Abstract
The functional integrity of the nucleus accumbens (NAC) core and shell is necessary for contextual cocaine-seeking behavior in the reinstatement animal model of drug relapse; however, the neuropharmacological mechanisms underlying this phenomenon are poorly understood. The present study evaluated the contribution of metabotropic glutamate receptor subtype 1 (mGluR1) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor populations to drug context-induced reinstatement of cocaine-seeking behavior. Rats were trained to lever press for un-signaled cocaine infusions in a distinct context followed by extinction training in a different context. Cocaine-seeking behavior (non-reinforced active lever pressing) was then assessed in the previously cocaine-paired and extinction contexts after JNJ16259685 (mGluR1 antagonist: 0.0, 0.6, or 30 pg/0.3 μl/hemisphere) or CNQX (AMPA/kainate receptor antagonist: 0.0, 0.03, or 0.3 μg/0.3 μl/hemisphere) administration into the NAC core, medial or lateral NAC shell, or the ventral caudate-putamen (vCPu, anatomical control). JNJ16259685 or CNQX in the NAC core dose-dependently impaired contextual cocaine-seeking behavior relative to vehicle. Conversely, CNQX, but not JNJ16259685, in the lateral or medial NAC shell attenuated, whereas CNQX or JNJ16259685 in vCPu failed to inhibit, this behavior. The manipulations failed to alter instrumental behavior in the extinction context, general motor activity, or food-reinforced instrumental behavior in control experiments. Thus, glutamate-mediated changes in drug context-induced motivation for cocaine involve distinct neuropharmacological mechanisms within the core and shell subregions of the NAC, with the stimulation of mGlu1 and AMPA/kainate receptors in the NAC core and the stimulation of AMPA/kainate, but not mGlu1, receptors in the NAC shell being necessary for this phenomenon.
Keywords: Cocaine, Context, Ionotropic glutamate receptor, Metabotropic glutamate receptor, Nucleus accumbens, Reinstatement
Functional imaging studies have demonstrated that self-reports of cue-induced cocaine craving are positively correlated with enhanced neural activity in the nucleus accumbens (NAC; Kilts et al. 2001; 2004; Volkow et al. 2010). Consistent with these clinical findings, the functional integrity of the NAC core and shell is necessary for drug context-induced cocaine-seeking behavior in the extinction/reinstatement animal model of drug relapse (Fuchs, Ramirez & Bell 2008). More specifically, glutamate neurotransmission in the NAC is critical for the renewal of drug-seeking behavior induced by exposure to environmental contexts paired previously with repeated drug exposure, in the presence of discrete cocaine-paired conditioned stimuli (Bossert et al. 2006); however, the contribution of specific NAC glutamate receptor subpopulations to this phenomenon or to context-induced reinstatement has not been investigated. Thus, the present study evaluated the putative role of metabotropic glutamate receptor subtype 1 (mGluR1) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors in the core, medial shell, and lateral shell subregions of the NAC as well as in the ventral caudate-putamen (vCPu) in drug context-induced reinstatement of cocaine-seeking behavior.
The mGluR1 is a member of the group I mGluRs family, similar to mGluR5 (Bird & Lawrence 2009; Kenny & Markou 2004). The study of mGluR1 function has been impeded by the unavailability of receptor selective antagonists. Recent studies, however, indicate that mGluR1 populations are relatively dense in the NAC (Bonsi et al. 2008; Martin et al. 1992; Mitrano & Smith 2007), and they potentially contribute to drug-induced behaviors by regulating intracellular calcium signaling and multiple forms of neural plasticity (Anwyl 2009; Bonsi et al. 2008; Grueter, McElligott & Winder 2007; Olive 2009). In particular, mGluR1s exhibit cocaine-induced changes in function, cellular expression, and localization in the NAC core and shell and these neuroadaptations persist even after prolonged cocaine abstinence (Ben-Shahar et al. 2009; Mitrano, Arnold & Smith 2008; Swanson et al. 2001). Stimulation of mGluR1s in the dorsal hippocampus (DH) is necessary for drug context-induced reinstatement of cocaine-seeking behavior (Xie et al. 2010). However, little is known about the putative contribution of striatal mGluR1 populations to this behavior.
Similar to mGluR1s, AMPA/kainate receptors exert a critical regulatory role over drug-induced behaviors. Consistent with this, administration of AMPA into the NAC core or shell is sufficient to elicit drug-seeking behavior (Ping et al. 2008; Suto et al. 2004). Inhibition of the surface expression, or pharmacological antagonism, of AMPA/kainate receptors in the NAC core or shell inhibits drug-primed reinstatement of drug-seeking behavior (Cornish & Kalivas 2000; Famous et al. 2008; LaLumiere & Kalivas 2008). Similarly, AMPA/kainate receptor antagonism in the NAC core impairs explicit cue-induced reinstatement of extinguished cocaine- and heroin-seeking behaviors (Bäckström & Hyytiä 2007; LaLumiere & Kalivas 2008). However, it has yet to be investigated whether the stimulation of AMPA/kainate receptor subpopulations in the NAC is also necessary for drug context-induced reinstatement of cocaine-seeking behavior.
To explore these questions, an ABA experimental design was used in the present study to evaluate the hypothesis that mGlu1 and AMPA/kainate receptors within the core and shell of the NAC as well as in the vCPu differentially regulate the ability of a previously drug-paired context to reinstate cocaine-seeking behavior, in the absence of discrete cocaine-paired conditioned stimuli. The medial and lateral shell subregions of the NAC were investigated separately because neurons in these subregions exhibit distinct morphology and connectivity (Ikemoto 2007; Meredith et al. 1992; Voorn et al. 2004) and may contribute differently to drug context-induced cocaine-seeking behavior. In these localization experiments, the dose-dependent effects of the highly potent mGluR1 subtype-selective antagonist JNJ16259685 and the AMPA/kainate receptor selective antagonist CNQX on drug context-induced reinstatement of cocaine-seeking behavior were assessed. In addition, the effects of JNJ16259685 and CNQX on food-reinforced instrumental responding as well as on general locomotor activity were also examined in order to discriminate the putative effects of these pharmacological manipulations on drug context-induced incentive motivation for cocaine and motor performance.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles-River, N = 144; 250–275 g) were individually housed in a temperature- and humidity-controlled vivarium on a reversed light/dark cycle. Rats were maintained on 20–25 g of rat chow per day and ad libitum water. The housing and treatment of the rats followed the guidelines of the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources, Commission on Life Sciences 1996) and were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Food training
In order to expedite the acquisition of cocaine self-administration, rats were first trained to lever press on a fixed ratio (FR) 1 schedule of food reinforcement (45 mg pellets; Purina, Richmond, IN, USA) in standard sound-attenuated operant-conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA, USA) during a single 16-h overnight training session. The chambers were equipped with two retractable levers and a food pellet dispenser between the levers. During the session, lever presses on one (active) lever resulted in delivery of one food pellet only. Lever presses on the second (inactive) lever had no programmed consequences. The contextual stimuli used for subsequent conditioning were not present.
Surgery
Forty-eight hours after food training, rats were anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.3 mg/kg, respectively, intraperitoneal). Intravenous catheters were constructed as described previously (Fuchs et al. 2007; 2008) and surgically implanted into the right jugular vein. The catheter ran subcutaneously and exited posterior to the shoulder blades. Immediately after the catheter surgery, the rats were placed into a stereotaxic instrument (Stoelting, Wood Dale, IL, USA). Stainless steel guide cannulae (26 G, Plastics One) were aimed at the NAC core (AP +1.4, ML +/−3.1, DV −4.8), medial shell (AP +1.3, ML +/−2.5, DV −5.9), lateral shell (AP +2.0, ML +/− 2.6, DV −7.0), or the ventral caudate-putamen (vCPu; anatomical control region: AP +1.4, ML +/−3.1, DV −3.8). Coordinates are shown in mm relative to bregma (Paxinos and Watson, 1997). Three stainless steel screws and dental acrylic anchored the guide cannulae to the skull. Stylets (Plastics One) and Tygon caps sealed the cannulae and catheters, respectively, in order to prevent occlusion. Rats were given 5 days for post-operative recovery before the experiments started.
Following surgery, catheters were flushed through once daily with 0.1 ml of an antibiotic solution of cefazolin (100.0 mg/ml, Schein Pharmaceutical, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Baxter Healthcare, Deerfield, IL, USA). During self-administration training, catheters were flushed through with an additional 0.1 ml of heparinized saline (10 U/ml) prior to each session. Catheter patency was periodically verified by infusing 0.1 ml of propofol (10 mg/ml, intravenous; Abbot Labs., North Chicago, IL, USA), which produces rapid loss of muscle tone.
Cocaine self-administration training
Cocaine self-administration training was conducted during daily 2-h sessions during the rats’ dark cycle and continued until a rat reached the acquisition criterion (i.e., ≥ 10 infusions self-administered/session on a minimum of 10 training days). Rats (N = 112) were trained to press a lever under an FR 1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.15 mg/0.05 ml per infusion; National Institute on Drug Abuse, Research Triangle Park, NC, USA) with a 20-s timeout period.
Rats were randomly assigned to receive self-administration training in operant-conditioning chambers equipped with two distinctly different sets of multi-modal contextual stimuli. Context 1 contained a continuous red house light (0.4 fc brightness), intermittent pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine-scented air freshener strip, and wire mesh floor (26 cm × 27 cm). Context 2 contained an intermittent white stimulus light above the inactive lever (1.2 fc brightness; 2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener strip, and a slanted ceramic tile wall that bisected the bar floor (19 cm × 27 cm). Rats had no exposure to these contextual stimuli prior to cocaine self-administration training. These stimuli were presented throughout each session independent of responding, as in our previous studies (Fuchs et al. 2005; 2007; 2008).
Extinction and reinstatement testing
Rats received daily 2-h extinction training sessions in a context distinctly different from the cocaine-paired context for a minimum of seven consecutive days. During the sessions, lever responses were recorded, but had no programmed consequences. Rats were adapted to the microinfusion procedure prior to placement into the chamber on extinction day 4. To this end, injection cannulae were inserted bilaterally into the guide cannulae to a depth 1 mm below the tip of the guide cannulae. The injectors were left in place for 4 min, but no fluid was infused. Once rats reached the extinction criterion (≤ 25 active lever responses/session on at least 2 consecutive days), they received two test sessions in the previously cocaine-paired context and two test sessions in the extinction context. Prior to each test session, rats received bilateral microinfusions of one dose of the selective mGluR1 antagonist JNJ16259685 (0.6 or 30 pg/0.3 μl/hemisphere), which displays a greater than 1,000-fold selectivity for mGluR1s over mGluR5s in vitro (IC50 < 19 nM; Fukunaga, Yeo & Batchelor 2007; Lavreysen et al. 2004), or 0.1 % DMSO vehicle (0.3 μl/hemisphere) into the NAC core, medial or lateral NAC shell, or ventral caudate-putamen (vCPu). Doses for JNJ16259685 were selected based on our preliminary experiments, which examined the effects of 0.6 pg-1.5 ng doses of JNJ16259685, and our previous study, in which JNJ16259685 inhibited contextual cocaine-seeking behavior after infusion into the DH (Xie et al. 2010). Separate groups of rats received bilateral microinfusions of one dose of the selective AMPA/kainate receptor antagonist CNQX (0.03 or 0.3 μg/0.3 μl/hemisphere) or phosphate-buffered saline (PBS; 0.3 μl/hemisphere) into the same brain regions. These doses of CNQX have been used to demonstrated the role of NAC AMPA/kainate receptors in cocaine self-administration and other forms of cocaine-seeking behavior (Bäckström and Hyytiä 2007; Bell, Duffy & Kalivas 2000; Cornish & Kalivas 2000; Famous et al. 2008; Park et al. 2002). The microinfusions were administered over 2 min, and the injectors were left in the guide cannulae for 1 min before and after the infusion. The order of testing in the previously cocaine-paired versus extinction contexts and treatment order (JNJ16259685 or CNQX, vehicle) were counterbalanced based on active lever responding during the last three cocaine self-administration sessions. Between test sessions, rats received a minimum of two additional extinction training sessions until they re-obtained the extinction criterion (described above). During each test session, responses on the active and inactive levers were recorded for 1 h, but had no programmed consequences. The use of 1-h test sessions minimized extinction learning in the cocaine-paired context thus permitting two test sessions per subject.
Locomotor activity and food-reinforced instrumental behavior
Pharmacological manipulations may produce motor side effects that alter the expression of motivated behavior. To assess this, effects of JNJ16259685 and CNQX on general activity and instrumental motor performance were examined.
Two locomotor activity test sessions were conducted using a partial within-subjects design, at least 72 h after the last reinstatement test session. Before testing, rats received bilateral microinfusions of one dose of JNJ16259685 or CNQX or vehicle into the target brain region using the infusion procedures described above, with the assignment to treatment group and treatment order randomized. Horizontal locomotor activity was measured in novel Plexiglas chambers (42 × 20 × 20 cm high), as described previously (Fuchs et al. 2007; 2008). The total number of photobeams breaks was recorded by a computerized activity system (San Diego Instruments, San Diego, CA, USA) during each 1-h test session.
Experimentally naïve rats (N = 32) were trained to lever press for food pellets (45 mg, Purina) during daily 2-h sessions in context 1 or 2. As in our previous study (Xie et al. 2010), the rats received free access to an additional 100 food pellets in their home cages 1-h before each session in order to elicit similar lever response rates – thus similar sensitivity to the rate-altering effects of the glutamate antagonists – as those seen in the cocaine reinstatement experiments. During all training and test sessions, active lever presses resulted in the delivery of a single food pellet (45 mg, Purina) under an FR 1 reinforcement schedule with a 20-s timeout period. Inactive lever presses had no programmed consequences. After active lever responding stabilized (i.e., ≤ 20% variability across two consecutive sessions), three 1-h test sessions were conducted using a counterbalanced within-subject test design. Before testing, rats received bilateral microinfusions of one dose of JNJ16259685 or CNQX or vehicle into the target brain region using the infusion procedure described above. Between test sessions, rats received a minimum of two food self-administration training sessions.
Histology
Rats were overdosed using ketamine and xylazine (66.6 and 1.3 mg/kg intravenous or 199.8 and 3.9 mg/kg intraperitoneal, respectively, depending on catheter patency). The brains were dissected out, sectioned, and stained with cresyl violet (Kodak, Rochester, NY). The most ventral point of each cannula track was mapped onto schematics from the rat brain atlas (Paxinos & Watson 1997).
Data analysis
Data were analyzed using mixed factorial or repeated measures analyses of variance (ANOVAs) where appropriate. Significant ANOVA main and interaction effects were further investigated using Tukey tests, when appropriate. Alpha was set at 0.05.
RESULTS
Histology
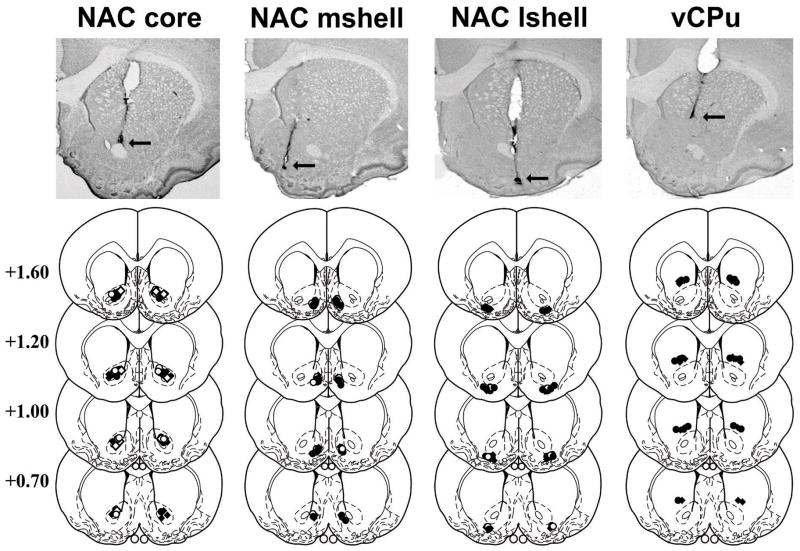
Schematics illustrating cannula placement and photomicrographs showing representative cannula tracts are shown in Fig. 1. The target brain regions were defined as the NAC core, NAC medial shell (i.e., NAC shell region medial relative to the NAC core), NAC lateral shell (i.e., NAC shell region ventral to the NAC core and lateral to the medial NAC shell), and vCPu (i.e., striatal region dorsally adjacent to the NAC core). The most ventral point of the infusion cannula tracks was located within the NAC core (N = 33), NAC medial shell (N = 31), NAC lateral shell (N = 31), or vCPu (N = 17) bilaterally in all cocaine-trained rats and within the NAC core (N = 16), NAC medial shell (N = 7), or NAC lateral shell (N = 9) in all food-trained rats. Furthermore, high power microscopy did not reveal any evidence of abnormal tissue damage (i.e., extensive cell loss or gliosis) at the infusion site. The resulting sample sizes for each treatment group are reported in the figure captions for figures 2–5.
Fig. 1.
Schematic and photographic representation of injection cannula placements within the nucleus accumbens core (NAC core), medial shell (NAC mshell), lateral shell (NAC lshell), and ventral caudate-putamen (vCPu). The arrows identify the most ventral point of the infusion cannula tracts on representative cresyl violet-stained sections. On the schematics from the rat brain atlas of Paxinos and Watson (1997), filled diamonds and open diamonds represent the most ventral point of the cannula tracts for JNJ162596850-treated rats in the cocaine-trained and food-trained groups, respectively. Filled circles and open circles represent the most ventral point of the cannula tracts for CNQX-treated rats in the cocaine-trained groups and food-trained groups, respectively. Numbers indicate the distance from bregma in millimeters.
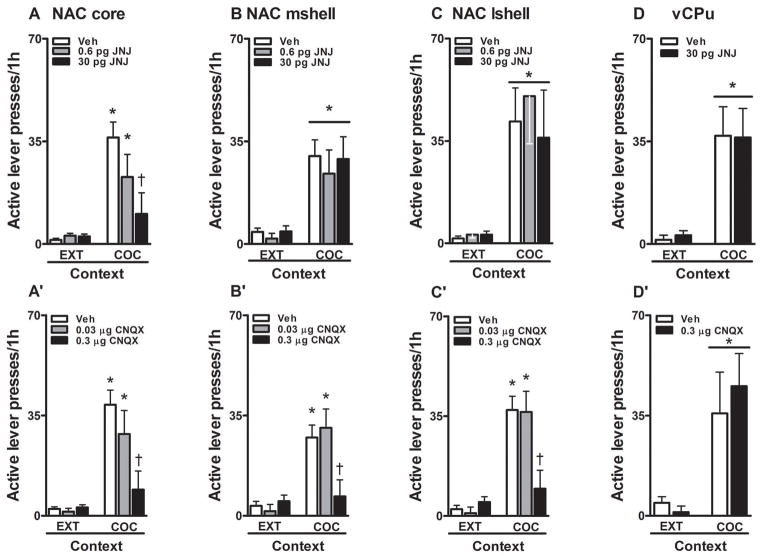
Fig. 2.
Subregion-specific effects of JNJ16259685 and CNQX on non-reinforced active lever responses (mean/1h ± SEM) during testing in the extinction (EXT) and previously cocaine-paired contexts (COC). JNJ16259685 or vehicle was infused bilaterally into the NAC core (A: N = 7–15/dose), NAC mshell (B: N = 7–15/dose), NAC lshell (C: N = 7–15/dose), or vCPu (D: N = 9/dose) before testing. In separate groups, CNQX or vehicle was infused bilaterally into the NAC core (A′: N = 7–18/dose), NAC mshell (B′: N = 7–16/dose), NAC lshell (C′: N = 7–16/dose), or vCPu (D′: N = 8/dose) before testing. Asterisks represent significant difference relative to responding in the extinction context (Panel A and A′–C′: ANOVA context simple main effect, Tukey test, p < 0.05; Panel B–D and D′: ANOVA context main effect, p < 0.05). Daggers represent significant difference relative to vehicle treatment (ANOVA treatment simple main effect, Tukey test, p < 0.05).
Cocaine Self-administration
All NAC core-, medial shell-, lateral shell-, and vCPu-cannulated groups exhibited stable responding on the active lever during the last three self-administration training days with a within-subject variability of < 10% in daily cocaine intake. Collapsed across groups, the mean numbers of active lever responses was 55.25 ± 3.60, and the mean daily cocaine intake (± SEM) was approximately 12.52 ± 0.45 mg/kg per session (25.04 ± 0.90 infusions). There was no pre-existing difference between the groups in active or inactive lever responding during the last three days of cocaine self-administration training (F < 1, data not shown).
Extinction
Upon removal of cocaine reinforcement, active and inactive lever responding gradually declined in all the NAC core-, medial shell-, lateral shell-, and vCPu-cannulated groups (all time main effects, F(6, 666) = 26.78–74.74, p = 0.0001). There was no pre-existing difference between the groups in active or inactive lever responding during the first seven days of extinction training (all cannula location main and interaction effects, (F < 1) or in the mean number of daily sessions (± SEM; 7.36 ± 0.05) needed to reach the extinction criterion (F < 1). Collapsed across groups, the average active and inactive lever responding (± SEM) decreased from 58.14 ± 4.64 and 8.44 ± 1.11 on the first day of extinction training to 7.59 ± 0.64 and 1.43 ± 0.25 on the last day of extinction training.
Site-specific effects of mGlu1 and AMPA/kainate receptor antagonism on drug context-induced reinstatement of cocaine seeking
Exposure to the previously cocaine-paired context reinstated active lever responding in rats following intracranial (NAC core, medial NAC shell, lateral NAC shell, or vCPu) vehicle pretreatment regardless of treatment order and treatment history (see Supplementary Fig. 1). Therefore, data were collapsed across treatment order and treatment history to form one vehicle condition per experiment (Fig. 2). Administration of JNJ16259685 or CNQX into the NAC core, medial NAC shell, lateral NAC shell, or vCPu did not alter inactive lever responding in either the cocaine-paired or the extinction context, relative to vehicle (see Supplementary Fig. 2).
NAC core
Intra-NAC core JNJ16259685 treatment differently attenuated active lever responding as a function of testing context and dose (Fig. 2A). Following pre-treatment with vehicle or the 0.6 pg dose of JNJ16259685, exposure to the cocaine-paired context increased active lever responding relative to responding in the extinction context (treatment × context interaction effect, F(2, 27) = 4.85, p = 0.01, Tukey test, p < 0.05; context main effect, F(1, 27) = 28.05, p = 0.0001; treatment main effect, F(2, 27) = 3.87, p = 0.03). The 0.6 pg dose of JNJ16259685 failed to alter active lever responding in either the extinction or cocaine-paired context relative to vehicle. In contrast, the 30 pg dose decreased active lever responding in the cocaine-paired context (Tukey test, p < 0.05), without altering active lever responding in the extinction context, relative to vehicle. As a result, active lever responding in the cocaine-paired context was not different from that in the extinction context.
Similar to JNJ16259685, intra-NAC core CNQX treatment differently attenuated active lever responding as a function of testing context and dose (Fig. 2A′). Following pre-treatment with vehicle or the 0.03 μg dose of CNQX, exposure to the cocaine-paired context increased active lever responding relative to responding in the extinction context (treatment × context interaction effect, F(2, 33) = 6.29, p = 0.005, Tukey test, p < 0.05; treatment main effect, F(1, 33) = 6.40, p = 0.004; context main effect, F(1, 33) = 33.80, p = 0.0001). The 0.03 μg dose of CNQX failed to alter active lever responding in the extinction or cocaine-paired context relative to vehicle. In contrast, the 0.3 μg dose decreased active lever responding in the cocaine-paired context (Tukey test, p < 0.05), without altering active lever responding in the extinction context, relative to vehicle. As a result, active lever responding in the cocaine-paired context was not different from that in the extinction context.
NAC medial shell
Following pre-treatment with vehicle, exposure to the previously cocaine-paired context increased active lever responding relative to responding in the extinction context (context main effect, F(1, 27) = 33.99, p = 0.0001, Fig. 2B). Furthermore, intra-NAC medial shell administration of 0.6 or 30 pg dose of JNJ16259685 failed to alter active lever responding in either context relative to vehicle (treatment × context interaction effect, F(2, 27) = 0.26, p = 0.77; treatment main effect, F(2, 27) = 0.17, p = 0.84).
In contrast to JNJ16259685, intra-NAC medial shell CNQX treatment differently attenuated active lever responding as a function of testing context and dose (Fig. 2B′). Following pre-treatment with vehicle or the 0.03 μg dose of CNQX into the NAC medial shell, exposure to the cocaine-paired context increased active lever responding relative to responding in the extinction context (treatment × context interaction effect, F(2, 29) = 5.86, p = 0.007, Tukey test, p < 0.05; treatment main effect, F(2, 29) = 3.43, p = 0.03; context main effect, F(1, 29) = 29.15, p = 0.0001). The 0.03 μg dose of CNQX failed to alter active lever responding in the extinction or cocaine-paired context relative to vehicle. In contrast, the 0.3 μg dose decreased active lever responding in the cocaine-paired context (Tukey test, p < 0.05), without altering responding in the extinction context, relative to vehicle. As a result, active lever responding in the cocaine-paired context was not different from that in the extinction context.
NAC lateral shell
Following pre-treatment with vehicle, exposure to the previously cocaine-paired context increased active lever responding relative to responding in the extinction context in the JNJ16259685 treatment groups (context main effect, F(1, 27) = 23.67, p = 0.0001, Fig. 2C). Furthermore, administration of 0.6 or 30 pg dose of JNJ16259685 into the NAC lateral shell failed to alter active lever responding in either context relative to vehicle (treatment × context interaction effect, F(2, 27) = 0.46, p = 0.63; treatment main effect, F(2, 27) = 0.43, p = 0.64).
In contrast to JNJ16259685, intra-NAC lateral shell CNQX treatment differently attenuated active lever responding as a function of testing context and dose (Fig. 2C′). Following pre-treatment with vehicle or the 0.03 μg dose of CNQX into the NAC lateral shell, exposure to the cocaine-paired context increased active lever responding relative to responding in the extinction context (treatment × context interaction effect, F(2, 29) = 8.51, p = 0.001, Tukey test, p < 0.05; treatment main effect, F(2, 29) = 4.22, p = 0.02; context main effect, F(1, 29) = 50.72, p = 0.0001). The 0.03 μg dose of CNQX failed to alter active lever responding in the extinction or cocaine-paired context relative to vehicle. In contrast, the 0.3 μg dose decreased active lever responding in the cocaine-paired context (Tukey test, p < 0.05), without altering responding in the extinction context, relative to vehicle. As a result, active lever responding in the cocaine-paired context was not different from that in the extinction context.
vCPu
Following pre-treatment with vehicle, exposure to the previously cocaine-paired context increased active lever responding relative to responding in the extinction context in the JNJ16259685 treatment group (context main effect, F(1, 16) = 24.40, p = 0.0001, Fig. 2D). Furthermore, the 30 pg dose of JNJ16259685, a dose behaviorally effective in the NAC core, did not alter active lever responding in either context relative to vehicle (treatment × context interaction effect, F(1, 16) = 0.02, p = 0.88; treatment main effect, F(1, 16) = 0.01, p = 0.95).
Similarly, following pre-treatment with vehicle, exposure to the previously cocaine-paired context increased active lever responding relative to responding in the extinction context in the CNQX treatment group (context main effect, F(1, 14) = 12.80, p = 0.003, Fig. 2D′). However, intra-vCPu administration of the 0.3 μg dose of CNQX, a dose behaviorally effective in the NAC core, did not alter active lever responding relative to vehicle in either context (treatment × context interaction effect, F(1, 14) = 0.36, p = 0.55; treatment main effect, F(1, 14) = 0.09, p = 0.76).
Effects of intra-NAC core JNJ16259685 treatment on locomotor activity and food-reinforced instrumental behavior
Intra-NAC core JNJ16259685 treatment selectively attenuated drug context-induced reinstatement of cocaine-seeking behavior. Therefore, we investigated the possible performance-impairing side effects of this manipulation on locomotor activity in a novel context and on instrumental behavior reinforced by food.
Intra-NAC core administration of JNJ16259685 did not alter locomotor activity in a novel context relative to vehicle (Fig. 3A). The number of photobeam breaks decreased during the locomotor activity test session (time main effect, F(2, 54) = 60.85, p = 0.0001; 20-min interval 1 > 2–3, Tukey test, p < 0.05). Furthermore, the 0.6 or 30 pg dose of JNJ16259685 did not alter the number of photobeam breaks relative to vehicle (time × treatment interaction effect, F(4, 54) = 1.03, p = 0.40; treatment main effect, F(2, 27) = 1.40, p = 0.26).
Fig. 3.
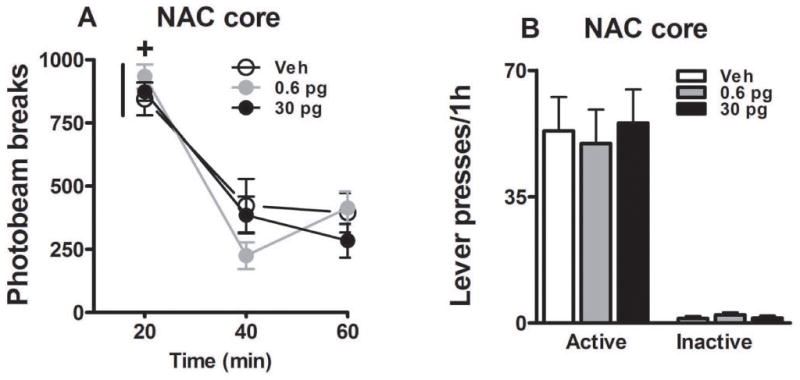
Locomotor activity in a novel context (A; mean photobeam breaks/1h ± SEM) and food-reinforced instrumental behavior in contexts 1 or 2 (B; mean active and inactive lever presses/1h + SEM). JNJ16259685 or vehicle was administered bilaterally into the NAC core (Panel A: N = 7–8/dose; Panel B: N = 8) before testing. Plus sign represents significant difference relative to all other time points (ANOVA time simple main effect, Tukey test, p < 0.05).
Intra-NAC core administration of JNJ16259685 did not alter food-reinforced instrumental behavior in context 1 or 2, relative to vehicle (Fig. 3B). Following vehicle pretreatment, the rate of active lever pressing for food reinforcement (Mean ± SEM; 53.25 ± 9.36) was similar to the rate of non-reinforced active lever pressing observed during the reinstatement test in the cocaine experiment. Furthermore, the 0.6 or 30 pg dose of JNJ16259685 did not alter food-reinforced active (F(2, 21) = 0.09, p = 0.91) or inactive lever responding (F(2, 21) = 0.62, p = 0.55), relative to vehicle.
Effects of CNQX treatment on locomotor activity and food-reinforced instrumental behavior
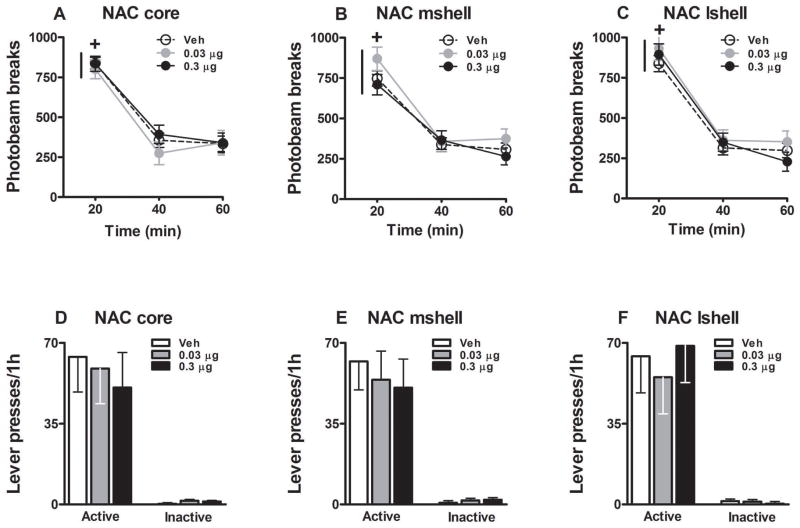
The possible performance-impairing side effects of intra-NAC core or shell CNQX treatment were also investigated. Administration of CNQX into the NAC core, medial NAC shell, or lateral NAC shell did not alter locomotor activity in a novel context relative to vehicle (Fig. 4A, 4B, 4C). The number of photobeam breaks decreased during the locomotor activity test session (all time main effects, F(2, 58-66) = 92.81–102.36, p = 0.0001; 20-min interval 1 > 2–3, Tukey tests, p < 0.05). Furthermore, the 0.03 or 0.3 μg dose of CNQX failed to alter the number of photobeam breaks relative to vehicle (all treatment main and treatment x time interaction effects, F(2-4, 29-66) = 0.32–1.01, p = 0.38–0.84).
Fig. 4.
Locomotor activity (A–C; mean photobeam breaks/1h ± SEM) in a novel context and food-reinforced instrumental behavior in contexts 1 or 2 (D–F; mean active and inactive lever presses/1h + SEM). CNQX or vehicle was administered bilaterally into the NAC core (Panel A: N= 7–11/dose; D: N = 8), NAC mshell (Panel B: N = 7–9/dose; E: N = 7), or NAC lshell (Panel C: N = 7–9/dose; F: N = 9) before testing. Plus signs represent significant difference relative to all other time points (ANOVA time simple main effect, Tukey test, p < 0.05).
Administration of CNQX into the NAC core, medial NAC shell, or lateral NAC shell did not alter food-reinforced instrumental behavior in context 1 or 2, relative to vehicle (Fig. 4D, 4E, 4F). Following vehicle pretreatment administered into the NAC core, medial NAC shell, or lateral NAC shell, the rate of active lever pressing for food reinforcement (Mean ± SEM = 63.87 ± 15.20; 62.00 ± 12.38; 64.22 ± 15.88, respectively) was similar to the rate of non-reinforced active lever pressing during the reinstatement test in the cocaine experiment. However, the 0.03 or 0.3 μg dose of CNQX did not alter food-reinforced active (F(2, 18-24) = 0.19–0.24, p = 0.78–0.82) or inactive lever responding (F(2, 18-24) = 0.40–1.66, p = 0.21–0.67), relative to vehicle.
DISCUSSION
The fundamental role of NAC glutamate neurotransmission in context-induced drug-seeking behavior is supported by the previous finding that activation of presynaptic inhibitory group II mGluRs in the NAC core or shell attenuates the context-induced renewal of heroin-seeking behavior in the presence of discrete drug-conditioned stimuli (Bossert et al. 2006). The present study extends this line of research by demonstrating that glutamate is also critical for the ability of a drug-paired context to reinstate cocaine-seeking behavior, in the absence of discrete drug-conditioned stimuli, via the stimulation of mGlu1 and AMPA/kainate receptors in a subregion-specific manner within the striatum. Consistent with this conclusion, JNJ16259685-induced antagonism of mGluR1s or CNQX-induced antagonism of AMPA/kainate receptors in the NAC core dose-dependently attenuated the expression of drug context-induced cocaine-seeking behavior relative to vehicle. Conversely, CNQX, but not JNJ16259685, administered into the lateral or medial NAC shell attenuated, furthermore CNQX or JNJ16259685 administered into vCPu failed to alter, this behavior. These manipulations did not have an effect on instrumental performance in the extinction context, general locomotor activity, or food-reinforced instrumental behavior in control experiments. Together, these findings suggest that stimulation of mGlu1 and AMPA/kainate receptors in the NAC core and stimulation of AMPA/kainate, but not mGlu1, receptors in the NAC shell is necessary for drug context-induced motivation for cocaine. Furthermore, it will be important to determine whether these effects can be generalized to contextual motivation produced by other drugs of abuse as well as natural reinforcers.
Contribution of NAC mGluR1s to drug context-induced cocaine-seeking behavior
Remarkably, despite the ubiquitous expression of mGluR1s throughout the NAC (Mitrano & Smith 2007), the present study revealed that stimulation of mGluR1s in the NAC core, but not the shell, is critical for context-induced reinstatement of cocaine-seeking behavior. The subregion-specific effect of JNJ16259685 in the NAC may stem from differences in the physiology of, and cocaine-induced neuroplasticity in, mGluR1 populations within the NAC core versus shell. Subpopulations of mGluR1s may display unique electrophysiological properties in the NAC depending on their localization, as elsewhere in the brain. For instance, stimulation of mGluR1s initiates depolarization in the substantia nigra pars reticulata, whereas it fails to do so in the subthalamic nucleus (Awad et al. 2000; Marino et al. 2001). Moreover, the subregion-specific contributions of mGluR1s may arise from differential regulatory changes produced by cocaine self-administration in distinct mGluR1 populations within the NAC core and shell. Following cocaine self-administration and extinction of cocaine-seeking behavior, expression of the group I mGluR subtype mGluR5 and of the scaffolding protein Homer1b/c is decreased in the NAC shell (Ghasemzadeh et al. 2009). Given the extensive co-localization of mGluR1s and mGluR5s (Mitrano and Smith 2007), it is possible that the negative effect of JNJ16259685 in the NAC shell was due to cocaine-induced decreases in mGluR1 density or transduction efficiency.
The effect of JNJ16259685 on context-induced reinstatement of cocaine-seeking behavior appears to be mediated by inhibition of mGluR1 receptor signaling within the NAC core (Fukunaga et al. 2007; Lavreysen et al. 2004; Xie et al. 2010), but it is hard to ascribe this effect to a specific cell subpopulation or receptor subtype. First, mGluR1s are expressed both in medium spiny neurons and cholinergic and parvalbumin-containing fast-spiking interneurons within the NAC (Mitrano & Smith 2007). In addition, a large portion of mGluR1s is extrasynaptic (Mitrano & Smith 2007) and is likely activated by extrasynaptic glutamate or glutamate released by glial cells (Baker et al. 2002; Cho & Bannai, 1990; Danbolt 2001; Patel et al. 2004; Rothstein et al. 1996). Second, mGluR1s are localized both postsynaptically on dendrites and spines (Mitrano & Smith 2007) as well as presynaptically on glutamatergic terminals (Awad et al. 2000; Fotuhi et al. 1993). As a result, presynaptic mGluR1s may have contributed to the effects of JNJ16259685, resulting in decreases in synaptic glutamate release (Swanson et al. 2001) and, subsequently, in altered stimulation of ionotropic and metabotropic pre- and postsynaptic glutamate receptors. Thus, overall, mGluR1s are in the position to critically modulate inputs to, outputs from, and signal processing within the NAC core, and future studies will be necessary in order to ascertain the exact neurochemical and physiological mechanisms by which mGluR1s mediate the reinstatement of drug context-induced cocaine-seeking behavior.
Contribution of AMPA/kainate receptors in the NAC to drug context-induced cocaine-seeking behavior
Unlike the effects of JNJ16259685, the behavioral effects of CNQX were not NAC subregion specific. This was not likely due to the spread of CNQX from the NAC core to the shell. In support of this, a slightly larger volume (5.0 nmol/0.4 μl/hemisphere) of CNQX administered into the NAC core, but not the NAC shell, inhibits defensive behavior in rats (da Cunha et al 2008). Furthermore, CNQX administered into the vCPu, a region dorsally adjacent to the NAC core, failed to alter cocaine-seeking behavior in the present study. Overall, these findings suggest that stimulation of AMPA/kainate receptor populations in both the NAC core and shell is necessary for drug context-induced reinstatement of cocaine-seeking behavior and extend upon previous literature that demonstrated a critical role for AMPA/kainate receptors in the NAC in drug-primed and explicit cue-induced reinstatement of extinguished cocaine-seeking behavior (Bäckström & Hyytiä 2007; Famous et al. 2008). It should be noted that, adjacent to the NAC shell, the olfactory tubercle has also been implicated in the rewarding and primary reinforcing effects of cocaine, morphine, and intracranial self-administration (Di Ciano, Robbins & Everitt 2008; Fibiger et al. 1987; Ikemoto 2003; 2007; Kornetsky, Huston-Lyons & Porrino 1991; Sellings, McQuade & Clarke 2006). Thus, it cannot be ruled out that AMPA/kainite receptor antagonism in the olfactory tubercle may have partially contributed to the effects of CNQX on context-induced reinstatement of cocaine-seeking behavior in the present study.
The absence of different effects in the lateral versus medial NAC shell suggests that AMPA/kainate receptor populations in these subregions are not functionally distinct with respect to drug context-induced cocaine-seeking behavior. This parallels the finding that D1 dopamine receptor populations in the lateral and medial NAC shell are equally critical for drug context-induced heroin-seeking behavior (Bossert et al. 2007), despite differences between the lateral and medial NAC shell in morphology and connectivity (Ikemoto 2007; Meredith et al. 1992; Voorn et al. 2004).
The behavioral effects of CNQX likely arose from the inhibition of striatal neuronal activity or of dopamine input into the NAC core and shell. Specifically, CNQX-induced antagonism of postsynaptic AMPA/kainate receptors within the NAC likely supresses excitatory postsynaptic potentials, current, and depolarization in medium spiny neurons (Lape & Dani 2004; Tarazi et al. 1998). Similarly, antagonism of presynaptic AMPA/kainate receptors on dopamine terminals within the NAC attenuates dopamine release (Pap & Bradberry 1995; Russell 2003; Tarazi et al 1998), which is necessary for drug context-induced heroin and ethanol-seeking behaviors via the stimulation of dopamine D1 receptors in the NAC core and shell (Bossert et al. 2007; Chaudhri, Sahuque & Janak 2009). Therefore, AMPA/kainate receptor stimulation likely contributes to drug context-induced reinstatement of cocaine-seeking behavior as well.
mGluR1 and AMPA/kainate receptors in the relapse circuitry
In summary, the present study expands our understandings of the critical role of glutamatergic neurotransmission in drug context-induced reinstatement of drug-seeking behavior by demonstrating that stimulation of mGlu1 and AMPA/kainate receptors in the NAC core and AMPA/kainate receptors in the NAC shell is necessary for this phenomenon. Importantly, these glutamate receptors operate in conjunction with other receptor families and as part of a larger corticolimbic neural circuitry.
The present study demonstrated that both mGluR1s and AMPA/kainate receptors are critical to drug context-induced cocaine-seeking behavior within the NAC core. While it is not clear what mechanisms underlie this phenomenon, it is possible that mGlu1 and AMPA/kainate receptors interact to regulate context-induced cocaine-seeking behavior. In support of this, mRNAs for the mGluR1 and for AMPA receptor subunits are co-expressed in striatal neurons (Ghasemzadeh et al. 1996). Hence, stimulation of mGlu1 and AMPA/kainate receptors may activate intersecting intracellular signaling pathways within the same striatal neuron. Future studies will be necessary to investigate the signaling molecules that contribute to the effects of mGlu1 and AMPA/kainate receptor stimulation in the NAC core on drug context-induced reinstatement of cocaine-seeking behavior.
Functional interactions between mGluR1 or AMPA/kainate receptors and dopamine receptors are well documented (Chiamulera et al. 2001; David & Abraini 2003; Swanson & Kalivas 2000; Vezina & Kim 1999; Anderson et al. 2008) and may also contribute to drug context-induced cocaine-seeking behavior. The exact mechanisms underlying interactions between mGluR1 and dopamine receptors remain poorly understood but could result from the activation of converging intracellular signaling pathways (David & Abraini 2003; Voulalas et al. 2005). In fact, dopamine receptor stimulation in the NAC may be necessary for the ability of mGluR1s to depolarize neurons, as in the globus pallidus (Poisik, Smith & Conn 2007). Similar to mGluR1 receptors, AMPA/kainate receptors and dopamine receptors are co-expressed on neurons within the NAC (Bernard, Somogyi & Bolam 1997; Glass et al. 2008). Moreover, stimulation of D1-like dopamine receptors promotes GluR1 AMPA subunit phosphorylation and AMPA receptor cell-surface expression in the NAC (Anderson et al. 2008; Chao et al. 2002; Mangiavacchi & Wolf 2004), and it is necessary for context-induced ethanol or heroin-seeking behaviors (Bossert et al. 2007; Chaudhri et al. 2009). Therefore, future studies will need to investigate whether interactions between dopamine and glutamate in the NAC are necessary for drug context-induced reinstatement of cocaine-seeking behavior.
The critical sources of glutamate to the NAC core and shell have yet to be determined but the most likely glutamatergic afferents to the NAC are the medial prefrontal cortex (mPFC), lateral orbitofrontal cortex (lOFC), dorsal hippocampus (DH), ventral hippocampus (VH), and basolateral amygdala (BLA) given that these brain regions regulate drug context-induced cocaine-seeking behavior (Fuchs et al. 2005; 2007; Lasseter et al. 2009; 2010). Anatomical studies demonstrate that glutamatergic fibers from these brain regions converge in the NAC (Amaral & Witter 1995; Goto & O’Donnell 2002; Groenewegen et al. 1999; O’Donnell & Grace 1995; van Groen & Wyss 1990). lOFC and BLA projection neurons innervate both the NAC core and shell (Groenewegen, Wright & Uylings 1997; McDonald 1991). Conversely, the dorsal mPFC and DH preferentially project to the NAC core, while the ventral mPFC and VH preferentially project to the NAC shell (Brog et al. 1993; Pennartz et al. 1994; Sesack et al. 1989; Kelley & Domesick 1982; Groenewegen et al. 1987). Paralleling the topographical connectivity between the PFC and NAC, the dmPFC-NAC core glutamatergic projection is necessary for drug-primed and cue-induced reinstatement of drug-seeking behavior, whereas the vmPFC-NAC shell glutamatergic projection is critical for extinction learning that suppresses drug-seeking behavior (LaLumiere & Kalivas 2008; McFarland, Lapish & Kalivas 2003; Peters, LaLumiere & Kalivas 2008). Together, these different glutamatergic subcircuits and glutamate receptor populations may provide for distinct contributions by the NAC core and shell to drug context-induced reinstatement of cocaine-seeking behavior. Hence, the exploration of these may aid in the development of a systemic approach to drug addiction treatment and relapse prevention.
Supplementary Material
Acknowledgments
The authors thank John Tobben, Kate Cowhey, Albert Newsome, Portia West and Amy Zipursky for excellent technical assistance. This work was supported by National Institute on Drug Abuse (NIDA) grant R01 DA017673 and NIDA R01 grant supplement to promote diversity in health-related research (DA017673-S1).
Footnotes
AUTHORS CONTRIBUTION
X.X and R.A.F designed the experiments and wrote the manuscript. X.X., H.C.L., D.R.R., K.L.P., and A.W. conducted the experiments, analyzed the data, and provided critical feedback on an earlier version of the manuscript. All authors reviewed the content and approved the final version of the manuscript for publication.
The authors declare to have no conflict of interest.
References
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Academic; Los Angeles, CA: 1995. pp. 443–493. [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–53. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–40. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–9. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology. 2008;55:392–5. doi: 10.1016/j.neuropharm.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–63. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002;81:984–92. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207:303–14. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Cho Y, Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J Neurochem. 1990;55:2091–7. doi: 10.1111/j.1471-4159.1990.tb05800.x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha IC, de Nazareth AM, Vargas JC, Ferraz A, Neto JM, Paschoalini MA, Faria MS. The microinjection of AMPA receptor antagonist into the accumbens shell failed to change food intake, but reduced fear-motivated behaviour in free-feeding female rats. Behav Brain Res. 2008;193:243–7. doi: 10.1016/j.bbr.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- David HN, Abraini JH. Blockade of the locomotor stimulant effects of amphetamine by group I, group II, and group III metabotropic glutamate receptor ligands in the rat nucleus accumbens: possible interactions with dopamine receptors. Neuropharmacology. 2003;44:717–27. doi: 10.1016/s0028-3908(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–25. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–70. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC, LePaine FG, Jakubovic A, Phillips AG. The role of dopamine in intracranial self-stimulation of the ventral tegmental area. J Neurosci. 1987;7:3888–3896. doi: 10.1523/JNEUROSCI.07-12-03888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Sharp AH, Glatt CE, Hwang PM, von Krosigk M, Snyder SH, Dawson TM. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–12. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–56. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga I, Yeo CH, Batchelor AM. Potent and specific action of the mGlu1 antagonists YM-298198 and JNJ16259685 on synaptic transmission in rat cerebellar slices. Br J Pharmacol. 2007;151:870–6. doi: 10.1038/sj.bjp.0707286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Sharma S, Surmeier DJ, Eberwine JH, Chesselet MF. Multiplicity of glutamate receptor subunits in single striatal neurons: an RNA amplification study. Mol Pharmacol. 1996;49:852–9. [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–71. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM. Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol. 2008;210:750–761. doi: 10.1016/j.expneurol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Timing-dependent limbic-motor synaptic integration in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:13189–13193. doi: 10.1073/pnas.202303199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–44. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23:9305–11. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–72. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Huston-Lyons D, Porrino LJ. The role of the olfactory tubercle in the effects of cocaine, morphine and brain-stimulation reward. Brain Res. 1991;541:75–81. doi: 10.1016/0006-8993(91)91076-d. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Dani JA. Complex response to afferent excitatory bursts by nucleus accumbens medium spiny projection neurons. J Neurophysiol. 2004;92:1276–84. doi: 10.1152/jn.00066.2004. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–81. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeing behavior in rats. Neuroscience. 2010;171:830–9. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nóbrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–72. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–71. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21:7001–12. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Agolia R, Arts MP, Groenewegen HJ, Zahm DS. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–62. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Arnold C, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocaine-treated rats. Neuroscience. 2008;154:653–66. doi: 10.1016/j.neuroscience.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–989. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap A, Bradberry CW. Excitatory amino acid antagonists attenuate the effects of cocaine on extracellular dopamine in the nucleus accumbens. J Pharmacol Exp Ther. 1995;274:127–33. [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Warren BA, Rhoderick JF, Bridges RJ. Differentiation of substrate and non-substrate inhibitors of transport system xc(−): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology. 2004;46:273–84. doi: 10.1016/j.neuropharm.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Los Angeles: CA: 1997. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res. 2008;1215:173–82. doi: 10.1016/j.brainres.2008.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisik OV, Smith Y, Conn PJ. D1- and D2-like dopamine receptors regulate signaling properties of group I metabotropic glutamate receptors in the rat globus pallidus. Eur J Neurosci. 2007;26:852–62. doi: 10.1111/j.1460-9568.2007.05710.x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Russell VA. In vitro glutamate-stimulated release of dopamine from nucleus accumbens core and shell of spontaneously hypertensive rats. Metab Brain Dis. 2003;18:161–8. doi: 10.1023/a:1023819220840. [DOI] [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther. 2006;317:1178–87. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–59. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–52. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–14. [PubMed] [Google Scholar]
- Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Localization of ionotropic glutamate receptors in caudate-putamen and nucleus accumbens septi of rat brain: comparison of NMDA, AMPA, and kainate receptors. Synapse. 1998;30:227–35. doi: 10.1002/(SICI)1098-2396(199810)30:2<227::AID-SYN13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302:515–28. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Vezina P, Kim JH. Metabotropic glutamate receptors and the generation of locomotor activity: interactions with midbrain dopamine. Neurosci Biobehav Rev. 1999;23:577–89. doi: 10.1016/s0149-7634(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–74. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci. 2005;25:3763–73. doi: 10.1523/JNEUROSCI.4574-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.