Abstract
Cushing's disease (CD) is a rare disorder characterized by the overproduction of adrenocorticotropic hormone due to a pituitary adenoma that ultimately stimulates excessive cortisol secretion from the adrenal glands. Prior to the detection of pituitary adenomas, various clinical signs of CD such as central obesity, moon face, hirsutism, and facial plethora are usually already present. Uncontrolled hypercortisolism is associated with metabolic, cardiovascular, and psychological disorders that result in increased mortality. Hence, the early detection and treatment of CD are not only important but mandatory. Because its clinical manifestations vary from patient to patient and are common in other obesity-related conditions, the precise diagnosis of CD can be problematic. Thus, the present set of guidelines was compiled by Korean experts in this field to assist clinicians with the screening, diagnoses, and treatment of patients with CD using currently available tests and treatment modalities.
Keywords: Adrenocorticotropic hormone, Corticotropin-releasing hormone, Pituitary ACTH hypersecretion, Cushing's disease, Inferior petrosal sinus sampling, Pasireotide, Pituitary adenomas, Transsphenoidal approach
INTRODUCTION
Cushing's disease (CD) is associated with increased risks of cardiovascular, metabolic, and respiratory disorders, psychiatric complications, osteoporosis, and infections, which all lead to increased rates of morbidity and mortality [1]. These issues originate from underlying hypercortisolism, which results in the manifestation of the cardinal signs of CD prior to the detection of pituitary problems. Because the complications associated with hypercortisolism may be followed by long-lasting morbidity even after successful treatment, the early detection and treatment of CD are not only important but mandatory.
A precise differential diagnosis of CD from Cushing's syndrome (CS) or from ectopic adrenocorticotropic hormone (ACTH)-secreting tumors is somewhat problematic due to common symptoms and signs [2]. Until recently, inferior petrosal sinus sampling (IPSS) with corticotropin-releasing hormone (CRH) was performed to stimulate ACTH secretion and allow for the discrimination between CD and ectopic ACTH-secreting tumors [3,4,5,6,7,8,9]; however, IPSS is currently performed using desmopressin [10,11], because of its stable supply and lower cost. Additionally, an 8-mg overnight dexamethasone suppression test (DMST) is now employed rather than the classic 5-day DMST, because the latter requires an inconveniently long testing period [12,13,14,15].
The surgical resection of a pituitary adenoma is first-line therapy for CD [16,17,18,19,20], while pharmacotherapy is a second-line strategy used to control recurrent or sustained CD [21]. Al-though ketoconazole, which suppresses cortisol synthesis at the level of the adrenal gland, is a convenient and effective drug used to control hypercortisolism, it was withdrawn from the market due to concerns of hepatotoxicity [22]. Thus, currently, there are few drugs available that can effectively control hyper-cortisolism with high efficacy and few side effects after sur-gery. More recently, a novel somatostatin analog called pasire-otide has shown significant efficacy in reducing cortisol pro-duction via inhibition of ACTH secretion in patients with CD [23]. Fortunately for CD patients, pasireotide will become available in Korea in the near future. Thus, several Korean experts in this field have reviewed the optimal strategies for diagnosis and management of CD, and the recommendations are compiled in the present set of guidelines.
CLINICAL FEATURES
CD is a serious condition associated with high rates of morbidity and mortality that result from excessive levels of systemic glucocorticoids [1]. The classic features of CD include central obesity, moon face, hirsutism, and facial plethora, but the clinical manifestations of CD vary from patient to patient and are common among other conditions, such as simple obesity and pseudo-CS [2]. Prolonged and inappropriately high glucocorticoid levels are associated with systemic abnormalities in metabolic (central obesity, weight gain, hyperglycemia, dyslipidemia, hypokalemia), hormonal (menstrual irregularity or amenorrhea, delayed puberty), musculoskeletal (proximal muscle wasting and weakness, osteoporosis), cardiovascular (hypertension, venous thromboembolism), dermatological (easy bruising, facial plethora, purple striae), neuropsychological (insomnia, depression, irritability), cognitive, and immunological (increased frequency of infections) functions [24]. Of these various clinical symptoms, the most discriminating signs and symptoms are easy bruising, facial plethora, proximal myopathy, and purple striae [25].
SCREENING TESTS
Diagnostic tests for CS aim to identify excessive secretion of cortisol, loss of diurnal variation, and decreased function of the negative feedback mechanism involved in the pituitary-adrenal axis. Screening tests for CD primarily focus on identifying loss of diurnal variation and the presence of excessive cortisol secretion, while a diagnosis of CD is made via confirmatory testing or localization of the lesion. It is recommended that screening and diagnostic tests for CS should be performed under controlled conditions to avoid stressful or pathological situations that could create unspecific activation of the pituitary-adrenal axis. Additionally, the possibility of iatrogenic CS must be excluded by confirming the patient's past history of steroid drug use.
Testing for diurnal variation in ACTH and cortisol levels
Cortisol is secreted by the adrenal gland in a pulsatile manner in accordance with the pulsating secretion of ACTH. The secretion of cortisol displays diurnal variations, with the maximum levels (6.5 to 25.4 µg/dL [range, 180 to 700 nmol/L]) in early morning and minimum level (<3.6 µg/dL [100 nmol/L]) at midnight. Because the diurnal variations characteristic of normal persons are absent in patients with CD, testing by random collection of blood is not recommended [26]. Additionally, it is recommended that diurnal cortisol levels be checked more than twice.
24-Hour urine-free cortisol test
The 24-hour urine free cortisol (UFC) test is a highly sensitive method of testing [2,27]. If the 24-hour UFC level exceeds a normal range, it is defined as a positive finding. The normal ranges are as follows: >90 µg/dL per 24 hours as measured by a radioimmunoassay or >50 µg/dL per 24 hours as measured by denaturing high-performance liquid chromatography/immunochemiluminometric assay. The 24-hour urine creatinine levels and urine volume should also be checked to assess the adequacy of urine collection [28,29].
It is recommended that this test be performed at least 2 to 3 times to minimize diagnostic errors, and further interpretation of the results may also be necessary in some situations. For example, if the glomerular filtration rate of the patient is <60 mL/min, then the amount of free cortisol in the urine may be reduced. Additionally, excessive amounts of cortisol can saturate the circulating cortisol-binding globulin (CBG) moieties and result in detectable increases in free-filtered cortisol in the urine. When conducted on hospitalized patients, the sensitivity and specificity of the 24-hour UFC test are better than those of the 1-mg overnight DMST test [27].
Late night salivary cortisol and serum cortisol tests
Under physiological conditions, serum cortisol concentrations reach a nadir at midnight or late at night. Salivary cortisol levels display a very high correlation with serum cortisol levels, which makes this measure a convenient method for the measurement of cortisol concentrations without inflicting stress on outpatients. For these tests, saliva should be collected during sleep or between the hours of 23:00 and 24:00, and it is recommended that the levels of salivary cortisol be checked more than twice. If the concentration of salivary cortisol at room temperature exceeds the cutoff value of 0.13 µg/dL (3.6 nmol/L) [30,31], it is defined as a positive finding. Both the sensitivity and specificity of this test are approximately 95% [26]. A "sleeping" midnight serum cortisol level of <1.8 µg/dL (50 nmol/L) effectively excludes a diagnosis of CS, while an "awake" midnight serum cortisol level of >7.5 µg/dL (207 nmol/L) is highly suggestive of CS [2,30]. However, the late night salivary cortisol test is not typically available in Korea.
Overnight DMST
The overnight DMST is the most widely used screening test for CS and CD [31,32]. In this test, 1 mg dexamethasone is administered orally between the hours of 23:00 and 24:00, and blood is collected between the hours of 08:00 and 09:00 on the following day to measure serum cortisol levels. If the serum cortisol concentration is >1.8 µg/dL (50 nmol/L), it is defined as a positive finding. In the case of obese patients and some patients suffering from depression and/or alcohol withdrawal syndrome, further attention is needed, because cortisol is not being suppressed in these situations.
Desmopressin stimulation test
The desmopressin stimulation test [33,34] depends upon the increase in ACTH release that occurs in 85% of patients with CD following intravenous injection of desmopressin. In this test, patients are intravenously administered 4 µg desmopressin, and blood is collected over a period of up to 2 hours at 30-minute intervals. If the serum ACTH level increases by >50%, the finding is defined as positive. This test is useful for determining ACTH levels following surgery for CD, rather than for the discrimination of CD, because the V3 receptor that binds desmopressin is found in 30% of tumors outside the pituitary gland that secrete ACTH.
CONFIRMATORY TESTS
Four tests may be used for the differential diagnosis of CD.
Sella magnetic resonance imaging
The sella magnetic resonance imaging (MRI) is an essential radiological tool for the diagnosis of CD necessary to assess the anatomical structure of the pituitary gland prior to surgery and to confirm whether pituitary adenomas are present. Compared with IPSS or a positron emission tomography scan, a sella MRI scan has the highest level of accuracy for tumor localization, with a positive predictive value of 86% [35]. In studies involving Korean patients with CD, concordant results between MRI scans and intraoperative surgical biopsies were observed in 77.8% of cases [36].
However, CD is frequently caused by adenomas that are too small to be detected by classic MRI scans. For example, more than 18% of patients with CD have microadenomas that are not visible on MRI scans [37]. Because the contrast enhancement of pituitary adenomas can be gradual or rapid after contrast administration, the use of dynamic contrast-enhanced MRI scans are recommended rather than classic MRI scans, which are obtaining only T1- and T2-weighted images before and after contrast administration. For cases in which the tumor is located in the far anterior aspect or far posterior aspect of the pituitary gland, the use of dynamic gadolinium-enhanced imaging with simultaneous acquisition of coronal and sagittal planes is helpful for the localization of a pituitary adenoma [38]. If pituitary adenomas are not detected using the aforementioned MRI procedures, half-dose contrast MRI scans or spoiled gradient recalled (SPGR) acquisition associated with the steady-state technique could be helpful. When MRI is executed using a lower injection dose of the contrast medium (0.05 mmol/kg gadolinium, for example), it becomes easier to distinguish microadenoma from normal pituitary tissue. [39]. Additionally, the use of SPGR MRI images with 1-mm sections facilitates detection of microadenomas, compared with spin echo MRI scans [40,41].
CRH stimulation test
In the majority of CD patients, secretion of ACTH is promoted by CRH and desmopressin [3,4,5,6,7,8,9]. The CRH stimulation test can be used to confirm the responses of serum ACTH and cortisol levels after intravenous injection of 100 µg CRH in the morning after fasting; however, a definitive cutoff value for this test to distinguish between CD and ectopic ACTH secretion (EAS) has yet to be established. Moreover, the responses of serum ACTH and cortisol are different between ovine and human CRH [9]. Nevertheless, increases in the serum ACTH and cortisol levels >50% within 30 minutes of CRH administration could suggest the presence of CD [9]. In Korea, the CRH stimulation test is not usually employed due to its high cost; typically, desmopressin is used to stimulate the secretion of ACTH. However, the cutoff values for the desmopressin stimulation tests that can provide an accurate differential diagnosis have yet to be determined.
IPSS using desmopressin or CRH
IPSS is the most important test for the differentiation of CD from EAS in patients diagnosed with CS [42,43]. According to a study that assessed Korean patients with CD using IPSS, 100% of patients tested positively for CD [36]. IPSS is a method that compares ACTH levels by collecting blood from peripheral vessels and both sides of the inferior petrosal sinus (IPS) after promotion of ACTH secretion via either desmopressin or CRH administration; the interpretation of the findings does not differ with regard to the stimulator used [10]. When desmopressin is used as the stimulator, the optimal injection dose is 10 µg [11] and is administered intravenously over a period of 15 seconds [10]. When CRH is used as the stimulator, the optimal intravenous injection dose is 100 µg [44].
Sampling should be executed from the bilateral IPS and peripheral vessels prior to and several times within the 10-minutes period following administration of the ACTH stimulator. CD is suggested by IPS/peripheral (IPS/P) ACTH ratios >2 prior to administration of the stimulator or by ratios >3 following administration of the stimulator. An ACTH ratio from both sides of the petrosal sinus of >1.4 following administration of the stimulator may imply lateralization of the ACTH-secreting tumors in the pituitary gland [45]. However, the primary purpose of IPSS is to distinguish between CD and EAS, because the presence of lateralization during this procedure is concordant with intraoperative biopsies in only 38.9% of Korean patients with CD [36]. Additionally, it is possible to obtain false negative results due to venous anomalies of the petrosal sinus or to the inexperience of the personnel performing the procedure. In such cases, it is possible to confirm whether or not the catheterization was successful by using prolactin values [46]; IPSS results that show an increase in prolactin levels >1.8-fold relative to the peripheral prolactin value indicate successful catheterization. A normalized ratio >1.3 ([dominant peak post-stimulation IPS/P ACTH]/[ipsilateral basal IPS/P prolactin]) implies the presence of CD, while a value <0.8 implies EAS [47].
8-mg overnight DMST
An 8-mg overnight DMST is very useful for the differential diagnosis of CD from EAS. Relative to the traditional high-dose DMST method, which requires the administration of dexamethasone over a 2-day period, the 8-mg overnight DMST is widely used due to its convenient drug administration and lack of requirement of a 24-hour urine test. Additionally, the overnight DMST has a sensitivity of 95%, which was reported to be superior to that of the traditional high-dose DMST test [12,13]. In the overnight DMST, an 8-mg dose of dexamethasone is administered between 23:00 and 24:00, and the blood cortisol level is measured at 8:00 on the following day. CD is inferred when serum cortisol levels are decreased by >50% from basal levels or if the serum cortisol level is <5 µg/dL [12,13,14,15].
TREATMENT
The treatment for CD aims to ameliorate any associated clinical conditions, improve any accompanying disorders through normalization of biochemical indices related to hypercortisolism, and minimize the recurrence rate. Treatments can be categorized as surgical therapy, medical management, and radiotherapy.
Surgical therapy
The transsphenoidal approach (TSA) is the primary treatment recommended for patients with CD, because it is associated with a complete remission rate of 65 to 90% for microadenomas, although the rate of remission for macroadenomas is lower than 65% [16]. While transsphenoidal microsurgery is the most frequently employed surgical method for CD patients, endoscopic surgery has also been implemented fairly widely in recent years. A head-to-head comparison of the prognoses for these two surgical methods has yet to be conducted, but no major differences in their surgical outcomes have been reported [17]. A total or partial hypophysectomy can be performed if the microadenomas are not easily visible, but this procedure has a relatively low remission rate (70%) as well as high frequencies of hypopituitarism and surgical complications [18].
The recurrence rate of microadenomas after TSA is 5 to 10% after 5 years and 10 to 20% after 10 years. Patients younger than 25 years were reported to experience recurrence more frequently [18,19]. Compared with microadenomas, macroadenomas have a higher recurrence rate (12 to 45%) and an earlier recurrence point (49 months vs. 16 months, respectively) [20]. However, there are a number of favorable prognostic factors related to surgery, including the fact that microadenomas are detectable on an MRI, the non-invasion of the dura mater or cavernous sinus, the confirmation of ACTH-secreting adenomas in pathological tissue, low serum cortisol levels after surgery, and a prolonged period of hypocortisolism [16].
A majority of CD patients require supplementary glucocorticoid therapy (optimal dose; 12 to 15 mg/m2) following surgery to correct hypocortisolism. Although the dosage can be increased if the hypocortisolism is severe, it should be reduced during the first month following surgery to a supplementary dose if possible. This supplementary glucocorticoid administration can be terminated if the morning serum cortisol level or stimulated serum cortisol is >18 µg/dL [16].
Pharmacotherapy
Centrally acting drugs (tumor-directed therapy)
Pasireotide (SOM230, Signifo, Abingdon, UK) suppresses the secretion of ACTH due to its affinity for somatostatin receptor subtype 5, which primarily manifests itself in cortisol-secreting adenomas. Pasireotide is over 40-fold more effective than octreotide [48]. After taking pasireotide, 15 to 26% of CD patients showed normalization of urine cortisol, 50 to 76% of the patients displayed a significant reduction in urine cortisol, and there was a reduction in adenoma size 12 months after the initiation of treatment [23]. Additionally, this study observed that when treatment was continued for 2 years, cortisol levels were reduced even further and were accompanied by improved clinical symptoms. The most typical side effect of pasireotide is hyperglycemia, which is manifested by drug-induced suppression of insulin and incretin secretion [23]. Pasireotide was recently approved for administration in Europe in the event of CD recurrence following surgery or if a surgical procedure cannot be used to treat CD.
Cabergoline is an agonist of dopamine D2 receptors, which are found in more than 70% of cortisol-secreting adenomas [49]. In a study on the long-term administration of cabergoline (more than 2 years at a maximum dose of 3.5 mg/week), hypercortisolism was normalized in 25 to 37% of patients, but the escape phenomenon was observed after several years of continuous administration [50].
Temozolomide is a methyl-triazenoimidazole-carboxamide derivative, and its effect as a DNA alkylating agent is dependent on its activation of the enzyme O6-methylguanine-DNA methyltransferase. Temozolomide is thought to be potentially effective against invasive CD based on the results of a study showing a reduction in the Ki-67 index due to softening and accelerated differentiation of adenomas [51]. However, the use of this drug is not yet approved in Korea.
Adrenal steroidogenesis blockers
Mitotane (o,p'-DDD) suppresses the synthesis of cortisol in the adrenal gland via decreases in the mitochondrial side-chain cleavage enzyme (CYP11A1), 11β-hydroxylation (CYP11B1), and 18-hydroxylation (CYP11B2), as well as a reduction in bioavailable steroids via promoted steroid metabolism [52]. Although remission rates of approximately 72 to 83% were observed with gradual effects within 2 to 3 months of treatment in patients with CD, only 30% of these patients remained in remission for up to 3 years after the cessation of mitotane treatment [53]. During mitotane administration, it is meaningless to measure total serum cortisol levels, because CBG levels are increased by its administration, and additionally, supplementary steroid therapy may be necessary due to the development of hypocortisolism [53]. Mitotane can be administered to women of childbearing age, because it can reverse hypogonadism and infertility. However, the drug accumulates in adipose tissues for up to 2 years after the cessation of treatment due to its lipophilic nature; therefore, it is advisable to avoid pregnancy for up to 5 years after treatment has ended [53]. Mitotane can be purchased through the Korea Orphan Drug Center (KODC).
Ketoconazole reduces steroid synthesis via its ability to suppress various steroid synthesizing enzymes such as 11β-hydroxylase, 17-hydroxylase, and 18-hydroxylase [22]. Reduced cortisol levels were found in 30 to 80% of patients after the daily administration of 400 to 1,200 mg ketoconazole, and normalization of urine cortisol levels was observed in 48% of 200 patients [22]. The escape phenomenon has been observed in some patients following treatment with ketoconazole, and its main side effects include hepatotoxicity, disturbances of the digestive system, and hypogonadism in men [21]. Currently, the production of ketoconazole is prohibited in the USA and Europe due to its hepatotoxicity. Ketoconazole is not approved for the treatment of CD in Korea but it can be purchased from the KODC.
Metyrapone inhibits the activities of 11-β hydroxylase, which is the final enzyme involved in the synthesis of cortisol and has the advantage of rapid activity onset within 2 hours of administration without tachyphylaxis [21]. Although it reduces cortisol levels in 75% of patients with CD, metyrapone pro duces a variety of side effects stemming from excessive levels of androgen and mineralocorticoid precursors due to an increase in ACTH levels, which arises from a loss of negative feedback [21]. Its use in Korea is not currently approved.
Etomidate, which is a type of anesthetic, suppresses the actions of 17-hydroxylase and 11-β hydroxylase [54]. Due to its rapid activity onset, etomidate can be administered by intravenous injection to patients in the very dangerous acute stages of CS. Etomidate displays its maximum effects approximately 5 hours after the initiation of continuous intravenous infusion, but it is possible that hypocortisolism could develop 24 hours later [54].
LCI699 is a recently developed drug with the ability to inhibit 11β-hydroxylase and 18-hydroxylase powerfully; clinical studies of this drug are being conducted currently [55]. When LCI699 was administered to 11 patients with CD over a 10-week period, the urine cortisol levels of 10 patients were normalized, ACTH levels increased more than 2-fold in five patients, and hypokalemia manifested in four patients [55].
Although cyproheptadine (a serotonin antagonist), valproic acid (a γ-aminobutyric acid uptake inhibitor), octreotide, peroxisome proliferator-activated receptor-γ (PPARγ) agonists (rosiglitazone, pioglitazone), and retinoic acid have displayed varying degrees of effectiveness in animal experiments, these drugs have had insignificant effects in humans. Although fluconazole is only 1% as effective as ketoconazole, this drug can be used for CD, because it suppresses 11β-hydroxylase and 17-hydroxylase [21].
Glucocorticoid receptor antagonists
Mifepristone has previously been used as an orally administered contraceptive, but it is the only glucocorticoid receptor antagonist currently approved for reducing hyperglycemia. A previous study found that the administration of mifepristone led to clinical improvements in 87% of 43 CD patients, and that it can be administered to patients with acute severe CD or patients awaiting radiation therapy [56]. However, there are no biochemical parameters that can be used to monitor its effects, and an overdose of mifepristone can cause hypocortisolism, aggravated high blood pressure or hypokalemia, or endometrial hyperplasia [56].
Combination therapy
Combination drug therapy may be initiated for the purpose of maximizing drug efficacies by using smaller doses of each individual drug to minimize potential side effects. Combination therapy of pasireotide, cabergoline, and ketoconazole was used to treat 17 patients with CD. In that study, pasireotide was administered for 1 month, and if urine cortisol levels did not normalize, then cabergoline was also administered. If after another month of treatment with these two drugs, urine cortisol levels were not normalized, then ketoconazole treatment was added. Remission was observed in approximately 90% of the study participants.
When pasireotide and cabergoline were co-administered in another study, the escape phenomenon typically associated with cabergoline was not observed [57]. Another study initially administered cabergoline to 12 patients with CD, followed by administration of low-dose ketoconazole to nine patients whose urine cortisol levels had not normalized after 6 months. Six of these nine patients experienced remission after an additional 6 months [58]. When the adrenal-blocking drugs mitotane, ketoconazole, and metyrapone were co-administered as the initial treatment, the urine cortisol levels of the majority of the patients normalized within the first 3 days of treatment with no serious side effects [59]. Thus, combination therapy can be attempted in patients with severe CD during the acute stages rather than performing a bilateral adrenalectomy.
Radiotherapy
Radiation-based therapies include the use of fractionated radiotherapy and stereotactic radiotherapies, such as Gamma Knife surgery (GKS), CyberKnife, and proton-beam therapy.
Fractionated external beam radiotherapy
Although fractionated external beam radiotherapy can be considered if CD is sustained after surgery, its effects only begin to manifest after an average of 2 years, and improvements are observed in only 50 to 70% of patients within 3 to 5 years [60]. Additionally, hypopituitarism is observed in approximately 40% of patients treated with this procedure, and this is accompanied by secondary brain tumors in 1 to 2% of patients [60].
Gamma Knife surgery
The effects of GKS begin to emerge as early as 6 months after surgery, and remission is observed in 70 to 95% of patients with CD treated by this procedure [61]. Hypopituitarism manifests in approximately 30% of patients, but the data concerning its side effects on the cerebrovascular system or cognitive impairments are currently insufficient [61].
Remission after surgery
The standard assessments for remission after surgery are as follows [16]: (1) if the morning serum cortisol levels the 1st week after surgery are <2 µg/dL, then CD is considered to be in remission; if the levels of serum cortisol are >5 µg/dL for up to 6 weeks after surgery, then additional tests should be performed; and if the levels of serum cortisol are 2 to 5 µg/dL, then close monitoring is necessary due to the high probability of remission; (2) UFC levels should be measured if the results of the serum cortisol analyses are ambiguous, and if the 24-hour UFC level is <20 µg, then CD is in a state of remission; if the 24-hour UFC level is in the range of 20 to 100 µg (normal range), the result is ambiguous; and if the 24-hour UFC level is above the normal range, CD is persistent; and (3) because serum cortisol levels sometimes decrease gradually, it is important to verify whether cortisol levels are at a minimum prior to initiation of any additional treatment.
Secondary treatments
In the event of recurrent or residual CD after surgery, secondary treatments such as repeat TSA, medical therapy, radiotherapy, or bilateral adrenalectomy can be implemented [16]. Although repeat surgery is associated with a high risk of hypopituitarism, repeat TSA has success rates of 50 to 70% and these rates improve if the microadenomas are visible. It is more advantageous to implement surgery for remnant adenomas as early as possible if the patient is in an active disease stage, but the surgical procedure could be delayed by approximately 4 to 6 weeks, since some patients improve gradually after surgery. Although a bilateral adrenalectomy induces the most rapid improvements in hypercortisolism among surgical methods, it can also induce permanent hypocortisolism and necessitate steroid therapy for the rest of a patient's life. Additionally, serum ACTH assessments and MRI tracking must accompany the bilateral adrenalectomy due to the risk of Nelson's syndrome. Therefore, this may be considered for patients who have recurrent CD, for whom surgical, medical, and radiation-based therapies have failed, or for those who are intolerant of the side effects of these treatment modalities.
Progress of complications following remission
Various complications, such as risks within the cardiovascular system, hypercoagulability, bone density decline, psychiatric problems, and cognitive impairments, are only partially ameliorated in patients following CD treatment [21]. The cardiovascular risks are greater in a CD patient than in a normal person even 5 years after a complete remission, and the extent of recovery from these complications is inversely proportional to the duration of exposure to hypercortisolism. Furthermore, the patient's quality of life continues to deteriorate even after remission, and this trend is worse for patients with hypopituitarism.
COMORBIDITY AND MORTALITY
The mortality rate of CD patients is 4-fold higher than that of the general population. Diabetes mellitus and high blood pressure are adverse prognostic factors for patients with CD [62], and even when CD has been treated, patients display a higher mortality rate than do those with non-functional pituitary tumors. This demonstrates that excessive exposure to cortisol is associated with an increased mortality rate [63]. CD may also be accompanied by metabolic diseases, cardiovascular diseases, various infections, and/or psychiatric diseases related to hypercortisolism [64].
CD is also characterized by high risks of osteoporosis and fractures. When a bone density test in CD patients was conducted using dual energy X-ray absorptiometry, the prevalence of osteoporosis was found to be approximately 40% [65]. That same study found a tendency towards reduced bone density in CD patients compared with normal persons of the same age regardless of whether CD began during childhood or adulthood. Thus, the long-term treatment of osteoporosis needs to be implemented in CD patients, because the risks of reduced bone density and damage to the spine are high, even after remission of the disease [66].
Hypercortisolism induces insulin resistance which, in turn, results in the manifestation of impaired glucose tolerance and diabetes mellitus in patients with CD. The prevalence rate of diabetes in patients with CD is 20 to 50% [67], and the neutral fat and cholesterol levels of patients with hypercortisolism are increased due to increased levels of very low density lipoprotein (VLDL) and low density lipoprotein [68,69]. Not only does cortisol directly influence the synthesis of VLDL and the generation of free fatty acids, it also causes insulin resistance via the presence of excessive glucocorticoid levels and may influence the development of dyslipidemia. Insulin resistance and dyslipidemia are risk factors of cardiovascular diseases that remain potential health threats for a minimum of 5 years after CD has been in complete remission [70].
The majority of CD patients also have high blood pressure related to hypercortisolism. Cardiovascular diseases are the most prevalent causes of death in patients with CD, and it is not easy to restore cortisol to normal levels in these patients. Even when cortisol levels have been normalized, the risk of cardiovascular diseases remains very high due to sustained damage to the blood vessels and the development of atherosclerotic plaques. It has been known since the early 1970s that patients with CS have a high risk of deep venous thrombosis. Glucocorticoids increase the concentrations of factor VIII, factor IX, and the von Willebrand factor, promote the generation of thrombin, and accelerate coagulation (as do surgical procedures and obesity), which results in an increase in the likelihood of thrombosis.
Additionally, there is a high incidence of nephrolithiasis among patients with CD, which is thought to be the result of calcium metabolism disorders and hemodynamic disorders that stem from hypercortisolism.
Complications due to severe infections are seen in 42% of CD patients who have not undergone treatment. Immunodysfunction manifests itself due to hypercortisolism which, in turn, results in frequent opportunistic infections. Numerous psychiatric disturbances are also associated with CD. Depression is the most frequently encountered psychiatric disturbance and is followed by not only possible sleep, manic, and anxiety disorders but also cognitive impairments. The expression and extent of these psychiatric disturbances vary and are not related to the degree of hypercortisolism. In fact, the brain volumes of CD patients tend to be reduced, but this deficit is partially recovered when cortisol levels are normalized.
CONCLUSIONS
CD is caused by the overproduction of ACTH due to pituitary adenomas that ultimately stimulate excessive cortisol production by the adrenal glands. Hypercortisolism is associated with increased risks of cardiovascular diseases, thromboembolism, musculoskeletal impairments, immunosuppression, and diabetes mellitus, which all result in long-lasting morbidity even after successful treatment. Hence, the early detection and treatment of CD are not only important but mandatory.
In the present set of guidelines, Korean experts summarized the current evidence regarding CD and provided recommendations for the screening, diagnosis, and treatment of this disorder. Furthermore, a standard proposal for these measures was prepared from the point of view of a precise diagnostic approach that entails the implementation of screening and confirmatory tests for CD as well as treatments using newly developed drugs. The screening and confirmatory test are summarized in Table 1.
Table 1. Modified diagnostic criteria for the diagnosis of Cushing's disease according to a nationwide survey of patients with Cushing's syndrome in Korea (2000).
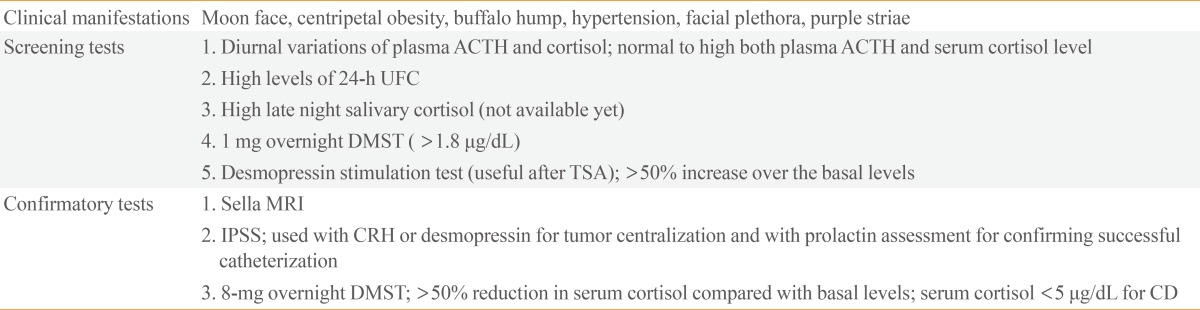
ACTH, adrenocorticotropic hormone; DMST, dexamethasone suppression test; TSA, transsphenoidal approach; MRI, magnetic resonance imaging; IPSS, Inferior petrosal sinus sampling; CRH, corticotropin-releasing hormone.
Although TSA is the first-line treatment option for CD, secondary treatments, including repeated TSA administration, should be considered in the event of recurrent or residual CD after surgery. Radiosurgery (GKS or CyberKnife) is an example of additional second-line treatment approach for remnant or recurrent tumors. Adjuvant medical treatment aims to block ACTH release at the pituitary (pasireotide, dopamine agonists) or adrenal levels (mitotane, ketoconazole, metyrapone, LCI699) while a glucocorticoid receptor antagonist (mifepristone) is generally required to correct hyperglycemia after surgery. Recently, ketoconazole is no longer available worldwide due to concerns regarding its hepatotoxicity, but a new ACTH-blocking drug, pasireotide, will soon be available to treat patients who fail or are ineligible for surgical therapy. Bilateral adrenalectomy may be considered the final treatment modality for patients with refractory CD.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367:1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 2.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimondo G, Paccotti P, Minetto M, Termine A, Stura G, Bergui M, Angeli A, Terzolo M. The corticotrophin-releasing hormone test is the most reliable noninvasive method to differentiate pituitary from ectopic ACTH secretion in Cushing's syndrome. Clin Endocrinol (Oxf) 2003;58:718–724. doi: 10.1046/j.1365-2265.2003.01776.x. [DOI] [PubMed] [Google Scholar]
- 4.Nieman LK, Chrousos GP, Oldfield EH, Avgerinos PC, Cutler GB, Jr, Loriaux DL. The ovine corticotropin-releasing hormone stimulation test and the dexamethasone suppression test in the differential diagnosis of Cushing's syndrome. Ann Intern Med. 1986;105:862–867. doi: 10.7326/0003-4819-105-6-862. [DOI] [PubMed] [Google Scholar]
- 5.Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB., Jr A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1993;77:1308–1312. doi: 10.1210/jcem.77.5.8077325. [DOI] [PubMed] [Google Scholar]
- 6.Tsagarakis S, Tsigos C, Vasiliou V, Tsiotra P, Kaskarelis J, Sotiropoulou C, Raptis SA, Thalassinos N. The desmopressin and combined CRH-desmopressin tests in the differential diagnosis of ACTH-dependent Cushing's syndrome: constraints imposed by the expression of V2 vasopressin receptors in tumors with ectopic ACTH secretion. J Clin Endocrinol Metab. 2002;87:1646–1653. doi: 10.1210/jcem.87.4.8358. [DOI] [PubMed] [Google Scholar]
- 7.Orth DN, DeBold CR, DeCherney GS, Jackson RV, Alexander AN, Rivier J, Rivier C, Spiess J, Vale W. Pituitary microadenomas causing Cushing's disease respond to corticotropin-releasing factor. J Clin Endocrinol Metab. 1982;55:1017–1019. doi: 10.1210/jcem-55-5-1017. [DOI] [PubMed] [Google Scholar]
- 8.Newell-Price J, Morris DG, Drake WM, Korbonits M, Monson JP, Besser GM, Grossman AB. Optimal response criteria for the human CRH test in the differential diagnosis of ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2002;87:1640–1645. doi: 10.1210/jcem.87.4.8357. [DOI] [PubMed] [Google Scholar]
- 9.Pecori Giraldi F, Invitti C, Cavagnini F Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone test in the diagnosis of ACTH-dependent Cushing's syndrome: a reappraisal. Clin Endocrinol (Oxf) 2001;54:601–607. doi: 10.1046/j.1365-2265.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 10.Deipolyi AR, Hirsch JA, Oklu R. Bilateral inferior petrosal sinus sampling with desmopressin. J Neurointerv Surg. 2013;5:487–488. doi: 10.1136/neurintsurg-2012-010437. [DOI] [PubMed] [Google Scholar]
- 11.Scott LV, Medbak S, Dinan TG. ACTH and cortisol release following intravenous desmopressin: a dose-response study. Clin Endocrinol (Oxf) 1999;51:653–658. doi: 10.1046/j.1365-2265.1999.00850.x. [DOI] [PubMed] [Google Scholar]
- 12.Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB., Jr A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1994;78:418–422. doi: 10.1210/jcem.78.2.8106630. [DOI] [PubMed] [Google Scholar]
- 13.Aytug S, Laws ER, Jr, Vance ML. Assessment of the utility of the high-dose dexamethasone suppression test in confirming the diagnosis of Cushing disease. Endocr Pract. 2012;18:152–157. doi: 10.4158/EP11179.OR. [DOI] [PubMed] [Google Scholar]
- 14.Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1997;82:1780–1785. doi: 10.1210/jcem.82.6.3991. [DOI] [PubMed] [Google Scholar]
- 15.Findling JW, Raff H. Diagnosis and differential diagnosis of Cushing's syndrome. Endocrinol Metab Clin North Am. 2001;30:729–747. doi: 10.1016/s0889-8529(05)70209-7. [DOI] [PubMed] [Google Scholar]
- 16.Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93:2454–2462. doi: 10.1210/jc.2007-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starke RM, Reames DL, Chen CJ, Laws ER, Jane JA., Jr Endoscopic transsphenoidal surgery for cushing disease: techniques, outcomes, and predictors of remission. Neurosurgery. 2013;72:240–247. doi: 10.1227/NEU.0b013e31827b966a. [DOI] [PubMed] [Google Scholar]
- 18.Hammer GD, Tyrrell JB, Lamborn KR, Applebury CB, Hannegan ET, Bell S, Rahl R, Lu A, Wilson CB. Transsphenoidal microsurgery for Cushing's disease: initial out come and long-term results. J Clin Endocrinol Metab. 2004;89:6348–6357. doi: 10.1210/jc.2003-032180. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing's disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 2005;63:549–559. doi: 10.1111/j.1365-2265.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 20.De Tommasi C, Vance ML, Okonkwo DO, Diallo A, Laws ER., Jr Surgical management of adrenocorticotropic hormone-secreting macroadenomas: outcome and challenges in patients with Cushing's disease or Nelson's syndrome. J Neurosurg. 2005;103:825–830. doi: 10.3171/jns.2005.103.5.0825. [DOI] [PubMed] [Google Scholar]
- 21.Feelders RA, Hofland LJ. Medical treatment of Cushing's disease. J Clin Endocrinol Metab. 2013;98:425–438. doi: 10.1210/jc.2012-3126. [DOI] [PubMed] [Google Scholar]
- 22.Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, Caron P, Luca F, Donadille B, Vantyghem MC, Bihan H, Delemer B, Raverot G, Motte E, Philippon M, Morange I, Conte-Devolx B, Quinquis L, Martinie M, Vezzosi D, Le Bras M, Baudry C, Christin-Maitre S, Goichot B, Chanson P, Young J, Chabre O, Tabarin A, Bertherat J, Brue T. Ketoconazole in Cushing's disease: is it worth a try? J Clin Endocrinol Metab. 2014;99:1623–1630. doi: 10.1210/jc.2013-3628. [DOI] [PubMed] [Google Scholar]
- 23.Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM Pasireotide B2305 Study Group. A 12-month phase 3 study of pasireotide in Cushing's disease. N Engl J Med. 2012;366:914–924. doi: 10.1056/NEJMoa1105743. [DOI] [PubMed] [Google Scholar]
- 24.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams textbook of endocrinology. 12th ed. Philadelphia: Elsevier Saunders; 2011. pp. 492–494. [Google Scholar]
- 25.Ross EJ, Linch DC. Cushing's syndrome: killing disease: discriminatory value of signs and symptoms aiding early diagnosis. Lancet. 1982;2:646–649. doi: 10.1016/s0140-6736(82)92749-0. [DOI] [PubMed] [Google Scholar]
- 26.Newell-Price J, Trainer P, Perry L, Wass J, Grossman A, Besser M. A single sleeping midnight cortisol has 100% sensitivity for the diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf) 1995;43:545–550. doi: 10.1111/j.1365-2265.1995.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 27.Newell-Price J. Diagnosis/differential diagnosis of Cushing's syndrome: a review of best practice. Best Pract Res Clin Endocrinol Metab. 2009;23(Suppl 1):S5–S14. doi: 10.1016/S1521-690X(09)70003-X. [DOI] [PubMed] [Google Scholar]
- 28.Nieman LK, Cutler GB., Jr The sensitivity of the urine free cortisol measurement as a screening test for Cushing's syndrome (abstract 822); Program of the 72nd Annual Meeting of the Endocrine Society; 1990 Jun 20-23; Atlanta, GA. Atlanta: Endocrine Society; 1990. p. 111. [Google Scholar]
- 29.Bope ET, Kellerman RD, Conn HF. Conn's current therapy 2014. Philadelphia: Elsevier Saunders; 2014. pp. 687–774. [Google Scholar]
- 30.Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM. Accuracy of diagnostic tests for Cushing's syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab. 2008;93:1553–1562. doi: 10.1210/jc.2008-0139. [DOI] [PubMed] [Google Scholar]
- 31.Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 32.Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 33.Malerbi DA, Mendonca BB, Liberman B, Toledo SP, Corradini MC, Cunha-Neto MB, Fragoso MC, Wajchenberg BL. The desmopressin stimulation test in the differential diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf) 1993;38:463–472. doi: 10.1111/j.1365-2265.1993.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 34.Moro M, Putignano P, Losa M, Invitti C, Maraschini C, Cavagnini F. The desmopressin test in the differential diagnosis between Cushing's disease and pseudo-Cushing states. J Clin Endocrinol Metab. 2000;85:3569–3574. doi: 10.1210/jcem.85.10.6862. [DOI] [PubMed] [Google Scholar]
- 35.Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing's disease. J Clin Endocrinol Metab. 2013;98:2285–2293. doi: 10.1210/jc.2012-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JS, Lee SK, Kim SH, Lee EJ, Kim SH. Intraoperative multiple-staged resection and tumor tissue identification using frozen sections provide the best result for the accurate localization and complete resection of tumors in Cushing's disease. Endocrine. 2011;40:452–461. doi: 10.1007/s12020-011-9499-5. [DOI] [PubMed] [Google Scholar]
- 37.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955–4962. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]
- 38.Lee HB, Kim ST, Kim HJ, Kim KH, Jeon P, Byun HS, Choi JW. Usefulness of the dynamic gadolinium-enhanced magnetic resonance imaging with simultaneous acquisition of coronal and sagittal planes for detection of pituitary microadenomas. Eur Radiol. 2012;22:514–518. doi: 10.1007/s00330-011-2291-3. [DOI] [PubMed] [Google Scholar]
- 39.Portocarrero-Ortiz L, Bonifacio-Delgadillo D, Sotomayor-Gonzalez A, Garcia-Marquez A, Lopez-Serna R. A modified protocol using half-dose gadolinium in dynamic 3-Tesla magnetic resonance imaging for detection of ACTH-secreting pituitary tumors. Pituitary. 2010;13:230–235. doi: 10.1007/s11102-010-0222-y. [DOI] [PubMed] [Google Scholar]
- 40.Batista D, Courkoutsakis NA, Oldfield EH, Griffin KJ, Keil M, Patronas NJ, Stratakis CA. Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with cushing disease. J Clin Endocrinol Metab. 2005;90:5134–5140. doi: 10.1210/jc.2004-1778. [DOI] [PubMed] [Google Scholar]
- 41.Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, Nieman LK. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. 2003;88:1565–1569. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 42.Doppman JL, Oldfield E, Krudy AG, Chrousos GP, Schulte HM, Schaaf M, Loriaux DL. Petrosal sinus sampling for Cushing syndrome: anatomical and technical considerations. Work in progress. Radiology. 1984;150:99–103. doi: 10.1148/radiology.150.1.6316418. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield EH, Chrousos GP, Schulte HM, Schaaf M, McKeever PE, Krudy AG, Cutler GB, Jr, Loriaux DL, Doppman JL. Preoperative lateralization of ACTH-secreting pituitary microadenomas by bilateral and simultaneous inferior petrosal venous sinus sampling. N Engl J Med. 1985;312:100–103. doi: 10.1056/NEJM198501103120207. [DOI] [PubMed] [Google Scholar]
- 44.Deipolyi AR, Hirsch JA, Oklu R. Bilateral inferior petrosal sinus sampling. J Neurointerv Surg. 2012;4:215–218. doi: 10.1136/neurintsurg-2011-010033. [DOI] [PubMed] [Google Scholar]
- 45.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 46.Findling JW, Kehoe ME, Raff H. Identification of patients with Cushing's disease with negative pituitary adrenocorticotropin gradients during inferior petrosal sinus sampling: prolactin as an index of pituitary venous effluent. J Clin Endocrinol Metab. 2004;89:6005–6009. doi: 10.1210/jc.2004-1378. [DOI] [PubMed] [Google Scholar]
- 47.Sharma ST, Raff H, Nieman LK. Prolactin as a marker of successful catheterization during IPSS in patients with ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96:3687–3694. doi: 10.1210/jc.2011-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batista DL, Zhang X, Gejman R, Ansell PJ, Zhou Y, Johnson SA, Swearingen B, Hedley-Whyte ET, Stratakis CA, Klibanski A. The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J Clin Endocrinol Metab. 2006;91:4482–4488. doi: 10.1210/jc.2006-1245. [DOI] [PubMed] [Google Scholar]
- 49.Pivonello R, Ferone D, de Herder WW, Kros JM, De Caro ML, Arvigo M, Annunziato L, Lombardi G, Colao A, Hofland LJ, Lamberts SW. Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab. 2004;89:2452–2462. doi: 10.1210/jc.2003-030837. [DOI] [PubMed] [Google Scholar]
- 50.Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A. The medical treatment of Cushing's disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. 2009;94:223–230. doi: 10.1210/jc.2008-1533. [DOI] [PubMed] [Google Scholar]
- 51.Dillard TH, Gultekin SH, Delashaw JB, Jr, Yedinak CG, Neuwelt EA, Fleseriu M. Temozolomide for corticotroph pituitary adenomas refractory to standard therapy. Pituitary. 2011;14:80–91. doi: 10.1007/s11102-010-0264-1. [DOI] [PubMed] [Google Scholar]
- 52.Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J, Abbas H, Legmann P, Bertagna X, Bertherat J. Efficiency and tolerance of mitotane in Cushing's disease in 76 patients from a single center. Eur J Endocrinol. 2012;167:473–481. doi: 10.1530/EJE-12-0358. [DOI] [PubMed] [Google Scholar]
- 53.Fleseriu M. Medical management of persistent and recurrent cushing disease. Neurosurg Clin N Am. 2012;23:653–668. doi: 10.1016/j.nec.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Preda VA, Sen J, Karavitaki N, Grossman AB. Etomidate in the management of hypercortisolaemia in Cushing's syndrome: a review. Eur J Endocrinol. 2012;167:137–143. doi: 10.1530/EJE-12-0274. [DOI] [PubMed] [Google Scholar]
- 55.Bertagna X, Pivonello R, Fleseriu M, Zhang Y, Robinson P, Taylor A, Watson CE, Maldonado M, Hamrahian AH, Boscaro M, Biller BM. LCI699, a potent 11beta-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab. 2014;99:1375–1383. doi: 10.1210/jc.2013-2117. [DOI] [PubMed] [Google Scholar]
- 56.Castinetti F, Brue T, Conte-Devolx B. The use of the glucocorticoid receptor antagonist mifepristone in Cushing's syndrome. Curr Opin Endocrinol Diabetes Obes. 2012;19:295–299. doi: 10.1097/MED.0b013e32835430bf. [DOI] [PubMed] [Google Scholar]
- 57.Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, Zelissen PM, van Heerebeek R, de Jong FH, van der Lely AJ, de Herder WW, Hofland LJ, Lamberts SW. Pasireotide alone or with cabergoline and ketoconazole in Cushing's disease. N Engl J Med. 2010;362:1846–1848. doi: 10.1056/NEJMc1000094. [DOI] [PubMed] [Google Scholar]
- 58.Vilar L, Naves LA, Azevedo MF, Arruda MJ, Arahata CM, Moura ESL, Agra R, Pontes L, Montenegro L, Albuquerque JL, Canadas V. Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing's disease. Pituitary. 2010;13:123–129. doi: 10.1007/s11102-009-0209-8. [DOI] [PubMed] [Google Scholar]
- 59.Kamenicky P, Droumaguet C, Salenave S, Blanchard A, Jublanc C, Gautier JF, Brailly-Tabard S, Leboulleux S, Schlumberger M, Baudin E, Chanson P, Young J. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96:2796–2804. doi: 10.1210/jc.2011-0536. [DOI] [PubMed] [Google Scholar]
- 60.Estrada J, Boronat M, Mielgo M, Magallon R, Millan I, Diez S, Lucas T, Barcelo B. The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing's disease. N Engl J Med. 1997;336:172–177. doi: 10.1056/NEJM199701163360303. [DOI] [PubMed] [Google Scholar]
- 61.Castinetti F, Nagai M, Dufour H, Kuhn JM, Morange I, Jaquet P, Conte-Devolx B, Regis J, Brue T. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing's disease. Eur J Endocrinol. 2007;156:91–98. doi: 10.1530/eje.1.02323. [DOI] [PubMed] [Google Scholar]
- 62.Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf) 1994;40:479–484. doi: 10.1111/j.1365-2265.1994.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 63.Dekkers OM, Biermasz NR, Pereira AM, Roelfsema F, van Aken MO, Voormolen JH, Romijn JA. Mortality in patients treated for Cushing's disease is increased, compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007;92:976–981. doi: 10.1210/jc.2006-2112. [DOI] [PubMed] [Google Scholar]
- 64.Bertagna X, Guignat L, Groussin L, Bertherat J. Cushing's disease. Best Pract Res Clin Endocrinol Metab. 2009;23:607–623. doi: 10.1016/j.beem.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing's disease: clinical and health-related quality of life aspects. Eur J Endocrinol. 2012;167:311–326. doi: 10.1530/EJE-11-1095. [DOI] [PubMed] [Google Scholar]
- 66.Faggiano A, Pivonello R, Filippella M, Di Somma C, Orio F, Jr, Lombard G, Colao A. Spine abnormalities and damage in patients cured from Cushing's disease. Pituitary. 2001;4:153–161. doi: 10.1023/a:1015362822901. [DOI] [PubMed] [Google Scholar]
- 67.Biering H, Knappe G, Gerl H, Lochs H. Prevalence of diabetes in acromegaly and Cushing syndrome. Acta Med Austriaca. 2000;27:27–31. doi: 10.1046/j.1563-2571.2000.200106.x. [DOI] [PubMed] [Google Scholar]
- 68.Taskinen MR, Nikkila EA, Pelkonen R, Sane T. Plasma lipoproteins, lipolytic enzymes, and very low density lipoprotein triglyceride turnover in Cushing's syndrome. J Clin Endocrinol Metab. 1983;57:619–626. doi: 10.1210/jcem-57-3-619. [DOI] [PubMed] [Google Scholar]
- 69.Melanson KJ, McInnis KJ, Rippe JM, Blackburn G, Wilson PF. Obesity and cardiovascular disease risk: research update. Cardiol Rev. 2001;9:202–207. doi: 10.1097/00045415-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, Marzullo P, Cerbone G, Siciliani M, Lombardi G. Persistence of increased cardiovascular risk in patients with Cushing's disease after five years of successful cure. J Clin Endocrinol Metab. 1999;84:2664–2672. doi: 10.1210/jcem.84.8.5896. [DOI] [PubMed] [Google Scholar]


