Abstract
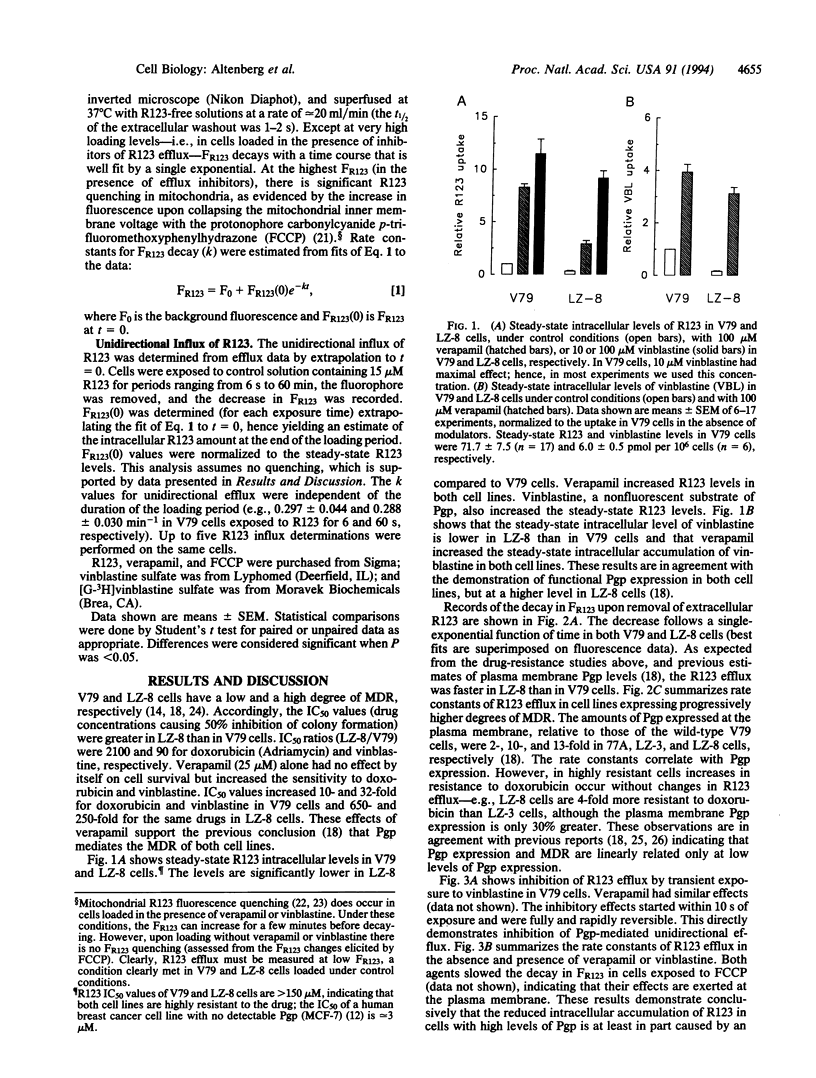
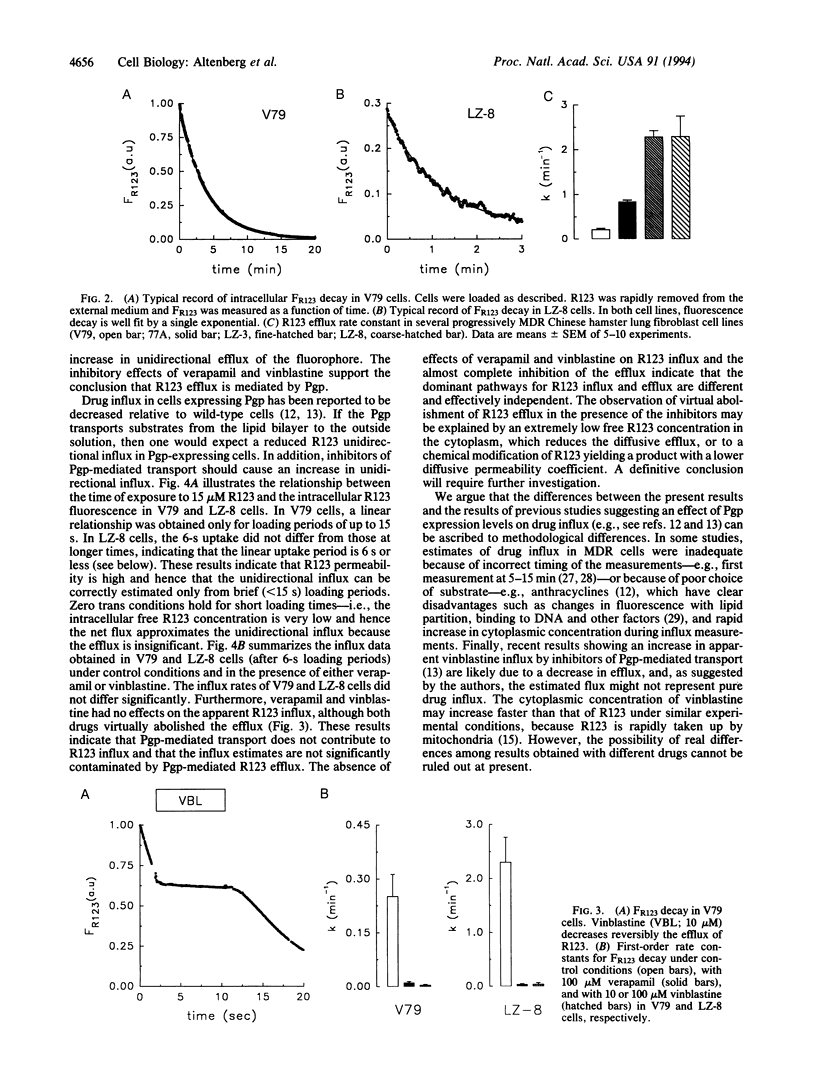
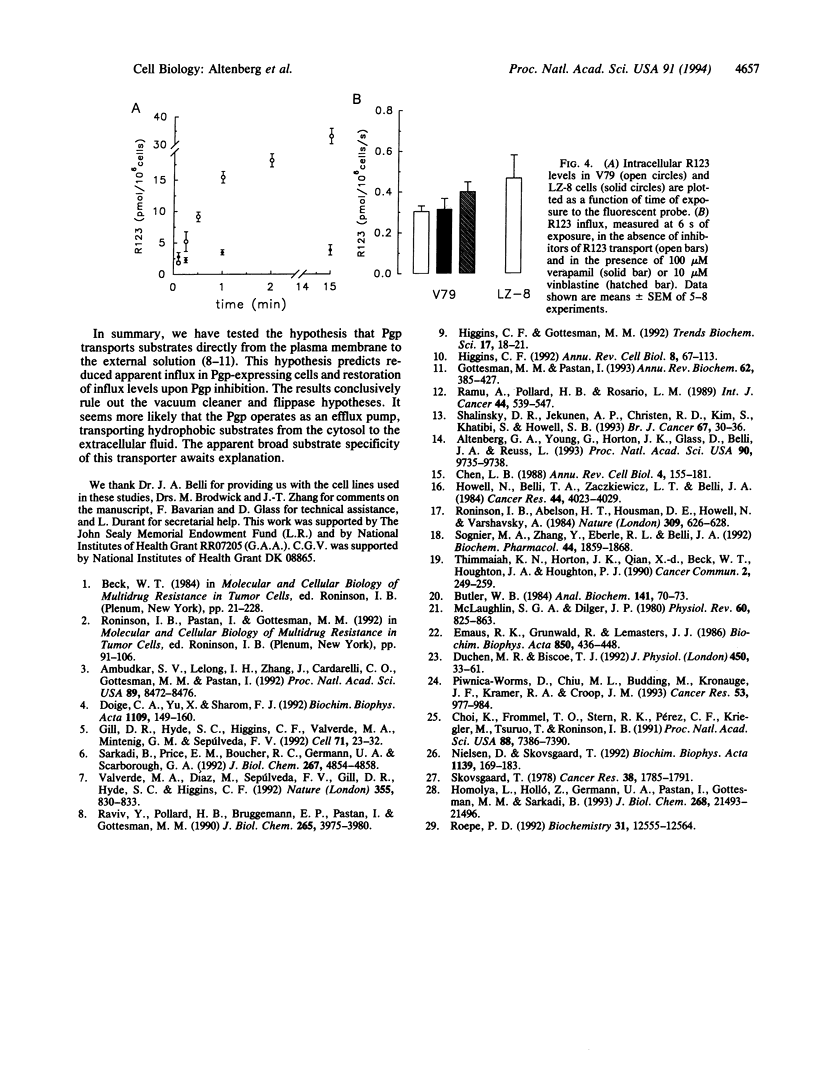
P-glycoprotein (Pgp), a plasma membrane protein overexpressed in multidrug-resistant tumor cells, is an ATPase thought to actively export cytotoxic drugs. It has been proposed that Pgp transports drugs directly from the lipid bilayer to the external medium ("vacuum cleaner" hypothesis). A possible mechanism for this model is that the Pgp is a flippase--i.e., it catalyzes the translocation of hydrophobic substrates from the inner to the outer leaflet of the cell membrane. Two immediate predictions of the vacuum cleaner and flippase hypotheses are that the apparent unidirectional influx of substrate should be less in Pgp-expressing than in Pgp-lacking cells and that this difference should be abolished by inhibition of the Pgp. We used Chinese hamster fibroblasts with different levels of Pgp expression to measure true unidirectional fluxes of rhodamine 123 (R123), a Pgp-transported fluorescent dye that accumulates in mitochondria (hence, its cytosolic concentration remains low at short times after external addition). The unidirectional efflux of R123 was proportional to the level of Pgp expression and was reduced by Pgp inhibitors. The unidirectional influx of R123 was the same in sensitive and resistant cells--i.e., independent of the level of Pgp expression and insensitive to inhibitors of R123 efflux. From these results, we rule out the vacuum cleaner and flippase hypotheses and conclude that Pgp extracts the actively transported substrates from the cytosol and not from the plasma membrane.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenberg G. A., Young G., Horton J. K., Glass D., Belli J. A., Reuss L. Changes in intra- or extracellular pH do not mediate P-glycoprotein-dependent multidrug resistance. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9735–9738. doi: 10.1073/pnas.90.20.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar S. V., Lelong I. H., Zhang J., Cardarelli C. O., Gottesman M. M., Pastan I. Partial purification and reconstitution of the human multidrug-resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8472–8476. doi: 10.1073/pnas.89.18.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. B. Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal Biochem. 1984 Aug 15;141(1):70–73. doi: 10.1016/0003-2697(84)90426-3. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Choi K., Frommel T. O., Stern R. K., Perez C. F., Kriegler M., Tsuruo T., Roninson I. B. Multidrug resistance after retroviral transfer of the human MDR1 gene correlates with P-glycoprotein density in the plasma membrane and is not affected by cytotoxic selection. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7386–7390. doi: 10.1073/pnas.88.16.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doige C. A., Yu X., Sharom F. J. ATPase activity of partially purified P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Biochim Biophys Acta. 1992 Aug 24;1109(2):149–160. doi: 10.1016/0005-2736(92)90078-z. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Biscoe T. J. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992 May;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Homolya L., Holló Z., Germann U. A., Pastan I., Gottesman M. M., Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993 Oct 15;268(29):21493–21496. [PubMed] [Google Scholar]
- Howell N., Belli T. A., Zaczkiewicz L. T., Belli J. A. High-level, unstable adriamycin resistance in a Chinese hamster mutant cell line with double minute chromosomes. Cancer Res. 1984 Sep;44(9):4023–4029. [PubMed] [Google Scholar]
- McLaughlin S. G., Dilger J. P. Transport of protons across membranes by weak acids. Physiol Rev. 1980 Jul;60(3):825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Nielsen D., Skovsgaard T. P-glycoprotein as multidrug transporter: a critical review of current multidrug resistant cell lines. Biochim Biophys Acta. 1992 Jul 7;1139(3):169–183. doi: 10.1016/0925-4439(92)90131-6. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Chiu M. L., Budding M., Kronauge J. F., Kramer R. A., Croop J. M. Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res. 1993 Mar 1;53(5):977–984. [PubMed] [Google Scholar]
- Ramu A., Pollard H. B., Rosario L. M. Doxorubicin resistance in P388 leukemia--evidence for reduced drug influx. Int J Cancer. 1989 Sep 15;44(3):539–547. doi: 10.1002/ijc.2910440328. [DOI] [PubMed] [Google Scholar]
- Raviv Y., Pollard H. B., Bruggemann E. P., Pastan I., Gottesman M. M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J Biol Chem. 1990 Mar 5;265(7):3975–3980. [PubMed] [Google Scholar]
- Roepe P. D. Analysis of the steady-state and initial rate of doxorubicin efflux from a series of multidrug-resistant cells expressing different levels of P-glycoprotein. Biochemistry. 1992 Dec 22;31(50):12555–12564. doi: 10.1021/bi00165a003. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992 Mar 5;267(7):4854–4858. [PubMed] [Google Scholar]
- Shalinsky D. R., Jekunen A. P., Alcaraz J. E., Christen R. D., Kim S., Khatibi S., Howell S. B. Regulation of initial vinblastine influx by P-glycoprotein. Br J Cancer. 1993 Jan;67(1):30–36. doi: 10.1038/bjc.1993.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovsgaard T. Mechanisms of resistance to daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Jun;38(6):1785–1791. [PubMed] [Google Scholar]
- Sognier M. A., Zhang Y., Eberle R. L., Belli J. A. Characterization of adriamycin-resistant and radiation-sensitive Chinese hamster cell lines. Biochem Pharmacol. 1992 Nov 3;44(9):1859–1868. doi: 10.1016/0006-2952(92)90082-t. [DOI] [PubMed] [Google Scholar]
- Thimmaiah K. N., Horton J. K., Qian X. D., Beck W. T., Houghton J. A., Houghton P. J. Structural determinants of phenoxazine type compounds required to modulate the accumulation of vinblastine and vincristine in multidrug-resistant cell lines. Cancer Commun. 1990;2(7):249–259. doi: 10.3727/095535490820874308. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]



