Abstract
Background
Anthracycline-induced cardiotoxicity and myocardial dysfunction may be associated with apoptosis. Caspase 3 catalyzes a terminal step in apoptosis, and its expression may serve as a marker of cardiomyocyte apoptosis. We synthesized 18F-CP18, a caspase-3 substrate and evaluated cardiac 18F-CP18 uptake in a mouse model of anthracycline cardiotoxicity.
Methods and Results
For 12 weeks, mice were injected with doxorubicin, 3 mg/kg/week, or vehicle (control). Left ventricular fractional shortening was quantified by echocardiography. CP18 uptake after intravenous injection of 250 μCi of 18F-CP18, 24 hours post-doxorubicin treatment was quantified by microPET, autoradiography, and gamma counting. Apoptosis was assessed by enzymatic assay of myocardial caspase 3 and TUNEL staining of tissue sections. Compared with controls, at 6 and 12 weeks of doxorubicin treatment, fractional shortening was reduced (20.7%±2.5% versus 31%±3.5%, P=0.010; and 20.3%±3.1% versus 32.4%±2.1%, P=0.011). Doxorubicin treatment was associated with increased 18F-CP18 uptake in %ID/g by gamma counting from 0.36±0.01 (week 1) to 0.78±0.01 (week 12), P=0.003. A similar increase in 18F-CP18 uptake was observed by microPET (0.41±0.04 versus 0.73±0.1, P=0.014) and autoradiography (1.1±0.3 versus 2.8±0.2 P=0.001). Caspase 3 enzymatic activity and apoptosis by TUNEL staining were also increased after 12 weeks of doxorubicin compared with weeks 1 and 3. CP18 uptake in controls was relatively unchanged at weeks 1, 3, and 12.
Conclusions
In a mouse model of cardiotoxicity, doxorubicin treatment is associated with increased myocardial caspase 3 expression and an increase in CP18 uptake. 18F-CP18 may be useful for detection of anthracycline-induced myocardial apoptosis.
Keywords: cardiotoxicity, caspase-3, doxorubicin
Anthracyclines are an effective class of drugs for treating a broad spectrum of cancers types, including leukemia, breast, and lung cancers; however, the use of this family of drugs is associated with cardiotoxic side effects, which can lead to both acute and chronic forms of cardiomyopathy in treated patients.1–4 Although the exact causative mechanism of anthracycline-related cardiomyopathy is not fully understood, induction of extrinsic and intrinsic apoptotic pathways in heart tissue is implicated in cardiomyocyte loss.5–9 All apoptotic cells express caspase 3, a key enzyme that catalyzes the final step in apoptotic cascade. Because of its role as the executioner caspase, caspase 3 expression is considered a surrogate marker for apoptotic activity.10,11 Noninvasive detection of cardiomyocyte apoptosis in patients undergoing anthracycline-based chemotherapy may potentially serve as a valuable tool for cardiac risk stratification. Several imaging probes have been developed for detection of apoptosis; however, none of them have been approved for clinical use in the United States. We have designed and synthesized an 18F-labeled tetrapeptidic caspase substrate 18F-CP18, containing the caspase 3–specific peptide recognition sequence D-E-V-D.12 18F-CP18 was labeled using a click radiochemistry approach, and this tracer has been evaluated in preclinical xenograft tumor models for apoptosis imaging.12–14 A polyethylene glycol and galactose moiety were attached to facilitate transport across cell membranes and maintain optimal pharmacokinetic properties in vivo. Mechanistically, this peptide substrate analog crosses intact cell membranes, and once inside the cell, the substrate is cleaved in the presence of activated caspase 3, resulting in the accumulation of the polar 18F-radiolabeled DEVD metabolite in the cytoplasm of apoptotic cells. In this study, we sought to examine myocardial uptake of this tracer in a mouse model of chronic anthracycline-induced cardiotoxicity and determine its capacity to identify altered myocardial function.
Methods
Animal Model
Animal studies were approved by the Institutional Animal Care and Use Committee of Siemens MIBR. To generate the chronic model of doxorubicin-induced cardiotoxicity, 6- to 8-week-old C57BL6/6J mice were treated with intraperitoneal injection of 3 mg/kg body weight of doxorubicin weekly for 12 weeks.15,16 Control mice received weekly injections of saline. Control and treated mice were euthanized at weeks 1, 3, 6, and 12 after the first injection. At each time point, 3 to 6 mice from control or doxorubicin treatment group were used for echocardiography, ex vivo PET, caspase 3 assay, TUNEL staining, and gamma counting. Sequential measurements of ejection fraction or tracer uptake at different time points in the same animal were not performed.
18F-CP18 and 18F-CP32 Syntheses
CP18 a caspase 3–specific substrate and CP32, a tetrapeptide with the same amino acid composition as CP18 but with a different sequence (E-D-D-V) which is not cleaved by caspase 3, were synthesized and labeled with 18F by click chemistry. 18F-labeling was performed as previously described on an automated synthesis module with minor hardware modifications to accommodate the volatility of 18F-fluoropentyne, including equipping all vents with a charcoal trap.12 Cyclotron-produced aqueous 18F-fluoride ion (1.0–2.0 Ci) was passed through an anion exchange resin cartridge (Waters QMA®) to retain the 18F-fluoride and sequester the 18O-H2O for recycling. The 18F-fluoride was eluted from the cartridge into the primary reaction vessel using a solution of aqueous potassium carbonate (3 mg dissolved in water [0.4 mL]). A solution of Kryptofix® 222 (20 mg) dissolved in acetonitrile (1 mL) was added to the aqueous 18F-fluoride in the primary reaction vessel. The aqueous 18F-fluoride mixture was dried by heating the solution between 68°C and 95°C under a simultaneous reduction of pressure (250 mbar) under a stream of argon. After the mixture was dried, a solution of the tosylate precursor pent-4-yn-1-yl 4-methylbenzenesulfonate (20 mg, 84 μmol), dissolved in tetrahydrofuran (0.5 mL), was added to the reaction vessel containing the anhydrous 18F-fluoride. The vessel was heated at 110±5°C for 3 minutes. The resulting 18F-fluoropentyne was then distilled from the reaction vessel and bubbled into a secondary reaction container. This container contained a premixed solution of the CP18 azide precursor1 or CP32 azide precursor3 CuSO4 (0.1 mol/L, 300 μL), Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (15 mg, 28.2 μmol), and sodium ascorbate (40 mg, 201 μmol) dissolved in DMF (0.1 mL) and MeOH (0.1 mL). The reaction was stirred at room temperature for 30 minutes. The crude reaction mixture was then diluted with water (4.5 mL) and transferred to a high performance liquid chromatography load loop (5 mL) followed by purification via chromatographic separation. Using a semipreparative high performance liquid chromatography column (Phenomenex Gemini, C18, 5μ, 10×250 mm) at a flow rate of 5 mL/min, a linear step gradient was used using the following solvents: 5% MeCN:95% aqueous TFA (0.05%) for 5 minutes, 10% MeCN:90% aqueous TFA (0.05%) for 5 minutes, 15% MeCN:85% aqueous TFA (0.05%) for 5 minutes, 20% MeCN:80% aqueous TFA (0.05%) for 5 minutes, and 25% MeCN:75% aqueous TFA (0.05%). The products eluted between 28 and 35 minutes from the time of injection. The purified fraction collected from the high performance liquid chromatography column were then diluted with water (30±10 mL) and captured onto a C18 SepPak cartridge. TheC18 SepPak cartridge was then washed with water (10 mL), followed by an elution with ethanol (0.5–1.0 mL) to release either 18F-CP18 or 18F-CP32 from the C18 cartridge (Figure 1). The samples were then diluted with sterile water to afford a final formulation of 10% ethanol in water (v/v). The radiosynthesis was typically accomplished within 90 minutes from the end of bombardment in an average radiochemical yield of 40% (decay corrected, 18F-CP18) and 42% (decay corrected, 18F-CP32). The average specific activity was 175 GBq/μmol (18F-CP18) and 359 GBq/μmol (18F-CP32).
Figure 1.
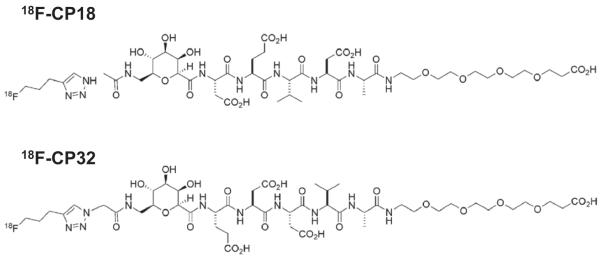
The upper panel represents the chemical structure of 18F-CP18 (amino acid sequence: DEVD) and the lower panel represents the chemical structure of 18F-CP32 (amino acid sequence: EDDV).
Assessment of Left Ventricular Function
Transthoracic echocardiography was performed with a 7.5 MHz transducer (GE) and images were acquired with a portable Vivid q (GE). For echocardiographic imaging, 3 mice per time point (treated with doxorubicin or control for 1 week, 3 weeks, 6 weeks) were anesthetized with 2% isoflurane/98% oxygen immediately before euthanasia. A parasternal short-axis view was obtained for M-mode imaging at the papillary muscle level, and left ventricular (LV) function was assessed by averaging 3 independent M-mode images acquired over 2 consecutive beats. Images were analyzed offline with EchoPAC (GE). LV internal dimension at end-diastole (LVIDD) and LV internal dimension at end-systole (LVISD) were measured by leading edge method according to the American society of Echocardiography. Fractional shortening (FS), a surrogate marker of LV ejection fraction, was calculated as FS (%)=100×[(LVIDD–LVISD)/LVIDD].17
Cardiac Tissue Uptake Studies
Animals were anesthetized with 2% isoflurane/98% oxygen, and 200 μCi of 18F-CP18 was administered via bolus intravenous tail vein injection. Animals were allowed to regain consciousness during the 1 hour uptake time and were deeply anesthetized with 200 mg/kg of ketamine and 20 mg/kg of xylazine injected intraperitoneally. Transcardial perfusion was performed with saline, and the heart was dissected free from surrounding tissue. For tissue biodistribution studies, muscle (quadriceps femoris), the liver, heart, mesenteric fat, and kidneys were removed from 12 mice. Tissue samples were weighed, and counted in a Wallac Wizard Gamma counter (Perkin-Elmer, Waltham, MA). Radioactivity of excised tissue was measured with a gamma counter. Background counts were subtracted, and radioactive decay was corrected to the time of injection. The radioactivity that accumulated in the tissue during a 60 minute period after injection of 18F-CP18 was expressed as the percent of injected dose per gram of tissue (%ID/g).
Caspase Assay
Cell pellets of tissue homogenates of the myocardium were first lysed in T-PER buffer (Fisher, Pittsburgh, PA). A fluorogenic caspase-3 substrate, Ac-DEVD-AFC (AnaSpec, CA), was added to the lysate in a 1 mmol/L DTT-containing 2× reaction buffer (150 mmol/L MaCl, 50 mmol/L HEPES, 5 mmol/L EDTA, 1 mmol/L DTT, 10% glycerol, pH 7.0). The fluorogenic reaction was detected and quantified using a SpectraMax M2 plate reader (Molecular Devices, CA) at 405 nm wavelength every 5 minutes for 2 hours. Caspase-3 activity was calculated as the amount of fluorescent free-AFC detected and expressed as nmol per minute per mg protein. Protein concentrations were measured by Bradford assay (Bio-rad, CA).
MicroPET Imaging
For qualitative assessment of tracer uptake with in vivo microPET imaging, mice (2 each at 1, 6, and 12 weeks after doxorubicin treatment) were anesthetized with 3% isoflurane/97% oxygen and placed on a heated scanner bed in an INVEON Multimodality scanner (Siemens) for imaging 1 hour after injection of 200 μCi of 18F-CP18 via intravenous tail vein injection. A CT scan was performed (80 kVp and images were reconstructed from 270 projections) for anatomic registration followed by a 15-minute static PET scan with an INVEON Multimodality scanner (Siemens Knoxville, TN). Images were reconstructed using filtered back projections without attenuation, scatter, or dead-time corrections with a pixel size of 0.77×0.77×0.79 mm. PET and CT images were coregistered on the basis of anatomic landmarks using an Inveon Research Workplace software (Siemens, Knoxville, TN).
Ex Vivo MicroPET Imaging
For ex vivo microPET imaging, mice were euthanized and a laparotomy was performed 1 hour after injection of 200 μCi of 18F-CP18. An INVEON Multimodality scanner (Siemens) was used for micro-PET imaging for 30 minutes. Quantification of tracer uptake was performed by visually drawing regions of interest (ROI) measuring 2.0 mm3 in the heart, and the corresponding activity values in %ID/g were quantified using the INVEON Research Workplace software (Siemens).
Autoradiography
Hearts were embedded and frozen in optimal cutting temperature (OCT) compound, and frozen tissue was cut into 10 μm sections using a cryotome (Leica Microsystems, Bannockburn, IL). Autoradiographic images were obtained by placing the tissue sections on an imaging plate (FujiFilm, Tokyo, Japan) and exposed overnight at −80°C. CP18 uptake was quantified by measuring count densities using a FLA-7000 scanner (FujiFilm, Tokyo, Japan), image analysis software (Image J Software, NIH), and expressed as PSL/mm2. Adjacent sections were used for histological staining studies.
Histological Analysis
TUNEL staining of tissues sections were performed using the In Situ Cell Death Detection Kit-TMR red (Roche, Germany) by following manufacturer’s instructions. Briefy, sections were fixed in Safefix II instrument (Fisher Scientific, Pittsburg, PA) and permeabilized using 0.1% TritonX-100 and 0.1% sodium citrate in PBS. The sections were incubated in reaction mixture containing TdT and Tetra methyl rhodamine (TMR)-conjugated nucelotides for 1 hour at 37°C. DAPI counterstaining was used to visualize cell nuclei. Both TMR (red) and DAPI (blue) fluorescence were visualized and imaged using an Axio Imager D2 microscope (Carl Zeiss Inc, Jena, Germany).
Statistical Analysis
Results were expressed as mean±SD, and the median and interquartile ranges were used when data were presented in figures. Nonparametric tests were used for statistical analysis using statistical software (SPSS, Version 21; IBM, Armonk NY). Kruskal–Wallis test was used to compare multiple groups and Mann–Whitney U test was used to compare differences between any 2 individual groups. No adjustments were made for multiple comparisons. Spearman’s rank correlation coefficient was used to evaluate the dependence between CP18 uptake by autoradiography and apoptotic activity by TUNEL assay
Results
LV Function
Compared with baseline, left ventricular function given by FS (FS=32.3±2.1%) showed a gradual decrease after 3 weeks (29.3±2.5%), 6 weeks (20.7±2.5%), and 12 weeks (20.3±3.1%), P=0.001, of doxorubicin treatment. Untreated control mice treated with vehicle alone did not manifest any significant changes in FS compared with at baseline (32.3±4%), week 3 (31.7±3.8%), week 6 (31±3.5%), or week 12 (32.4±2.1%), P=0.18. When compared with untreated control mice, FS was significantly decreased in mice treated with doxorubicin at weeks 6 (P=0.011) and 12 (P=0.01). FS was relatively similar between control and doxorubicin-treated mice after 1 week (P=0.24) and 3 weeks (P=0.09) of treatment.
Relative Myocardial Uptake by Gamma Counting
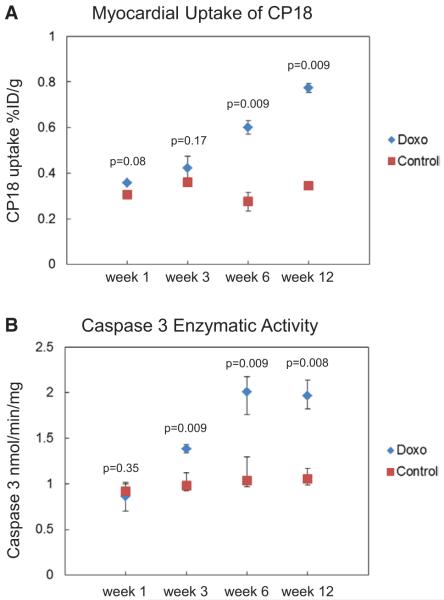
Myocardial uptake of 18F-CP18 in doxorubicin-treated mice (n=5 per time point), quantified by gamma counting and expressed as %ID/g, steadily increased from 0.36±0.009 at baseline to 0.43±0.07 at week 3, 0.59±0.07 at week 6, and 0.78±0.01 at week 12, P=0.001 (Figure 2A). In contrast, relative 18F-CP18 uptake remained unchanged from baseline: 0.31±0.02 at week 3; 0.35±0.004 at week 6; 0.28±0.07 at week 12; 0.34±0.005 in untreated control mice, P=0.06. Comparing relative myocardial uptake between untreated control and treated mice at each time point, there was a significant increase in 18F-CP18 uptake at week 6 and week 12 in doxorubicin-treated mice compared with untreated control (Figure 2A). The relative tissue distribution of 18F-CP18 in a separate group of untreated control mice (n=12) 1 hour after injection showed the highest uptake in the kidney measuring 7.6%±0.9%ID/g followed by the liver (0.27%±0.002%ID/g). Uptake in the muscle, heart, brain, and blood measured 0.21±0.0001, 0.36±0.006, 0.05±0.0001, and 0.11±0.002%ID/g, respectively.
Figure 2.
A, Myocardial uptake of 18F-CP18 was quantified by gamma counting of the heart explanted from doxorubicin-treated (Doxo) mice and mice treated with vehicle (control). Uptake of both myocardium measured in %ID/g. Data points represent median and interquartile range of myocardial uptake from 5 mice each at weeks 1, 3, 6, and 12 of treatment expressed as %ID/g. B, Enzymatic activity of caspase 3 was measured in homogenized samples of myocardium in control and Doxo mice (n=5), and data points represent median and interquartile range of myocardial caspase activity in 5 mice each at weeks 1, 3, 6, and 12 of treatment expressed in nmol/min/mg of tissue.
Caspase 3 Activity
Enzymatic caspase 3 activity increased steadily from week 1 to week 12 of doxorubicin treatment (P=0.001; n=5 per time point; Figure 2B). The first detectable increase in caspase 3 enzymatic activity from baseline (0.9±0.3 nmol/min/mg) was seen at week 3 of doxorubicin treatment (1.4±0.09 nmol/min/mg), progressively peaked at week 6 (1.9±0.4 nmol/min/mg, P<0.05), and remained increased above baseline at week 12 of doxorubicin treatment (1.9±0.3 nmol/min/mg). In control mice injected with vehicle, caspase 3 activity remained relatively unchanged with enzymatic activities that ranged from 1.0±0.1 nmol/min/mg at baseline to 1.2±0.3 nmol/min/mg and 1.1±0.2 nmol/min/mg at weeks 6 and 12 (P=0.11), respectively. Caspase 3 activity increased as a function of doxorubicin treatment compared with untreated control mice at week 3 (P=0.009), week 6 (P=0.009), and week 12 (P=0.008).
Quantification of Myocardial Uptake of CP18 by Ex Vivo MicroPET
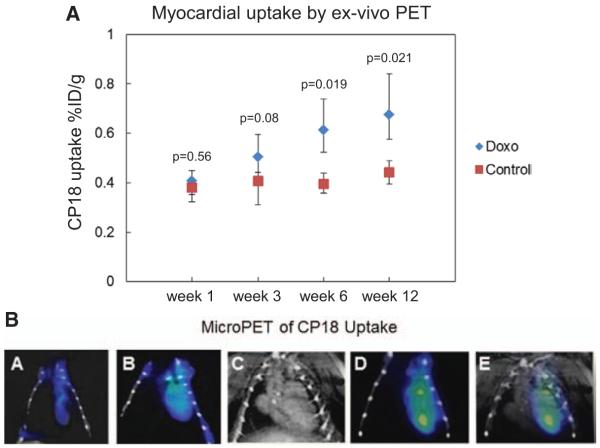
18F-CP18 uptake by the myocardium was quantified in specific regions of interest (ROI) in the ex vivo microPET images and expressed as %ID/g of myocardium in 5 mice at each time point. The absolute values of 18F-CP18 uptake increased from 0.41%±0.04% ID/g at week 1 to 0.73%±0.1%ID/g at week 12 in doxorubicin-treated mice, P=0.014. In contrast, 18F-CP18 uptake was relatively unchanged-with 0.39%±0.06%ID/g at week 1 and 0.43%±0.07%ID/g at week 12 in untreated controls, P=0.34 (Figure 3A). Comparing relative myocardial uptake between untreated control and doxorubicin-treated mice at each time point, there was a significant increase in 18F-CP18 uptake at week 6 (P=0.019) and week 12 in doxorubicin-treated mice (P=0.021). Visual assessment of in vivo microPET performed in doxorubicin-treated mice at week 1, 6, and 12 after treatment with doxorubicin showed a gradual increase in CP18 uptake in the region corresponding to the heart. There was negligible tracer uptake after 1 week of treatment with a mild increase in tracer uptake at 6 weeks after doxorubicin treatment (Figure 3B, panels a and b) and a more moderate increase in tracer uptake at 12 weeks after treatment (Figure 3B, panels d and e).
Figure 3.
A, 18F-CP18 uptake by the myocardium was quantified in regions of interest (ROI) in the ex vivo microPET images obtained 1 h post injection of 200 μCi of 18F-CP18 and expressed as a ratio of %ID/g of myocardium. Individual data points represent median and interquartile range of myocardial CP18 uptake activity in 5 mice each at weeks 1, 3, 6, and 12 of treatment. B, In vivo microPET imaging was performed in doxorubicin-treated (Doxo) mice at week 1, 6, and 12 of treatment using an INVEON Multimodality scanner. a and b, In vivo microPET images from Doxo mice for 1 week and 6 weeks, respectively. c–e, Imaging performed at 12 weeks of doxorubicin treatment; the CT angiogram (c), microPET showing prominent myocardial uptake of 18F-CP18 at week 12 of treatment with doxorubicin (d), and coregsitration of microPET with CT angiogram (e).
Absolute Myocardial Uptake
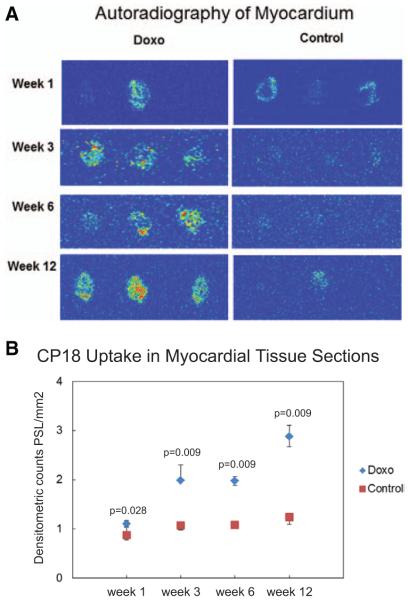
The absolute tracer uptake of 18F-CP18 in myocardial tissue sections was assessed visually by signal intensity (Figure 4A) and quantified from digital autoradiographic images expressed as PSL/mm2. There was a steady increase of 18FCP18 uptake in the myocardium (averaged over 3–4 myocardial sections in each of 3 mice per time point) from week 1 to week 12 of doxorubicin treatment, P=0.001. 18F-CP18 uptake remained essentially similar from week 1 to week 12 in control mice, P=0.06. 18F-CP18 uptake increased as early as week 3 of doxorubicin treatment (2.21±0.4) compared with untreated control mice treated with vehicle only (1.14±0.04), P=0.009 (Figure 4B). This relative increase in 18F-CP18 uptake detected by autoradiography in doxorubicin-treated mice compared with controls was sustained at 6 weeks (2.0±0.4 versus 1.1±0.1; P=0.009) and 12 weeks (2.8±0.2 versus 1.4±0.5; P=0.009).
Figure 4.
A, Heart explanted from doxorubicin-treated (Doxo) mice and mice treated with vehicle (control) was fixed and 10 μm sections of myocardium were placed on an imaging plate and exposed overnight at −80°C for autoradiography. Doxorubicin treatment for 3, 6, and 12 weeks was associated with increase in 18F-CP18 uptake of myocardial tissue sections compared with minimal 18F-CP18 uptake seen in control. Regions in red represent foci with high uptake, yellow and green represent intermediate uptake, and blue represents background. Each slide contains myocardial sections from the base, mid, and distal segments of the heart from 1 individual animal. B, 18F-CP18 uptake was quantified by measuring count densities using an FLA-7000 scanner. An image analysis software was used to quantify signal intensity of 18F-CP18 retained in the myocardium and expressed as PSL/mm2. Data points represent median and interquartile range of 18F-CP18 uptake in 3 to 4 myocardial tissue sections from 3 mice each at weeks 1, 3, 6, and 12 of treatment.
TUNEL Staining
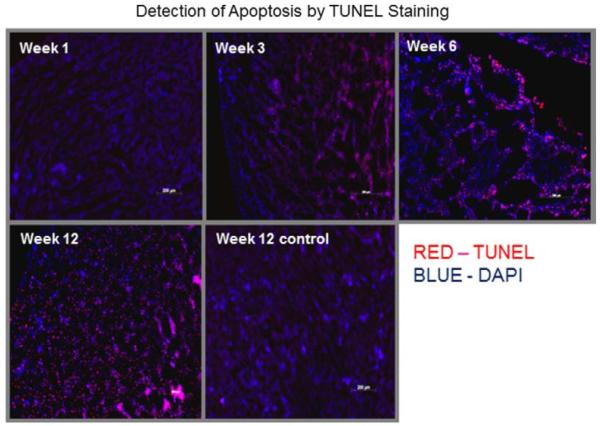
Apoptosis was assessed histologically by TUNEL staining; TUNEL-positive apoptotic nuclei and DAPI-stained nuclei were counted at ×200 magnification and averaged in 4 heart sections from each of 3 mice at weeks 1, 3, 6, and 12 after doxorubicin treatment (Figure 5). An apoptotic index was generated by counting the % TUNEL-positive cells among the DAPI-positive cells from each section. Compared with week 1 (8.5%±4.4%), there was an increase in apoptosis with doxorubicin treatment at week 3 (16.0%±1.2%) with a continued increase of TUNEL staining at weeks 6 (38.5%±3.6%) and week 12 (89.8%±6.7%), P=0.008. The extent of apoptosis remained at a baseline level in the control group treated over 12 weeks (8.7%±2.4% at week 1 versus 9.7%±0.1% at week 12;, P=0.05). A comparison between the degree of apoptosis and autoradiographic uptake of 18F-CP18 in tissue sections derived from doxorubicin-treated mice at 1, 3, 6, and 12 weeks exhibited a linear relationship with a good correlation (R2=0.76, P=0.008; Figure 6).
Figure 5.
TUNEL staining of tissue sections were performed using the in situ cell death detection kit-TMR red. TUNEL-positive apoptotic nuclei and DAPI-stained nuclei were visualized at ×200 magnification. Representative sections obtained from mice at weeks 1, 3, 6, and 12 after doxorubicin treatment and a contrasting section from a control mouse treated with vehicle are shown.
Figure 6.
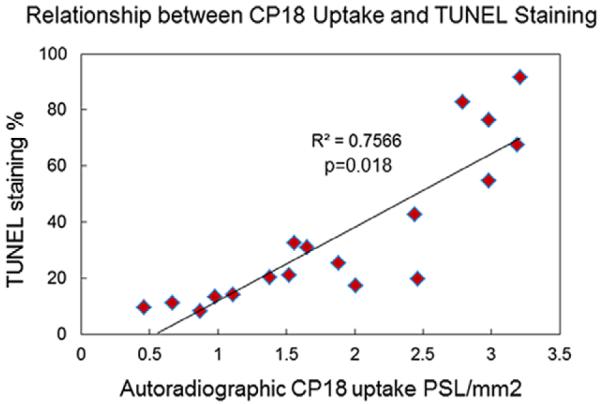
Comparison of CP18 uptake by autoradiography with apoptosis by TUNEL staining. 18F-CP18 uptake quantified by measuring count densities and expressed as PSL/mm2 was compared with the % of TUNEL-positive apoptotic nuclei among DAPI-stained nuclei per high power field. The association between CP18 uptake and TUNEL staining of adjacent myocardial 5 μm sections from 4 individual mice treated with doxorubicin for 1 week, 3 weeks, 6 weeks, and 12 weeks, respectively, was given by a Spearman’s rank correlation coefficient.
CP32 Uptake
We sought to demonstrate that 18F-CP18 uptake reflects increased caspase 3 activity and is not caused by nonspecific uptake and diffusion of the tracer into myocytes damaged by doxorubicin. We therefore evaluated 18F-CP32, a related compound that is not a substrate of caspase 3. 18F-CP32 has the same amino acid composition as 18F-CP18, but with a different sequence, EDDV, that is not recognized by caspase 3. As a result, caspase 3 is unable to cleave the polyethylene glycol moiety from 18F-CP32 and 18F-CP32 is free to diffuse in and out of myocytes. One would not expect 18F-CP32 to accumulate in the myocardium unless the tracer was retained nonspecifically in myocytes damaged by doxorubicin. Myocardial 18F-CP32 uptake measured by gamma counting 60 minutes after injection of the tracer in mice (n=3) treated with doxorubicin for 6 weeks was 0.31±0.009 %ID/g. This is essentially similar to 18F-CP32 uptake in untreated controls (n=3) 0.28±0.07 %ID/g and is significantly lower than 18F-CP18 uptake (0.59±0.07 %ID/g) measured in mice treated with doxorubicin for 6 weeks.
Discussion
This is the first preclinical study of a novel caspase 3 tracer used for noninvasive detection of apoptosis in a mouse model of cardiotoxicity. We have used a mouse model of cardiotoxicity to demonstrate preferential uptake and retention of this tracer in apoptotic myocardium. In this mouse model of chronic doxorubicin-induced cardiomyopathy, we demonstrate that apoptosis may be detectable by this tracer as early as 3 weeks after treatment in mice with 3 mg/kg body weight of doxorubicin. We have demonstrated that increase in apoptosis detected by myocardial accumulation of 18F-CP18 can be confirmed by both an enzymatic Caspase 3 assay and histologically by a TUNEL assay. These preliminary studies suggest that apoptosis detected by 18F-CP18 tracer uptake and confirmed histologically may precede the onset of echocardio-graphically detectable LV dysfunction.
Myocardial apoptosis has been implicated in ischemic heart disease, nonischemic cardiomyopathy, and chemotherapy-induced cardiotoxicity. Given the potential importance of apoptosis imaging, several apoptosis-based imaging probes have been developed, including annexin-V, synaptotagmin I, various isatin-based probes, and hydrophobic cation-based probes, such as 18F-fluorobenzyl triphenylphosphonium (18F FBnTP),13 all of which target different steps in the apoptotic cascade18–22; however, no apoptosis PET imaging agent has been approved for routine clinical use in the United States. We designed CP18 to serve as a substrate-based caspase-3 imaging agent.12 Using in vivo PET imaging, we previously demonstrated a preferential uptake of 18F-CP18 in tissue expressing elevated levels of caspase 3 activity.12,14 In this study, we sought to test the potential utility of this tracer to detect myocardial apoptosis. We chose a mouse model of cardiotoxicity to evaluate myocardial uptake of this novel radiotracer.
Mouse models of cardiotoxicity have been used previously to detect differential expression of genes that may be associated with doxorubicin-induced myocardial dysfunction.15,23 We used a similar mouse model for our investigation, and our echocardiographic findings are similar to what has been reported previously with respect to the time of onset of myocardial dysfunction with doxorubicin treatment.15,23 We detected reductions in myocardial function at week 6 and week 12; however, doxorubicin treatment was not associated with a significant decrease in LV function at week 1 and 3. Although there was no significant reduction in LV function at 3 weeks of doxorubicin treatment, there was a small but measurable increase in caspase 3 expression reflected by increased caspase 3 enzymatic activity, increase in CP18 uptake detected by autoradiography, and increase in apoptosis detected by TUNEL assay. Apoptosis has been postulated to be one of the principal mechanisms by which doxorubicin induces cardiac cell death, thereby resulting in myocardial dysfunction. Previous studies in a similar mouse model of doxorubicin cardiotoxicity have shown increased myocardial apoptosis and caspase 3 expression.15,23,24 Our findings are similar to what has previously been described in the literature using this model. We show that increase in CP18 uptake measured by multiple techniques, including gamma counting, PET imaging, and autoradiography, parallel the increase in caspase 3 enzymatic activity. We also demonstrate that increase in apoptosis detected by a histochemical technique, TUNEL staining, is reflected by a similar increase in CP18 uptake in tissue sections that were nearly identical to the ones used for TUNEL staining. We observed that CP18 uptake detected by autoradiography was heterogeneous. This heterogeneity in uptake suggests that doxorubicin induced caspase 3 expression, and apoptosis may be nonuniform. There are reports of regional heterogeneity in apoptosis between the subepicardium and the subendocardium during the regression of hypertensive left ventricular hypertrophy with antihypertensive medical therapy in a spontaneusly hypertensive rat model of hypertension.25 Although to our knowledge, this phenomenon has not been reported in mouse and rat models of cardiotoxocity, it is conceivable that a similar process is involved in apoptosis induced by cardiotoxic chemotherapy, and our findings need to be confirmed by independent studies.
An early increase in caspase 3 activity was also associated with an increase in 18F-CP18 uptake by autoradiography. Our findings suggest that an increase in apoptosis may precede the onset of detectable LV dysfunction; however, more sensitive echocardiographic parameters of LV dysfunction—that were not measured in these studies—such as global longitudinal strain, may have already been abnormal at week 1 and 3 of doxorubicin treatment. Therefore, temporal changes in 18FCP18 uptake will need to be compared with changes in more sensitive echocardiographic parameters, including regional and global longitudinal strain in future studies to evaluate the incremental value of molecular imaging of apoptosis in predicting LV dysfunction. Our ex vivo techniques, including autoradiography, gamma counting, and ex-vivo PET, suggest that an increase in 18F-CP18 uptake induced by doxorubicin is correlated with apoptosis as reflected by an increase in caspase 3 expression by TUNEL assay, However, our preliminary findings would have to be confirmed with in vivo PET imaging in models of myocardial dysfunction in preclinical and in clinical studies.
To confirm caspase 3–dependent specific myocardial uptake of 18F-CP18, an alternate scrambled peptide tracer, 18FCP32, not cleaved by capsase 3, was tested in the same animal model. If tracer uptake was the result of nonspecific entry of the tracer into damaged myocardium, we would expect to find increased myocardial uptake of 18F-CP32 in doxorubicin-treated animals. Instead, we found a preferential uptake of 18F-CP18 uptake with doxorubicin treatment and an absence of 18F-CP32 uptake in the myocardium after 6 weeks of doxorubicin treatment. This strongly suggests that increased 18F-CP18 uptake in the myocardium of animals treated with doxorubicin is caused by increased caspase 3–mediated cleavage of 18F-CP18 and preferential intracellular retention of the tracer rather than nonspecific influx of 18F-CP18 into cells that are damaged by cardiotoxicity.
Limitations
Small sample sizes and the inherent variability between individual animals is a limitation of our approach. Our study was conducted in a small animal model of cardiotoxicity, and our observations may not be applicable to clinical subjects affected by doxorubicin-induced cardiomyopathy. Although our results suggest that, in this mouse model, 18F-CP18 uptake may be useful for detecting myocardial damage, this hypothesis will have to be tested in prospective studies in clinical subjects to confirm its imaging effectiveness in humans. Our in vivo images have potential contamination from the blood pool signal. Although this increase in the blood pool signal was not observed in the in vivo images obtained after 1 and 6 weeks of doxorubicin treatment, additional in vivo imaging studies are required to establish both the temporal changes in myocardial uptake of CP18 and to confirm the regional heterogeneity of CP18 uptake that was observed with autoradiography. It is likely that imaging performed 2 hours after injection of the tracer may be more optimal for in vivo microPET, and our studies do not examine optimal timing of PET imaging to clearly visualize myocardial uptake by in vivo microPET. Our study shows that an increase in apoptosis and 18F-CP18 uptake precedes a reduction in LV systolic function; however, these are preliminary observations and do not unequivocally establish CP18 uptake as a prognostic marker of future LV dysfunction. Additional studies are needed to further characterize the time course of detectable changes in LV function and apoptosis by including intermediate time points. We terminated our observations at week 12 of doxorubicin treatment; additional time points would be necessary to evaluate whether 18F-CP18 uptake may be used for detecting long-term myocardial damage at later time points after discontinuation of doxorubicin treatment.
Conclusions
18F-CP18 may be potentially useful for noninvasive imaging of apoptosis in cardiovascular disease. In a mouse model, doxorubicin-induced cardiotoxicity is characterized by increased apoptosis and caspase 3 activity with an associated increase in myocardial 18F-CP18 uptake.
CLINICAL PERSPECTIVE.
Cardiomyocyte apoptosis is a key mechanism by which anthracyclines induce cardiotoxicity, which manifests as a reduction in left ventricular ejection fraction anywhere from several weeks to several years after treatment. Early detection of apoptosis may allow identification of patients who might benefit from dose adjustments of chemotherapy and initiation of potentially cardioprotective medications, including beta blockers, angiotensin-converting enzyme inhibitors, and statins. We have synthesized 18F-CP18, a 18F-labeled substrate of caspase 3, a cytosolic protein that catalyzes the final enzymatic step in apoptosis. 18F-CP18 selectively accumulates in cells with high caspase 3 activity, and 18F-CP18 uptake may serve as a surrogate marker for apoptosis. In our preclinical study of 18F-CP18 uptake in a mouse model of chronic doxorubicin-induced cardiotoxicity, we found that a progressive increase in myocardial dysfunction with 12 weeks of doxorubicin treatment was associated with an increase in both the biochemical activity of caspase 3 and 18F-CP18 uptake in the myocardium. Preliminary findings show that an increase in 18F-CP18 uptake may precede a detectable decrease in left ventricular function by echocardiography. Based on these preliminary studies, we propose that 18F-CP18 may be a useful agent for noninvasive monitoring of myocardial apoptosis induced by anthracycline treatment and therefore warrants further preclinical and clinical investigation.
Acknowledgments
We thank Ms Jeanna Arteaga, BS, for technical support.
Sources of Funding Siemens Molecular Imaging. Dr Balaji Tamarappoo was supported by the American Heart Association Clinical Research Program (11CRP5050015) and the National Institute of Health CTSA KL2 grant (2KL2TR000440).
Footnotes
Disclosures All the authors of the article state that they do not have any conflict of interests to disclose. Helen Su, Natalia Gorodny, Dr.Luis Felipe Gomez, Dr. Umesh Gangadharmath, Dr. Fanrong Mu, Dr. Gang Chen, Dr. Joseph Walsh, Dr. Katrin Szardenings and Dr. Hartmuth Kolb were employees of Siemens Molecular Imaging.
References
- 1.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 2.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol. 2013;20:443–464. doi: 10.1007/s12350-013-9707-1. doi: 10.1007/s12350-013-9707-1. [DOI] [PubMed] [Google Scholar]
- 4.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 5.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 6.Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234-235:119–124. [PubMed] [Google Scholar]
- 7.Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000;102:572–578. doi: 10.1161/01.cir.102.5.572. [DOI] [PubMed] [Google Scholar]
- 8.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 9.Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [PubMed] [Google Scholar]
- 10.Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, Chen G, Ren X, Kranias EG. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, Miyauchi T, Yamaguchi I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006;101:151–158. doi: 10.1254/jphs.fp0050980. [DOI] [PubMed] [Google Scholar]
- 12.Su H, Chen G, Gangadharmath U, Gomez LF, Liang Q, Mu F, Mocharla VP, Szardenings AK, Walsh JC, Xia CF, Yu C, Kolb HC. Evaluation of [(18)F]-CP18 as a PET imaging tracer for apoptosis. Mol Imaging Biol. 2013;15:739–747. doi: 10.1007/s11307-013-0644-9. doi: 10.1007/s11307-013-0644-9. [DOI] [PubMed] [Google Scholar]
- 13.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Xia CF, Chen G, Gangadharmath U, Gomez LF, Liang Q, Mu F, Mocharla VP, Su H, Szardenings AK, Walsh JC, Zhao T, Kolb HC. In vitro and in vivo evaluation of the caspase-3 substrate-based radiotracer [(18)F]-CP18 for PET imaging of apoptosis in tumors. Mol Imaging Biol. 2013;15:748–757. doi: 10.1007/s11307-013-0646-7. doi: 10.1007/s11307-013-0646-7. [DOI] [PubMed] [Google Scholar]
- 15.Yi X, Bekeredjian R, DeFilippis NJ, Siddiquee Z, Fernandez E, Shohet RV. Transcriptional analysis of doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2006;290:H1098–H1102. doi: 10.1152/ajpheart.00832.2005. doi: 10.1152/ajpheart.00832.2005. [DOI] [PubMed] [Google Scholar]
- 16.Papoian T, Lewis W. Adriamycin cardiotoxicity in vivo. Selective alterations in rat cardiac mRNAs. Am J Pathol. 1990;136:1201–1207. [PMC free article] [PubMed] [Google Scholar]
- 17.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res. 1995;76:907–914. doi: 10.1161/01.res.76.5.907. [DOI] [PubMed] [Google Scholar]
- 18.Chen DL, Zhou D, Chu W, Herrbrich PE, Jones LA, Rothfuss JM, Engle JT, Geraci M, Welch MJ, Mach RH. Comparison of radiolabeled isatin analogs for imaging apoptosis with positron emission tomography. Nucl Med Biol. 2009;36:651–658. doi: 10.1016/j.nucmedbio.2009.03.008. doi: 10.1016/j.nucmedbio.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madar I, Huang Y, Ravert H, Dalrymple SL, Davidson NE, Isaacs JT, Dannals RF, Frost JJ. Detection and quantification of the evolution dynamics of apoptosis using the PET voltage sensor 18F-fluorobenzyl triphenyl phosphonium. J Nucl Med. 2009;50:774–780. doi: 10.2967/jnumed.108.061283. doi: 10.2967/jnumed.108.061283. [DOI] [PubMed] [Google Scholar]
- 20.Reshef A, Shirvan A, Akselrod-Ballin A, Wall A, Ziv I. Small-molecule biomarkers for clinical PET imaging of apoptosis. J Nucl Med. 2010;51:837–840. doi: 10.2967/jnumed.109.063917. doi: 10.2967/jnumed.109.063917. [DOI] [PubMed] [Google Scholar]
- 21.Yagle KJ, Eary JF, Tait JF, Grierson JR, Link JM, Lewellen B, Gibson DF, Krohn KA. Evaluation of 18F-annexin V as a PET imaging agent in an animal model of apoptosis. J Nucl Med. 2005;46:658–666. [PubMed] [Google Scholar]
- 22.Zhu X, Li Z, Zhao M. Imaging acute cardiac cell death: temporal and spatial distribution of 99mTc-labeled C2A in the area at risk after myocardial ischemia and reperfusion. J Nucl Med. 2007;48:1031–1036. doi: 10.2967/jnumed.106.037754. doi: 10.2967/jnumed.106.037754. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 24.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 25.Teng BS, Dam TV, Moreau P, Hamet P, DeBlois D. Apoptosis during regression of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 1999;34:229–235. doi: 10.1161/01.hyp.34.2.229. [DOI] [PubMed] [Google Scholar]