.g., age, sex, and diabetes mellitus duration) were different in each study. To evaluate the methodological qualities of the included studies, we used the Newcastle-Ottawa scale. The Newcastle-Ottawa scale assessment score of most studies was >6 (mean: 6.71; standard deviation: 1.83) and 2 studies got less than 6 because of too few data sources or due to methodological design. All the results are presented in Table 2.
Table 2.
Quality assessment of included studies#.
| Author, year | Quality assessment criteria | |||
|---|---|---|---|---|
| Selection | Comparability | Outcome/exposure | Overall quality | |
| Henricsson M., 1997 | *** | ** | *** | 8 |
| Bertram B., 1997 | *** | * | ** | 6 |
| Klein R., 1995 | *** | ** | *** | 8 |
| Leske M.C., 2003 | *** | * | ** | 6 |
| Aroca P.R., 2004 | ** | * | ** | 5 |
| Romero-Aroca P., 2006 | *** | ** | ** | 7 |
| Lee S.J., 2006 | *** | ** | ** | 7 |
| Hirai F.E., 2008 | ** | * | ** | 5 |
| Shen L.Q., 2008 | *** | ** | ** | 7 |
| Liu L.Y., 2007 | *** | ** | ** | 7 |
| Fong D.S., 2009 | *** | ** | *** | 8 |
| Motola D., 2012 | *** | * | ** | 6 |
| Idris I., 2012 | *** | ** | *** | 8 |
| Bertelsen G., 2013 | *** | * | ** | 6 |
the methodological qualities of the included studies were assessed using the Newcastle-Ottawa scale.
Insulin use and risk of macular edema
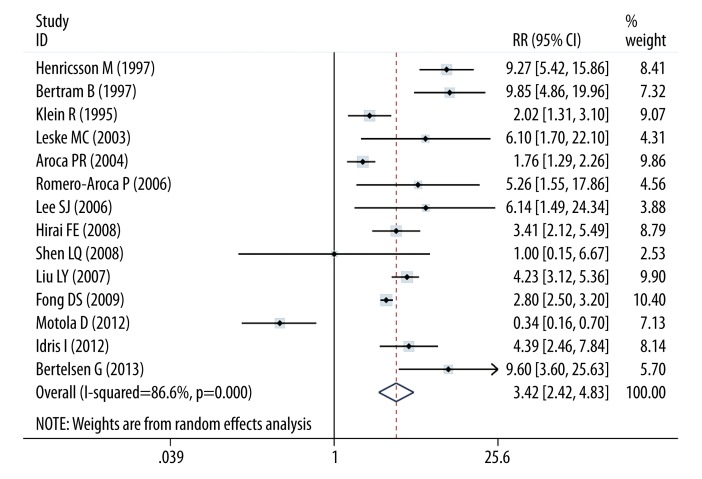
Figure 2 shows the relationship between insulin use and risk of macular edema. In a random-effects meta-analysis, the use of insulin was related with increased risk of macular edema (RR, 3.416; 95% CI, 2.417–4.829; I2, 86.6%). Table 3 shows the effects of insulin use and edema risk in subgroup analysis by adjustment status, study type, country, diabetes mellitus types, and duration. Analysis that just included the high-quality studies showed that insulin use increased that risk of macular edema (RR, 2.728; 95% CI, 1.881–3.955; I2=77.7%). Increased incidence of macular edema was observed in cohort studies (RR, 4.509; 95% CI, 3.100–6.559; I2, 77.7%) but not in case-control studies (RR, 1.455; 95% CI, 0.520–4.066; I2, 95.9%). When subgroup analyses were conducted according to the study designs, significant associations were detected in prospective studies (RR, 3.85; 95% CI, 2.637–5.620; I2=83.5) and retrospective studies (RR, 2.420; 95% CI, 0.867–6.753; I2=91.3). When the data source was considered, the population-based (RR, 2.726; 95% CI, 1.709–4.349; I2=82.7) and hospital based studies (RR, 4.934; 95% CI, 2.475–9.837; I2=91.4) showed a significant association between insulin use and risk of macular edema. However, the results were not changed in the subgroup analyses by follow-up duration and number of adjustment factors.
Figure 2.
Forest plot of insulin use and risk of diabetic macular edema. The size of the shaded square is proportional to the percent weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled ORs. A random-effects model was obtained.
Table 3.
Subgroup analysis of insulin use and macular edema incidence with combined RR.
| No. of studies | Pooled estimate | Tests of heterogeneity | |||
|---|---|---|---|---|---|
| RR | 95% CI | P value | I2 (%) | ||
| All studies | 14 | 3.416 | 2.417 to 4.829 | <0.001 | 86.6 |
| High-quality studies (scores ≥7) | 10 | 2.728 | 1.881 to 3.955 | <0.001 | 77.7 |
| Study design | |||||
| Cohort | 11 | 4.509 | 3.100 to 6.559 | <0.001 | 77.0 |
| Case-control | 3 | 1.455 | 0.520 to 4.066 | <0.001 | 95.9 |
| Data source | |||||
| Population based | 9 | 2.726 | 1.709 to 4.349 | <0.001 | 82.7 |
| Hospital based | 5 | 4.934 | 2.475 to 9.837 | <0.001 | 91.4 |
| Geographic location | |||||
| Europe | 6 | 5.560 | 2.579 to 11.985 | <0.001 | 89.8 |
| Asia | 2 | 4.288 | 3.287 to 5.592 | 0.607 | 0.0 |
| North America | 6 | 1.928 | 1.087 to 3.420 | <0.001 | 86.0 |
| DM type | |||||
| T2DM | 12 | 4.520 | 2.444 to 8.362 | <0.001 | 85.4 |
| T1DM and T2DM | 2 | 2.486 | 1.510 to 4.094 | <0.001 | 90.6 |
| Follow-up duration | |||||
| ≤5 years | 11 | 3.567 | 2.297 to 5.539 | <0.001 | 89.1 |
| >5 years | 3 | 3.026 | 1.918 to 4.774 | 0.076 | 61.3 |
| Design | |||||
| Prospective | 8 | 3.850 | 2.637 to 5.620 | <0.001 | 83.5 |
| Retrospective | 5 | 2.420 | 0.867 to 6.753 | <0.001 | 91.4 |
| Number of adjustment factors | |||||
| n ≤5 confounders | 8 | 3.663 | 1.842 to 7.283 | <0.001 | 91.7 |
| n ≥6 confounders | 6 | 3.136 | 2.284 to 4.308 | 0.03 | 59.4 |
RR – relative risk; CI – confidence interval.
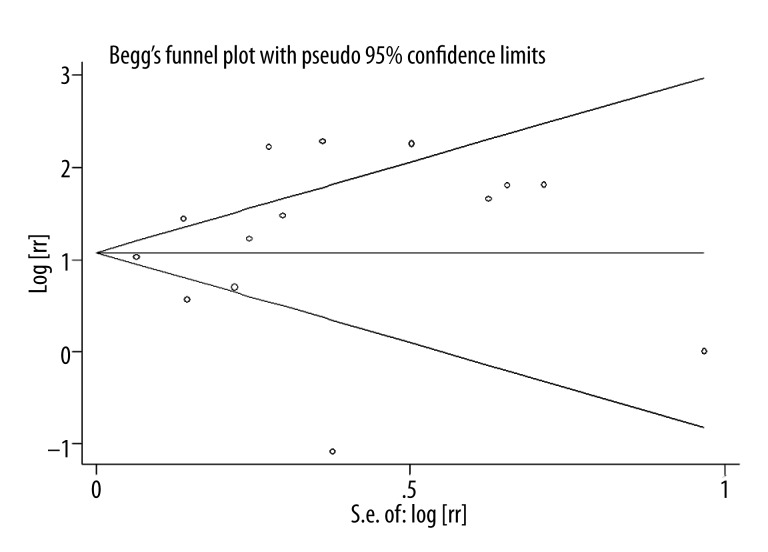
A significant heterogeneity was observed when all the 14 studies were included (I2, 86.6%; P<0.866). However, the heterogeneity was significant when the study design, data source, follow-up duration, and number of the adjusted factors were accounted for. However, when the subgroup analyses was conducted by location, we found that the studies conducted in Asia showed no significant association between insulin use and risk of macular edema (I2=0.0%, P=0.607). However, considering that only 2 of the studies in this meta-analysis were in Asian populations, the relatively low number of included studies might be the reason for the non-significant result. A sensitivity analysis was conducted after 2 studies that got Newcastle-Ottawa Scale <6 were excluded and no change was observed (RR, 2.728; 95% CI, 1.881–3.955; I2, 77.7%). No significant publication bias was found in the 14 selected studies, see Figure 3 (Begg’s funnel plot, symmetrical; Begg’s test, P for bias=0.381; Begg’s test, P for bias=0.606).
Figure 3.
Funnel plot of all the included studies.
Discussion
To the best of our knowledge, this is the first meta-analysis evaluating the association between insulin use and macular edema risk. The present meta-analysis included 14 observational studies currently available (11 cohort studies and 3 case-control studies), involving a total of 202 905 participants. There was statistically significant heterogeneity among the 14 included studies investigating the association between insulin use and macular edema risk, so a random-effects model was chosen over a fixed-effects model. Finally, we found that insulin use significantly increase the macular edema risk. Sensitivity analysis indicated that the omission of any 1 study did not alter the magnitude of observed effect, suggesting the stability of our findings. Moreover, the results of Begg’s test and Egger’s test did not support the existence of major publication bias.
In general, insulin use is one of the most important therapies for diabetes mellitus [30,31]. Insulin provides a better effect in the hypoglycemic effect and it can protect the insular function [32]. However, in the long-term outcome of patients with diabetes, we found that insulin use is associated with increased risk of various cancers with the development of insulin use. For example, in a meta-analysis using data from 12 published epidemiologic studies (7 case-control and 5 cohort studies) published before January 2014, it was reported that insulin use increased the risk of colorectal cancer [33]. Findings of significantly harmful effects of insulin, reported mainly in case-control studies, may stem from study design differences and number of included studies. Several studies reported that insulin use increased the risk of diabetic retinopathy. In this prospective, non-interventional, cross-sectional case-control study, 729 subjects from Los Angeles County University of Southern California Medical Center (LAC + USC), Los Angeles, CA, were enrolled. It was found that insulin use increased the risk of proliferative diabetic retinopathy (OR, 1.85; 95% CI, 1.13–3.03) [34]. Considering that diabetic macular edema is a common cause of visual impairment in diabetic patients, the association between insulin use and risk of macular edema needs advanced research [35]. In both case-control and cohort studies, there are strong and independent associations between insulin use and risk of macular edema [22–24]. There are various biases in observational studies. Recently, there have been no randomized controlled trials investigating the effect of insulin use on the incidence of macular edema. In this meta-analysis, through pooling 14 observational studies, we found that insulin use is a risk factor for macular edema.
The pathogenesis of diabetic macular edema remains to be completely defined because it is caused by a complex pathological process with many contributing factors [36]. Dysfunction of the inner and outer retinal barriers leads to accumulation of sub- and intra-retinal fluid in the inner and outer plexiform layers [37]. Vascular endothelial growth factor (VEGF) has generally been accepted as the main factor that disrupts the inner blood-retinal barrier (BRB) function, making it an important target for pharmaceutical intervention [38,39]. A study in diabetic mice found that insulin treatment resulted in increased vascular leakage, apparently mediated by betacellulin and signaling via the epidermal growth factor (EGF) receptor. In addition, treatment with EGF receptor inhibitors reduced retinal vascular leakage in diabetic mice on insulin. These findings provide unique insight into the role of insulin signaling in mediating retinal effects in diabetes, and open new avenues for treating the retinal complications of diabetes mellitus [40]., An in vitro study showed that PLGF-1 induced a reversible decrease in transepithelial resistance and enhanced tritiated insulin flux. These effects were specifically abolished by an antisense oligonucleotide directed at VEGF receptor 1. Exposure of ARPE-19 cells to hypoxic conditions or to insulin induced upregulation of PLGF-1 expression along with increased transcellular permeability. The PLGF-1-induced RPE cell permeability involved the MEK signalling pathway [41].
The strengths of this study are: (1) we adopted a relatively comprehensive literature search strategy in the acquisition of studies to consider for inclusion. To avoid missing includable articles, we searched the database with keywords of “insulin” and “antihyperglycemic” in combination with “macular edema”. (2) Most of the studies included in this meta-analysis demonstrated relatively high quality. The results of the sensitivity analysis and the publication bias detection suggest that the conclusions of the present study are quite robust (3). We performed consummate analyses, including detailed subgroup analyses and sensitivity analyses. The careful analyses provide more detailed data on the relation between insulin use and risk of diabetic macular edema.
As with any meta-analysis of observational studies, ours has several limitations. First, some of the studies followed a case-control study design and thus had recall and selection bias, which are inherent in retrospective studies. This study is limited in that, although subgroup analysis by study design was conducted, the robustness was limited by including too few cohort studies. Second, data on the formulations and methods of insulin use are limited in this study. There points all indicate the need for further well-designed studies.
Conclusions
The results from this meta-analysis of observational studies demonstrate that insulin use is a risk factor for diabetic macular edema. However, available data are still sparse, and in-depth analyses of the assessed associations in the context of additional longitudinal studies are highly desirable to enable more precise estimates and a better understanding of the role of insulin use in incidence of diabetic macular edema.
Footnotes
Source of support: Departmental sources
References
- 1.Raskin P, Rendell M, Riddle MC, et al. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–32. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Raman R, Rachapalli SR, Kulothungan V, et al. Estimating the rate of non-participation and its influence on the study results: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study report 32. Ophthalmic Res. 2011;45:79–86. doi: 10.1159/000319237. [DOI] [PubMed] [Google Scholar]
- 4.Robaszkiewicz J, Chmielewska K, Figurska M, et al. Triple therapy: Phaco-vitrectomy with ILM peeling, retinal endophotocoagulation, and intraoperative use of Bevacizumab for diffuse diabetic macular edema. Med Sci Monit. 2012;18(4):CR241–51. doi: 10.12659/MSM.882624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das UN. Is lipoxins A4 a better alternative to anti-VEGF and anti-TNF-alpha antibody to prevent and treat age-related macular degeneration, diabetic macular edema and retinopathy? Med Sci Monit. 2012;18(1):LE1–2. doi: 10.12659/msm.882187. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima H, Mizukawa K, Kiryu J. Factors associated with visual recovery after sub-Tenon injection of triamcinolone acetonide in diabetic macular edema. Clin Ophthalmol. 2012;6:1307–14. doi: 10.2147/OPTH.S34631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studnicka J. [The diabetic macular edema – new possibilities of the treatment]. Cesk Slov Oftalmol. 2012;68:61–63. [in Czech] [PubMed] [Google Scholar]
- 8.Ford JA, Elders A, Shyangdan D, et al. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review. BMJ. 2012;345:e5182. doi: 10.1136/bmj.e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho M, D’Amico DJ. Transconjunctival 25-gauge pars plana vitrectomy and internal limiting membrane peeling for chronic macular edema. Clin Ophthalmol. 2012;6:981–89. doi: 10.2147/OPTH.S33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127:1175–82. doi: 10.1001/archophthalmol.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zapata MA, Badal J, Fonollosa A, et al. Insulin resistance and diabetic macular oedema in type 2 diabetes mellitus. Br J Ophthalmol. 2010;94:1230–32. doi: 10.1136/bjo.2009.171702. [DOI] [PubMed] [Google Scholar]
- 12.Hirai FE, Knudtson MD, Klein BE, Klein R. Clinically significant macular edema and survival in type 1 and type 2 diabetes. Am J Ophthalmol. 2008;145:700–6. doi: 10.1016/j.ajo.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen LQ, Child A, Weber GM, et al. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126:793–99. doi: 10.1001/archopht.126.6.793. [DOI] [PubMed] [Google Scholar]
- 14.Liu LY, Dong FT, Li H. [Relationship between the classification of diabetic macular edema and its related factors]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:797–802. [in Chinese] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Robertson-Malt S, Malt GN, Farquhar V, Greer W. Heparin versus normal saline for patency of arterial lines. Cochrane Database Syst Rev. 2014;2010;5:CD007364. doi: 10.1002/14651858.CD007364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henricsson M, Nilsson A, Janzon L, Groop L. The effect of glycaemic control and the introduction of insulin therapy on retinopathy in non-insulin-dependent diabetes mellitus. Diabet Med. 1997;14:123–31. doi: 10.1002/(SICI)1096-9136(199702)14:2<123::AID-DIA306>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Bertram B. [Prevalence of patients with diabetes mellitus without and with retinopathy in an ophthalmology practice]. Ophthalmologe. 1997;94:401–4. doi: 10.1007/s003470050133. [in German] [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 22.Leske MC, Wu SY, Hennis A, et al. Incidence of diabetic retinopathy in the Barbados Eye Studies. Ophthalmology. 2003;110:941–47. doi: 10.1016/S0161-6420(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 23.Aroca PR, Salvat M, Fernandez J, Mendez I. Risk factors for diffuse and focal macular edema. J Diabetes Complications. 2004;18:211–15. doi: 10.1016/S1056-8727(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Aroca P, Fernandez-Ballart J, Almena-Garcia M, et al. Nonproliferative diabetic retinopathy and macular edema progression after phacoemulsification: prospective study. J Cataract Refract Surg. 2006;32:1438–44. doi: 10.1016/j.jcrs.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Choi MG. Association of manganese superoxide dismutase gene polymorphism (V16A) with diabetic macular edema in Korean type 2 diabetic patients. Metabolism. 2006;55:1681–88. doi: 10.1016/j.metabol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Fong DS, Contreras R. Glitazone use associated with diabetic macular edema. Am J Ophthalmol. 2009;147:583–86. doi: 10.1016/j.ajo.2008.10.016. e581. [DOI] [PubMed] [Google Scholar]
- 27.Motola D, Piccinni C, Biagi C, et al. Cardiovascular, ocular and bone adverse reactions associated with thiazolidinediones: a disproportionality analysis of the US FDA adverse event reporting system database. Drug Saf. 2012;35:315–23. doi: 10.2165/11596510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012;172:1005–11. doi: 10.1001/archinternmed.2012.1938. [DOI] [PubMed] [Google Scholar]
- 29.Bertelsen G, Peto T, Lindekleiv H, et al. Tromso eye study: prevalence and risk factors of diabetic retinopathy. Acta Ophthalmol. 2013;91:716–21. doi: 10.1111/j.1755-3768.2012.02542.x. [DOI] [PubMed] [Google Scholar]
- 30.Takahara M, Shiraiwa T, Katakami N, et al. Efficacy of Adding Once-Daily Insulin Glulisine in Japanese Type 2 Diabetes Patients Treated with Insulin Glargine and Sitagliptin. Diabetes Technol Ther. 2014 doi: 10.1089/dia.2014.0075. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Maria Rotella C, Pala L, Mannucci E. Role of insulin in the type 2 diabetes therapy: past, present and future. Int J Endocrinol Metab. 2013;11:137–44. doi: 10.5812/ijem.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tibaldi J. Achieving glycemic goals with addition of incretin-based therapies to insulin in patients with type 2 diabetes mellitus. Am J Med Sci. 2014;347:491–501. doi: 10.1097/MAJ.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 33.Yin S, Bai H, Jing D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients: a systemic review and meta-analysis. Diagn Pathol. 2014;9:91. doi: 10.1186/1746-1596-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nittala MG, Keane PA, Zhang K, Sadda SR. Risk Factors for Proliferative Diabetic Retinopathy in a Latino American Population. Retina. 2014;34(8):1594–99. doi: 10.1097/IAE.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aref AA, Scott IU. Management of macular edema secondary to branch retinal vein occlusion: an evidence-based update. Adv Ther. 2011;28:28–39. doi: 10.1007/s12325-010-0089-3. [DOI] [PubMed] [Google Scholar]
- 36.White NH, Sun W, Cleary PA, et al. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes. 2010;59:1244–53. doi: 10.2337/db09-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N, Xu X, Zou H, et al. The status of diabetic retinopathy and diabetic macular edema in patients with type 2 diabetes: a survey from Beixinjing District of Shanghai city in China. Ophthalmologica. 2008;222:32–36. doi: 10.1159/000109276. [DOI] [PubMed] [Google Scholar]
- 38.Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology. 2009;116:461–67. doi: 10.1016/j.ophtha.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy. The Beijing Eye Study 2006. Graefes Arch Clin Exp Ophthalmol. 2008;246:1519–26. doi: 10.1007/s00417-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto M, Cutler A, Shen B, et al. Inhibition of EGF signaling protects the diabetic retina from insulin-induced vascular leakage. Am J Pathol. 2013;183:987–95. doi: 10.1016/j.ajpath.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto N, de Kozak Y, Jeanny JC, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–70. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]