Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disorder with unknown cause and no effective treatment. The incidence of and mortality from IPF increase with age, suggesting that advanced age is a major risk factor for IPF. The mechanism underlying the increased susceptibility of the elderly to IPF, however, is unknown. In this study, we show for the first time that the protein level of plasminogen activator inhibitor 1 (PAI-1), a protease inhibitor which plays an essential role in the control of fibrinolysis, was significantly increased with age in mouse lung homogenate and lung fibroblasts. Upon bleomycin challenge, old mice experienced augmented PAI-1 induction and lung fibrosis as compared to young mice. Most interestingly, we show that fewer (myo)fibroblasts underwent apoptosis and more (myo)fibroblasts with increased level of PAI-1 accumulated in the lung of old than in young mice after bleomycin challenge. In vitro studies further demonstrate that fibroblasts isolated from lungs of old mice were resistant to H2O2 and tumor necrosis factor alpha-induced apoptosis and had augmented fibrotic responses to TGF-β1, compared to fibroblasts isolated from young mice. Inhibition of PAI-1 activity with a PAI-1 inhibitor, on the other hand, eliminated the aging-related apoptosis resistance and TGF-β1 sensitivity in isolated fibroblasts. Moreover, we show that knocking down PAI-1 in human lung fibroblasts with PAI-1 siRNA significantly increased their sensitivity to apoptosis and inhibited their responses to TGF-β1. Together, the results suggest that increased PAI-1 expression may underlie the aging-related sensitivity to lung fibrosis in part by protecting fibroblasts from apoptosis.
Keywords: Aging, Plasminogen activator inhibitor 1, Idiopathic Pulmonary Fibrosis, Fibroblast apoptosis
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive fatal lung disorder affecting approximately 85,000 Americans (Raghu and others 2006). The incidence of IPF increases with advanced age (Casanova and others 2009; Fell and others 2010; Fernandez Perez and others 2010; Raghu and others 2006) and the mortality from IPF is also significantly higher in old than in young patients (Araki and others 2003; Casanova and others 2009; Pinheiro and others 2008). Animal studies, although limited, have also shown that old animals are more sensitive than young animals to lung fibrosis with (Hecker and others 2014; Naik and others 2012; Redente and others 2011) or without (Calabresi and others 2007; Huang and others 2007b; Sueblinvong and others 2012) challenge. Together, these observations suggest that advanced age is a major risk factor for IPF. The mechanism underlying increased susceptibility of the elderly to IPF, however, is unknown.
Plasminogen activator inhibitor 1 (PAI-1) is a primary inhibitor of urokinase-type and tissue-type plasminogen activators (uPA and tPA, respectively), which convert plasminogen into plasmin, a serine proteinase involved in fibrinolysis as well as degradation of extracellular matrix (ECM) proteins. Therefore, PAI-1 plays a critical role in the regulation (inhibition) of fibrinolysis as well as ECM degradation. Studies from this lab and others have shown that PAI-1 is essential in the development of lung fibrosis (Chuang-Tsai and others 2003; Eitzman and others 1996; Hattori and others 2000; Huang and others 2012; Senoo and others 2010), although its role in the development of cardiovascular (Knier and others 2011; Weisberg and others 2005; Xu and others 2010) and liver (von Montfort and others 2010; Wang and others 2007) fibrosis is still debated. Importantly, PAI-1 expression is increased in the plasma of the elderly (Aillaud and others 1986; Cesari and others 2010), in several organs/tissues of old rodents (Liu and others 2011; Serrano and others 2009; Sueblinvong and others 2012), and in aging model, klotho mice (Eren and others 2014; Takeshita and others 2002). Whether and how increased PAI-1 contributes to the aging-related susceptibility to lung fibrosis, however, is unknown.
To elucidate the potential role of PAI-1 in aging-related susceptibility to lung fibrosis, we assessed, in this study, the age-related changes in PAI-1 protein level in mouse lung homogenates and lung fibroblasts as well as the sensitivities of young and old mice to bleomycin-induced lung fibrosis. Lung fibroblasts were isolated from young and old mice to further explore the potential mechanism whereby PAI-1 promotes fibrosis in aged mice. Our data suggest that increased PAI-1 expression may contribute to the augmented sensitivity of the elderly to lung fibrosis in part by increasing resistance of (myo)fibroblasts to apoptosis.
MATERIALS AND METHODS
Animal treatment and sample collection
Three- and eighteen-month old male C57BL/6 mice (National Institute of Aging) were challenged with 4 U/kg body weight of bleomycin, dissolved in 40 µl of saline, or saline alone by intranasal instillation and sacrificed 7 and 14 days after challenge. Bronchoalveolar lavage (BAL) was performed and then pulmonary artery vascular beds perfused as we have described previously (Liu and others 2012). After perfusion, left lung was fixed with 10% PBS buffered formalin and the rest of the lung was frozen immediately in liquid nitrogen. All procedures involving animals were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham and conducted at the UAB animal facilities under specific pathogen-free conditions.
Total and differential cell counts in BALF
BAL fluid (BALF) was spun down at 400 g for 10 min and the cells were uniformly suspended in saline. Total cell numbers were counted using a hemocytometer while differential cell counts determined after the cells were centrifuged onto a microscope slide using a CytoSpin and stained with Protocol HEMA3 (Protocol Cat # 123-869). Total of 500 cells were counted on each slide using oil immersion (100×) lens of Zeiss microscope and the differential cell count performed. The percentages of macrophages, neutrophils, and lymphocytes were calculated.
Western blot analysis
Lung tissues were homogenized in 0.25 M sucrose buffer containing protease inhibitor (Sigma P8340) and phosphatase inhibitor cocktails (Sigma, P5726) and then centrifuged at 3,000 × g, 4°C, for 10 min. Protein concentrations in the supernatants were measured using a BCA protein assay kit (Pierce, Rockford, IL). Fifty micrograms of protein were resolved on a 10% SDS-PAGE gel and electrophoretically transferred onto a PVDF membranes, which were probed with the following antibodies: TGF-β1 (R&D, MAB240), PAI-1 (Molecular Innovation, ASMPAI-GF), α-SMA (Biocare, CM001B), fibronectin (BD Biosciences, 610077), and β-actin (protein loading control), and then with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies. The protein bands were visualized using the ECL detection system (Amersham, Piscataway, NY), semi-quantified using Image J software, and normalized by β-actin.
Collagen staining
Collagen deposition was revealed by Masson’s Trichrome staining and quantified by quantitative morphometry techniques as we have described previously (Huang and others 2012).
Measurement of hydroxyproline
The hydroxyproline content in mouse lung was measured as we have described previously (Liu and others 2012). The results were calculated based on the standard curves generated using hydroxyproline reagent.
Detection of (myo)fibroblast apoptosis in mouse lung
Formalin-fixed, paraffin-embedded tissue slides were deparaffinized and rehydrated through graded concentrations of ethanol. Antigen retrieval was performed by steaming the slides for 8 min in the Antigen unmasking solution (Vector Lab, USA). The slides were then incubated with rabbit polyclonal anti-mouse FSP-1 antibody (Millipore, Cat No 07-2274) at 4°C overnight, followed by AMCA-conjugated anti-rabbit secondary antibody (blue color, Vector Lab, FI-1200) at 37°C for 30 min. After washing, sections were incubated with the reaction mixture containing deoxynucleotidyl transferase and FITC–dUDP at 37°C for 1 hour. Nuclei were stained with Propidium iodide (red color). Apoptotic (myo)fibroblasts were identified by co-localization of FSP-1 positive and TUNEL positive cells using epifluoresecent microscope (Nikon TE2000E-2) and analyzed using Image Pro Plus version 5.1.2 (Media Cybernetics). The numbers of (myo)fibroblasts (FSP-1 positive, blue color) and apoptotic (myo)fibroblasts [light blue color from overlay of blue color (FSP-1 positive) and yellow color (TUNEL positive)] were counted in 5 different areas of interstitial sections of the lung in each mouse, 5–6 mice per group.
Co-localization of cleaved caspase-3, an apoptosis marker, and α-SMA, a myofibroblast marker, was conducted to confirm myofibroblast apoptosis in young and old mouse lungs. Briefly, slides, after deparaffinized and rehydrated, were microwaved in 0.1 M citrate buffer (pH 6.0) for 5 min (350W) to retrieve antigens and then blocked with 1% BSA plus 5% goat serum. The slides were then incubated with rabbit polyclonal anti-cleaved caspase-3 (Millipore, 1:100 dilutions) and mouse monoclonal anti-mouse α-SMA (Biocare, 1:400 dilutions) antibodies at 4°C overnight. After washing, the slides were incubated with fluorescein-conjugated anti-rabbit (1:100 dilutions, green) and texas red-conjugated anti-mouse (1:100 dilutions, red) secondary antibodies. Nuclei were stained with DAPI (blue) and images taken using Nikon TE2000E-2.
Immunostaining of PAI-1 in mouse lung fibroblasts
To reveal PAI-1 expression in (myo)fibroblasts, formalin-fixed, paraffin-embedded tissue slides were deparaffinized and rehydrated as described above. The slides were then blocked with 2% BSA plus diluted (1:500) goat serum and incubated with mouse monoclonal anti-human PAI-1 antibody (Molecular Innovations, Cat No MA33H1F7) and rabbit polyclonal anti-mouse FSP-1 antibody (Millipore, Cat No 07-2274) at 4°C overnight. The sections were washed and then incubated with fluorescein-conjugated anti-mouse (Vector Lab, Cat No TI-2000, red color) and anti-rabbit (Vector Lab, Cat No FI-1000, green color) secondary antibodies. Images were taken using a Nikon Eclipse 90i microscope at 40 × magnification. (Myo)fibroblasts expressing high levels of PAI-1 [yellow color from overlay of green color (FSP-1) and red color (PAI-1)] were counted in five different areas of the lung in each mouse, 5–6 mice per group.
Isolation of lung fibroblasts
Lung fibroblasts were isolated from 3- and 22-month old male C57BL/6 mice as described previously (Bruce and others 1999). Briefly, the chest wall was opened under sterile conditions; the right ventricle was cannulated and pulmonary vasculature perfused with sterile PBS. The lungs were removed and minced to approximately 0.5 mm pieces and incubated in calcium and magnesium-free Hanks’ balanced salt solution containing 0.3 mg/ml type IV collagenase and 0.5 mg/ml trypsin at 37°C for 60 min, shaking every 10 minutes. After digestion, equal volume of Eagle's Minimum Essential Medium (EMEM; ATCC, 30-2003) containing 10% fetal bovine serum (FBF) was added and the tissue was disaggregated by filtration through sterile tissue grinder pestle mesh (Sigma Aldrich, T8279-2EA). The isolated cells were suspended in EMEM growth medium containing non-essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1500 mg/L sodium bicarbonate supplemented with 10% (v/v) FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco BRL) and cultured in a humidified atmosphere with 5% CO2 at 37° for 4 hours. Then, the cells were washed gently with EMEM to remove non-adherent cells and growth medium was replaced every 2–3 days. The passages 4 to 8 of primary fibroblasts were used for the experiments.
Flow cytometry analysis of fibroblast apoptosis in vitro
Apoptosis of cultured fibroblasts was analyzed by flow cytometry techniques using FITC annexin V Apoptosis Detection Kit II (BD Bioscience) as we have described previously (Huang and others 2012). Briefly, after treatment, the cells were trypsinized, spun down, and incubated with 1× annexin-binding buffer for 15 minutes, then with FITC annexin V and PI working solution at room temperature for another 15 minutes. Apoptotic cells were analyzed with flow cytometer at UAB Flow Cytometry facility.
siRNA transfection
Primary human lung fibroblasts (CCL-210 cells), purchased from American Type Culture Collection (ATCC, Manassas, VA), were seeded in 60 mm cell culture dish at the density of 4 × 105 cells/well. After reaching 60–80% confluence, cells were transfected with either PAI-1 siRNA (Santa Cruz, sc36179) or Non-target siRNA (Santa Cruz, control siRNA-A) according to the protocol provided by the manufacturer. Cells were cultured with complete medium containing serum for 24 hours before being treated with TGF-β1 or H2O2 in serum-free medium.
Statistical analysis
Data are presented as means ± SEM and evaluated by one-way analysis of variance (ANOVA). Statistical significance was determined post-hoc by Fisher LSD test wherein p<0.05 was considered significant.
RESULTS
Aging-dependent increase in PAI-1 expression in mouse lung tissue
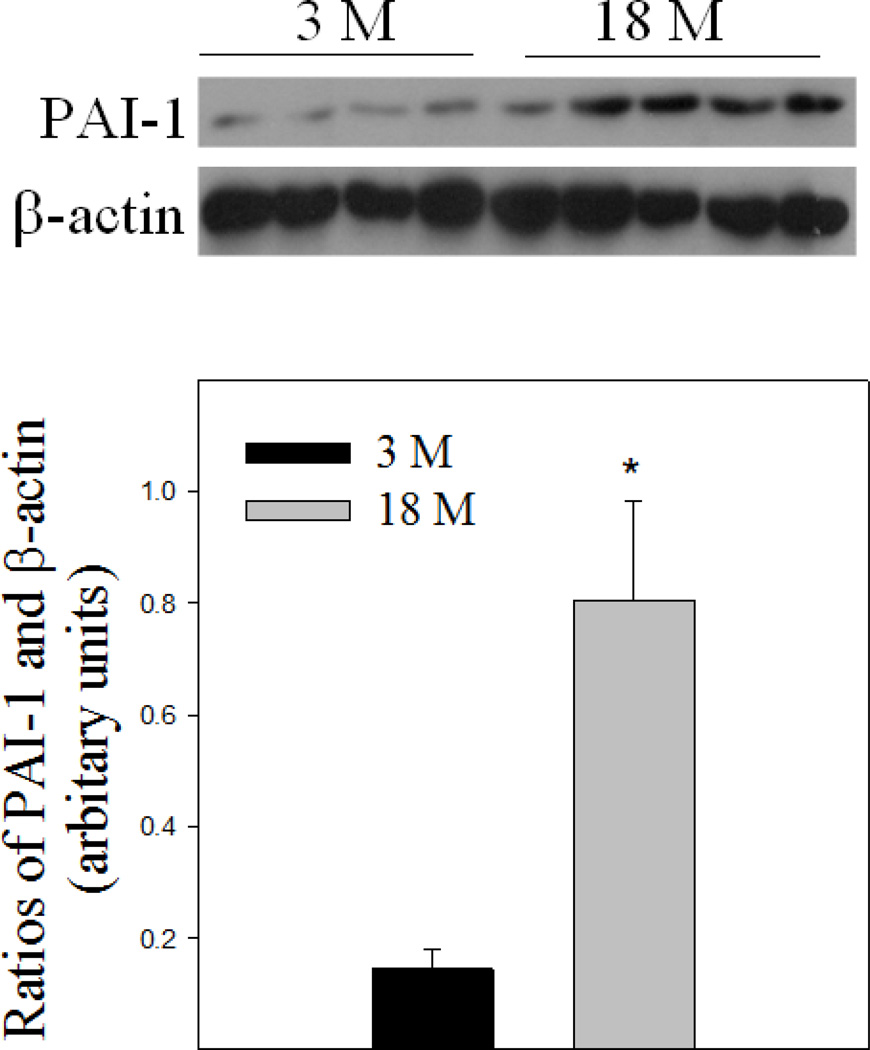
Although it has been reported that PAI-1 mRNA expression is increased in lung tissue of old mice (Sueblinvong and others 2012), whether its protein level is increased with age in the lung was unknown. To begin our understanding of the potential role of PAI-1 in aging-related susceptibility to lung fibrosis, we first examined PAI-1 protein in mouse lung tissue by Western analysis. The results show that 18-month old mice expressed higher level of PAI-1 protein in their lungs compared to 3-month old mice under unchallenged condition (Fig 1).
Fig 1. Age-dependent increase in PAI-1 protein level in mouse lung.
Top panel, representative Western blotting pictures; Bottom panel, semi-quantified Western blotting data. *Significantly different from 3-month old mice (p<0.05, n = 4–5).
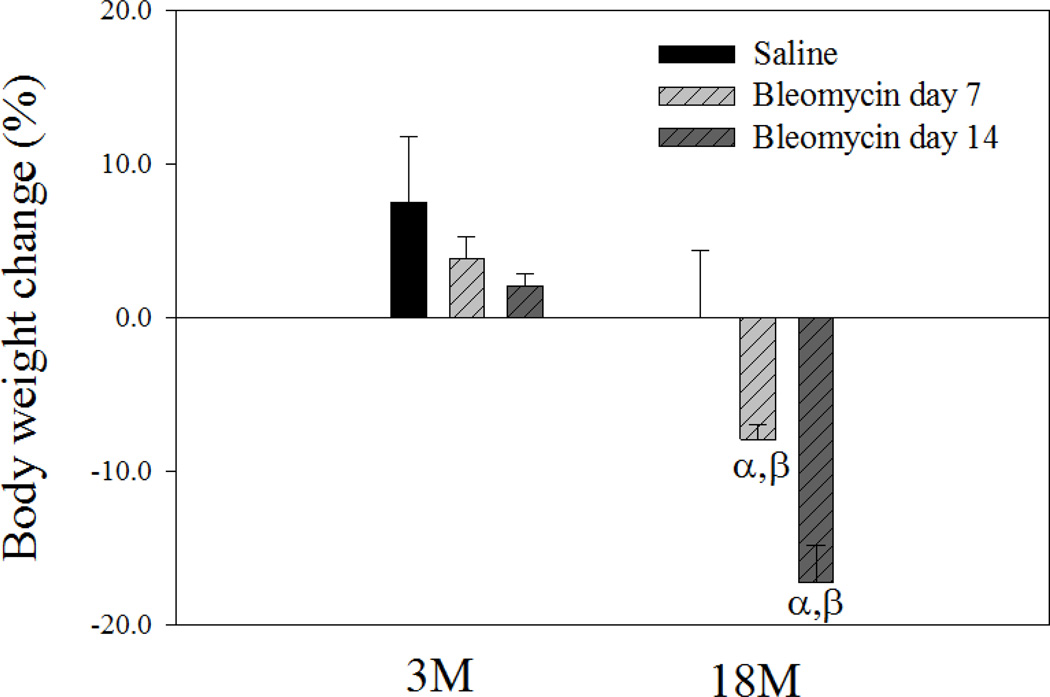
Age-dependent changes in mouse body weight and BALF cell counts upon bleomycin challenge
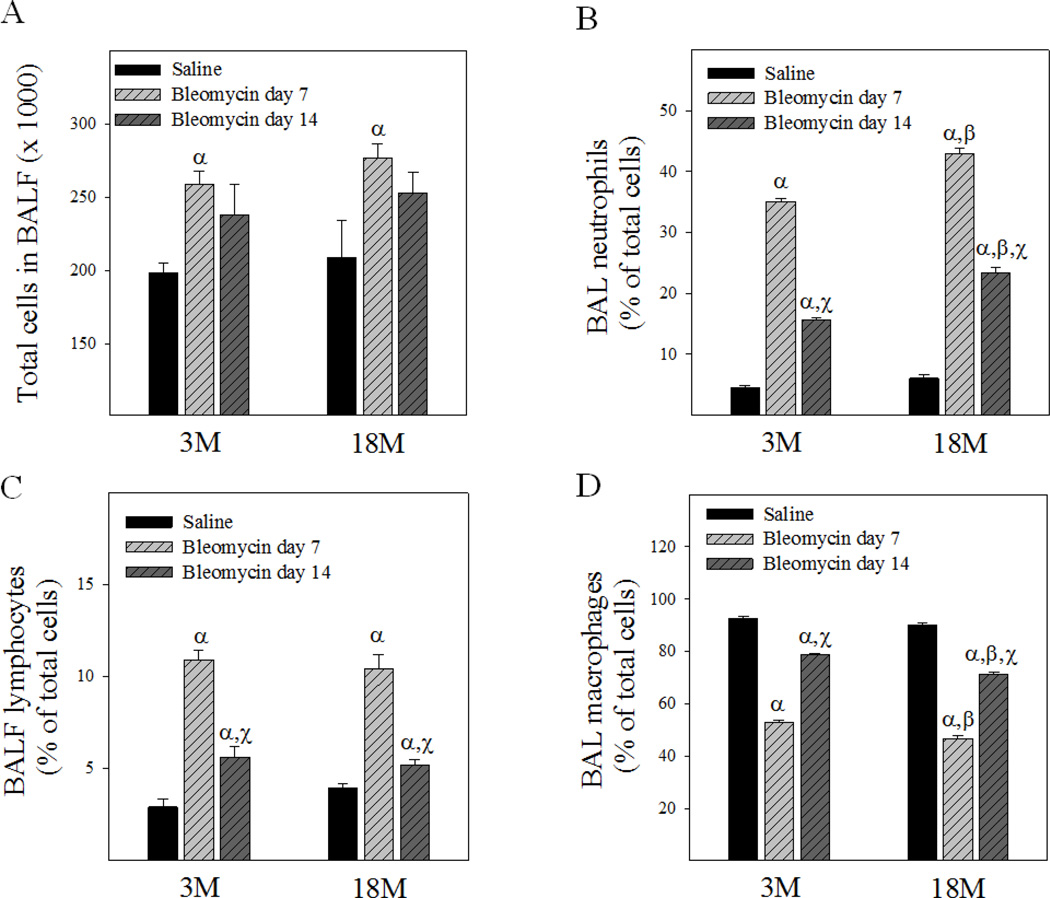
Changes in mouse body weight 7 and 14 days after bleomycin instillation were monitored to reveal general toxicity of bleomycin to young and old mice. As shown in Fig 2, bleomycin challenge significantly reduced the body weight of 18-month old, but not 3-month old, mice. BALF cell count data show, on the other hand, that bleomycin instillation significantly increased the total cell numbers in both young and old mice at day 7 although not at day 14 (Fig 3A). Differential cell count data further show that, although the numbers of lymphocytes and neutrophils were significantly increased and the percentages of macrophages decreased in both young and old mice 7 and 14 days after bleomycin challenge (Fig 3B&3C&3D), the increase in the counts of neutrophils as well as the decrease in the percentages of macrophages were greater in old than in young mice (Fig 3B&D). Consistent with the changes in total cell number, the counts of lymphocytes and neutrophils in BALF were also lower in day 14 compared to day 7 after bleomycin challenge, indicating that acute inflammation started to subside by day 14 in both young and old mice.
Fig 2. Age-dependent effect of bleomycin on mouse body weight.
Mice were instilled with bleomycin or saline as described in the Material and Method section. Body weights before instillation and 7 or 14 days after instillation were recorded. The average body weight before instillation was 29.8g for 3-month old and 32.7 for 18-month old mice. The percentages of changes in body weights after treatment were presented (the body weights of the saline groups were from 14 days after saline instillation). α, Significantly different from the same age saline control; β, significantly different from 3-month old mice treated with bleomycin for the same length of time (p < 0.05, n = 5).
Fig 3. Age-dependent effects of bleomycin on BALF cell counts.
Total and differential cell counts in BALF were determined as described in Material and Method section. A) Total cell numbers; B) Percentages of neutrophils; C) Percentages of lymphocytes; D) Percentages of macrophages in BALF. The data for the saline groups were from 14 days after saline instillation. α, Significantly different from the same age saline control; β, significantly different from 3-month old mice treated with bleomycin for the same length of time; χ, significantly different from the same aged mice treated with bleomycin for 7 days (p < 0.05, n = 5).
Age-dependent responses to bleomycin-induced lung fibrosis in mice
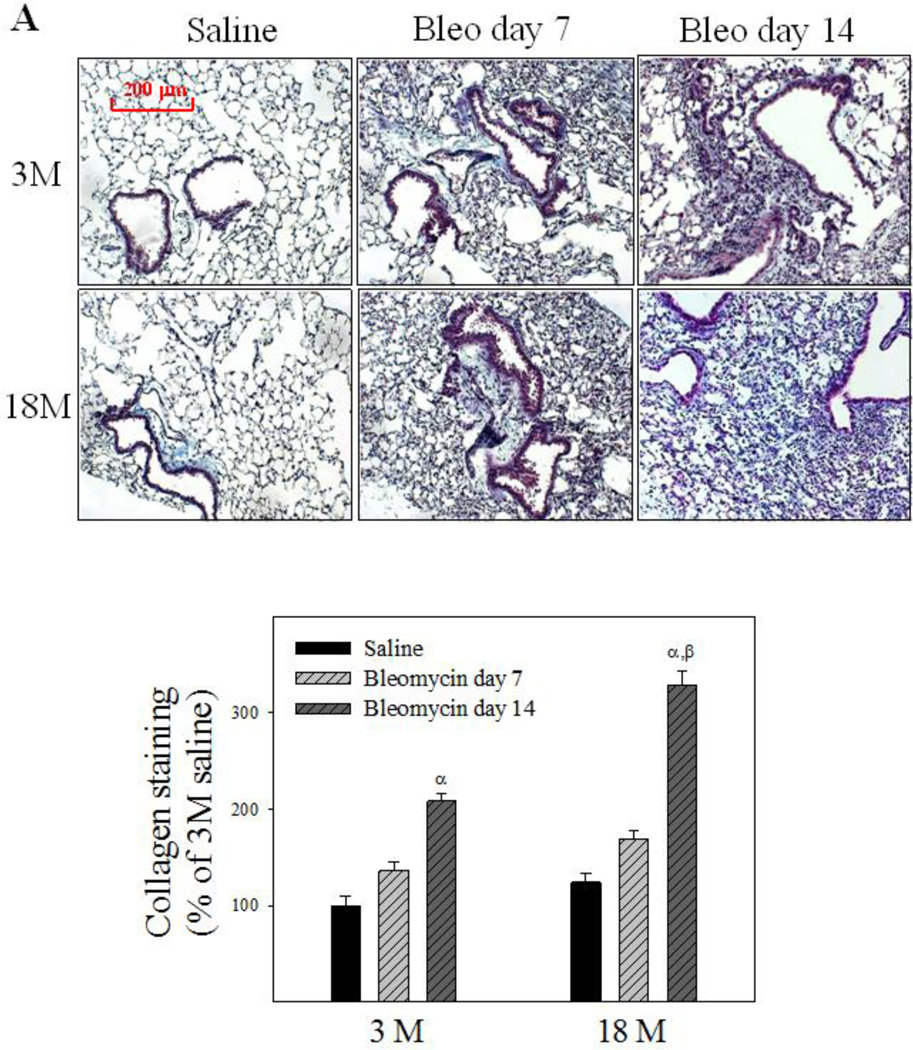
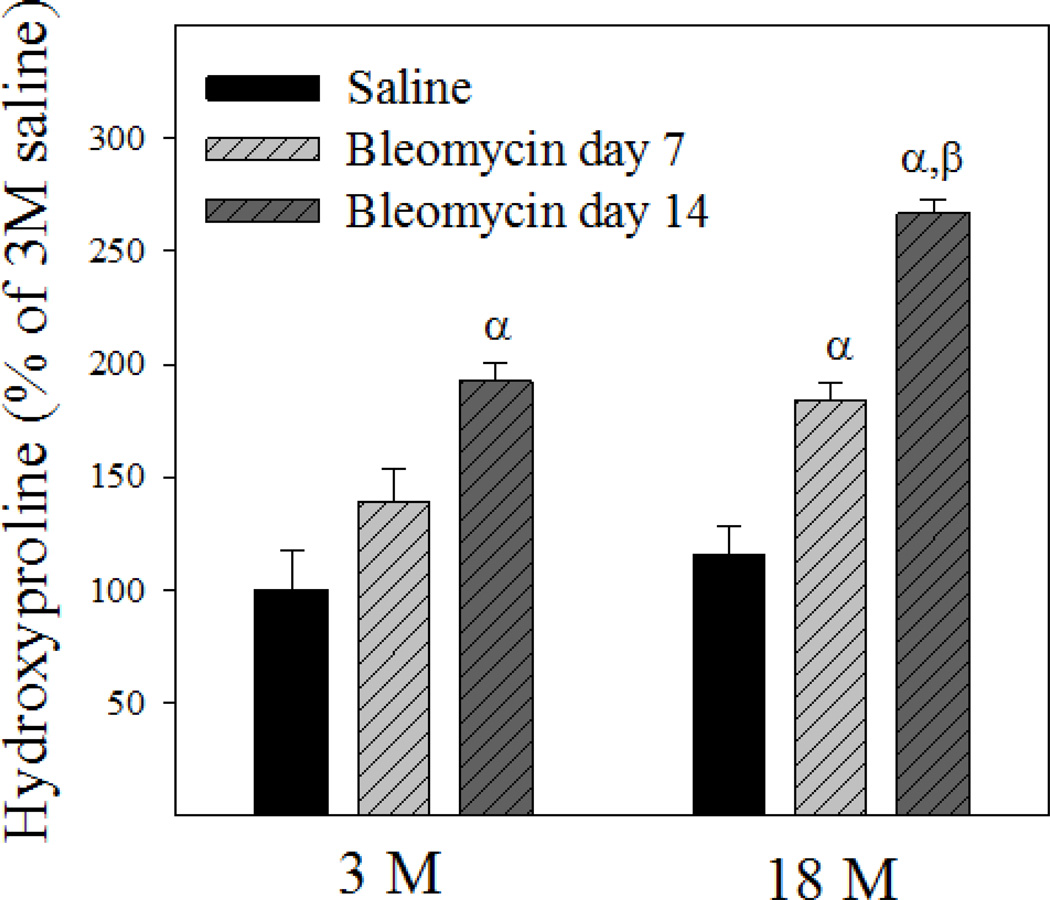
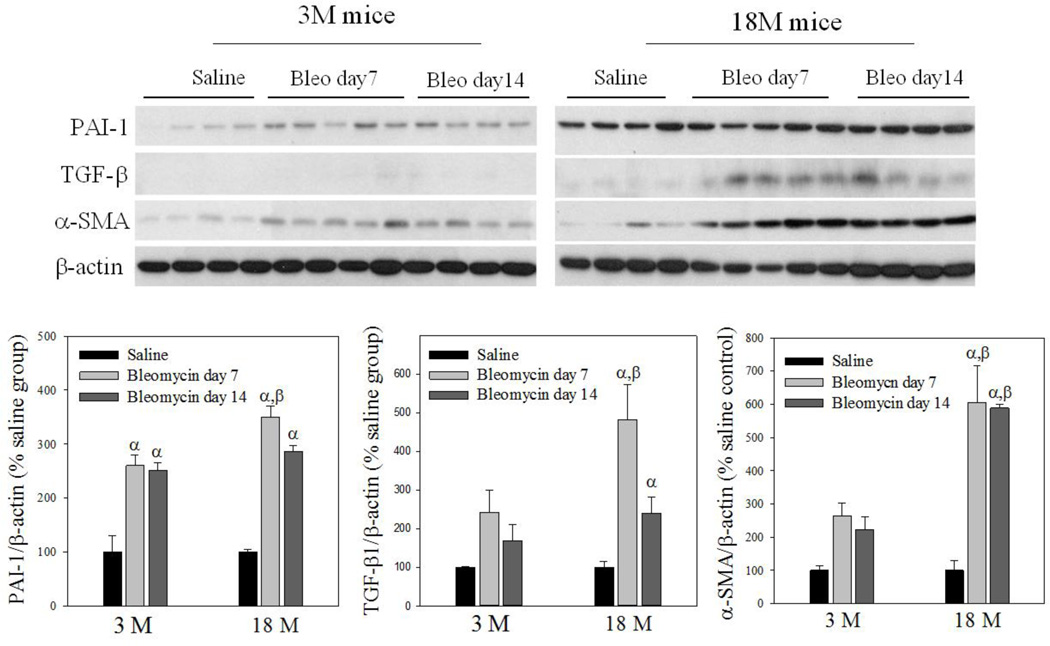
To reveal whether old mice are more sensitive to bleomycin-induced lung fibrosis, trichrome staining was performed and hydroxyproline content measured 7 and 14 days after bleomycin challenge. Trichrome staining results show that more collagen (blue color) was accumulated in the lung of old than in young mice at day 14 after bleomycin challenge (Fig 4A). Hydroxyproline data further show that more hydroxyproline was accumulated in the lung of old than in young mice at both time points after bleomycin challenge, although the basal levels of hydroxyproline (saline treated) were not significantly different between two age groups (23.64 ± 1.77 µg/mg verses 27.33 ± 3.01 µg/mg for 3- and 18-month old mice, respectively) (Fig 4B). Western analyses further indicate that 18-month old mice experienced augmented induction of PAI-1, TGF-β1, and α-SMA, compared to 3-month old mice, upon bleomycin challenge (Fig 4C), although the basal levels of TGF-β and α-SMA proteins were not significantly different between 3 and 18 month old mice (the densities of the bands relative to β-actin are: 100 ± 59 and 652 ± 179 for TGF-β1, 100 ± 13.5 and 103 ± 29.9 for α-SMA, for 3-month and 18-month old mice, respectively). Together, the data indicate that old mice are more sensitive to lung fibrotic challenge than young mice.
Fig 4. Age-dependent lung fibrotic responses in mice.
A) Trichrome staining of collagens: Top panels, representative trichrome staining pictures; Bottom panel, semi-quantified collagen staining data. B) Hydroxyproline content in mouse lung. C) Western analyses of proteins: Top panels, Western blotting pictures (β-actin was used as protein loading control); Bottom panels, semi-quantified Western blotting data expressed as percentage of the same age saline controls. α, Significantly different from the same age saline control; β, significantly different from 3-month old mice treated with bleomycin for the same length of time; χ, significantly different from the same aged mice treated with bleomycin for 7 days (p<0.05, n=4–6).
Age-dependent apoptosis resistance of (myo)fibroblasts in the lung of bleomycin challenged mice
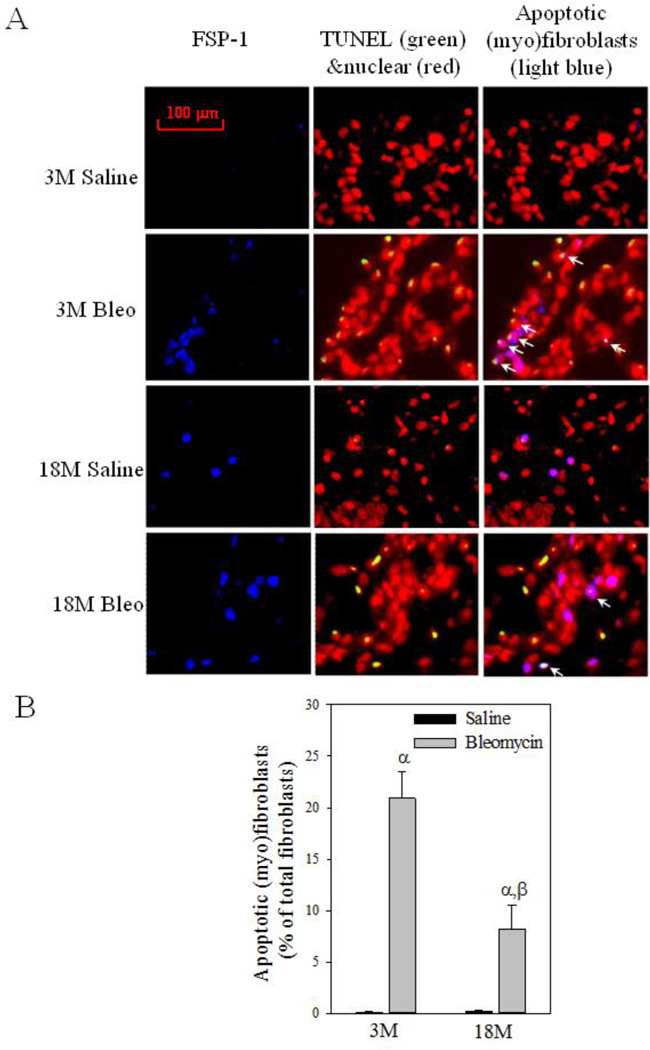
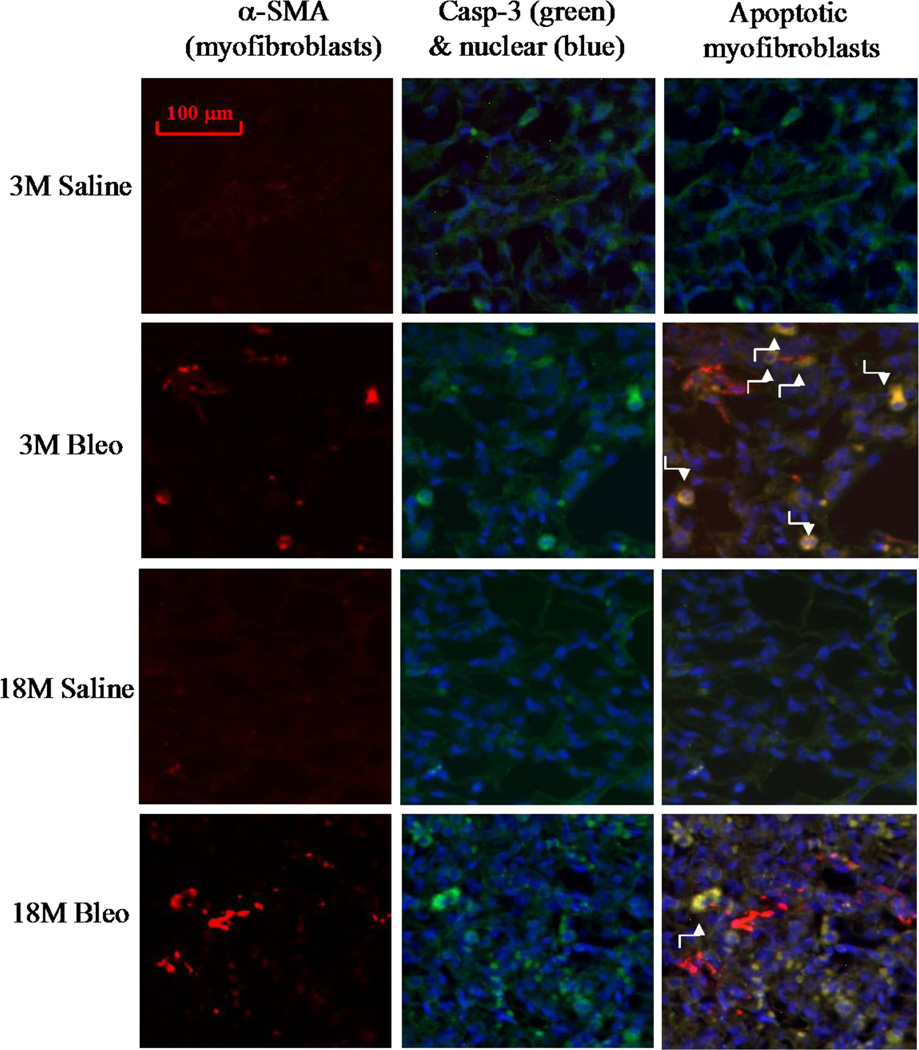
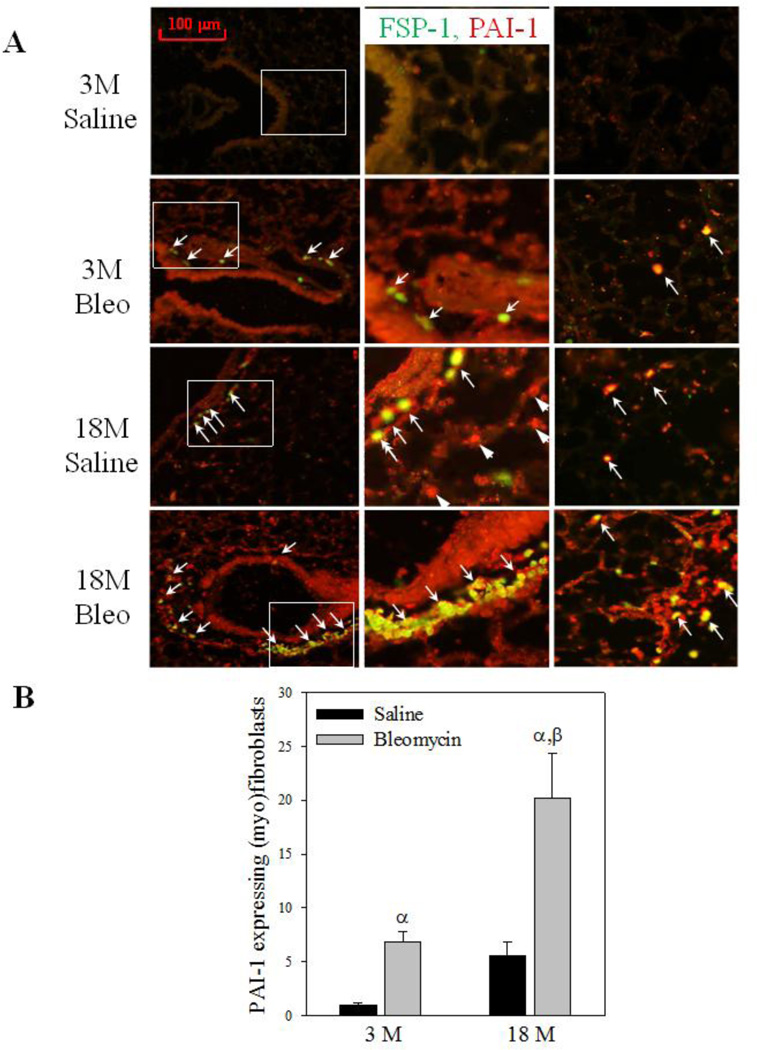
Increased resistance of myofibroblasts to apoptosis is believed to contribute importantly to the development of fibrosis (Hecker and others 2014; Thannickal and Horowitz 2006). To explore the mechanism underlying increased sensitivity of old mice to lung fibrosis, we assessed the numbers of PAI-1 expressing (myo)fibroblasts and apoptotic (myo)fibroblasts in lungs of young and old mice 14 days after bleomycin challenge. Co-localization of fibroblast specific protein 1 (FSP-1) and TUNEL staining (apoptotic) show that fewer (myo)fibroblasts underwent apoptosis (arrows pointed light blue color stained cells on the right panels) in the lung of 18-month old mice, compared to 3-month old mice, after bleomycin challenge (Fig 5A&B). Double immunostaining with antibodies to apoptosis marker cleaved caspase-3 and myofibroblast marker alpha smooth muscle actin (α-SMA) further confirmed the finding (Fig 5C). Associated with increased resistance to apoptosis, more (myo)fibroblasts with increased PAI-1 were accumulated in the lung of 18-month old mice compared to 3-month old mice with and without bleomycin challenge (Fig 6A central and right panels, arrows pointed, and Fig 6B). The data also suggest that, besides (myo)fibroblasts, PAI-1 expression is increased with age in other types of cells as well (Fig 6A central panels, arrowheads pointed).
Fig 5. (Myo)fibroblast apoptosis in the lung of young and old mice treated with bleomycin.
A) Left panels: representative pictures of immunostaining of mouse lung tissue with anti-fibroblast specific protein 1 (FSP-1) antibody (blue); Middle panels, representative overlay pictures of TUNEL (green) and nuclear (red) staining (yellow, TUNEL positive cells); Right panels: Overlay of FSP-1 (blue), TUNEL (green), and nuclear (red) staining (light blue color, TUNEL positive fibroblasts, arrows pointed). B) Quantitative data of apoptotic (myo)fibroblasts, expressed as the percentage of total (myo)fibroblasts as described in Material and Method section. α, Significantly different from the same age saline control; β, significantly different from 3-month old, bleomycin-treated mice (p<0.05, n=4–5). C) Co-localization of α-SMA (red), cleaved caspase-3 (green), and nuclear (blue) immunostaining. Yellow color stained cells (arrows pointed) are apoptotic myofibroblasts.
Fig 6. Age-dependent accumulation of PAI-1 expressing (myo)fibroblasts in mouse lung upon bleomycin challenge.
A) Double immunofluorescence staining of mouse lung slides with antibodies to PAI-1 (red) and FSP-1 (green). Yellow color stained cells are PAI-1 expressing fibroblasts (overlay of green FSP-1 and red PAI-1). Central column: enlarged view of the white boxes in the left column. Right column: lung parenchyma. B) Quantification of PAI-1 expressing (myo)fibroblasts, expressed as the percentage of total (myo)fibroblasts. α, Significantly different from the same age saline control; β, significantly different from 3-month old, bleomycin-treated mice (p<0.05, n=4–5).
Fibroblasts from the lung of old mice are resistant to apoptosis and more fibrogenic upon challenge in vitro
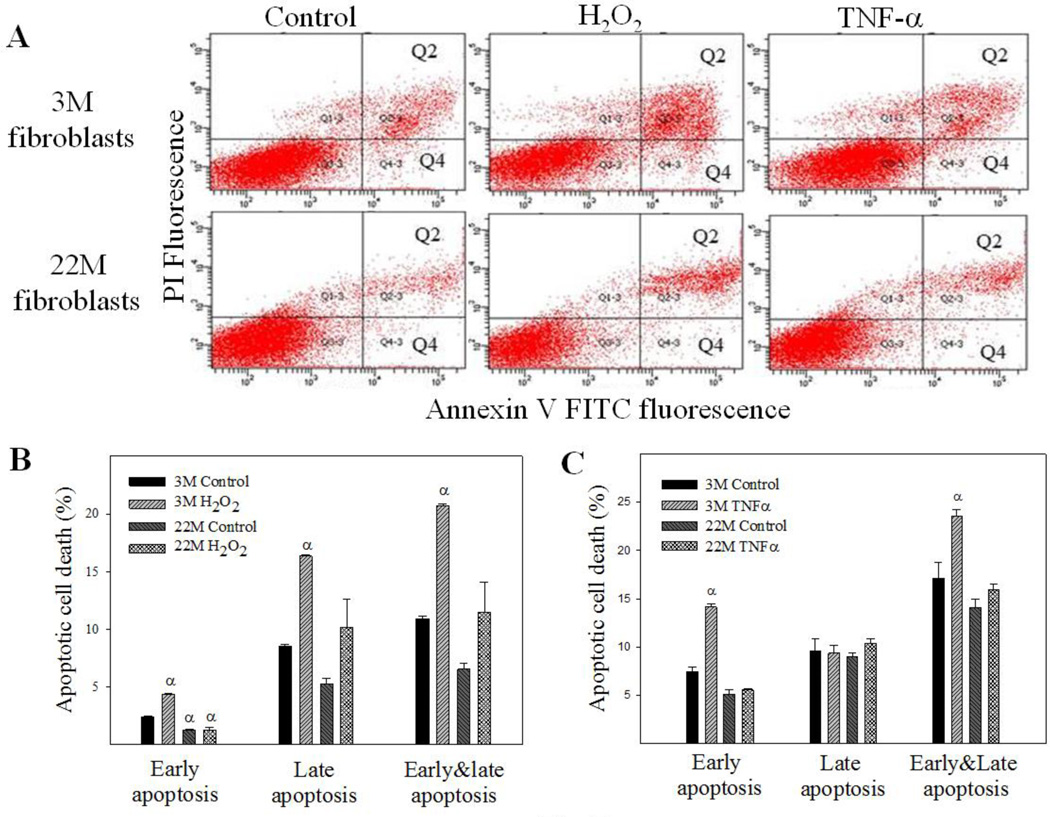
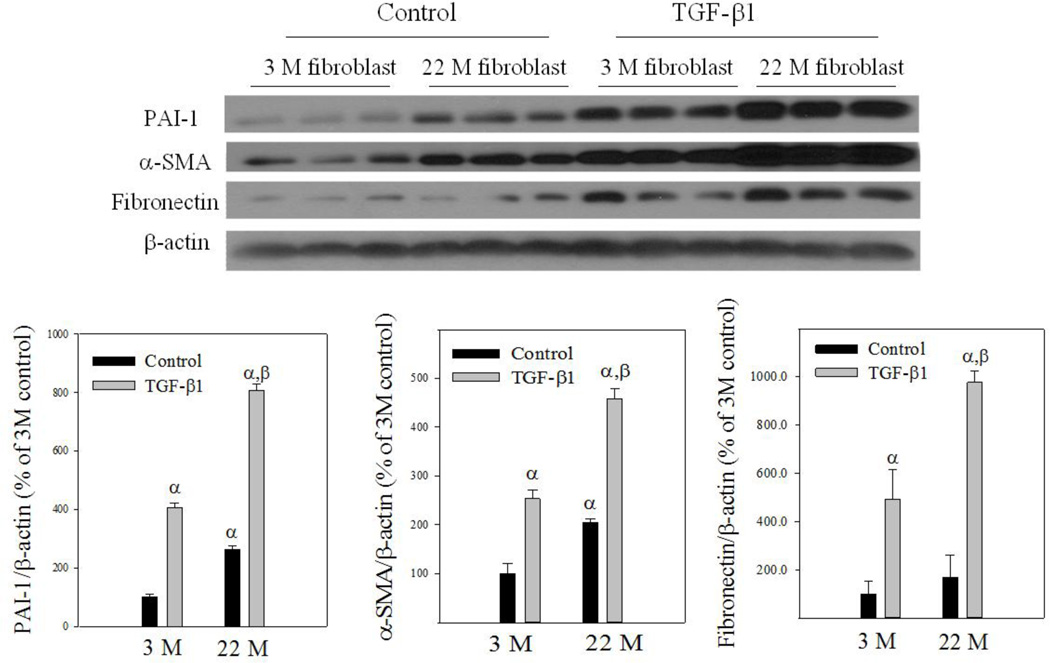
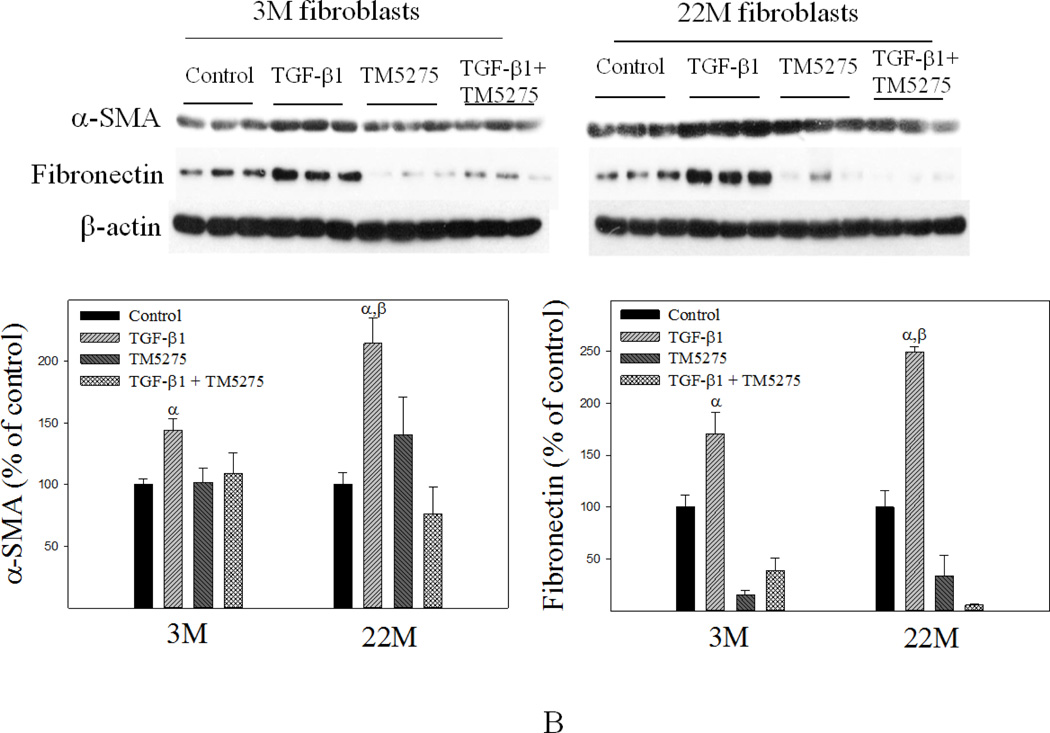
Apoptosis is induced through two distinct pathways, extrinsic, activated by death receptors, and intrinsic, triggered by various stress signals through releasing cytochrome C from mitochondria. Tumor necrosis factor alpha (TNF-α) and hydrogen peroxide (H2O2), which induce apoptosis through extrinsic and intrinsic pathways, respectively, have been shown to induce fibroblast apoptosis (Boucher and others 2010; Niederer and others 2012; Park and others 2014; Szigeti and others 2010; Zhang and others 2011). To confirm that fibroblasts from the lung of old mice are more resistant to apoptosis and fibrogenic than fibroblasts from young mice, lung fibroblasts were isolated from 3- and 22-month old mice and treated with H2O2, TNF-α, or TGF-β1. Flow cytometry data show that treatment with H2O2 significantly increased both early (labeled with annexin V only, Q4) and late (labeled with annexin V and propidium iodide, Q2) stages of apoptotic cell death in fibroblasts isolated from lungs of young, but not old, mice (Fig 7A&B). An increased resistance to TNF-α-induced apoptosis was also observed in lung fibroblasts isolated from old mice (Fig 7A&C). Western analyses further show that fibroblasts from old mice had augmented induction of PAI-1, α-SMA, and fibronectin upon TGF-β1 challenge, compared to fibroblasts from young mice (Fig 8).
Fig 7. Responses of lung fibroblasts from young and old mice to apoptotic stimuli in vitro.
Fibroblasts isolated from lungs of 3 month and 22 month old mice were challenged with 200 µM of H2O2 or 25 µM of TNF-α for 24 hours. Apoptosis was analyzed by flow cytometry. A) Representative flow cytometry graphic pictures. Q2: late apoptotic cell death labeled with both annexin V and propidium iodide; Q4: early apoptotic cell death labeled with annexin V only. B&C) Quantitative data of the early and late apoptotic cell death induced by H2O2 (B) or TNF-α (C). α, Significantly different from the corresponding 3M control fibroblasts (p<0.05, n=3).
Fig 8. Responses of lung fibroblasts from young and old mice to TGF-β1 in vitro.
Isolated lung fibroblasts were treated with 1 ng/ml TGF-β1 for 24 hours and then Western analyses were conducted with cell lysates. Top panel: representative Western blotting pictures; bottom panels, semi-quantified Western blotting data, expressed as the percentage of 3M control fibroblasts. α, Significantly different from 3M control group; β, significantly different from TGF-β1 treated 3M fibroblasts (p<0.05, n=3).
Inhibition of PAI-1 activity eliminated the aging-related apoptosis resistance and augmented fibrotic responses in lung fibroblasts
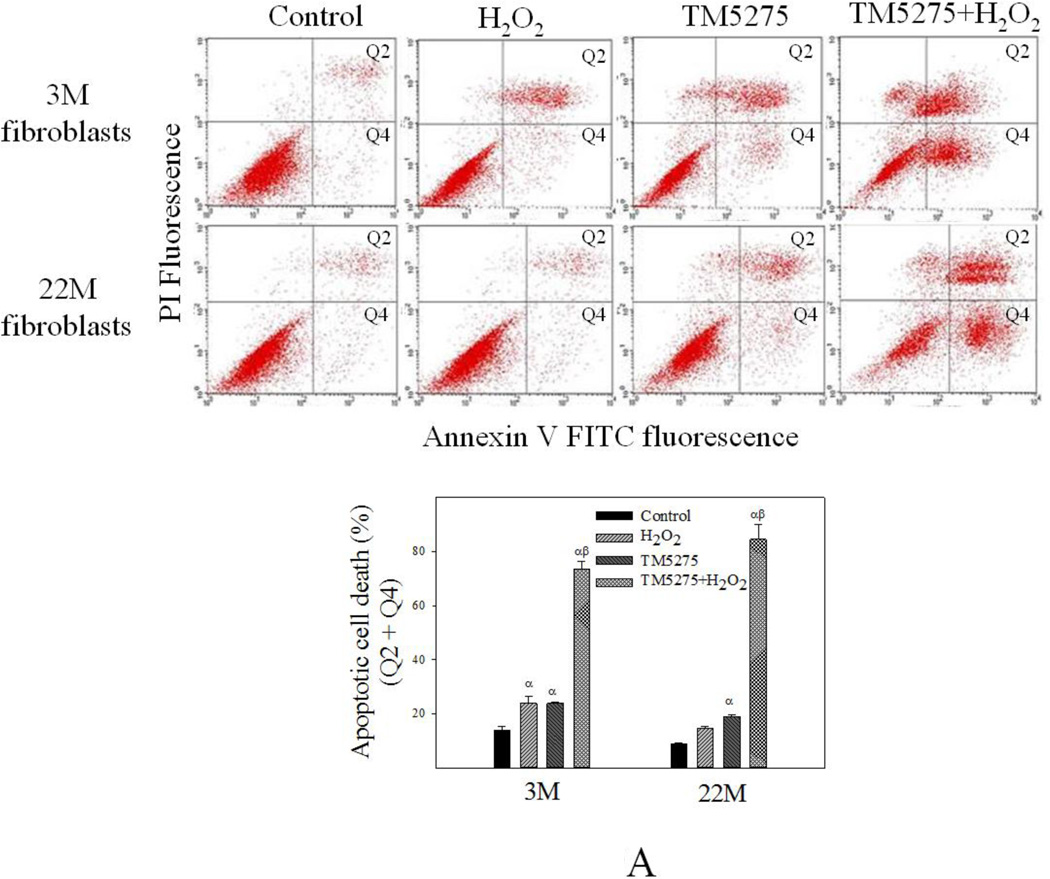
TM5275 is an orally effective small molecule PAI-1 inhibitor which has potent anti-thrombotic effects but does not interfere with other serpin/serine protease systems (Izuhara and others 2010; Yamaoka and others 2011). Our recent study showed that TM5275 treatment almost completely blocked TGF-β1-induced fibrotic responses in mouse lung in vivo and in human lung fibroblasts in vitro (Huang and others 2012). To determine whether increased PAI-1 expression/activity is responsible for the aging-related apoptosis resistance and TGF-β1 sensitivity, lung fibroblasts isolated from 3- and 22-month old mice were pre-treated with 75 µM TM5275 for 8 hours and then treated with H2O2 or TGF-β1 in the presence of TM5275 for another 24 hrs. The results show that inhibition of PAI-1 with TM5275 increased apoptosis sensitivity and reduced TGF-β1 responses in fibroblasts isolated from both young and old mice. Importantly, there was no significant difference between young and old fibroblasts in terms of their responses to apoptotic stimulus or TGF-β1 after inhibition of PAI-1 activity (Fig 9A&B), suggesting that increased PAI-1 may underlie the aging-related apoptosis resistance and TGF-β1 sensitivity of lung (myo)fibroblasts.
Fig 9. Inhibition of PAI-1 activity eliminated the aging-related increase in apoptosis resistance and TGF-β1 sensitivity in isolated fibroblasts.
Fibroblasts isolated from 3- and 22-month old mouse lungs were pretreated with 75 µM of PAI-1 inhibitor TM5275 for 8 hours and then treated with 200 µM of H2O2 or 1ng/ml TGF-β1 in the presence of TM5275 for 24 hours. (A) Effects of TM5275 on apoptotic response. Top panels: representative flow cytometry graphic pictures. Bottom panels: quantitative data of apoptotic cell death (early plus late stages). (B) Effects of TM5275 on the sensitivity of fibroblasts to TGF-β. Top panels: representative Western blotting pictures; Bottom panels, semi-quantified Western blotting data, expressed as the percentages of untreated control fibroblasts from same aged mice. α, Significantly different from the same age untreated control; β, significantly different from TGF-β1 treated 3M fibroblasts; (p<0.05, n=3–5).
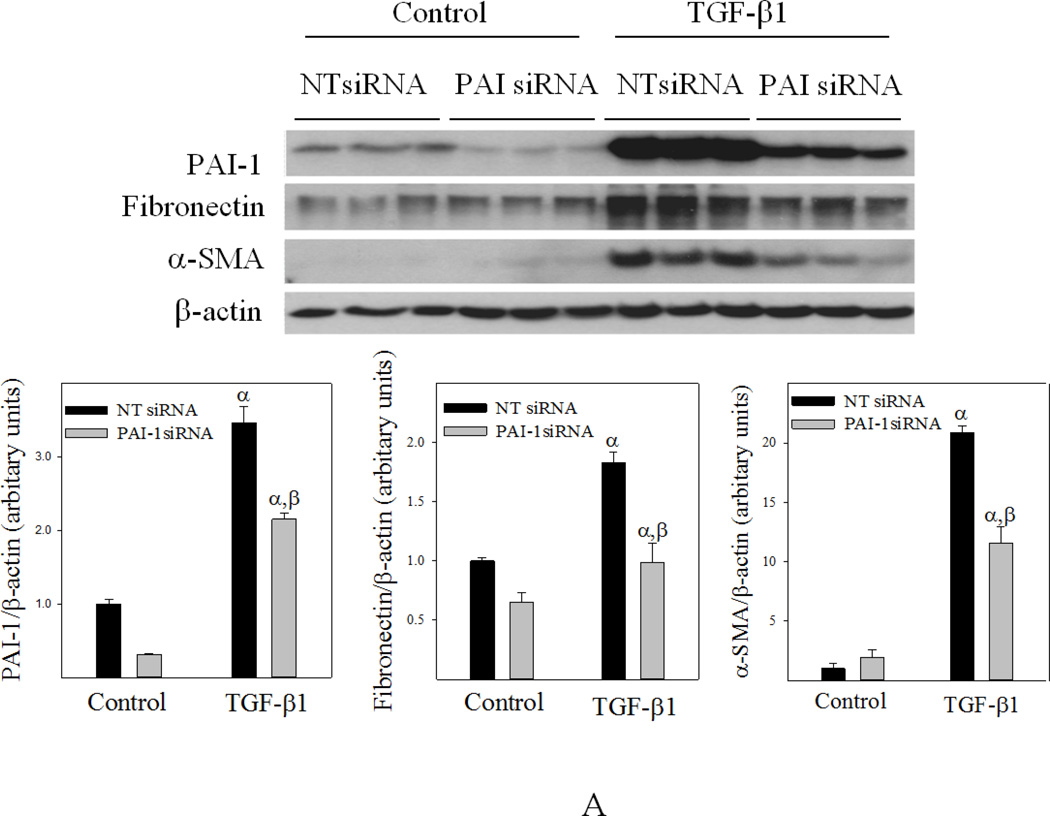
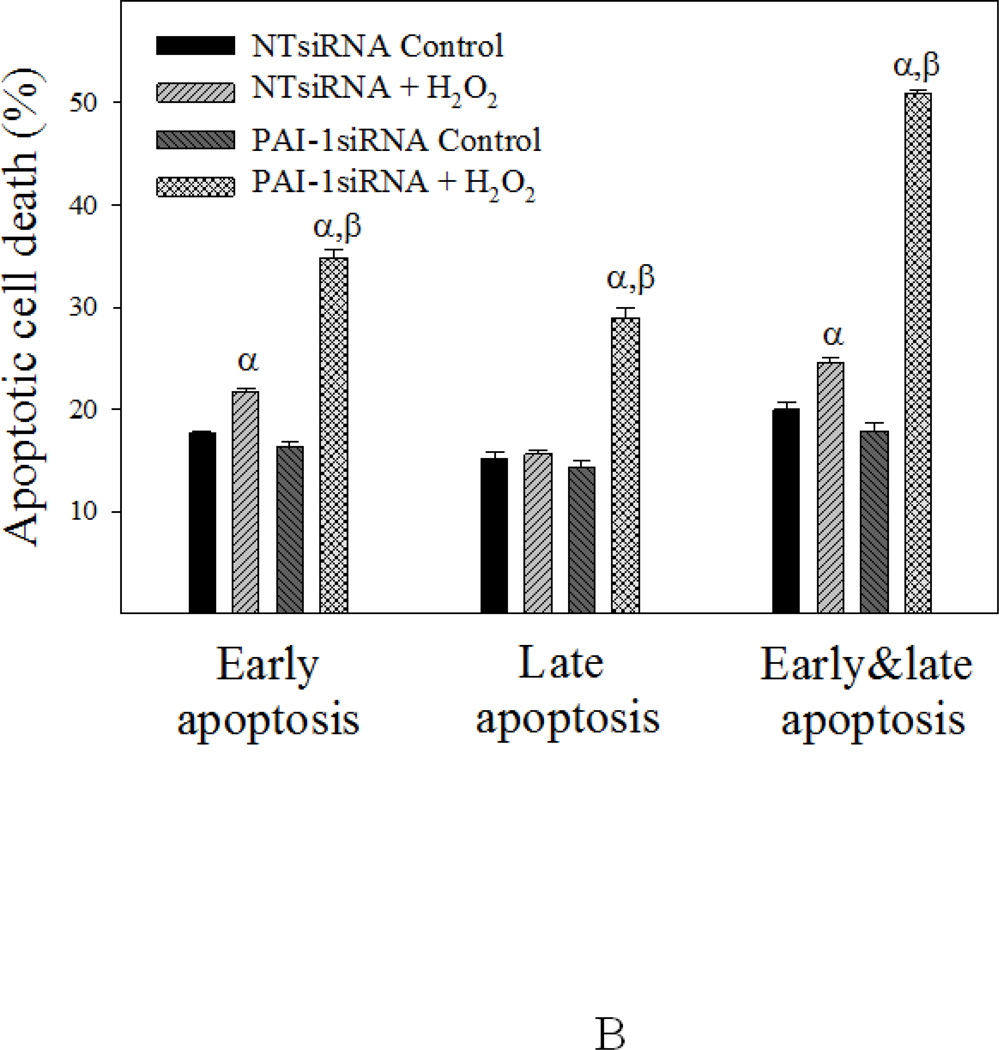
Knockdown of PAI-1 with PAI-1 siRNA increased apoptosis sensitivity and reduced fibrotic response of human lung fibroblasts
To further elucidate the role of PAI-1 in fibroblast apoptosis resistance and sensitivity to TGF-β1, primary human lung fibroblasts (CCL-210 cells) were transfected with PAI-1 siRNA or non-target siRNA (NTsiRNA) and then treated with H2O2 or TGF-β1 for 24 hours. The results show that transfection of PAI-1 siRNA significantly knocked down PAI-1 protein in CCL-210 cells with or without TGF-β1 treatment (Fig 10A). Most importantly, the data show that knockdown of PAI-1 with PAI-1 siRNA dramatically reduced TGF-β1-induced fibronectin and α-SMA expression (Fig 10A) and increased the sensitivity of CCL-210 cells to H2O2-induced apoptosis (Fig 10B).
Fig 10. Knockdown of PAI-1 with PAI-1 siRNA increased apoptosis sensitivity and reduced fibrotic responses in human lung fibroblasts.
Human lung fibroblasts (CCL-210 cells) were transfected with PAI-1 siRNA or non-target siRNA (NT-siRNA) and then treated with 1 ng/ml TGFβ-1 (A) or 200 µM H2O2 (B) for 24 hours. A) Western analyses of PAI-1, fibronectin, and α-SMA proteins. Top panel: representative Western blotting pictures; bottom panels, semi-quantified Western blotting data. B) Flow cytometry analysis of apoptosis. α, Significantly different from the corresponding untreated control fibroblasts; β, significantly different from NT-siRNA transfected TGF-β1 (A) or H2O2 (B) treated fibroblasts (p<0.05, n=6).
DISCUSSION
PAI-1, a primary inhibitor of uPA and tPA, has multi-functions besides inhibition of fibrinolysis. Studies from this lab and others have shown that PAI-1 plays a critical role in the development of lung fibrosis. Whether and how PAI-1 contributes to aging-related susceptibility to lung fibrosis, however, was unknown. In this study, we show for the first time that PAI-1 protein level was increased with age in mouse lung tissue homogenates and in lung fibroblasts, which was associated with increased fibrotic response in old mice and increased resistance of old fibroblasts to apoptosis in vivo and in vitro. Inhibition of PAI-1 activity with a small molecule PAI-1 inhibitor eliminated the aging-related apoptosis resistance and TGF-β1 sensitivity in isolated fibroblasts. Moreover, we show that knockdown of PAI-1 protein with PAI-1 siRNA in primary human lung fibroblasts significantly increased their sensitivity to apoptosis and inhibited their responses to TGF-β1. Together, our data suggest that increased PAI-1 expression may play an important role in the increased susceptibility of the elderly to lung fibrosis in part by protecting (myo)fibroblasts from apoptosis.
Besides inhibition of fibrinolysis, PAI-1, by interacting with vitronectin and uPA receptor (uPAR), also regulates cell adhesion, migration, and thereby inflammatory cell infiltration (Courey and others 2011; Huang and others 2003; Ichimura and others 2013; Poggi and others 2007; Roelofs and others 2008). It has been reported that treatment of experimental glomerulonephritis rats with mutant non-inhibitory PAI-1 proteins reduced ECM deposition in glomeruli, which was associated with a 46% decrease in the number of monocytes/macrophages (Huang and others 2003). It has also been shown that neutrophil influx was significantly lower in PAI-1 knockout than in wild-type mice in an acute pyelonephritis model (Roelofs and others 2008). In this study, we show that, associated with an increase in PAI-1 protein level, old mice experienced amplified response in neutrophil infiltration upon bleomycin challenge. Together, the data suggest that the aging-associated increase in PAI-1 expression may promote lung fibrosis in the elderly in part by facilitating inflammatory cell infiltration.
TGF-β is the most potent profibrogenic cytokine. It is believed in general that PAI-1 functions as a downstream effector of TGF-β. However, emerging evidence suggests that there is a positive feedback loop between PAI-1 and TGF-β1 (Matsuo and others 2005; Seo and others 2009). Masuo et al reported that PAI-1 transgenic mice expressed higher level of TGF-β1 mRNA upon unilateral ureteral obstruction than non-transgenic mice (Matsuo and others 2005). Huang et al also showed that treatment with mutant non-inhibitory PAI-1 proteins decreased TGF-β level in the kidney in an experimental glomerulonephritis model (Huang and others 2003). Seo et al further showed that knockout of the PAI-1 gene suppressed high glucose-induced TGF-β1 mRNA expression, whereas recombinant PAI-1 restored such inducibility in PAI-1 knockout mesangial cells (Seo and others 2009). They also showed that recombinant PAI-1 protein stimulated TGF-β promoter activity and that the induction of fibronectin and collagen I by recombinant PAI-1 was abrogated by TGF-β1 receptor inhibitor or anti-TGF-β antibody (Seo and others 2009). In a previous study, we showed that small molecule PAI-1 inhibitor TM5275 blocked TGF-β1 autocrine function as well as TGF-β1-induced collagen and α-SMA expression in mouse lung and in human lung fibroblasts (Huang and others 2012). In this study, we further show that inhibition of PAI-1 activity with TM5275 completely eliminated the augmented sensitivity of old fibroblasts to TGF-β1. Together, the data suggest that PAI-1 is not only a downstream effector of TGF-β but also a regulator of TGF-β’s fibrogenic activity. Therefore, the third potential mechanism whereby increased PAI-1 promotes lung fibrosis in old mice is to increase the sensitivity of fibroblasts to TGF-β.
It should be emphasized that although some studies have shown that aging per se leads to increased deposition of fibrotic tissue in the lung this seems to be species or strain specific. The collagen deposition in the lung was increased with age in rats (Calabresi and others 2007) but not in mice (Naik and others 2012; Redente and others 2011; Sueblinvong and others 2012). Huang et al further showed that aging-related accumulation of collagens in the lung occurred in 20 month old DBA/2J mice but not in 20 month old C57B6 mice, compared to young mice of corresponding strain (Huang and others 2007a). Consistent with the results reported by these investigators (Naik and others 2012; Redente and others 2011; Sueblinvong and others 2012), we show, in this study, that there was no significant increase in fibrotic tissue in the lung of 18-month old mice, compared to 3-month old mice, under unchallenged condition. The results suggest that an increase in PAI-1 expression per se may not lead to fibrosis, although it may increase the sensitivity of old animals to fibrotic stimuli.
It should also be stressed that although it has been well documented that PAI-1 contributes to the development of lung fibrosis (Eitzman and others 1996; Huang and others 2012; Senoo and others 2010; Zhang and others 2012), the profibrogenic function of PAI-1 in some other organs is still debated. Kaikita (Kaikita and others 2001) and Weisberg (Weisberg and others 2005) showed that inhibition of PAI-1 activity with a small molecule PAI-1 inhibitor or knockout of the PAI-1 gene attenuated aortic or vascular remodeling. Moriwaki (Moriwaki and others 2004) and Xu (Xu and others 2010) demonstrated, on the other hand, that knockout of the PAI-1 gene led to the development of heart fibrosis. Knier et al further showed that knockout of the PAI-1 gene attenuated kidney but potentiated heart fibrosis in a hypertension model (Knier and others 2011). Apparent divergent roles of PAI-1 in the development of liver fibrosis have also been observed (Beier and others 2011; von Montfort and others 2010; Wang and others 2007). The mechanism underlying the conflicting effects of PAI-1 on the development of fibrosis in different organs or in the same organ but induced by different stimuli remains unclear and warrants further investigation.
The mechanism whereby PAI-1 protects lung (myo)fibroblasts from apoptosis in old mice is unclear. Horowitz et al showed that plasminogen and plasmin induced apoptosis in cultured fibroblasts, which was associated with pericellular fibronectin proteolysis (Horowitz and others 2008). They also showed that PAI-1 protected fibroblasts from apoptosis induced by plasminogen but not by plasmin, suggesting that PAI-1 protects cultured fibroblasts from apoptosis by inhibiting plaminogen activation (Horowitz and others 2008). Zhang et al reported, on the other hand, that PAI-1 inhibited apoptosis of cultured lung fibroblasts by increasing intracellular Ca2+ concentration and thereby activation of caspase-3, pERK, and Akt (Zhang and others 2013). In a previous study, we showed that small molecule PAI-1 inhibitor TM5275 increased the protein level of p53 as well as the activity of Caspase-3/7, associated with an induction of apoptosis in cultured human lung fibroblasts (Huang and others 2012). Whether PAI-1 protects (myo)fibroblasts from apoptosis in old mouse lung through inhibiting plasmin activity, caspase-3 activity, or p53 expression remains to be determined. We also want to point out that although PAI-1 protects fibroblasts from apoptosis (Chang and others 2010; Horowitz and others 2008; Huang and others 2012; Zhang and others 2013), opposite effects have been observed in other types of cells (Abderrahmani and others 2012; Bajou and others 2008; Bhandary and others 2013; Chen and others 2008; Shetty and others 2012). Whether PAI-1 expression is increased with age in other types of cells in the lung and how PAI-1 regulates apoptosis sensitivity in these other types of cells is unknown and is under active investigation in this lab.
The mechanism underlying the aging-related increase in PAI-1 expression is also unclear. TGF-β is the most potent PAI-1 inducer. It has been reported that the expression of TGF-β and TGF-β receptor mRNAs in the lung was increased in 24-month old mice compared to 3-month old mice (Sueblinvong and others 2012). In contrast, Naik and Redente et al reported that there was no significant difference in the basal levels of TGF-β between young and old mice, although bleomycin induced more TGF-β in 12–18 month old mice than in 3–4 month old mice (Naik and others 2012; Redente and others 2011). Consistent with the results reported by Naik and Redente (Naik and others 2012; Redente and others 2011), we show, in this study, that there was no significant difference in the basal levels of TGF-β1 protein between 3- and 18-month old mice. Our results suggest that the increased PAI-1 expression in 18-month old mice may not be driven by TGF-β. Besides TGF-β, PAI-1 expression is induced by other growth factors and inflammatory cytokines including TNF-α, IL-1β, IL-6, and insulin/insulin-like growth factor (Dimova and Kietzmann 2008; Dong and others 2007; Hou and others 2004; Kruithof 2008). Interestingly, studies from this lab and others have shown that reactive oxygen species (ROS) mediate the induction of PAI-1 by many of these stimuli including TGF-β1, TNF-α, and IL-1 (Liu 2008; Liu and others 2010; Okada and others 1998; Oszajca and others 2008; To and others 2013; Vayalil and others 2007). As oxidative stress increases with age and is evident in many age-related diseases including IPF (Hecker and others 2014; Kinnula 2008; Kinnula and Myllarniemi 2008; Liu 2008), one potential mechanism underlying the aging-related increase in PAI-1 expression is increased oxidative stress. More studies are needed to address whether and how increased oxidative stress leads to increased PAI-1 expression during aging, which is beyond the scope of this study.
In summary, the data presented in this study suggest that increased PAI-1 may contribute importantly to the increased susceptibility of the elderly to lung fibrosis by protecting (myo)fibroblasts from apoptosis and increasing the sensitivity of (myo)fibroblasts to TGF-β1. More studies are needed to uncover the mechanism underlying aging-related increase in PAI-1 expression.
Highlights.
First to show that PAI-1 protein level is increased with age in mouse lung tissue and lung fibroblasts
First to show that apoptosis resistance of lung fibroblasts is associated with increased PAI-1 both in vivo and in vitro
Old mice experienced augmented PAI-1 induction and lung fibrosis, compared to young mice, upon bleomycin challenge
Inhibition of PAI-1 activity eliminated the aging-related apoptosis resistance and TGF-β1 sensitivity in isolated fibroblasts
Knockdown of PAI-1 with PAI-1 siRNA increased apoptosis sensitivity and suppressed TGF-β1 response in human lung fibroblasts
ACKNOWLEDGEMENTS
The work was supported by grants from the National Institute of Environmental Health Sciences NHLBI (5R01HL088141) as well as by a grant from the American Lung Association to Rui-Ming Liu. The authors would also like to thank Dr. Namasivayam Ambalavanan and Brain A. Halloran for their assistance in fluorescence microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abderrahmani R, Francois A, Buard V, Tarlet G, Blirando K, Hneino M, Vaurijoux A, Benderitter M, Sabourin JC, Milliat F. PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PloS one. 2012;7:e35740. doi: 10.1371/journal.pone.0035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aillaud MF, Pignol F, Alessi MC, Harle JR, Escande M, Mongin M, Juhan-Vague I. Increase in plasma concentration of plasminogen activator inhibitor, fibrinogen, von Willebrand factor, factor VIII:C and in erythrocyte sedimentation rate with age. Thrombosis and haemostasis. 1986;55:330–332. [PubMed] [Google Scholar]
- Araki T, Katsura H, Sawabe M, Kida K. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Intern Med. 2003;42:483–489. doi: 10.2169/internalmedicine.42.483. [DOI] [PubMed] [Google Scholar]
- Bajou K, Peng H, Laug WE, Maillard C, Noel A, Foidart JM, Martial JA, DeClerck YA. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell. 2008;14:324–334. doi: 10.1016/j.ccr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JI, Kaiser JP, Guo L, Martinez-Maldonado M, Arteel GE. Plasminogen activator inhibitor-1 deficient mice are protected from angiotensin II-induced fibrosis. Arch Biochem Biophys. 2011;510:19–26. doi: 10.1016/j.abb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandary YP, Shetty SK, Marudamuthu AS, Ji HL, Neuenschwander PF, Boggaram V, Morris GF, Fu J, Idell S, Shetty S. Regulation of Lung Injury and Fibrosis by p53-Mediated Changes in Urokinase and Plasminogen Activator Inhibitor-1. Am J Pathol. 2013;183:131–143. doi: 10.1016/j.ajpath.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Macotela Y, Bezy O, Mori MA, Kriauciunas K, Kahn CR. A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis. Science signaling. 2010;3:ra87. doi: 10.1126/scisignal.2001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. American journal of respiratory cell and molecular biology. 1999;20:228–236. doi: 10.1165/ajrcmb.20.2.3150. [DOI] [PubMed] [Google Scholar]
- Calabresi C, Arosio B, Galimberti L, Scanziani E, Bergottini R, Annoni G, Vergani C. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Experimental gerontology. 2007;42:1003–1011. doi: 10.1016/j.exger.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Casanova A, Giron RM, Molina M, Xaubet A, Ancochea J. Predictive factors for survival in patients with idiopathic pulmonary fibrosis. Med Clin (Barc) 2009;133:333–336. doi: 10.1016/j.medcli.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Cesari M, Pahor M, Incalzi RA. REVIEW: Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovascular Therapeutics. 2010;28:e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. The Journal of biological chemistry. 2010;285:8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Henry DO, Reczek PR, Wong MK. Plasminogen activator inhibitor-1 inhibits prostate tumor growth through endothelial apoptosis. Molecular cancer therapeutics. 2008;7:1227–1236. doi: 10.1158/1535-7163.MCT-08-0051. [DOI] [PubMed] [Google Scholar]
- Chuang-Tsai S, Sisson TH, Hattori N, Tsai CG, Subbotina NM, Hanson KE, Simon RH. Reduction in fibrotic tissue formation in mice genetically deficient in plasminogen activator inhibitor-1. Am J Pathol. 2003;163:445–452. doi: 10.1016/S0002-9440(10)63674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, Warnock M, Xue B, Cunningham AK, Lin Y, Goldklang MP, Simon RH, Lawrence DA, Sisson TH. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood. 2011;118:2313–2321. doi: 10.1182/blood-2010-12-324574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova EY, Kietzmann T. Metabolic, hormonal and environmental regulation of plasminogen activator inhibitor-1 (PAI-1) expression: lessons from the liver. Thrombosis and haemostasis. 2008;100:992–1006. [PubMed] [Google Scholar]
- Dong J, Fujii S, Imagawa S, Matsumoto S, Matsushita M, Todo S, Tsutsui H, Sobel BE. IL-1 and IL-6 induce hepatocyte plasminogen activator inhibitor-1 expression through independent signaling pathways converging on C/EBPdelta. American journal of physiology Cell physiology. 2007;292:C209–C215. doi: 10.1152/ajpcell.00157.2006. [DOI] [PubMed] [Google Scholar]
- Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. The Journal of clinical investigation. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR, Mutlu GM, Miyata T, Vaughan DE. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, Gross BH, Myers J, Travis WD, Colby TV, Toews GB, Flaherty KR. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2010;181:832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Perez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137:129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. The Journal of clinical investigation. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting nox4-nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen Activation Induced Pericellular Fibronectin Proteolysis Promotes Fibroblast Apoptosis. Am J Respir Cell Mol Biol. 2008;38:78–87. doi: 10.1165/rcmb.2007-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Eren M, Painter CA, Covington JW, Dixon JD, Schoenhard JA, Vaughan DE. Tumor Necrosis Factor {alpha} Activates the Human Plasminogen Activator Inhibitor-1 Gene through a Distal Nuclear Factor {kappa}B Site. J Biol Chem. 2004;279:18127–18136. doi: 10.1074/jbc.M310438200. [DOI] [PubMed] [Google Scholar]
- Huang K, Mitzner W, Rabold R, Schofield B, Lee H, Biswal S, Tankersley CG. Variation in senescent-dependent lung changes in inbred mouse strains. J Appl Physiol. 2007a;102:1632–1639. doi: 10.1152/japplphysiol.00833.2006. [DOI] [PubMed] [Google Scholar]
- Huang K, Rabold R, Schofield B, Mitzner W, Tankersley CG. Age-dependent changes of airway and lung parenchyma in C57BL/6J mice. J Appl Physiol. 2007b;102:200–206. doi: 10.1152/japplphysiol.00400.2006. [DOI] [PubMed] [Google Scholar]
- Huang WT, Vayalil PK, Miyata T, Hagood J, Liu RM. Therapeutic value of small molecule inhibitor to plasminogen activator inhibitor-1 for lung fibrosis. American journal of respiratory cell and molecular biology. 2012;46:87–95. doi: 10.1165/rcmb.2011-0139OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. The Journal of clinical investigation. 2003;112:379–388. doi: 10.1172/JCI18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura A, Matsumoto S, Suzuki S, Dan T, Yamaki S, Sato Y, Kiyomoto H, Ishii N, Okada K, Matsuo O, Hou FF, Vaughan DE, van Ypersele de Strihou C, Miyata T. A small molecule inhibitor to plasminogen activator inhibitor 1 inhibits macrophage migration. Arterioscler Thromb Vasc Biol. 2013;33:935–942. doi: 10.1161/ATVBAHA.113.301224. [DOI] [PubMed] [Google Scholar]
- Izuhara Y, Yamaoka N, Kodama H, Dan T, Takizawa S, Hirayama N, Meguro K, van Ypersele de Strihou C, Miyata T. A novel inhibitor of plasminogen activator inhibitor-1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. J Cereb Blood Flow Metab. 2010;30:904–912. doi: 10.1038/jcbfm.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–844. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]
- Kinnula VL. Redox imbalance and lung fibrosis. Antioxidants & redox signaling. 2008;10:249–252. doi: 10.1089/ars.2007.1912. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Myllarniemi M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxidants & redox signaling. 2008;10:727–738. doi: 10.1089/ars.2007.1942. [DOI] [PubMed] [Google Scholar]
- Knier B, Cordasic N, Klanke B, Heusinger-Ribeiro J, Daniel C, Veelken R, Hartner A, Hilgers KF. Effect of the plasminogen-plasmin system on hypertensive renal and cardiac damage. J Hypertens. 2011;29:1602–1612. doi: 10.1097/HJH.0b013e32834840e8. [DOI] [PubMed] [Google Scholar]
- Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thrombosis and haemostasis. 2008;100:969–975. [PubMed] [Google Scholar]
- Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxidants & redox signaling. 2008;10:303–319. doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RM, Choi J, Wu JH, Gaston Pravia KA, Lewis KM, Brand JD, Mochel NS, Krzywanski DM, Lambeth JD, Hagood JS, Forman HJ, Thannickal VJ, Postlethwait EM. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. The Journal of biological chemistry. 2010;285:16239–16247. doi: 10.1074/jbc.M110.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RM, van Groen T, Katre A, Cao D, Kadisha I, Ballinger C, Wang L, Carroll SL, Li L. Knockout of plasminogen activator inhibitor 1 gene reduces amyloid beta peptide burden in a mouse model of Alzheimer's disease. Neurobiology of aging. 2011;32:1079–1089. doi: 10.1016/j.neurobiolaging.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, Kavanagh TJ, Matthews QL, Postlethwait EM. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic Biol Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int. 2005;67:2221–2238. doi: 10.1111/j.1523-1755.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res. 2004;95:637–644. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]
- Naik PN, Horowitz JC, Moore TA, Wilke CA, Toews GB, Moore BB. Pulmonary fibrosis induced by gamma-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-beta. J Gerontol A Biol Sci Med Sci. 2012;67:714–725. doi: 10.1093/gerona/glr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer F, Trenkmann M, Ospelt C, Karouzakis E, Neidhart M, Stanczyk J, Kolling C, Gay RE, Detmar M, Gay S, Jungel A, Kyburz D. Down-regulation of microRNA-34a* in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis and rheumatism. 2012;64:1771–1779. doi: 10.1002/art.34334. [DOI] [PubMed] [Google Scholar]
- Okada H, Woodcock-Mitchell J, Mitchell J, Sakamoto T, Marutsuka K, Sobel BE, Fujii S. Induction of plasminogen activator inhibitor type 1 and type 1 collagen expression in rat cardiac microvascular endothelial cells by interleukin-1 and its dependence on oxygen-centered free radicals. Circulation. 1998;97:2175–2182. doi: 10.1161/01.cir.97.21.2175. [DOI] [PubMed] [Google Scholar]
- Oszajca K, Bieniasz M, Brown G, Swiatkowska M, Bartkowiak J, Szemraj J. Effect of oxidative stress on the expression of t-PA, u-PA, u-PAR, and PAI-1 in endothelial cells. Biochem Cell Biol. 2008;86:477–486. doi: 10.1139/O08-137. [DOI] [PubMed] [Google Scholar]
- Park JM, Lee JS, Lee KR, Ha SJ, Hong EK. Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence. Nutrients. 2014;6:3711–3726. doi: 10.3390/nu6093711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro GA, Antao VC, Wood JM, Wassell JT. Occupational risks for idiopathic pulmonary fibrosis mortality in the United States. Int J Occup Environ Health. 2008;14:117–123. doi: 10.1179/oeh.2008.14.2.117. [DOI] [PubMed] [Google Scholar]
- Poggi M, Paulmyer-Lacroix O, Verdier M, Peiretti F, Bastelica D, Boucraut J, Lijnen HR, Juhan-Vague I, Alessi MC. Chronic plasminogen activator inhibitor-1 (PAI-1) overexpression dampens CD25+ lymphocyte recruitment after lipopolysaccharide endotoxemia in mouse lung. Journal of Thrombosis and Haemostasis. 2007;5:2467–2475. doi: 10.1111/j.1538-7836.2007.02757.x. [DOI] [PubMed] [Google Scholar]
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- Redente EF, Jacobsen KM, Solomon JJ, Lara AR, Faubel S, Keith RC, Henson PM, Downey GP, Riches DW. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L510–L518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs JJTH, Teske GJD, Bonta PI, de Vries CJM, Meijers JCM, Weening JJ, van der Poll T, Florquin S. Plasminogen activator inhibitor-1 regulates neutrophil influx during acute pyelonephritis. Kidney Int. 2008;75:52–59. doi: 10.1038/ki.2008.454. [DOI] [PubMed] [Google Scholar]
- Senoo T, Hattori N, Tanimoto T, Furonaka M, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Yokoyama A, Kohno N. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax. 2010;65:334–340. doi: 10.1136/thx.2009.119974. [DOI] [PubMed] [Google Scholar]
- Seo JY, Park J, Yu MR, Kim YS, Ha H, Lee HB. Positive feedback loop between plasminogen activator inhibitor-1 and transforming growth factor-beta1 during renal fibrosis in diabetes. Am J Nephrol. 2009;30:481–490. doi: 10.1159/000242477. [DOI] [PubMed] [Google Scholar]
- Serrano R, Barrenetxe J, Orbe J, Rodriguez JA, Gallardo N, Martinez C, Andres A, Paramo JA. Tissue-specific PAI-1 gene expression and glycosylation pattern in insulin-resistant old rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1563–R1569. doi: 10.1152/ajpregu.00093.2009. [DOI] [PubMed] [Google Scholar]
- Shetty SK, Bhandary YP, Marudamuthu AS, Abernathy D, Velusamy T, Starcher B, Shetty S. Regulation of airway and alveolar epithelial cell apoptosis by p53-Induced plasminogen activator inhibitor-1 during cigarette smoke exposure injury. American journal of respiratory cell and molecular biology. 2012;47:474–483. doi: 10.1165/rcmb.2011-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueblinvong V, Neujahr DC, Mills ST, Roser-Page S, Ritzenthaler JD, Guidot D, Rojas M, Roman J. Predisposition for disrepair in the aged lung. The American journal of the medical sciences. 2012;344:41–51. doi: 10.1097/MAJ.0b013e318234c132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szigeti A, Hocsak E, Rapolti E, Racz B, Boronkai A, Pozsgai E, Debreceni B, Bognar Z, Bellyei S, Sumegi B, Gallyas F., Jr Facilitation of mitochondrial outer and inner membrane permeabilization and cell death in oxidative stress by a novel Bcl-2 homology 3 domain protein. The Journal of biological chemistry. 2010;285:2140–2151. doi: 10.1074/jbc.M109.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, "Klotho" mouse. Semin Thromb Hemost. 2002;28:545–554. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To M, Takagi D, Akashi K, Kano I, Haruki K, Barnes PJ, Ito K. Sputum plasminogen activator inhibitor-1 elevation by oxidative stress-dependent nuclear factor-kappaB activation in COPD. Chest. 2013;144:515–521. doi: 10.1378/chest.12-2381. [DOI] [PubMed] [Google Scholar]
- Vayalil PK, Iles KE, Choi J, Yi AK, Postlethwait EM, Liu RM. Glutathione suppresses TGF-beta-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1281–L1292. doi: 10.1152/ajplung.00128.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Montfort C, Beier JI, Kaiser JP, Guo L, Joshi-Barve S, Pritchard MT, States JC, Arteel GE. PAI-1 plays a protective role in CCl4-induced hepatic fibrosis in mice: role of hepatocyte division. Am J Physiol Gastrointest Liver Physiol. 2010;298:G657–G666. doi: 10.1152/ajpgi.00107.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Heuckeroth RO. PAI-1 deficiency reduces liver fibrosis after bile duct ligation in mice through activation of tPA. FEBS letters. 2007;581:3098–3104. doi: 10.1016/j.febslet.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, Vaughan DE, Brown NJ. Pharmacological Inhibition and Genetic Deficiency of Plasminogen Activator Inhibitor-1 Attenuates Angiotensin II/Salt-Induced Aortic Remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- Xu Z, Castellino FJ, Ploplis VA. Plasminogen activator inhibitor-1 (PAI-1) is cardioprotective in mice by maintaining microvascular integrity and cardiac architecture. Blood. 2010;115:2038–2047. doi: 10.1182/blood-2009-09-244962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka N, Kodama H, Izuhara Y, Miyata T, Meguro K. Structure-activity relationships of new N-acylanthranilic acid derivatives as plasminogen activator inhibitor-1 inhibitors. Chem Pharm Bull (Tokyo) 2011;59:215–224. doi: 10.1248/cpb.59.215. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Zheng M, Zhao J, Li YY, Huang Z, Li Z, Han J. Multiple death pathways in TNF-treated fibroblasts: RIP3- and RIP1-dependent and independent routes. Cell research. 2011;21:368–371. doi: 10.1038/cr.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Li WB, Wang WL, Liu J, Song SX, Bai LL, Hu YY, Yuan YD, Zhang M. siRNA against plasminogen activator inhibitor-1 ameliorates bleomycin-induced lung fibrosis in rats. Acta Pharmacol Sin. 2012 doi: 10.1038/aps.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Wang WL, Liu J, Li WB, Bai LL, Yuan YD, Song SX. Plasminogen activator inhibitor-1 promotes the proliferation and inhibits the apoptosis of pulmonary fibroblasts by Ca(2+) signaling. Thrombosis research. 2013;131:64–71. doi: 10.1016/j.thromres.2012.09.003. [DOI] [PubMed] [Google Scholar]