Abstract
Background
With the goal of improving clinical efficiency and effectiveness, programs to enhance care coordination are a major focus of health care reform.
Objective
To examine whether “care density”—a claims-based measure of patient sharing by office-based physicians—is associated with measures of quality. Care density is a proxy measure that may reflect how frequently a patient’s doctors collaborate.
Research Design
Cohort study using administrative databases from 3 large commercial insurance plans
Subjects
1.7 million adult patients; 31,675 with congestive heart failure, 78,530 with chronic obstructive pulmonary disease, and 240,378 with diabetes
Measures
Care density was assessed in 2008. Prevention Quality Indicators (PQIs), 30-day readmissions, and HEDIS quality indicators were measured in the following year.
Results
Among all patients, we found that patients with the highest care density density—indicating high levels of patient-sharing among their office-based physicians— had significantly lower rates of adverse events measured as PQIs compared to patients with low care density (Odds Ratio [OR] 0.88, 95% Confidence Interval [CI] 0.85–0.92). A significant association between care density and PQIs was also observed for patients with DM but not CHF or COPD. Diabetic patients with higher care density scores had significantly lower odds of 30-day readmissions (OR 0.68, 95%CI 0.48–0.97). Significant associations were observed between care density and HEDIS measures though not always in the expected direction.
Conclusions
In some settings, patients whose doctors share more patients had lower odds of adverse events and 30-day readmissions.
Keywords: Care coordination, performance measure, provider social networks, care density
INTRODUCTION
Care continuity and coordination has received increased attention as an important way to improve quality and to reduce costs. Multiple provisions of the Affordable Care Act (ACA) seek to align incentives to improve care coordination, through Accountable Care Organizations (ACOs), hospital incentive payments, bundled payments, and patient centered medical homes (PCMH).1, 2 A common objective of these programs is to encourage providers to improve processes of care, to provide patients with timely primary and preventive care, and to reduce spending on services such as potentially avoidable emergency care and hospital readmissions.3, 4
Pay for performance programs focus on several domains of care quality and health outcomes. Under the ACA, Medicare has begun reducing payments to hospitals with excess 30-day readmissions for index hospitalizations related to heart attacks, heart failure, or pneumonia.5 The ACA also supports the development of Accountable Care Organizations, whose aim is to improve the coordination of care, such as disease testing and management, across a range of provider settings.6, 7 Starting in 2015, Medicare will begin paying providers for chronic care management to assist with coordinating services for patients with multiple chronic conditions.8 Similar private-sector initiatives have emerged, with the aim of strengthening providers’ incentives to improve care quality, while reducing high-cost and potentially unnecessary care.9
The underlying premise of these programs is that physicians who coordinate services across different health care providers can promote a more efficient use of health services while improving health outcomes. It is difficult, however, to measure coordination and communication among providers on a large scale using available administrative data.10, 11 To help address this need, we previously developed a novel metric we term ‘care density,’ which uses health insurance claims data to measure how often a patient’s doctors share patients with one another.12, 13 Evidence suggests that doctors who frequently share patients in claims data (e.g. doctors who bill for the same patient’s care) are more likely to be part of a provider’s ‘social network’—they are more likely to obtain clinical advice and refer to one another.14 Sharing advice and referrals may reflect increased communication between providers and lead to more coordinated care.15 For example, among patients with congestive heart failure and diabetes, we found that patients who were treated by doctors who share a relatively large number of patients (i.e., had higher care density) tended to have lower total and inpatient costs of care and were less likely to have a hospitalization.12 Similarly, cancer survivors treated by doctors who shared more patients tended to have lower costs, and to receive (on some measures) a higher quality of care.13
This suggests that the care density indicator may be a useful measure of aspects of care coordination. To offer insights to policymakers, payers, and providers, an objective of our research is to gain an understanding of how variation in care density relates to process and quality measures for patients treated in the office-based settings. First, we investigate whether care density is correlated with delivery and outcome measures that are the focus of health reform initiatives. Second, we provide guidance about the value of using claims data to measure the density of provider patient-sharing networks as an indicator of levels of communication and integration.
Specifically, building on our prior work, this study examines whether higher care density predicts performance quality indicators in a general cohort of adults, and in subgroups of adults with congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and diabetes mellitus (DM). We examine 30-day hospital readmissions, quality indicators that measure the occurrence of potentially preventable inpatient admissions (termed “Prevention Quality Indicators”), and HEDIS quality measures related to disease screening and management.
METHODS
Data Sources
Administrative databases from 3 large commercial insurance plans were the primary data source. Plans were located in separate Census regions and ranged in size from 629,735 to 3,367,942 members. All plans were obtained from the IMS Health Plan Claims Database and represent a range of different product types including employer-sponsored insurance and Medicare Advantage. Because we calculated care density and all covariates from 2008 claims and examined outcomes in 2009, patients were required to be continuously enrolled for the two years.
We included all patients age 40 and older in order to create a more homogenous cohort as well as particular subsets of these patients: those with CHF, COPD, and DM. Diagnoses were based on Expanded Diagnostic Clusters (EDCs) as part of the Johns Hopkins Adjusted Clinical Group (ACG) Case-Mix Assessment System (version 9.0.1).
This study was exempted by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Quality metrics
Prevention Quality Indicators (PQI)
PQIs refer to episodes that may be potentially avoided through the timely receipt of primary or preventive care (see Appendix Table 1).16 We included a composite measure of having any versus no PQIs in 2009. In this formulation, having a PQI is considered a marker for worse care. We further included specific PQIs for CHF, COPD, and DM (which consisted of short-term complications, long-term complications, uncontrolled diabetes, and amputations).
HEDIS quality indicators
We used measures from the Healthcare Effectiveness Data and Information Set (HEDIS) to measure quality of care delivered in 2009.17 In the entire cohort, we included breast cancer screening (women age 42–69), cervical cancer screening (women age 40–64), and colon cancer screening (adults age 51–75). From each measure, patients are excluded if they are ineligible for screening using 2008 claims as described in Appendix Table 2 (e.g., if they have a history of the particular cancer type). Among patients with DM, we examined receipt of an eye exam, hemoglobin A1c testing, and low-density lipoprotein (LDL) testing in 2009.
Hospital readmissions within 30 days
We included the first hospital admission for patients admitted between January 1 and November 30, 2009 and examined whether each patient was readmitted within 30 days of discharge. Hospitals are increasingly being penalized for excessive readmissions, a potential indicator of poor quality.5
Care Density
Care density measures the extent of ‘patient-sharing’ among an individual’s ambulatory providers. The numerator of care density is the sum of shared patients among each pair of a patient’s outpatient doctors, and the denominator is the total number of pairs of outpatient doctors that a patient sees (see Appendix Figure 1 for an example). Care density was calculated using 2008 data.
A pair of doctors is considered to have shared a patient if they both billed for outpatient evaluation and management visits for a given patient. We excluded non-MD/DO providers (e.g., midwifes, podiatrists) and specialists who were unlikely to have direct patient contact (i.e. radiologists). The number of shared patients between a pair of providers was top-coded at 10 patients, which represents the 98th percentile of the distribution. Care density was calculated for each patient using all ambulatory visits and providers. Because physician identifiers were different between insurers, care density was calculated separately for each insurer.
Covariates
Age and gender were determined from the health plan membership files. Comorbidity was assessed using 32 different Aggregated Diagnostic Groups™ (ADGs) from the Johns Hopkins ACG® Case-Mix Assessment System.18 The count of different ADGs (morbidity types) for each patient was used as a measure of comorbidity.
We included a dummy indicator for each of the three different insurance plans. Across each plan, insurance products were classified as commercial, Medicare Advantage, and other/unknown payer. Insurance products were further designated according to their provider networks as being a HMO, PPO, and other/unknown. Because the number of outpatient visits may reflect comorbidity and influence the total number of doctors a patient has the opportunity to see, we included a variable for the total ambulatory visit count. We further included an indicator that a patient had at least one ambulatory visit with a primary care provider given the known associations with costs and outcomes and a variable for the usual provider of care (UPC), a commonly used measure of care fragmentation.11 UPC represents the percentage of outpatient visits to a patient’s plurality provider.
Statistical Analyses
We performed bivariate analyses to examine the association between care density and quality measures. Care density was categorized in plan-specific tertiles based on the sample distribution due to its non-linear distribution and in accordance with prior studies.12, 13 Care density cannot be calculated for patients who visit only a single doctor; we opted to retain these patients in a separate category for our analyses. Multivariable logistic regression models were constructed to assess whether care density was correlated with quality measures, adjusting for all patient and insurance-related factors (age, gender, comorbidity, insurance plan and product, number of outpatient visit, number of providers, whether the patient saw a PCP, and percentage of visits with the usual provider of care). Models were run separately for each quality measure.
We performed multiple sensitivity analyses. First, we examined patient-sharing among a more limited set of physicians who we hypothesized may be more important to care coordination and outcomes for patients with chronic disease. For CHF, we included primary care providers (internal medicine without subspecialty training, family practice, and general practice) and cardiologists. In one plan, visits to these providers comprised 88% of all cardiac-related visits and 63% of all visits. For COPD, we included primary care providers, pulmonologists, and cardiologists (87% of all pulmonary related visits and 66% of all visits). For DM, we included primary care providers, endocrinologists, cardiologists, and ophthalmologists (93% of all endocrine-related visits and 73% of all visits). Second, we tested whether our models were robust to different specifications of comorbidity. Specifically, we replaced the number of ADGs with the specific indicators for the 100 most frequent comorbidities in our study cohort. Third, because care density may have a differential impact among patients with high and low comorbidity, we tested for an interaction between comorbidity (divided into tertiles) and care density. Fourth, we excluded patients who had high comorbidity (ADG count ≥ 9) when calculating care density among patients with lower comorbidity. Because these high comorbidity patients may experience fragmented care, sharing high comorbidity patients may be less indicative that a pair of doctors knows one another. Lastly, we recalculated care density including both inpatient and outpatient providers.
RESULTS
Table 1 presents the descriptive characteristics of patients overall, and with DM, CHF, and COPD. Over 1.7 million patients are included in the overall sample, 31,675 in the CHF cohort, 78,530 with COPD and 240,378 with DM. The mean age was highest in the CHF sample (71.7) and lowest in the overall cohort (58.7). The majority of enrollees were in commercial payers (>90%) and approximately half were in PPO plans. Bivariate analyses showing the association between care density and patient characteristics are found in Appendix Table 3.
Table 1.
Descriptive characteristics of the overall study population and for patients with CHF, COPD, and DM.
| All patients |
CHF | COPD | DM | |
|---|---|---|---|---|
| N | 1,704,616 | 31,675 | 78,530 | 240,378 |
| Gender, % female | 59.8% | 44.5% | 52.5% | 49.4% |
| Age, mean (SD) | 58.7 (11.9) | 71.7 (11.4) | 65.8 (11.6) | 62.9 (11.2) |
| Number of ADGs, mean (SD) | 7.83 (3.55) | 10.98 (3.89) | 10.55 (3.81) | 8.73 (3.77) |
| Payer type distribution | ||||
| Commercial | 95.3% | 90.3% | 90.5% | 92.7% |
| Medicare | 4.2% | 8.5% | 8.5% | 6.5% |
| other/unknown | 0.5% | 1.2% | 1.0% | 0.8% |
| Product type distribution | ||||
| HMO | 5.4% | 2.2% | 4.3% | 4.7% |
| PPO | 52.9% | 50.5% | 48.6% | 51.7% |
| other/unknown | 41.7% | 47.3% | 47.1% | 43.6% |
| Mean number of MD/DOs seen during 2008 (SD) | 3.49 (1.81) | 4.93 (2.56) | 4.42 (2.35) | 4.06 (2.12) |
| Saw a Primary Care Provider during 2008, % | 81.6% | 78.5% | 82.8% | 82.2% |
| Percent of visits with usual provider of care during 2008, mean (SD) | 63.3 (25.4) | 53.5 (22.6) | 58.2 (24.2) | 60.4 (24.5) |
Tables 2 and 3 show the bivariate analyses between care density and outcomes. We observed significant differences in rates of 30-day rehospitalization among all patients (ranging from 5.0% among patients in the high care density tertile and 5.8% among patients in the low care density) and those with diabetes (5.9% in the high care density, 6.3% in the middle, and 6.2% in the low care density). For each patient cohort, the lowest rates of the PQI composite measures were found among patients in the high care density tertile. Significant differences were also observed among the disease-specific PQIs for patients with COPD and DM. With regard to HEDIS measures, we observed significant variation in rates of cancer screening across care density tertiles with the highest unadjusted rates of breast cancer screening among patients in the middle tertile and the lowest rates of cervical cancer screening among patients in the high tertile. There was also significant variation in DM HEDIS quality indicators. Contrary to our expectations, the lowest rates of hemoglobin A1c and LDL testing were observed among patients in the high care density tertile, and the highest rates of eye exams were found among patients in the middle care density tertile.
Table 2.
Bivariate analyses showing the association between care density and hospitalizations, 30-day readmissions, and Prevention Quality Indicators (PQIs) for the overall population and for patients with CHF, COPD, and DM
| All patients | CHF | COPD | DM | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Care Density Tertile | Care Density Tertile | Care Density Tertile | Care Density Tertile | |||||||||||||
| Low | Middle | High | p- value† |
Low | Middle | High | p- value† |
Low | Middle | High | p- value† |
Low | Middle | High | p- value† |
|
| N | 378,073 | 405,499 | 389,421 | 7,837 | 10,612 | 7,414 | 18,734 | 23,723 | 17,705 | 51,620 | 65,509 | 56,681 | ||||
| Mean care density (SD) | 1.61 (0.59) | 4.26 (0.95) | 8.78 (1.40) | <.001 | 1.77 (0.60) | 4.27 (0.94) | 8.31 (1.43) | <.001 | 1.72 (0.59) | 4.27 (0.95) | 8.48 (1.43) | <.001 | 1.70 (0.60) | 4.27 (0.94) | 8.63 (1.43) | <.001 |
| Hospitalization, % | 10.9% | 10.6% | 8.9% | <.001 | 36.2% | 36.8% | 33.9% | <.001 | 24.4% | 25.4% | 22.0% | <.001 | 17.5% | 17.8% | 14.1% | <.001 |
| 30-day rehospitalization, %* | 5.8% | 5.5% | 5.0% | <.001 | 7.0% | 6.5% | 6.6% | .772 | 6.9% | 6.3% | 5.9% | .177 | 6.2% | 6.3% | 5.9% | .029 |
| PQI Composite, %^ | 1.2% | 1.3% | 1.0% | <.001 | 6.1% | 6.1% | 5.6% | .295 | 3.6% | 3.7% | 3.0% | <.001 | 2.3% | 2.2% | 1.6% | <.001 |
| PQI Disease-Composite, % | n/a | n/a | n/a | 3.9% | 3.8% | 3.7% | .860 | 0.5% | 0.3% | 0.3% | .004 | 1.6% | 1.5% | 1.1% | <.001 | |
Among patients who had at least 1 hospital admission
See Appendix Table 1 for listing of PQIs. Note that lower scores are better.
Table 3.
Bivariate analyses showing the association between care density and HEDIS measures for the overall population and for patients with Diabetes
| Care Density Tertile | ||||
|---|---|---|---|---|
| Low | Middle | High | p-value† | |
| All patients | ||||
| Breast Cancer Screening, % | 52.9% | 56.6% | 54.5% | <.001 |
| Cervical Cancer Screening, % | 35.6% | 34.3% | 28.9% | <.001 |
| Colon Cancer Screening, % | 11.7% | 12.4% | 11.4% | <.001 |
| Diabetes | ||||
| HbA1c Test, % | 41.8% | 40.8% | 39.3% | <.001 |
| LDL Test, % | 38.7% | 38.1% | 36.5% | <.001 |
| Eye Exam, % | 30.0% | 33.8% | 31.4% | <.001 |
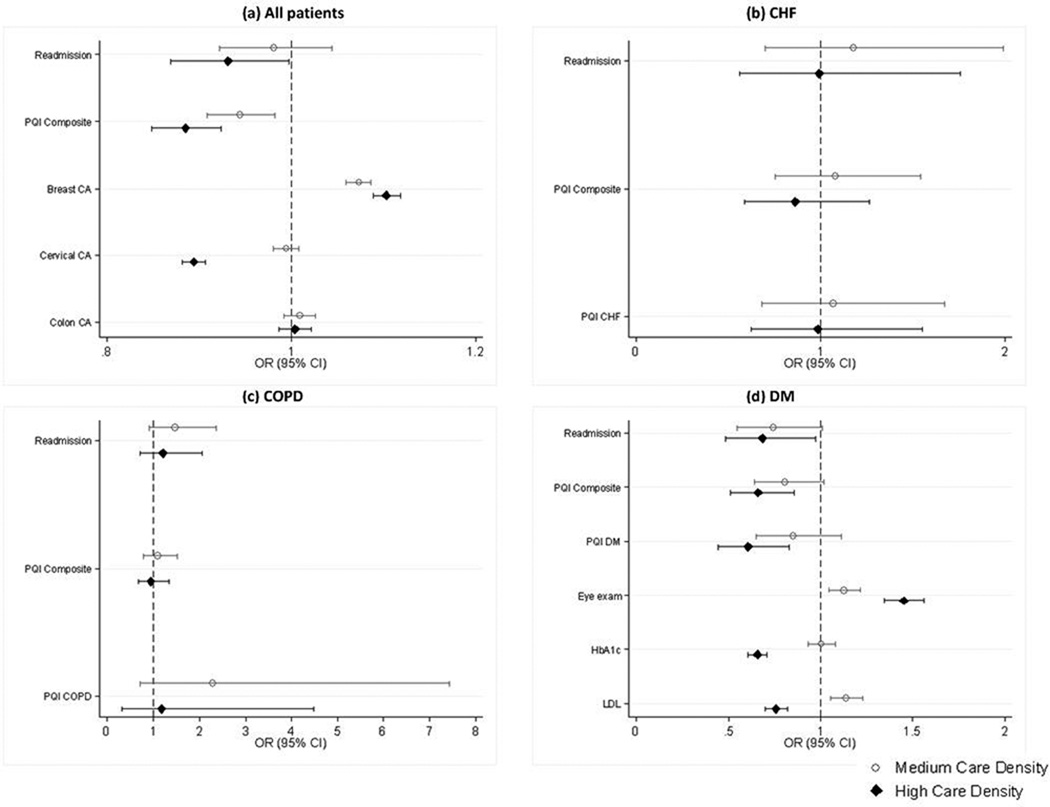
Figure 1 presents adjusted estimates of the association between care density and quality measures. Among all patients (Figure 1, panel a), we found that patients with the highest care density had significantly lower PQIs compared to patients with low care density (Odds Ratio [OR] 0.88, 95% Confidence Interval [CI] 0.85–0.92). There was no significant association between care density and rehospitalization. For the HEDIS measures, high care density was associated with higher odds of breast cancer screening (OR 1.10, 95%CI 1.08–1.11), lower odds of cervical cancer screening (OR 0.89, 95%CI 0.88–0.90) and no significant difference in colon cancer screening.
Figure 1.
Multivariable logistic regression analyses showing the association between care density and outcomes for (a) the entire cohort and patients with (b) CHF, (c) COPD, and (d) DM. The comparison group contains patients in the lowest tertile of care density. Analyses are adjusted for age, gender (except breast and cervical cancer screening), insurance plan and product type, comorbidity (ADGs), number of outpatient visits, number of providers, whether the patients saw a PCP, and the percentage of visits with the usual provider of care.
(Note: See Appendix Table 4 for detailed logistic regression results.)
For CHF and COPD, care density was not associated with readmissions, the PQI composite indicator, or the disease-specific PQI composite indicator (Figure 1, panels b and c). For patients with DM (figure 1, panel d), those with high care density had significantly lower odds of the 30-day readmission (OR 0.68, 95%CI 0.48–0.97), composite PQI (OR 0.66, 95%CI 0.51–0.85) and DM-specific PQI (OR 0.61, 95%CI 0.44–0.83) compared to patients with low care density. High care density was associated with higher odds of receiving an eye exam (OR 1.45, 95%CI 1.35–1.56) but lower odds of appropriate laboratory testing (OR for HbA1c 0.66, 95%CI 0.0.61–0.71; OR for LDL 0.76, 95%CI 0.70–0.82).
Qualitatively similar results were found when using a more limited set of providers for patients with the three diseases (Appendix Table 4), when we performed analyses separately by insurance plan (results not shown for confidentiality reasons), and when we replaced comorbidity count for specific comorbidity indicators (Appendix Table 5). We did find evidence of an interaction effect in which the impact of care density varied by patient comorbidity (Appendix Table 6). When we recalculated care density excluding patients with high comorbidity, we observed 95% of patients remained in the same care density tertile as before. Similarly, including both inpatient and outpatient providers in the construction of care density, 94% of patients remained in the same care density tertile.
DISCUSSION
Among a large sample of privately insured adults overall and among patients with DM, seeing office-based providers who share more patients with one another was associated with reduced rates of 30-day readmissions and lower odds of potentially avoidable complications (PQIs) in the subsequent year. Patient sharing as measured by care density was associated with some measures of quality, though not always in the expected direction; it was not associated with 30-day readmissions or potentially avoidable complications among patients with CHF or COPD. The results raise a number of important questions about the potential for using patient sharing to assess care coordination.
First, why might the association between patient sharing and outcomes differ across patient cohorts? CHF, COPD, and DM were selected because care coordination was postulated to be important in management and shown to be associated with disease costs.19 While it is unknown why the associations would vary across cohorts, it is possible that different types of provider relationships and aspects of continuity may be important.15 For example, the connections between inpatient and outpatient providers may be particularly important for readmissions for some patients whereas the care density measure used in this study focused on outpatient providers. Moreover, the all patient cohort and those with DM tended to have lower rates of composite PQIs and somewhat lower rates of readmissions. The inability to adequately adjust for disease severity (i.e. ejection fraction in CHF and peak flow in COPD) may have differentially impacted the associations with outcomes.
Second, why did we observe varying results for HEDIS quality measures? For the all patient cohort, we observed a positive association between care density and breast cancer screening and negative association with cervical cancer. Guidelines often recommend yearly or biennial breast cancer screening whereas cervical cancer screening is often recommended every 3 to 5 years, depending on past results and the use of human papillomavirus (HPV) co-testing.20 To the extent that cervical cancer screening is often performed yearly, this may represent overuse.21 In exploratory analyses, we added a variable for whether a patient had cervical cancer screening (baseline screening). We continued to find a significant negative association between high care density and screening, though the point estimate was reduced (OR 0.95, 95%CI 0.93–0.96). Our prior analysis of cancer survivors similarly measured diabetes quality metrics and found a significant association between high care density and greater odds of eye examination.13 In contrast to the present study which found that high care density was associated with lower rates of laboratory testing, we did not observe a significant association between care density and hemoglobin A1c testing among diabetic cancer survivors. The reasons for these discordant findings warrant close attention and may reflect, in part, that the previous study focused on older cancer survivors with fee-for-service Medicare as opposed to privately insured adults in the present study. Further work is needed to validate care density in a range of populations.
Importantly, though care density may reflect relationships between physicians, it is likely impacted by multiple factors including the structure of insurance networks (e.g., narrow versus broad networks), the structure of health care organizations (e.g., hospital affiliation, large or small practice size, use of an electronic health record), area-level effects (e.g., the number of physicians within a given area, the intensity of resource utilization within an area22), physicians’ propensity to refer (which has been shown to vary widely based on training, patient panels, and other factors23, 24), and characteristics of the underlying patient panels (e.g., extent of comorbidity). Investigating the extent to which care density may be affected by each of these factors and how they may influence the association between care density and outcomes may be promising next steps.
This paper has several limitations. First, as noted above, though we account for severity using morbidity types (ADGs), it is possible that there may be unobserved confounding by disease severity. Related to this, we do not have measures of family history which may impact cancer screening recommendations. Second, as noted above, we are unable to account for structural features of the relationships between providers. Third, our construction of quality metrics using claims data may be prone to measurement error. Fourth, we are unable to account for patient race/ethnicity and socioeconomic status. We are further unable to examine geographic features which may limit the availability providers and be associated with care density. Fifth, our data is from large insurers and may not be generalizable to smaller insurers. Related to this, we were unable to link providers across insurers (thus we created separate measures of care density for each of the three insurers). Each insurer likely represents a somewhat limited subset of a physician’s practice and we likely underestimate the amount of shared patients between a pair of doctors. Using all-payer datasets or incorporating data on structural features of a physician’s practice are potential next steps.
In conclusion, our results point to some significant associations between care density and 30 day readmissions, PQIs, and HEDIS quality metrics. Work is needed to further validate the impact of patient sharing in specific populations to gain a fuller understanding of which aspects of care coordination may be reflected by this care density metric and for which populations. If validated, this measure may present a useful tool to help identify patients at risk for poor outcomes and for facilitating improved communication and other interventions in support of better care coordination.
Supplementary Material
Acknowledgments
Funders: Dr. Pollack’s salary was supported by a career development award from the NIH National Cancer Institute and Office of Behavioral and Social Sciences Research (1K07CA151910-01A1). The funder had no role in the design and conduct off the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
This work was performed with support by faculty and staff at The Johns Hopkins University, where the ACG method was developed and is maintained. To help support research and development, The Johns Hopkins University receives royalties from health plans and other organizations that use the ACG software. A version of care density is included in the ACG software.
Footnotes
Conflict of Interest: The authors report no other conflict of interest.
REFERENCES
- 1.Meyers D, Peikes D, Genevro J, et al. The Roles of Patient-Centered Medical Homes and Accountable Care Organizations in Coordinating Patient Care. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [Google Scholar]
- 2.Chen C, Ackerly D. Beyond acos and bundled payments: Medicare’s shift toward accountability in fee-for-service. JAMA. 2014;311:673–674. doi: 10.1001/jama.2014.11. [DOI] [PubMed] [Google Scholar]
- 3.Orszag PR, Emanuel EJ. Health Care Reform and Cost Control. New England Journal of Medicine. 2010;363:601–603. doi: 10.1056/NEJMp1006571. [DOI] [PubMed] [Google Scholar]
- 4.Bodenheimer T. Coordinating care--a perilious journey through the health care system. N Engl J Med. 2008;358:1064–1071. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 5.Kocher RP, Adashi EY. Hospital readmissions and the affordable care act: Paying for coordinated quality care. JAMA. 2011;306:1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 6.Berwick DM. Launching Accountable Care Organizations — The Proposed Rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364:e32. doi: 10.1056/NEJMp1103602. [DOI] [PubMed] [Google Scholar]
- 7.Ginsburg PB. Spending to Save — ACOs and the Medicare Shared Savings Program. N Engl J Med. 2011;364:2085–2086. doi: 10.1056/NEJMp1103604. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. [accessed December 9, 2014];Fact sheets: Proposed policy and payment changes to the Medicare Physician Fee Schedule for Calendar Year 2015. Available from URL: http://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2014-Fact-sheets-items/2014-07-03-1.html.
- 9.McWilliams J, Landon BE, Chernew ME. CHanges in health care spending and quality for medicare beneficiaries associated with a commercial aco contract. JAMA. 2013;310:829–836. doi: 10.1001/jama.2013.276302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald K, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 11.Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006;63:158–188. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- 12.Pollack CE, Weissman G, Lemke KW, Hussey PS, Weiner JP. Patient sharing among physicians and costs of care: a netework analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–465. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack C, Frick K, Herbert R, et al. It’s who you know: patient-sharing, quality, and costs of cancer survivorship care. J Cancer Surviv. 2014;8:156–166. doi: 10.1007/s11764-014-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett ML, Landon BE, O'Malley AJ, Keating NL, Christakis NA. Mapping Physician Networks with Self-Reported and Administrative Data. Health Serv Res. 2011;46:1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saultz JW. Defining and Measuring Interpersonal Continuity of Care. Ann Fam Med. 2003;1:134–143. doi: 10.1370/afm.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agency for Health Care Research and Quality. [accessed July 14, 2014];Prevention Quality Indicators Technical Specifications - Version 4.5. 2014 Available from URL: http://www.qualityindicators.ahrq.gov/modules/PQI_TechSpec.aspx.
- 17.NCQA. [accessed July 14, 2014];HEDIS Measures. Available from URL: http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures.aspx.
- 18.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and Application of a Population-Oriented Measure of Ambulatory Care Case-Mix. Med Care. 1991;29:452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Hussey PS, Schneider EC, Rudin RS, Fox D, Lai J, Pollack C. Continuity and the costs of care for chronic disease. JAMA Int Med. 2014;174:742–748. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;156:880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 21.Mathias JS, Gossett D, Baker DW. Use of electronic health record data to evaluate overuse of cervical cancer screening. J Am Med Inform Assn. 2012;19:e96–e101. doi: 10.1136/amiajnl-2011-000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirovich B, Gallagher PM, Wennberg DE, Fisher ES. Discretionary Decision Making By Primary Care Physicians And The Cost Of U.S. Health Care. Health Aff. 2008;27:813–823. doi: 10.1377/hlthaff.27.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra A, Forrest CB, Lin CY. Dropping the Baton: Specialty Referrals in the United States. Milbank Q. 2011;89:39–68. doi: 10.1111/j.1468-0009.2011.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrest CB, Nutting PA, von Schrader S, Rohde C, Starfield B. Primary Care Physician Specialty Referral Decision Making: Patient, Physician, and Health Care System Determinants. Med Decis Making. 2006;26:76–85. doi: 10.1177/0272989X05284110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.