Abstract
Although sarcopenia is thought to underlie the manifestations of frailty, association of frailty with measures of body composition is underinvestigated.
Methods
Eighty hemodialysis patients were included in the study. Performance-based frailty (PbF) used gait speed over 20 feet and 5 sit-to-stand (1 point each for lowest quintile) for the physical components of the frailty phenotype plus exhaustion (Short Form-36 [SF-36] vitality score <55) and physical activity (lowest quintile of weekly kcal energy expenditure on leisure activity on the Physical Activity Scale for the Elderly questionnaire; 1 point). Function-based frailty (FbF) defined by questionnaire measures of physical functioning (SF-36 Physical Function score <75; 1 point), exhaustion, and physical activity as for PbF. A score of 2 or greater was defined as frail. Outcomes related to muscle size included muscle area of the contractile tissue of the anterior tibialis and quadriceps muscles using magnetic resonance imaging, phase angle using bioimpedance analysis, lean body mass using dual energy X-ray absorptiometry, and body mass index (BMI). Linear regression was used to analyze associations between frailty and muscle size, with and without sex and age covariates.
Results
Fifty-nine percent of individuals met PbF criteria, 63% met FbF criteria, and 55% met both. In univariate analysis, PbF and FbF were associated with smaller muscle area of the quadriceps, smaller phase angle, and higher BMI. Associations remained significant for the quadriceps after adjustment for age and sex. The magnitude of association of PbF with quadriceps muscle area was greater than 10 years of age (−30.3 cm2 P = .02 vs. −6.6 cm2 P < .0001) in multivariate analysis. There was no significant association between either measure of frailty and other measures of body composition after adjustment for age and sex.
Conclusion
Frailty was associated with measurements related to muscle size in a population of individuals with chronic kidney disease, a known contributor to muscle wasting.
Introduction
Muscle wasting leading to reduced physical functioning is thought to be one of the major underpinnings of frailty. Frailty has long been recognized as a syndrome of decreased reserve that predisposes to disability and other adverse outcomes.1,2 One phenotypic definition of frailty that includes at least 3 of the following, weight loss, weakness, exhaustion, slow gait, and low physical activity level, has gained wide acceptance and has been applied to several populations, including chronic kidney disease (CKD).3–5
Frailty is thought to result from the additive effects of chronic inflammation and acute insults (illness, injuries, major life events) accompanied by periods of limited activity including periods of bedrest.6 These processes are thought to ultimately lead to loss of muscle mass and poor physical functioning. Although the frailty phenotype is generally assumed to reflect muscle wasting, there has been little direct confirmation of this linkage. In a general population of elderly individuals, those who were frail had a lower muscle density measured by peripheral quantitative computerized tomography compared with nonfrail individuals.7 However, it remains unknown whether the association persists in the setting of chronic disease that might affect muscle size and function.
In the CKD population, decreased muscle cross-sectional area, strength, and physical functioning have been well described.8 Low exercise capacity among patients with CKD has been documented in several studies.9–12 Systemic inflammation, decreased protein synthesis,13 and decreased oxygen extraction14 have been described as playing a role in muscle wasting and dysfunction in CKD.15–21
Most studies of frailty in the CKD population have been based on self-reported function (function-based frailty; FbF) rather than on directly measured physical performance (performance-based frailty; PbF). The prevalence of FbF in the CKD population is quite high, reported to be 21% among participants in the National Health and Nutrition Examination Survey22 data and 67% among patients newly started on dialysis.4 The measurement of FbF has recently been suggested to overestimate the prevalence in the CKD population.23 We sought to determine whether frailty is associated with body composition in the expected way in the end-stage renal disease (ESRD) population in which disease-specific processes are affecting muscle size and function. We also examined FbF and PbF in the same study population to assess the extent to which FbF is associated with body composition and thus might be a reasonable alternative when components of PbF are unavailable or their measurement is not feasible.
Methods
Study Participants
We used baseline data from the Nandrolone and Exercise study (NEXT), which enrolled 80 patients undergoing hemodialysis for more than 3 months.24 The Committee on Human Subjects at the University of California–San Francisco and the Research and Development Committee of the San Francisco Veterans Affairs Medical Center approved the study. All subjects provided written informed consent.
PbF
PbF was defined using gait speed over 20 feet and the time required to perform 5 sit-to-stand maneuvers. Sit to stand was chosen as a measure of muscle strength because grip strength was not measured in the NEXT study. For each measure, a point was allocated if individuals were in the lowest quintile using normative data.25–28 Specifically, a point was allocated to the frailty score if gait speed was less than 0.8 m/second.25,28 For sit-to-stand measurement,29 a point was allocated if participants had a sit-to-stand time of greater than 14.5 seconds.26 The Short Form-36 SF-36 vitality score less than 55 (1 point)30 and the lowest quintile of weekly energy expenditure on leisure activity calculated from the Physical Activity Scale for the Elderly questionnaire using normative data (1 point)31 were also used, and a score of 2 or greater was considered frail.
FbF
FbF was defined using questionnaire measures of physical functioning, exhaustion, and physical activity as has been previously described and previously used.32 These included a SF-36 physical function score less than 75 (1 point), a SF-36 vitality score less than 55 (1 point),30 and the lowest quintile of weekly energy expenditure on leisure activity calculated from the Physical Activity Scale for the Elderly questionnaire using normative data.31 On the basis of previous evaluations in the dialysis population, the association of poor physical functioning to outcomes was similar to associations with exhaustion and low physical activity; thus, 1 point was allocated to physical function, a slight deviation from the Woods criteria.4,32,33 No baseline weight change data were available for inclusion in the definition of frailty. A score of 2 or greater was considered frail.
Body Composition
Body Mass Index
Baseline weight in kilograms and height in centimeters were collected as previously described.24 Body mass index (BMI) was calculated in kilograms per meter squared (kg/m2).
Phase Angle
Measures of body composition included bioimpedance analysis (BIA; RJL Spectrum III, RJL Systems, Clinton Township, MI) at 50 kHz. BIA introduces a low-amplitude current across electrodes placed on the dorsum of the hand and foot of participants. Resistance (R) and reactance (Xc) can then be used to estimate body composition.34,35 Phase angle (PA; the arc tangent of the Xc to R ratio) captures the relative contribution of Xc and R and can range in theory from 0 to 90° : 0° if the circuit is only resistive (a system with no cell membranes) and 90° if the circuit is only capacitive (a system of membranes with no fluid). Thus, higher phase angle indicates more intact cell membranes and higher body cell mass. BIA has been validated as a method of body composition analysis in several patient groups including dialysis patients.35,36
Lean Body Mass
Whole-body dual energy X-ray absorptiometry (Lunar DPX, Madison, WI) was used to measure lean body mass (LBM) and fat mass in kilograms. Measurements were made 1 hour after hemodialysis as previously described.24
Muscle Area
Magnetic resonance images of the leg were obtained on a day after a dialysis session. The right leg was studied except in cases in which there was hardware or previous injury that distorted leg anatomy. Muscle areas were measured using proton T1-weighted axial images acquired at 1.5 T with image parameters as follows: echo time of 14 milliseconds, field of view equal to 210 mm2, and matrix equal to 256 × 256. The single slice with largest area of the muscle of interest was selected for analysis. For the quadriceps, slices were centered around the midpoint between the kneecap and the femoral head, and slice thickness was 8 mm. For the anterior tibialis, a 31-cm-diameter extremity coil was used, and slices were 4 mm thick. A customized software program written in IDL (Research Systems, Inc., Boulder, CO) allowed for the separate quantitation of contractile and noncontractile components of the anterior compartment of the leg, which contains the ankle dorsiflexor muscles and the anterior compartment of the thigh containing the rectus femoris, vastus lateralis, vastus intermedius, and vastus medialis (collectively the quadriceps). The software produced the following output: total compartment cross sectional area (CSA) (cm2), contractile tissue CSA (variable of interest; cm2), noncontractile CSA (cm2), percent contractile tissue (contractile CSA/total CSA×100), signal intensity threshold value, and total number of pixels. Each participant’s image was analyzed 3 times, and the average values for each variable were recorded.
Statistical Methods
Characteristics by PbF and FbF were compared with nonfrail participants by t test for continuous variables and χ2 for categorical variables. BMI, muscle area, phase angle, and LBM were treated as continuous outcome variables. Linear regression analyses were performed with measures related to muscle size, including BMI, phase angle, LBM, and tibialis anterior and quadriceps muscle areas as the outcomes and frailty (by PbF and FbF in separate models) as the main predictor of interest. Potential covariates included age and sex because of their expected associations with frailty and body composition. Potential interactions among age, sex, and frailty were also tested. For each frailty definition, the univariate associations with body composition measures were modeled (Model 1), and then the association was sequentially adjusted for age (Model 2) and sex (Model 3). Interaction terms were included in the model if they had a P value of less than .1. Stata version 11 (College Station, TX) was used for all analyses.
Results
Study Participants
Most participants were male (63%) with a mean age of 55 ± 13 years. Fifty-eight percent of participants were African American (Table 1). Fifty percent of individuals had diabetes mellitus, and 92% had been previously diagnosed with hypertension. The median dialysis vintage was 26 (12, 52) months, and the mean Kt/V was 1.41 ± 0.3. The mean hemoglobin was 11.8 ± 1.5 g/dL and the mean albumin was 3.9 ± 0.5 g/dL.
Table 1.
Characteristics of Study Population by Performance-Based Frailty
| N = 80 | All Participants | Frail (n = 47) | Not Frail (n = 33) | P |
|---|---|---|---|---|
| Male | 63% | 55% | 72% | .04 |
| Mean age, y (SD) | 55 (13) | 58 (13) | 53 (12) | .04 |
| Race/ethnicity | .23 | |||
| White | 4% | 6% | 0% | |
| Black | 58% | 62% | 50% | |
| Asian | 26% | 21% | 33% | |
| Hispanic | 11% | 8% | 15% | .37 |
| Clinical characteristics | ||||
| Coronary artery disease | 45% | 51% | 36% | .23 |
| Previous or current tobacco use | 50% | 51% | 48% | .74 |
| CVA/TIA | 14% | 13% | 15% | .79 |
| PVD | 13% | 14% | 9% | .41 |
| CHF | 28% | 29% | 24% | .58 |
| Hypertension | 93% | 91% | 94% | .68 |
| Diabetes | 50% | 60% | 36% | .04 |
| Body composition | ||||
| BMI (kg/m2) | 27 (6.9) | 28.8 (7.7) | 24.9 (4.7) | .01 |
| Anterior tibialis muscle area (cm2)* | 9.7 (2.2) | 9.33 (2.4) | 10.2 (2.0) | .13 |
| Quadriceps muscle area (cm2)† | 47.4 (14.7) | 42.3 (9.6) | 53.0 (17.3) | .003 |
| Phase angle (°)‡ | 5.7 (1.5) | 5.24 (1.3) | 6.24 (1.6) | .006 |
| Lean body mass (kg) | 48.3 (11.1) | 47.2 (10.2) | 50.0 (12.4) | .32 |
BMI, body mass index; CHF, congestive heart failure; CVA, cerebral vascular accident; PVD, peripheral vascular disease; SD, standard deviation; TIA, transient ischemic attack.
n = 66 for analysis of anterior tibialis muscle area.
n = 62 for analysis of quadriceps muscle area.
n = 69 for analysis of phase angle.
Frailty
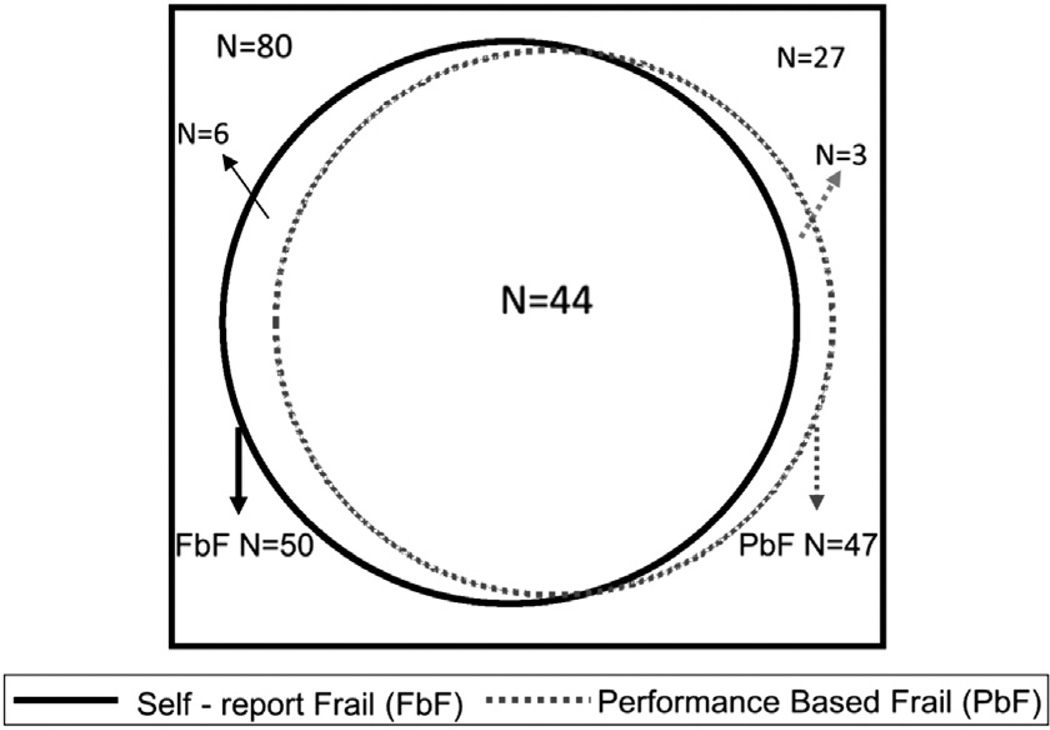
Fifty-four individuals (66%) met at least 1 definition of frailty, including 47 (59%) who met performance-based criteria for frailty, 50 (63%) who met function-based criteria, and 44 (55%) who met both (Fig. 1). Participants who were frail by PbF were older on average and more likely to be female and to have diabetes than individuals who were not frail on the basis of the PbF definition (Table 1); these associations were not statistically significant for FbF (Table 2). Most clinical characteristics of frail individuals did not differ from nonfrail individuals (Tables 1 and 2). There was no statistically significant difference in the proportion of individuals with hypertension, previous tobacco use, cerebral vascular accidents, or congestive heart failure among frail and nonfrail individuals.
Figure 1.
Proportion of individuals meeting either definition of frailty.
Table 2.
Characteristics of Study Population by Functional-Based Frailty
| N = 80 | All Participants | Frail (n = 50) | Not Frail (n = 30) | P |
|---|---|---|---|---|
| Male | 63% | 56% | 73% | .12 |
| Mean age, y (SD) | 55 (13) | 56 (13) | 54 (13) | .60 |
| Race/ethnicity | .13 | |||
| White | 4% | 6% | 0% | |
| Black | 58% | 62% | 50% | |
| Asian | 26% | 24% | 30% | |
| Hispanic | 11% | 6% | 20% | .06 |
| Clinical characteristics | ||||
| Coronary artery disease | 45% | 46% | 43% | .92 |
| Previous or current tobacco use | 50% | 56% | 40% | .13 |
| CVA/TIA | 14% | 14% | 13% | .90 |
| PVD | 13% | 14% | 10% | .57 |
| CHF | 28% | 28% | 26% | .89 |
| Hypertension | 93% | 92% | 93% | .82 |
| Diabetes | 50% | 52% | 46% | .64 |
| Body composition | ||||
| BMI (kg/m2) | 27 (6.9) | 28.4 (7.5) | 25.2 (5.1) | .04 |
| Anterior tibialis muscle area (cm2)* | 9.7 (2.2) | 9.3 (2.2) | 10.4 (2.0) | .06 |
| Quadriceps muscle area (cm2)† | 47.4 (14.7) | 44.0 (9.8) | 52.7 (19.0) | .02 |
| Phase angle (°)‡ | 5.7 (1.5) | 5.4 (1.5) | 6.1 (1.5) | .04 |
| Lean body mass (kg) | 48.3 (11.1) | 47.8 (10.7) | 50.0 (12.0) | .61 |
BMI, body mass index; CHF, congestive heart failure; CVA, cerebral vascular accident; PVD, peripheral vascular disease; SD, standard deviation; TIA, transient ischemic attack.
n = 66 for analysis of anterior tibialis muscle area.
n = 62 for analysis of quadriceps muscle area.
n = 69 for analysis of phase angle.
Body Composition Assessments
As expected, measures of body composition differed by sex and age. Mean area of the anterior compartment of the lower leg and quadriceps was greater in men than women (anterior tibialis 69.1 ± 15.5 vs. 53.6 ± 12.6, respectively, P < .0001; quadriceps 109.0 ± 21.9 vs. 82.3 ± 23.0, respectively P < .0001). Mean phase angle was greater in men (6.1 ± 1.5°) than women (4.7 ± 1.1°, P < .0001). Although LBM measures were greater in men than women (53.0 ± 9.5 kg vs. 40.0 ± 8.6 kg, respectively; P < .0001), there was no statistically significant difference in BMI between the two sexes. Likewise, age was associated with smaller measures of body composition for all measures (P < .05) except BMI (P = .88).
Association Between PbF and Body Composition
In bivariate analyses, PbF was associated with higher BMI, lower phase angle, and smaller muscle area of the quadriceps muscle (all P < .05; Table 3). LBM and anterior tibialis muscle area were smaller in PbF individuals, but the difference was not statistically significant. In multivariable analyses, PbF remained associated with quadriceps muscle area after adjustment for age, sex, and the interaction of PbF with age. The association of PbF with LBM became more pronounced with adjustment for age and sex (and an interaction with age and PbF similar to that observed for quadriceps muscle area), but it did not reach statistical significance.
Table 3.
Bivariate and Multivariable Regression Models of the Association of Performance-Based Frailty With Body Composition
| Anterior Tibialis Muscle Area |
Quadriceps Muscle Area |
Phase Angle |
Body Mass Index |
Lean Body Mass |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (cm2) |
P | Coefficient (cm2) |
P | Coefficient (cm2) |
P | Coefficient (cm2) |
P | Coefficient (cm2) |
P |
| Model 1: bivariate | ||||||||||
| PbF | −0.85 | .13 | −10.65 | .004 | −0.99 | .007 | 3.86 | .01 | −2.58 | .32 |
| Model 2: Model 1 + age | ||||||||||
| PbF | −1.22 | .60 | −34.44 | .02 | −2.23 | .13 | −2.65 | .69 | −19.54 | .08 |
| Age per 10 y | −0.59 | .08 | −7.03 | .001 | −0.60 | .005 | −1.25 | .20 | −4.64 | .004 |
| PbF × age, per 10 y | — | 4.92 | .06 | — | — | 3.4 | .08 | |||
| Model 3: Model 2 + sex | ||||||||||
| PbF | −1.08 | .60 | −30.28 | .02 | −1.49 | .27 | −2.96 | .66 | −15.80 | .08 |
| Sex | −2.1 | <.0001 | −10.70 | .001 | −1.15 | .001 | 1.09 | .51 | −12.33 | <.0001 |
| Age per 10 y | −0.54 | .08 | −6.60 | <.0001 | −0.51 | .01 | −1.32 | .18 | −3.88 | .004 |
| PbF × age, per 10 y | — | 4.64 | .05 | — | — | 3.11 | .06 | |||
PbF, performance-based frailty.
Association Between FbF and Body Composition
Mirroring PbF, in bivariate analyses, FbF was associated with higher BMI, lower phase angle, and smaller muscle area of the quadriceps muscle in bivariate analyses (all P < .05; Table 4). LBM and anterior tibialis muscle area were smaller in individuals who were frail on the basis of FbF than among those who were not, but the difference was not statistically significant. Multivariable analyses of the associations of FbF with body composition were similar to those of PbF after adjustment for age and sex.
Table 4.
Bivariate and Multivariable Regression Models of the Association of Self- Reported Functional-Based Frailty With Body Composition
| Anterior Tibialis Muscle Area |
Quadriceps Muscle Area |
Phase Angle |
Body Mass Index |
Lean Body Mass |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (cm2) |
P | Coefficient (cm2) |
P | Coefficient (cm2) |
P | Coefficient (cm2) |
P | Coefficient (cm2) |
P |
| Model 1 | ||||||||||
| FbF | −1.10 | .06 | −8.83 | .02 | −0.77 | .04 | 3.21 | .05 | −1.34 | .61 |
| Model 2 | ||||||||||
| FbF | −2.86 | .23 | −37.73 | .008 | −1.82 | .23 | −3.0 | .66 | −19.76 | .07 |
| Age per 10 y | −0.76 | .03 | −8.05 | <.0001 | −0.61 | .005 | −0.88 | .36 | −4.70 | .003 |
| PbF × age, per 10 y | — | 5.6 | .03 | — | — | 3.42 | .07 | |||
| Model 3 | ||||||||||
| FbF | −2.47 | .24 | −32.59 | .01 | −1.16 | .40 | −3.3 | .63 | −16.33 | .07 |
| Sex | −2.02 | <.0001 | −10.70 | .001 | −1.14 | .001 | 1.16 | .48 | −12.48 | <.0001 |
| Age per 10 y | −0.65 | .04 | −7.30 | <.0001 | −0.53 | .01 | −0.96 | .32 | −3.89 | .003 |
| FbF × age per 10 y | — | — | 5.09 | .03 | — | — | — | — | 3.23 | .04 |
FbF, functional-based frailty.
Discussion
In this study we set out to determine the extent to which both performance-based measures (PbF) and self-reported functioning-based measures (FbF) of frailty were associated with body composition in the setting of ESRD. We found that performance-based and function-based measures of frailty identified overlapping but nonidentical groups of patients in this cohort, with the percentage of frail individuals slightly greater using function-based criteria. We found an association between measures of body composition and both definitions of frailty in patients with ESRD.
Previous studies, such as the Invecchiare in Chianti study, have shown that frailty is linked to muscle mass and size in the general elderly population,37–40 but very few studies have tested this link in the setting of other known contributors to muscle wasting. Our results are similar to those of the Invecchiare in Chianti study, showing that the relationship between frailty and body composition is present even in an ESRD population in whom muscle mass and physical performance and function are limited and the prevalence of frailty is considerably higher. Furthermore, the magnitude of the association of frailty with quadriceps muscle size was impressive. In specific, the association between quadriceps muscle area and PbF was of greater magnitude than that of 10 years of age or of female sex.
Frailty was associated with smaller muscle size as estimated by quadriceps muscle area and phase angle in bivariate analyses, but it was not significantly associated with anterior tibialis muscle area or total body LBM. It is interesting to speculate that the anterior tibialis muscle may be less vulnerable to inactivity-related atrophy because it plays a large role in the mechanics of walking, requiring its regular use even in an inactive population. Variations in fluid balance among individuals on dialysis may alter the hydration status of lean tissue and thus introduce variability into the LBM measure that is not related to muscle size, obscuring any relationship with frailty.
The somewhat counterintuitive association of frailty with higher rather than lower BMI in univariate analysis has been reported by others41,42 and may be driven by decreased functioning, low physical activity levels, and greater inflammation among obese individuals, a situation that has been referred to as “sarcopenic obesity”.41,42
In analyses that also adjusted for age and sex, frailty remained independently associated with the cross-sectional area of the quadriceps muscles of these patients but not with phase angle. There are several potential explanations for the lack of independent association of frailty with phase angle. First, sex and age, particularly age, may be along the “causal pathway” of frailty. Because women have smaller muscles than men, reductions in muscle mass that occur in relation to aging, chronic disease, and other factors may be more likely to lead to frailty in women because less atrophy would be required to reach any threshold level of muscle mass that leads to poor physical function. Thus, adjusting for age and sex in our models may represent over-adjustment. On the other hand, because the magnitude and direction of the coefficients relating frailty to phase angle did not diminish with adjustment for age and sex, small sample size and limited power might be a reason for the lack of statistical significance. Finally, it is possible that frailty is not associated with muscle in this population, but we believe that the consistent results across measures even in the absence of statistical significance for some associations makes this possibility less likely.
The associations of frailty with body composition were similar regardless of the definition of frailty used. Results from the modeling of FbF with measures of body composition were similar to those using the PbF definition. The associations of frailty with quadriceps area were of similar magnitude as that of female sex and 10 years of age. There was a significant interaction between age and PbF, such that the association of age with quadriceps area was stronger among those who were not frail than among frail patients, perhaps confirming that these frailty phenotypes reflect more than just the effect of age on muscle size.
The similarity of these findings using either FbF or PbF is not surprising considering the large overlap of individuals identified using either definition of frailty. Thus, although larger studies are needed to confirm our findings, the similarities in the association of body composition with both definitions of frailty, combined with data showing that FbF is associated with bad outcomes among patients with ESRD,4 suggest that FbF is a reasonable surrogate for PbF when PbF is not available or practical.
Although our study showed an association between muscle atrophy and frailty, several limitations should be noted. First, in part because of the size of the study, this study may have been underpowered. Second, the patients in this study were not necessarily representative of the entire U.S. population with ESRD. However, we do not expect that associations between frailty and muscle size would systematically differ based on patient characteristics. Third, because this study is cross-sectional, directionality of the linkage between frailty and measures of body composition could not be determined. However, it is biologically plausible that chronic inflammation and acute physiologic insults such as illness, injuries, and possibly uremia or inactivity lead to small muscles, with the resultant weakness leading to the designation of frailty. On the other hand, the exhaustion that is part of frailty could also lead to inactivity and low muscle mass and weakness. Regardless of the cause of the association, low muscle mass is more difficult to measure than frailty, and small phase angle36,43 and frailty4 are associated with bad outcomes among those with ESRD, rendering frailty a potentially useful construct in the ESRD population. It remains to be seen whether measures of muscle size will add prognostic information above and beyond the designation of frailty.
In conclusion, we found that frailty is associated with smaller muscle cross-sectional area even in the setting of kidney disease. It is interesting to note that the relation of frailty with measures of quadriceps area was of comparable magnitude as associations with age and sex, which are key nonmodifiable determinants of muscle size. Because it is more feasible to measure frailty in large cohorts than it is to obtain direct measures of muscle size and body composition, a frailty designation could be used to identify patients likely to have muscle wasting who are at risk for poor outcomes and who could benefit from interventions aimed at improving muscle atrophy and poor physical function.
Practical Application
Identifying frail CKD patients who could benefit from interventions aimed at improving muscle atrophy and poor physical function may improve risk for poor outcomes.
References
- 1.Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45:265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 7.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergstrom J, Lindholm B, Lacson E, Jr, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial. 2000;13:163–175. doi: 10.1046/j.1525-139x.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 9.Beasley CR, Smith DA, Neale TJ. Exercise capacity in chronic renal failure patients managed by continuous ambulatory peritoneal dialysis. Aust N Z J Med. 1986;16:5–10. doi: 10.1111/j.1445-5994.1986.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 10.Painter P, Messer-Rehak D, Hanson P, Zimmerman SW, Glass NR. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron. 1986;42:47–51. doi: 10.1159/000183632. [DOI] [PubMed] [Google Scholar]
- 11.Moore GE, Brinker KR, Stray-Gundersen J, Mitchell JH. Determinants of VO2peak in patients with end-stage renal disease: on and off dialysis. Med Sci Sports Exerc. 1993;25:18–23. doi: 10.1249/00005768-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Padilla J, Krasnoff J, Da Silva M, et al. Physical functioning in patients with chronic kidney disease. J Nephrol. 2008;21:550–559. [PubMed] [Google Scholar]
- 13.Pupim LB, Flakoll PJ, Majchrzak KM, Aftab Guy DL, Stenvinkel P, Ikizler TA. Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int. 2005;68:1857–1865. doi: 10.1111/j.1523-1755.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 14.Marrades RM, Roca J, Campistol JM, et al. Effects of erythropoietin on muscle O2 transport during exercise in patients with chronic renal failure. J Clin Invest. 1996;97:2092–2100. doi: 10.1172/JCI118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato A, Ishida J, Endo Y, et al. Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol Dial Transplant. 2011;26:1967–1976. doi: 10.1093/ndt/gfq652. [DOI] [PubMed] [Google Scholar]
- 16.Cheema B, Abas H, Smith B, et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis. 2007;50:574–584. doi: 10.1053/j.ajkd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Cheema B, Abas H, Smith B, et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton) 2010;15:454–463. doi: 10.1111/j.1440-1797.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 18.Boivin MA, Battah SI, Dominic EA, et al. Activation of caspase-3 in the skeletal muscle during haemodialysis. Eur J Clin Invest. 2010;40:903–910. doi: 10.1111/j.1365-2362.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol. 2010;25:711–724. doi: 10.1007/s00467-009-1427-z. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wang XH, Wang H, Du J, Mitch WE. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol. 2010;21:419–427. doi: 10.1681/ASN.2009060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowe AV, McArdle A, McArdle F, et al. Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol Dial Transplant. 2007;22:1177–1183. doi: 10.1093/ndt/gfl721. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671.e2. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodial Int. 2013;17:41–49. doi: 10.1111/j.1542-4758.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 25.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohannon RW. Population representative gait speed and its determinants. J Geriatr Phys Ther. 2008;31:49–52. doi: 10.1519/00139143-200831020-00002. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 30.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 32.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 33.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erselcan T, Candan F, Saruhan S, Ayca T. Comparison of body composition analysis methods in clinical routine. Ann Nutr Metab. 2000;44:243–248. doi: 10.1159/000046691. [DOI] [PubMed] [Google Scholar]
- 35.Roubenoff R. Applications of bioelectrical impedance analysis for body composition to epidemiologic studies. Am J Clin Nutr. 1996;64(suppl):459S–462S. doi: 10.1093/ajcn/64.3.459S. [DOI] [PubMed] [Google Scholar]
- 36.Chertow GM. Estimates of body composition as intermediate outcome variables: are DEXA and BIA ready for prime time? J Ren Nutr. 1999;9:138–141. doi: 10.1016/s1051-2276(99)90052-3. [DOI] [PubMed] [Google Scholar]
- 37.Evans WJ, Paolisso G, Abbatecola AM, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. doi: 10.1007/s10522-010-9297-0. [DOI] [PubMed] [Google Scholar]
- 38.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 39.Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. doi: 10.1002/(sici)1097-4598(199706)20:6<679::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 41.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 42.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 43.Chertow GM, Jacobs DO, Lazarus JM, Lew NL, Lowrie EG. Phase angle predicts survival in hemodialysis patients. J Ren Nutr. 1997;7:204–207. [Google Scholar]