Abstract
Genetic and pharmacological studies of indoleamine 2,3-dioxygenase (IDO) have established this tryptophan catabolic enzyme as a central driver of malignant development and progression. IDO acts in tumor, stromal and immune cells to support pathogenic inflammatory processes that engender immune tolerance to tumor antigens. The multifaceted effects of IDO activation in cancer include the suppression of T and NK cells, the generation and activation of T regulatory cells and myeloid-derived suppressor cells, and the promotion of tumor angiogenesis. Mechanistic investigations have defined the aryl hydrocarbon receptor, the master metabolic regulator mTORC1 and the stress kinase Gcn2 as key effector signaling elements for IDO, which also exerts a non-catalytic role in TGF-β signaling. Small-molecule inhibitors of IDO exhibit anticancer activity and cooperate with immunotherapy, radiotherapy or chemotherapy to trigger rapid regression of aggressive tumors otherwise resistant to treatment. Notably, the dramatic antitumor activity of certain targeted therapeutics such as imatinib (Gleevec) in gastrointestinal stromal tumors has been traced in part to IDO downregulation. Further, antitumor responses to immune checkpoint inhibitors can be heightened safely by a clinical lead inhibitor of the IDO pathway that relieves IDO-mediated suppression of mTORC1 in T cells. In this personal perspective on IDO as a nodal mediator of pathogenic inflammation and immune escape in cancer, we provide a conceptual foundation for the clinical development of IDO inhibitors as a novel class of immunomodulators with broad application in the treatment of advanced human cancer.
Keywords: Tryptophan catabolism, Immunometabolism, Immune escape, Immune surveillance, Immunoediting, Cancer-associated inflammation
Introduction
Advanced metastatic cancer is the key challenge in cancer research, with existing therapies providing only limited benefit to those patients who present with disseminated disease at diagnosis or who relapse with drug-resistant disease after initial response. While it is clear that tumors display many immunogenic antigens, they manage to escape immune rejection by somehow evading, subverting or reprogramming the immune system. However, while immune escape is central to the development of a clinically relevant cancer, the mechanistic basis of this phenomenon is not yet fully understood, in part because its critical contributions to cancer were not accepted by the mainstream in the field of cancer research until relatively recently [1, 2]. An appropriately activated immune system can eradicate cancer, even when it is aggressive and disseminated, but spontaneous occurrences of such events in humans are rare. Cancer immunology is arguably the oldest field of cancer research, with roots in the nineteenth century, yet effective strategies to stimulate efficacious responses beyond a few patients has been elusive. With the recent description of mechanisms of tumoral immune suppression and escape that engender pathological immune tolerance, it is becoming clear that to ‘get on the gas’ of immune activation against tumors, it is necessary to ‘get off the brakes’ of tumor-associated immune suppression [1, 3]. One such ‘braking’ mechanism with considerable practical appeal focuses on the enzyme indoleamine 2,3-dioxygenase (IDO) which functions in tryptophan catabolism [4–10]. Figure 1 summarizes the various sites in tumors and tumor-draining lymph nodes (TDLN) where IDO is expressed and has been proposed to promote cancer.
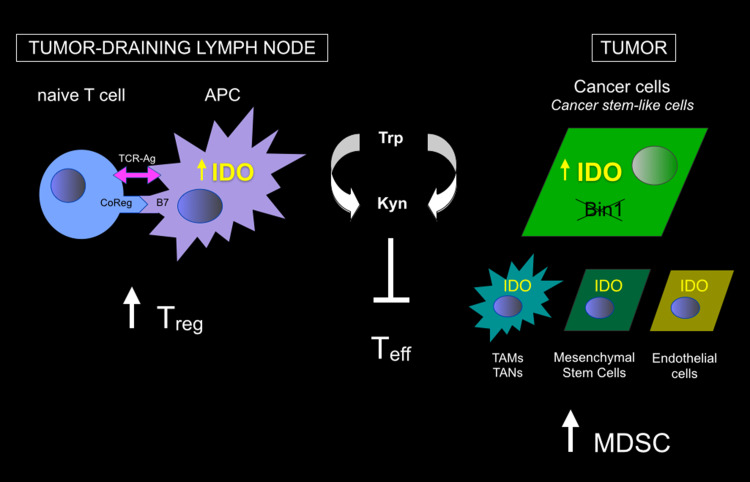
Fig. 1.
Sites of IDO expression and action in cancer. IDO expression has been documented in a variety of cells in tumors and tumor-draining lymph nodes (and other metastatic sites) including malignant cells as well as other stromal, vascular and immune cells indicated. Both tryptophan deprivation and kynurenine production mediated by IDO has been implicated in inflammatory processes and the generation of antigenic immune tolerance (immune escape). The figure summarizes the general effects that have been described on T cell function at each site. APC antigen-presenting cell (e.g., dendritic cell), MDSC myeloid-derived suppressor cell, TAM tumor-associated macrophage, TAN tumor-associated neutrophil, Teff T effector cell, Treg T regulatory cell
IDO and T cell suppression in cancer
The discovery of IDO was rooted in initial observations made in the 1950s in cancer patients where tryptophan catabolism was found to be elevated [11]. In the 1970s, the gene encoding IDO1 was the first interferon-activated gene to be described [12], but the impact of this association was obscure. In 1998, a conceptual breakthrough emerged from the work of Munn, Mellor and their colleagues suggesting that IDO might mediate immunosuppression based on the preferential sensitivity of T cells to tryptophan deprivation [13]. Briefly, they proposed that tryptophan deprivation would impair antigen-dependent T cell activation in microenvironments where IDO was active. Initial evidence supporting this concept was offered by studies of how immune tolerance to ‘foreign’ paternal antigens in pregnant mice could be reversed by the bioactive IDO inhibitor 1-methyl-tryptophan (1MT), the administration of which elicited MHC-restricted T cell-mediated rejection of allogeneic concepti [13, 14]. Subsequent studies developed this concept as a mechanism to defeat immune surveillance in cancer (reviewed in [8, 15]), supported by evidence that IDO activity could suppress T cells [16–18] and NK cells [19], and also that IDO was critical to support the formation and activity of Tregs [20] and myeloid-derived suppressor cells (MDSCs) [10].
Regulatory functions for both tryptophan depletion and catabolite production by IDO have been described, with a keen focus on regulatory roles in antigen-presenting dendritic cells (DC) where IDO is regulated by interferons, TLR ligands and other important immune signals [21]. IDO is produced in response to IFN-γ in endothelial cells, mesenchymal stromal cells, fibroblasts and various myeloid-derived cells including dendritic cells and macrophages [22]. For example, IDO expression in a small minority population of dendritic cells (DC) enables them to dominantly suppress effector T cell responses [23, 24]. The anergizing response of T cells to IDO-mediated tryptophan depletion requires the stress-response kinase GCN2, which is also required for IDO-induced differentiation of CD4+ T cells into Tregs [15]. Likewise, kynurenine and other tryptophan catabolites block T cell activation and trigger T cell apoptosis while also promoting the emergence of Tregs (through a TGF-β-dependent mechanism), with apparent synergistic effects of this effector arm with tryptophan deprivation [25]. Extending its breadth as an important immune regulator, IDO has been implicated in numerous diseases characterized by disordered immune control, including cancer, chronic viral infection, allergy and autoimmune and inflammatory diseases characterized by disordered immune control [26].
Indoleamine 2,3-dioxygenase is widely overexpressed in tumor cells where it is has been associated predominantly with poor prognosis [27, 28]. Mouse genetic studies suggest that IDO overexpression can be mediated by inactivation of Bin1, a tumor suppressor gene that is widely inactivated during cancer progression [5, 29]. Bin1 is among the most frequently attenuated genes in human cancer, due to aberrant RNA splicing patterns that eliminate its suppressor functions [30–34] or to altered gene methylation patterns that extinguish its expression [35–39]. While Bin1 inactivation produces cancer cell-intrinsic benefits to cell proliferation, motility and survival [29], in vivo studies argue that the most important consequence of Bin1 inactivation is the upregulation of IDO expression leading to T cell suppression [5].
IDO mediates pathogenic inflammatory processes needed to support cancer
The tissue microenvironment where a tumor arises poses a huge barrier to tumor development and progression. IDO has been found to act at multiple levels to create a more hospitable environment for tumor development and metastatic progression (Fig. 2). Genetic studies in mice indicate that IDO contributes a crucial function to the inflammatory tumor microenvironment. As shown originally in a classical model of DMBA + TPA-induced inflammatory skin carcinogenesis, IDO is activated normally in the skin and in TDLN as part of the chronic inflammation required for tumor emergence [40]. Studies in this model argued that thinking of IDO solely as a modifier of immune tolerance oversimplifies its role in cancer pathogenesis. IDO induction was integral to the inflammatory tissue microenvironment, even in the absence of cancer, and IDO loss had little impact on tumor formation in the absence of an inflammatory promoter [9]. Moreover, it was clear that IDO was dispensable for engraftment of established cancer cell lines that already had developed an effective immunoediting route, unlike transgenic models where the route must be developed and will vary between individual mice. Interestingly, bone marrow transplant experiments argued that the key source of IDO function required for tumor initiation was non-hematopoietic cells, in support of the evidence that Bin1 deficiency in cancer cells is sufficient to facilitate IDO-mediated immune escape by a cell-autonomous mechanism [9]. Thus, IDO appears to function proximally as an element of ‘cancer-associated’ inflammatory processes that tilt the immune system toward tumor support. At a broader level, these studies of IDO suggested that mediators of immune escape and cancer-associated inflammation may be genetically synonymous, at least during the initial stages of tumor development [41].
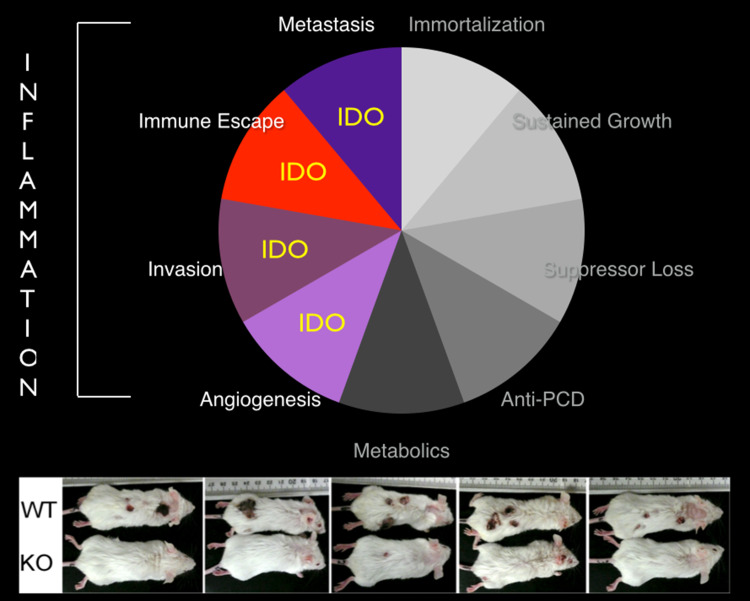
Fig. 2.
IDO programs a pathogenic inflammatory state that supports multiple traits of cancer progression. IDO is highlighted in traits where its role has been functionally implicated in preclinical and clinical studies. The panel below shows the outcome of a regimen of inflammatory skin carcinogenesis in wild-type (WT) and IDO1-deficient (KO) mice, illustrating the essential contributions of IDO function [40]
Other observations from Ido1-deficient mice strengthen the concept that IDO exerts a proximal influence on inflammation that is too subtle to understand as simply immunosuppressive. If IDO were a solely immunosuppressive enzyme, inflammation might be expected to run rampant in Ido1-deficient mice where this presumptive check is no longer in place. However, Ido1 deficiency does not produce such effects, in contrast to deficiency of an immunosuppressive function like CTLA-4. Moreover, the inflammation elicited in Ido1-deficient mice by treatment with a pro-inflammatory agent was not discernibly different than in wild-type control animals receiving the same treatment [9]. So, rather than IDO simply being an immunosuppressive counterbalance in inflammatory reactions, a more nuanced interpretation is required in which IDO shapes the inflammatory pathogenicity of the tissue microenvironment.
The degradation of normal cellular physiology leading to malignancy involves acquisition of the cell-intrinsic traits of immortalization, growth sufficiency, insensitivity to growth inhibitory signals and resistance to apoptosis, along with the cell-extrinsic traits of angiogenesis, invasive capability, metastatic capacity and immune escape. In this context, immune escape mechanisms utilized by tumors, such as IDO induction, have been postulated to be a terminal feature of the immunoediting process, which comprises the three distinct phases of elimination, equilibrium and escape [42]. However, a contrarian argument has also been made that tumoral immune escape is not a late event driven by selective pressure, but instead develops as an early, integral component of the tumorigenic process [43]. The multistage aspect of the DMBA/TPA carcinogenesis protocol described above provided us with a unique opportunity to investigate this question with regard to the role of IDO induction in the contextual setting of de novo tumor development. The immunoediting postulate would require that there be at least some nascent tumor present for IDO to be induced. Instead, however, TPA treatment alone was sufficient to induce IDO in the proximal lymph nodes [40]. Because these mice were never exposed to DMBA-based tumor initiation, this elevation of IDO occurred in the absence of cancer, as TPA alone is not able to drive the development of neoplasia in the absence of an initiating agent. This outcome, therefore, was more in line with IDO elevation being an early event driven by TPA-elicited inflammation, rather that a late event driven by immune selection.
Other studies extend the notion that IDO acts in a proximal manner to program pathogenic inflammatory processes which then go on to direct antigenic tolerization in the adaptive immune system at a more distal level. In one study, ectopic modulation of IDO in murine breast cancer cells influenced T cell responses in immunocompetent mice, but also affected primary tumor growth and metastasis in immunodeficient scid/beige mice which lack T, B and NK cells [44]. Thus, these pathogenic effects of IDO overexpression could not be readily interpreted as mediated solely by adaptive immunological mechanisms. The conceptual realization that IDO acts as an integral component of the inflammatory milieu is supported additionally by evidence of a role in supporting other pathogenicities associated with chronic inflammation. For example, IDO-mediated tryptophan degradation is elevated in rheumatoid arthritis and systemic lupus erythematosus patients, suggesting a role for increased IDO activity in promoting autoimmune disease [45, 46]. These observations have some direct corroborative support from studies in the KxB/N spontaneous mouse model of arthritis [47]. In the KxB/N model, IDO activity is elevated at disease onset, and administration of the IDO inhibitor 1MT results in alleviation of joint inflammation, with 1MT-treated animals exhibiting minimal synovial expansion and fewer infiltrating inflammatory cells [48]. In this setting, 1MT treatment did not affect levels of T regulatory cells or Th1/Th2/Th17 cytokines, but it did greatly diminish the autoreactive B cell response, indicative of a role for IDO upregulation in supporting the development of autoimmune disease by supporting the activation of autoreactive B cells. In conjunction with results from cancer models, these results argue strongly that IDO contributes to pathogenic forms of chronic inflammation in a manner that is more complex than simply acting as an immunosuppressive brake.
IDO activation drives tumor angiogenesis and metastasis
More recent findings derived from mouse models of lung and metastatic breast carcinoma further elucidate how IDO contributes to cancer development by altering the inflammatory milieu [10]. Ido1-deficient mice exhibited reduced primary or metastatic pulmonary tumor burdens in each model that were associated with improved survival. In each model, IDO deficiency was associated with an attenuation of IL-6 that limited the accumulation and T cell suppressor function of MDSC known to be critical. The importance of these findings to metastasis was shown by restoration of IL-6 levels, which overcame the MDSC impairment and allowed pulmonary metastases to progress at the same rate as Ido1-competent mice [10]. The implication that IL-6 serves as a key regulator of tumor growth downstream of IDO has therapeutic implication as increased IL-6 levels are associated with recurring tumors in patients [49]. In yet another clue that IDO contributes beyond adaptive immune control, Ido1-deficient mice displayed an angiogenic defect in lungs even in the absence of tumors. Together, these studies highlight a more complex and nuanced interpretation of what tryptophan catabolism means to a developing tumor, extending beyond adaptive immunoregulation to inflammatory programming, metastasis and angiogenesis.
IDO regulatory and effector pathways in cancer
IDO is subject to complex regulation in tumor, tumor stromal cells and immune cells, with the inputs on its expression reinforcing the concept of IDO as a modifier of inflammatory states. Downstream of IDO, functional studies have defined three effector pathways that mediate the effects of IDO activity on T cell function, two related to tryptophan deprivation—Gcn2 activation and mTOR repression—and a third involving kynurenine, the product of tryptophan catabolism by IDO (Fig. 3). All of these effector pathways are linked to suppression of T cell-mediated immunity in the tumor microenvironment, as discussed below.
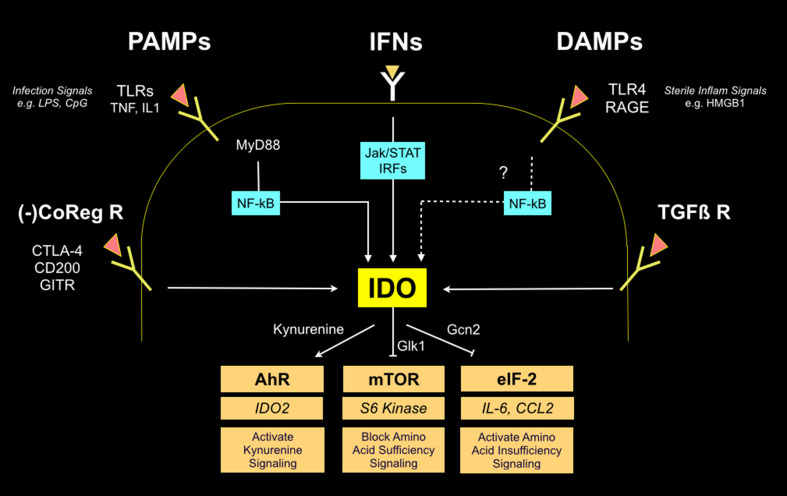
Fig. 3.
IDO regulatory and effector signaling pathways. Multiple upstream-acting regulatory pathways include cell surface receptors which act through intermediary transcription factors (light blue boxes) to stimulate IDO transcription. IDO is also controlled at the level of protein stability by SOCS3 (not shown). Three downstream-acting effector pathways mediate effects of IDO activity (tan boxes), leading to stimulation of AhR by kynurenine binding along with mTOR and eIF-2 suppression due to tryptophan deprivation. Key mediators in each pathway are shown, including IDO2 elevation (via AhR), S6 kinase suppression (via mTOR suppression), and IL-6 and CCL2 elevation (via eiF-2 modulation)
Regulatory pathways
IDO was the first interferon-regulated gene to be described, and it is strongly upregulated by both type I and type II interferons in tumor cells, tumor stromal cells and immune cells, particularly in DC where IDO function has been studied intensively (as reviewed elsewhere, e.g., [26, 50] and in excellent detailed reviews in the literature by Fallarino, Grohmann, Puccetti and colleagues and by Munn, Mellor and colleagues). Briefly, IFN, PAMP and DAMP all broadly activate IDO transcription through canonical and non-canonical NFkB and Jak/STAT pathways, with additional contributions to IDO expression provided by PKC and TGF-β signaling pathways associated with cancerous inflammations or non-inflammatory contexts, respectively [9, 51]. In regulatory DC, IDO is upregulated by reverse signaling from negative-acting T cell co-regulatory receptors, such as CTLA-4, CD200 and GITR, thereby conferring the critical contributions of tryptophan catabolism to the generation of antigenic tolerance [20, 52–54]. In particular, IDO activation by these co-regulatory pathways is critical for the generation of tolerance to tumor antigens, contributing powerfully as a positive modifier with therapeutic implications [55, 56].
In DC, type I and II interferons act at a central interface between IDO and other components of inflammation and immunity. TLR9 ligands such as CpG were found to induce IDO expression in a subset of DCs through a type 1 interferon-dependent signaling pathway [57]. Interactions with immune cells are also implicated in IDO regulation. The first of these interactions to be characterized was an intriguing reverse signaling mechanism described for the inhibitory T cell co-receptor CTLA-4, which is constitutively expressed on T regulatory (Treg) cells. By binding to B7 ligands CD80 and CD86 on DC, CTLA-4 elicits an IFN-γ-dependent induction of IDO [52]. The stimulatory T cell co-receptor CD28 also binds the same B7 ligands but fails to similarly induce IDO because of the concomitant induction of IL-6 which interferes with IFN-γ-elicited STAT signaling through SOCS3 upregulation [58]. Similarly, CD40, CD200 and GITR all induce IDO by related reverse signaling mechanisms which share the non-canonical NF-κB pathway as a common point of convergence [59].
TGF-β was initially reported to antagonize IFNγ-mediated induction of IDO expression [60]. These experiments, carried out in fibroblasts, run counter to immunosuppressive activity ascribed to TGF-β but parallel its ability to antagonize positively regulated targets of IFN-γ. More recently, a positive relationship between TGF-β and IDO was reported in DC, suggesting that the regulatory impact of TGF-β on IDO expression may be complex and contextual. In these experiments, autocrine TGF-β sustained the activation of IDO in a tolerogenic subpopulation of CD8+ DC, while exogenous TGF-β could convert immunogenic CD8- DCs into tolerogenic cells in conjunction with induction of IDO [61]. In this milieu, it was found that even DCs that lack expression of IDO could be rendered tolerogenic by exposure to tryptophan catobolites produced by IDO-expressing cells [62] as part of a feedforward expansion of IDO-elicited immune suppression described as ‘infectious tolerance’ [63]. From work to date, it is clear that IDO is involved in the maintenance of a stable regulatory phenotype in DC and that IDO exerts a tonic, non-enzymic function that contributes to TGF-β-driven tolerance in non-inflammatory contexts [64].
Effector pathways: kynurenine as ligand for its pro-inflammatory receptor AhR
Work to define critical downstream effector signals has focused mainly on the proximal effects that IDO-expressing cells have on T cell function, whether in the tumor and tumor-draining lymph nodes (or other metastatic niches), as a result of local tryptophan deprivation and/or kynurenine production. Kynurenine production resulting from IDO-mediated tryptophan catabolism is widely recognized as one of the elements which mediates the immunosuppressive effects of IDO [50, 65]. Precisely how kynurenine contributes to inflammatory programming was unclear prior to the identification by Platten and colleagues of the aryl hydrocarbon receptor (AhR) as a physiological receptor for kynurenine [66]. This connection links the fields of toxicology, immunology and cancer biology and may help explain why tryptophan consumption assists pathogenic inflammatory programming and drives malignant progression. In activating AhR, kynurenine not only mediates an effector signaling pathway from IDO but also another tryptophan-catabolizing enzyme, TDO2, in driving cancer growth [66]. Kynurenine binding to AhR is essential to generate T regulatory cells that suppress adaptive immunity [67]. In binding AhR, kynurenine triggers nuclear translocation of this receptor, licensing activation of its target genes. A broad literature implicates AhR in immune regulation, inflammation and carcinogenesis [68] in the same vein that IDO has been implicated [41], and elevated levels of AhR correspond with poor prognosis in cancer patients [66].
The discovery that kynurenine is an endogenous ligand for AhR helps explain the selection for tryptophan consumption mediated by IDO during tumor development, because kynurenine binding to AhR provides a mechanism to help tumors program a pathogenic inflammation in their microenvironment that can tilt it from an antagonist role to a facilitator role (i.e., from immunosurveillance toward immune escape). Upon binding its ligand, AhR locates to the nucleus to activate transcription of a set of pro-inflammatory target genes. In myeloid cells, one of these target genes is the IDO-related gene IDO2 [69, 70], which interacts genetically with IDO and supports Treg formation [71]. In connecting tryptophan consumption to AhR activation, this discovery also helps explain why immune escape and tryptophan consumption may be integrally connected in cancer [41].
Effector pathways: Gcn2 activation elevates IL-6 and CCL2
In a nutrient-deprived tissue microenvironment, such as that which occurs within tumors, tryptophan degradation by IDO may cause a local tryptophan deficiency that leads to the accumulation of uncharged tryptophan-tRNA in cells in the locale. This event may activate GCN2, a stress-response kinase that is simulated by elevations in uncharged tRNA and that limits or alters protein translation in response to this condition. Notably, T cells where GCN2 is genetically disrupted are not susceptible to IDO-mediated suppression of proliferation in vitro or in vivo, and these T cells cannot be anergized by IDO-expressing DC [72]. Further, IDO-expressing DC can induce the immunosuppressive activity pre-existing T regulatory cells (Treg), but this effect is abolished by genetic disruption of GCN2. Thus, one critical downstream effector pathway for IDO to blunt T cell-mediated tumor immunity involves GCN2 activation in T cells, which allows them to respond to tryptophan deprivation manifested in a local tissue microenvironment. GCN2 blunts protein translation by phosphorylating the initiation factor eIF-2α, attenuating its activity and preventing readout of most RNA transcripts. However, some RNA transcripts become preferentially translated, including LIP, an isoform of the immunoregulatory transcription factor NF-IL6 (also known as CEBP-β), which goes on to stimulate translation of IL-6 and other immunoregulatory cytokines [73]. The relevance of this pathway is documented in vivo in tumor-bearing animals, where IDO genetic deficiency leads to reduced IL-6 production, a factor that is causally related to tumor outgrowth and metastasis [10]. The consequences of GCN2 activation by IDO in this regard may differ between cell types, since the effect of IDO on IL-6 production through this pathway can be repressive or inductive [10, 72], but in mouse models of cancer, it appears that IDO supports IL-6 production, and this effect is critical for MDSC function and malignant progression [10].
Effector pathways: mTOR suppression and autophagy
Genetic studies in our laboratory suggested that the role of GCN2 in detecting tryptophan deprivation and stimulating IL-6 production was insufficient for inflammation-driven cancers [40, 74], implying the operation of additional cancer-relevant pathways downstream of IDO. In considering other effector mechanisms, we hypothesized that IDO would suppress the master metabolic regulator mTOR (mTORC1), which monitors not only energy status via AMPK but also the status of essential amino acids [75, 76]. Indeed, in cells harboring an inducible IDO gene, we demonstrated [74] that IDO-mediated catabolism of tryptophan inhibits mTORC1 as well as the T cell receptor regulatory kinase PKC-Θ, both of which are regulatory targets of the master amino acid-sensing kinase GLK1 (also known as MAP4K3) acting upstream of mTORC1 [77]. Thus, tryptophan deprivation generated by IDO activation is read out in two distinct effector pathways, one of which is activated by tryptophan insufficiency (GCN2) and the other of which is blocked by tryptophan insufficiency (mTOR/PKC-Θ via presumptive GLK1 blockade). As expected, mTORC1 suppression by IDO was sufficient to trigger autophagy, as shown by LC3 processing and relocalization in cells, and this effect could be reversed by tryptophan restoration which relieved mTOR blockade [74]. The finding that IDO blocked mTORC1 and stimulated autophagy distinct from Gcn2 control advances understanding of IDO function in the many settings where mTOR acts as a pivotal immune regulator. Further, this work provides a novel conceptual perspective on IDO by suggesting its analogy to the mTOR inhibitor rapamycin and by revealing how IDO can trigger autophagy to anergize T cells in the tumor microenvironment.
IDO inhibitors for cancer therapy
IDO has a number of appealing features as a target for small-molecule drug development. First, IDO is a single-chain catalytic enzyme with a well-defined biochemistry. Unlike many proposed therapeutic targets in cancer, this means that IDO is readily tractable for the discovery and development of small-molecule inhibitors. Second, other tryptophan-catabolizing enzymes (TDO2, IDO2 and TPH) are structurally distinct and/or relatively restricted in their pattern of expression and substrate specificity, mitigating ‘‘off-target’’ issues posed by novel agents. Third, bioactive and orally bioavailable ‘lead’ inhibitors exist which can serve as useful tools for preclinical validation studies. Fourth, Ido1-deficient mice are viable and healthy [78], and their careful examination argues that while IDO inhibitors will exhibit some side effects, they are unlikely to pose unmanageable mechanism-based toxicities [79]. Fifth, pharmacodynamic evaluation of IDO inhibitors can be performed by examining blood serum levels of tryptophan and kynurenine, the chief substrate and downstream product of the IDO reaction, respectively. Lastly, small-molecule inhibitors of IDO offer logistical and cost advantages compared to biological or cell-based therapeutic alternatives to modulating T cell immunity. In pursuing the therapeutic opportunity offered by IDO inhibitors, many studies have documented preclinical evidence of potent anticancer effects. More recently, several approved and experimental drugs that block IDO expression have been described and found to exert their anticancer effects in part through IDO disruption. Figure 4 provides an overview of approaches to block IDO in cancer at the level of effector signaling, enzymatic inhibition or expression blockade, as discussed in turn below. There are no published reports of the clinical experience with IDO inhibitors to date. However, there are over a dozen clinical trials which are enrolling patients, including for various drug combinations, especially for indoximod and INCB024360 which represent the primary clinical leads being evaluated (http://clinicaltrials.gov/ct2/results?term=IDO&Search=Search).
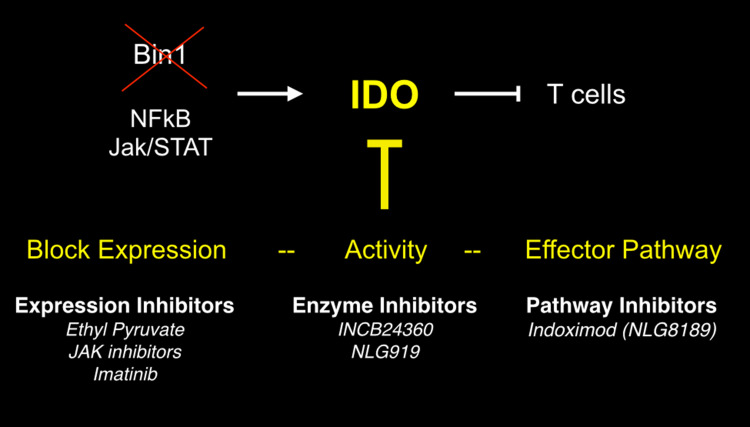
Fig. 4.
Strategies for blocking IDO function in cancer. Attenuation of the tumor suppressor Bin1 in malignant cells relieves suppression of IDO transcription through effects on Jak/STAT and NF-kB dependent pathways. IDO upregulation by this mechanism or the stimulatory mechanisms presented in Fig. 3 manifests IDO as a target for cancer therapy, through expression blockade, enzymatic inhibition or effector signal blockade
Indoximod (D-1MT; also known as NLG8189)
By far, the IDO inhibitor most employed in the literature is the simple racemic compound 1-methyl-d,l-tryptophan (1MT). Evidence offered initially in the early 2000s showed that 1MT could partly retard the growth of cancer cells engrafted into syngeneic hosts [27, 80]. However, these initial studies only weakly addressed whether IDO inhibition could exert meaningful therapeutic effects based on restoring tumor immunity. In spontaneously arising aggressive mammary tumors in the MMTV-neu/HER2 transgenic mouse model of breast cancer, we found that 1MT had little effect on tumor outgrowth but that it could dramatically empower the efficacy of a variety of chemotherapeutic agents, triggering stable regressions of otherwise mainly recalcitrant tumors [5]. Regressions did not reflect drug–drug interaction, that is, by acting to raise the effective dose of the cytotoxic agent, because efficacy was increased in the absence of increased side effects. Most importantly, immunodepletion of CD4+ or CD8+ T cells abolished the combinatorial antitumor effect, and similar results were obtained by structurally different IDO inhibitors, confirming the expectation that they acted by de-repressing T cell-mediated antitumor immunity [5].
Unexpectedly, we later traced the majority of the antitumor activity of 1MT to the D racemer rather than the L racemer, only the latter of which inhibits the activity of the recombinant or cell-expressed IDO enzyme [7]. Factors in choosing to clinically translate D-1MT instead of L-1MT included the greater potency of D-1MT in relieving IDO-mediated suppression of T cell proliferation in mixed lymphocyte reactions involving human IDO + plasmacytoid DCs; the superior preclinical pharmacology and toxicology of D-1MT; the superior antitumor activity relative to L-1MT in our preclincal models (both as a single agent or in combination with chemotherapy); and the genetic validity of targeting the IDO pathway based on the loss of D-1MT antitumor activity in Ido1-nullizygous mice [7]. While others have documented antitumor properties of L-1MT in certain cancer models, our own experience in various aggressive transgenic and graft cancer models has been equivocal. In contrast, our results with D-1MT helped propel it onto a list of select immunotherapeutic agents identified by an NCI workshop panel in 2008 as having high potential for use in cancer therapy [81]. First-in-man Phase I trials were initiated later that year, and at present, D-1MT—now termed indoximod in ongoing clinical studies—is being evaluated in Phase II combination drug trials in breast or prostate cancer patients with taxotere or the DC vaccine sipuleucel-T, respectively (http://clinicaltrials.gov/ct2/results?term=IDO&Search=Search).
Insofar as 1MT is a racemic compound, it was necessary to choose a single molecular species for clinical testing. For the reasons noted above, D-1MT was selected instead of L-1MT but this choice generated some controversy given the enigmatic mechanism of action of D-1MT. Using classical in vitro assays that employ recombinant IDO1 enzyme and the non-physiological reductant methylene blue, L-1MT acts as a weak catalytic inhibitor. In contrast, under the same conditions, D-1MT exerts little if any effect as an IDO1 catalytic inhibitor [7]. In cell-based assays, we found that D-1MT could inhibit IDO2 activity [69]. While these findings have been challenged in other systems [82–84], they gained recent in vivo support in a study of Ido2-deficient mice [85] where the D-1MT mechanism of action was found to rely genetically only upon the IDO2 enzyme [85]. It is important to appreciate that the use of non-physiological reductants in IDO biochemical reactions may impact interpretations of D-1MT action, especially for IDO2 which displays weaker catalytic activity, given evidence that non-physiological reductants differentially affect inhibitor binding and activity when compared to physiological reductants used in the reactions (R.Metz, unpublished observations). In summary, questions concerning D-1MT as a direct inhibitor of IDO enzymes [86] must be tempered by concerns about the use of non-physiological reductants in enzyme assays.
Investigations of the basis for D-1MT activity led us to identify mTORC1 suppression as an IDO effector mechanism and resuscitation of mTORC1 activity in tryptophan-depleted conditions as a likely drug mechanism of action [74]. Specifically, we found that D-1MT acts as a high-potency tryptophan mimetic in reversing mTORC1 inhibition and autophagic induction by IDO, even though D-1MT is insufficient to charge tryptophan-tRNA and therefore to rescue protein translation or return Gcn2 to a quiescent state. Figure 5 summarizes the discovery of D-1MT as a tryptophan mimetic that is peculiar to the mTORC1 pathway. Strikingly, D-1MT relieved mTOR suppression by IDO at even higher potency than L-tryptophan itself (i.e., at lower concentrations) [74], acting at nanomolar concentrations consistent with the clinical pharmacodynamics observed in patient responses in Phase I trials (H. Soliman and N. Vahanian, pers. comm.). Whether this activity is distinct or coupled to IDO2 inhibitory activity is not known at present. Other implications of this discovery are discussed in detail elsewhere [74, 87], but as summarized, they provide timely insight into the unique mechanism of action of D-1MT relative to frank enzymatic inhibitors of IDO.
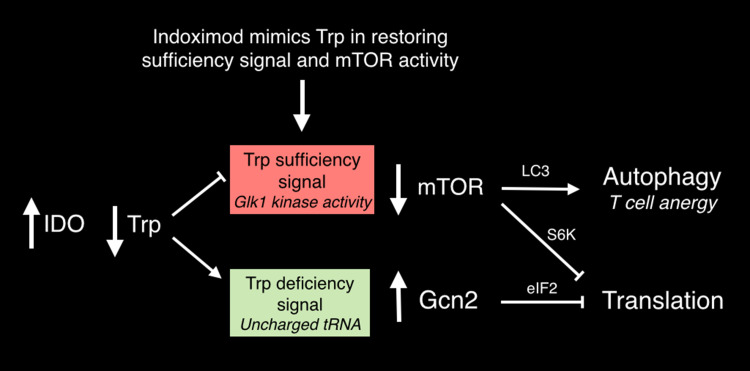
Fig. 5.
Tryptophan deprivation by IDO generates signals sensed by distinct amino acid sufficiency and deficiency pathways. Trp deficiency is sensed by the integrated stress kinase GCN2 that inhibits eIF-2α and alters translation. Through a distinct pathway, the lack of Trp sufficiency causes mTOR to be inactivated, leading to autophagy via LC3 de-repression and translational blockade via S6 kinase inactivation. D-1MT acts as a peculiar mimetic of Trp in the sufficiency pathway, thereby functionally reversing the effects of IDO on mTOR. The figure is modified from Metz et al. [74]
Our discovery that D-1MT reverses IDO-mediated suppression of mTORC1 offers explanative power with regard to a recent preclinical demonstration that D-1MT can safely leverage the antitumor properties of antibodies disrupting CTLA4, PD1 or GITR [55]. As noted above, CTLA4 engagement elevates IDO in DC by reverse signaling in a manner that is critical for generation of CTLA4-mediated immune tolerance. Building upon the observation that melanoma-bearing Ido-deficient mice exhibited a relative increase in overall survival when treated with any of these immune checkpoint antibodies, it was demonstrated that D-1MT strongly leveraged their efficacy in rejecting IDO-expressing and non-expressing poorly immunogenic tumors, emphasizing the importance of the inhibitory effects of both tumor-derived and host-derived IDO. The effects were T cell-dependent in enhancing infiltration of tumor-specific T effector cells, with a marked increase in the effector-to-regulatory T cell ratios in the tumors. These findings were particularly interesting in light of evidence that the positive T cell co-regulatory receptor ICOS is elevated in T effector cells infiltrating melanomas with favorable survival outcomes [88], insofar as mTORC1 activation elevates cell surface expression of ICOS [89]. Thus, D-1MT may leverage immune checkpoint therapy by increasing a prognostically favorable pharmacodynamic marker in mTORC1-mediated ICOS expression in tumor-infiltrating tumor-specific T effector cells. Overall, these results illustrate the immunosuppressive role of IDO in the context of immunotherapies targeting immune checkpoints, and they provide a strong incentive to clinically evaluate combinations with indoximod/D-1MT or other IDO inhibitors, irrespective of IDO expression by the tumor cells.
The ability of D-1MT to resuscitate IDO-mediated mTORC1 blockade has additional translational implications. First, since D-1MT selectively targets the mTOR effector pathway, it may display greater safety margins than enzymatic inhibitors which block all effector signals [79]. Second, since D-1MT may resuscitate mTORC1 activity depressed by any tryptophan catabolic enzyme implicated in immune escape (IDO, IDO2, TDO or TPH), its use may be broadly applicable in any cancer where one or more of these enzymes are overexpressed. This quality may also help defeat the likelihood of compensatory responses in patient tumors, reducing opportunities for resistance. Third, the different mechanism of action of D-1MT may suggest its combination with enzymatic inhibitors of IDO, IDO2, TDO or TPH which are in preclinical and clinical development. Lastly, the definition of mTORC1 and PKC-Θ as candidate pharmacodynamic markers for D-1MT/indoximod responses in patients may be useful in advancing its evaluation in human trials, addressing a pressing clinical need. In this regard, we note that the concentrations at which D-1MT affects mTORC1 and PKC-Θ are consistent with the clinical pharmacokinetics of D-1MT/indoximod documented in human trials [90]. Overall, D-1MT may prove a useful and fascinating probe of the role of mTORC1 in T cell responses in cancer, as discussed further elsewhere [87].
Enzyme inhibitors
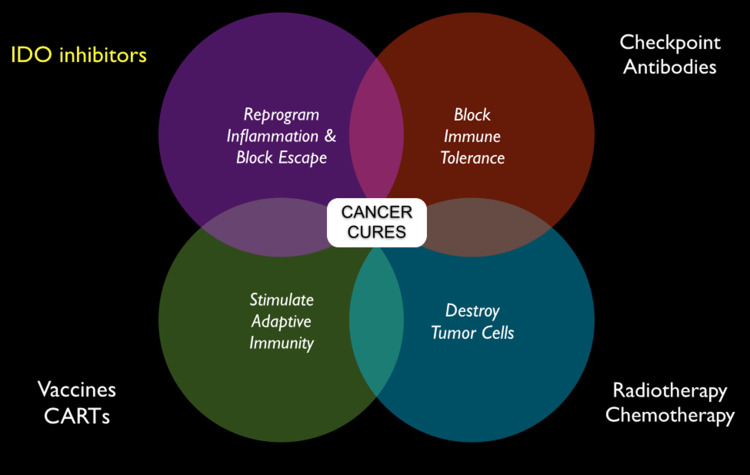
With the initiation of clinical trials of D-1MT concerns regarding its inhibitory effects on IDO catalytic activity prompted the development of pharmacologically superior IDO inhibitory compounds. Proposed models for the processes at work in the active site have been developed based on mechanistic studies [91]. The publication of an X-ray crystal structure for IDO complexed with a simple inhibitor [92] has facilitated this work. A number of groups have screened chemical collections for novel inhibitors as reviewed in detail elsewhere (e.g., [28, 50].). Figure 6 captures the theme of this review in suggesting the flexible use of IDO inhibitors to reprogram inflammation and block immune escape as applied in combination with traditional radiotherapy and chemotherapy, checkpoint inhibitors and active immunotherapeutic interventions.
Fig. 6.
Immunochemotherapy of the future with IDO inhibitors. Multiple applications are suggested in various combination with tolerance blockade (immune checkpoint inhibitors), active immunotherapeutic interventions (vaccines or CART [chimeric antigen receptor T cells]), and classical/targeted chemotherapy or radiotherapy
Two IDO catalytic inhibitors that have entered clinical trials are INCB024360 and NLG919, both of which are tryptophan competitive for binding to the enzyme. INCB024360, which began Phase I trials for advanced malignancies in 2010, is an orally available hydrozyamidine that competitively blocks the degradation of tryptophan to kynurenine by IDO with an IC50 of approximately 72 nM [93]. Oral administration in mice and dogs reduced kynurenine levels in the plasma as well as in tumors and tumor-draining lymph nodes [94]. In several mouse models, INCB024360 delayed tumor growth in wild-type mice, but not in nude mice or Ido1-/- mice, indicating not only that this drug targets IDO1 but that it also mediates its antitumor effects through the immune system [93, 94]. The preclinical in vivo data complement in vitro experiments showing that INCB024360 does not inhibit IDO2 or TDO2 activity [93]. An important mechanistic observation is the ability of INCB024360 to increase the survival and decrease the apoptosis of DCs, suggesting that this drug may improve the number of functional DCs thereby allowing T cells to be more effectively primed against tumor cell antigens [93]. NLG919 has recently emerged from preclinical development and has only recently entered Phase I trials. This compound is an IDO1-selective orally bioavailable inhibitor with nanomolar potency. It displays immune-dependent monotherapy activity and leverages the antitumor properties of chemotherapy such as other IDO inhibitors; indoximod has been observed to safely leverage the efficacy of NLG919 (M. Mautino, pers. communication). The clinical pharmacodynamics of INCB024360 and NLG919 are readily assessed in patients by determining blood serum kynurenine/tryptophan ratioes, based on preclinical evidence that they not only relieve IDO-mediated tryptophan deprivation but also IDO-mediated kynurenine production (i.e., target all effector pathways).
Our studies of Ido1-deficient mice over several years suggest the potential for gastrointestinal and cardiac side effects from the use of potent IDO enzyme inhibitors [79], information which may assist the design and conduct of clinical investigations. The most striking phenotype observed to date was calcification of the cardiac endometrium proximal to the right ventricle. This phenotype was 30 % penetrant, specific to Ido1 deficiency on the BALB/c strain background and sexually dimorphic in nature [79]. Additionally, we observed that administration of complete Freund’s adjuvant containing Toll-like receptor ligands known to induce IDO caused acute pancreatitis in Ido1-deficient mice [79], with implications for the design of planned combination studies of IDO inhibitors with cancer vaccines. Further, in an established model of hyperlipidemia, we found that IDO deficiency caused a dramatic elevation in serum triglycerides [79], suggesting a possible risk to elderly patients who may have occult unstable cardiovascular plaque. Lastly, Ido1-deficient mice displayed increased sensitivity to induction of acute colitis, with a marked elevation in tumor incidence, multiplicity and staging in animals subjected to a regimen of inflammatory colon carcinogenesis [79]. These findings suggested risks of colitis in the short term and colon carcinoma in the longer term in patients who may receive IDO inhibitors as part of their therapy. Here, we note that administration of D-1MT/indoximod has never been observed to produce any of these phenotypes, consistent with a different mechanism of action than enzymatic inhibitors. These risks should be monitored clinically during clinical development.
IDO peptides as vaccines
One interesting aspect of IDO that has been reported by Andersen and colleagues is that its enzyme appears to be spontaneously recognized by specific CD8+ T cells that are present in humans [95]. Indeed, IDO-reactive CD8+ T cells have been found to act as specific CTLs that can recognize and kill IDO-expressing cells [96]. Intriguingly, IDO2 may share these features [97]. These observations have prompted the hypothesis that IDO peptides might be exploited as an anticancer vaccine. This possibility was examined recently in a Phase I clinical trial, where early evidence was obtained of long-lasting disease stabilization and a partial response against liver metastasis in metastatic lung cancer patients vaccinated with an IDO-derived peptide, in the absence of notable toxicity [98].
IDO expression inhibitors
Several recent studies suggest alternative targeting strategies for IDO blockade at the level of gene expression (upstream inhibition). Certain NSAIDs indirectly block IDO expression by inhibiting COX2, which through the production of PGE2 acts to stimulate IDO activity [99]. This COX2-IDO regulatory connection has antitumor implications which have been analyzed in a preclinical model of breast cancer treatment with COX2 inhibitors [100], illustrating its therapeutic relevance in principle. T regulatory cell functions disrupted by COX2 inhibition have also been found to be mediated by IDO inhibition, possibly contributing to the anticancer properties of COX-2 inhibitors [101]. In pursuing the role of NF-kB in promoting IDO transcription, we studied the mechanism of action of the anti-inflammatory compound ethyl pyruvate, which was shown previously to inhibit NF-κB activity. Notably, ethyl pyruvate was a potent inhibitor of IDO expression that could produce robust antitumor response relying upon both IDO targeting and T cell activity [102].
In a similar vein, a recent study has linked the therapeutic effects of imatinib (Gleevec) in gastrointestinal stromal tumors (GIST) to an inhibition of IDO expression. Specifically, it was found that IDO targeting was crucial for the antitumor properties of imatinib in an immunocompetent mouse model, based on evidence that oncogenic Kit signaling could interfere with antitumor T cell responses by inducing IDO expression [103]. This finding is striking, because it lends credence to the notion that IDO targeting may already be providing benefit in the context of imatinib treatment of GIST. Given evidence that the Ras/PKC pathway drives IDO expression in inflammatory models of cancer [9, 10, 40], it is tempting to speculate along the same lines that IDO inhibition may be responsible for some of the therapeutic activity of certain tyrosine kinase and Raf inhibitors which interface with these pathways. Indeed, the future would seem to be bright for combining IDO inhibitors and targeted drugs that interfere with immune escape pathways in tumor cells, in creating drug combinations that act effectively as immunochemotherapy.
Acknowledgments
Work in the authors’ laboratories has been supported by grants from the NIH, Department of Defense Breast and Lung Cancer Research Programs, Susan G. Komen for the Cure and the W.W. Smith Trust with additional support from NewLink Genetics Corporation, Sharpe-Strumia Foundation, Dan Green Foundation, Lankenau Medical Center Foundation and the Main Line Health System. C. Smith was the recipient of a Postdoctoral Fellowship through the US Department of Defense Breast Cancer Research Program.
Conflict of interest
G.C. Prendergast, R. Metz and A.J. Muller state a conflict of interest as shareholders and G.C. Prendergast also a grant recipient and a member of the scientific advisory board for New Link Genetics Corporation, a biopharmaceutical company that has licensed IDO intellectual property for clinical development from the Lankenau Institute of Medical Research, as described in U.S. Patents Nos. 7705022, 7714139, 8008281, 8058416, 8383613, 8389568, 8436151, 8476454 and 8586636. The other authors state no conflict of interest.
Abbreviations
- 1MT
1-Methyl-tryptophan
- AhR
Aryl hydrocarbon receptor (kynurenine receptor)
- CCL-2
Myeloid attraction cytokine (also known as MCP-1) which binds to receptors CCR2 and CCR4 and causes basophils and mast cells to release their granules
- eIF-2α
Master regulatory eukaryotic translation initiation factor
- Gcn2
Starvation-induced kinase that phosporylates and suppresses eIF-2α
- GLK1
A kinase also known as MAP4K3 that responds to amino acid sufficiency by activating mTORC1
- IDO
Indoleamine 2,3-dioxygenase (also known as IDO1)
- IDO2
Distinct gene encoding an IDO-related enyzme with weaker tryptophan catabolic activity
- IFN-γ
Interferon-γ
- MDSC
Myeloid-derived suppressor cells
- INCB024360
A specific small-molecule inhibitor of IDO1 enzymatic activity in clinical trials
- Indoximod
D racemer of 1-methyl-tryptophan (D-1MT), an IDO pathway inhibitor in clinical trials that relieves IDO-mediated suppression of the mTORC1 pathway (also known as NLG8189)
- mTORC1
Mammalian target of rapamycin complex-1, a master cell growth regulatory kinase
- NLG919
A specific small-molecule inhibitor of IDO1 enzymatic activity in clinical trials
- NK
Natural killer immune cells
- PGE-2
Pro-inflammatory prostaglandin produced by activation of COX-2 which may rely on IDO function for its pro-cancerous activity
- PKC-θ
Protein kinase C variant that phosphorylates and limits the function of the T cell receptor
- TDLN
Tumor-draining lymph node
- TLR
Toll-like receptor (infection/inflammation-associated PAMP receptor)
- TGF-β
Transforming growth factor-β
- TPA
12-O-tetradecanoylphorbol-13-acetate (pro-inflammatory chemical also known as PMA)
- Treg
T regulatory cells
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eleventh Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 14th-16th May, 2013. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Prendergast GC, Jaffee EM. Cancer immunologists and cancer biologists: why we didn’t talk then but need to now. Cancer Res. 2007;67(8):3500–3504. doi: 10.1158/0008-5472.CAN-06-4626. [DOI] [PubMed] [Google Scholar]
- 2.Prendergast GC. Immunological thought in the mainstream of cancer research: past divorce, recent remarriage and elective affinities of the future. Oncoimmunology. 2012;1(6):793–797. doi: 10.4161/onci.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prendergast GC, Jaffee EM, editors. Cancer immunotherapy: immune suppression and tumor growth. 2. New York: Academic Press; 2013. [Google Scholar]
- 4.Muller AJ, Prendergast GC. Marrying immunotherapy with chemotherapy: why say IDO? Cancer Res. 2005;65(18):8065–8068. doi: 10.1158/0008-5472.CAN-05-2213. [DOI] [PubMed] [Google Scholar]
- 5.Muller AJ, DuHadaway JB, Sutanto-Ward E, Donover PS, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunomodulatory target of the tumor suppressor gene Bin1, potentiates cancer chemotherapy. Nature Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 6.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6(8):613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 7.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27(28):3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 9.Muller AJ, DuHadaway JB, Chang MY, Ramalingam A, Sutanto-Ward E, Boulden J, Soler AP, Mandik-Nayak L, Gilmour SK, Prendergast GC. Non-hematopoietic expression of IDO is integrally required for inflammatory tumor promotion. Cancer Immunol Immunother. 2010;59(11):1655–1663. doi: 10.1007/s00262-010-0891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C, Chang MY, Parker KH, Beury DW, Duhadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ. IDO Is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2(8):722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyland E, Williams DC. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder. Biochem J. 1956;64(3):578–582. doi: 10.1042/bj0640578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci USA. 1981;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 14.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 15.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 17.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196(4):459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, Moretta L, Moretta A, Vitale M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108(13):4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 20.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 21.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8(1):74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 22.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop LD, Metz R, Muller AJ. IDO in inflammatory programming and immune suppression in cancer. In: Gabrilovich DI, Hurwitz AA, editors. Tumor-induced immune suppression. New York: Springer; 2014. [Google Scholar]
- 23.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24(5):242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 24.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 25.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 26.Katz JB, Muller AJ, Metz R, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 27.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van Den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Newton RC, Friedman SM, Scherle PA. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9(8):938–952. doi: 10.2174/156800909790192374. [DOI] [PubMed] [Google Scholar]
- 29.Prendergast GC, Muller AJ, Ramalingam A, Chang MY. BAR the door: cancer suppression by amphiphysin-like genes. Biochim Biophys Acta. 2009;1795(1):25–36. doi: 10.1016/j.bbcan.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge K, DuHadaway J, Du W, Herlyn M, Rodeck U, Prendergast GC. Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proc Natl Acad Sci USA. 1999;96:9689–9694. doi: 10.1073/pnas.96.17.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pineda-Lucena A, Ho CS, Mao DY, Sheng Y, Laister RC, Muhandiram R, Lu Y, Seet BT, Katz S, Szyperski T, Penn LZ, Arrowsmith CH. A structure-based model of the c-Myc/Bin1 protein interaction shows alternative splicing of Bin1 and c-Myc phosphorylation are key binding determinants. J Mol Biol. 2005;351(1):182–194. doi: 10.1016/j.jmb.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14(3):185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anczukow O, Rosenberg AZ, Akerman M, Das S, Zhan L, Karni R, Muthuswamy SK, Krainer AR. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol. 2012;19(2):220–228. doi: 10.1038/nsmb.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golan-Gerstl R, Cohen M, Shilo A, Suh SS, Bakacs A, Coppola L, Karni R. Splicing factor hnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 2011;71(13):4464–4472. doi: 10.1158/0008-5472.CAN-10-4410. [DOI] [PubMed] [Google Scholar]
- 35.Barekati Z, Radpour R, Lu Q, Bitzer J, Zheng H, Toniolo P, Lenner P, Zhong XY. Methylation signature of lymph node metastases in breast cancer patients. BMC Cancer. 2012;12:244. doi: 10.1186/1471-2407-12-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radpour R, Barekati Z, Kohler C, Lv Q, Burki N, Diesch C, Bitzer J, Zheng H, Schmid S, Zhong XY. Hypermethylation of tumor suppressor genes involved in critical regulatory pathways for developing a blood-based test in breast cancer. PLoS ONE. 2011;6(1):e16080. doi: 10.1371/journal.pone.0016080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radpour R, Kohler C, Haghighi MM, Fan AX, Holzgreve W, Zhong XY. Methylation profiles of 22 candidate genes in breast cancer using high-throughput MALDI-TOF mass array. Oncogene. 2009;28(33):2969–2978. doi: 10.1038/onc.2009.149. [DOI] [PubMed] [Google Scholar]
- 38.Kuznetsova EB, Kekeeva TV, Larin SS, Zemlyakova VV, Khomyakova AV, Babenko OV, Nemtsova MV, Zaletayev DV, Strelnikov VV. Methylation of the BIN1 gene promoter CpG island associated with breast and prostate cancer. J Carcinog. 2007;6:9. doi: 10.1186/1477-3163-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna ES, Tamayo P, Cho YJ, Tillman EJ, Mora-Blanco EL, Sansam CG, Koellhoffer EC, Pomeroy SL, Roberts CW. Epigenetic inactivation of the tumor suppressor BIN1 drives proliferation of SNF5-deficient tumors. Cell Cycle. 2012;11(10):1956–1965. doi: 10.4161/cc.20280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, Prendergast GC, Mellor AL. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105(44):17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prendergast GC, Metz R, Muller AJ. Towards a genetic definition of cancer-associated inflammation: role of the IDO pathway. Am J Pathol. 2010;176(5):2082–2087. doi: 10.2353/ajpath.2010.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Willimsky G, Czeh M, Loddenkemper C, Gellermann J, Schmidt K, Wust P, Stein H, Blankenstein T. Immunogenicity of premalignant lesions is the primary cause of general cytotoxic T lymphocyte unresponsiveness. J Exp Med. 2008;205(7):1687–1700. doi: 10.1084/jem.20072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levina V, Su Y, Gorelik E. Immunological and nonimmunological effects of indoleamine 2,3-dioxygenase on breast tumor growth and spontaneous metastasis formation. Clin Dev Immunol. 2012;2012:173029. doi: 10.1155/2012/173029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertovaara M, Hasan T, Raitala A, Oja SS, Yli-Kerttula U, Korpela M, Hurme M. Indoleamine 2,3-dioxygenase activity is increased in patients with systemic lupus erythematosus and predicts disease activation in the sunny season. Clin Exp Immunol. 2007;150(2):274–278. doi: 10.1111/j.1365-2249.2007.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroecksnadel K, Winkler C, Duftner C, Wirleitner B, Schirmer M, Fuchs D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(3):334–337. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 47.Mandik-Nayak L, Allen PM. Initiation of an autoimmune response: insights from a transgenic model of rheumatoid arthritis. Immunol Res. 2005;32(1–3):5–13. doi: 10.1385/IR:32:1-3:005. [DOI] [PubMed] [Google Scholar]
- 48.Scott GN, DuHadaway J, Pigott E, Ridge N, Prendergast GC, Muller AJ, Mandik-Nayak L. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J Immunol. 2009;182(12):7509–7517. doi: 10.4049/jimmunol.0804328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kita H, Shiraishi Y, Watanabe K, Suda K, Ohtsuka K, Koshiishi Y, Goya T. Does postoperative serum interleukin-6 influence early recurrence after curative pulmonary resection of lung cancer? Ann Thorac Cardiovasc Surg Off J Assoc Thorac Cardiovasc Surg Asia. 2011;17(5):454–460. doi: 10.5761/atcs.oa.10.01627. [DOI] [PubMed] [Google Scholar]
- 50.Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem. 2011;18(15):2257–2262. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol. 2012;42(8):1932–1937. doi: 10.1002/eji.201242572. [DOI] [PubMed] [Google Scholar]
- 52.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 53.Fallarino F, Bianchi R, Orabona C, Vacca C, Belladonna ML, Fioretti MC, Serreze DV, Grohmann U, Puccetti P. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200(8):1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13(5):579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 55.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210(7):1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175(9):5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 58.Orabona C, Belladonna ML, Vacca C, Bianchi R, Fallarino F, Volpi C, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: silencing suppressor of cytokine signaling 3 expression in dendritic cells turns CD28-Ig from immune adjuvant to suppressant. J Immunol. 2005;174(11):6582–6586. doi: 10.4049/jimmunol.174.11.6582. [DOI] [PubMed] [Google Scholar]
- 59.Puccetti P. On watching the watchers: IDO and type I/II IFN. Eur J Immunol. 2007;37(4):876–879. doi: 10.1002/eji.200737184. [DOI] [PubMed] [Google Scholar]
- 60.Yuan W, Collado-Hidalgo A, Yufit T, Taylor M, Varga J. Modulation of cellular tryptophan metabolism in human fibroblasts by transforming growth factor-beta: selective inhibition of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase gene expression. J Cell Physiol. 1998;177(1):174–186. doi: 10.1002/(SICI)1097-4652(199810)177:1<174::AID-JCP18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 61.Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181(8):5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 62.Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177(1):130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 63.Belladonna ML, Orabona C, Grohmann U, Puccetti P. TGF-beta and kynurenines as the key to infectious tolerance. Trends Mol Med. 2009;15(2):41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 65.McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, Mellor AL. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. 2012;249(1):135–157. doi: 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 67.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 70.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375(3):331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Metz R, Smith C, Duhadaway JB, Chandler P, Baban B, Merlo LM, Pigott E, Keough MP, Rust S, Mellor AL, Mandik-Nayak L, Muller AJ, Prendergast GC. IDO2 is critical for IDO1-mediated T cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014 doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Hu HM, Tian Q, Baer M, Spooner CJ, Williams SC, Johnson PF, Schwartz RC. The C/EBP bZIP domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem. 2000;275(21):16373–16381. doi: 10.1074/jbc.M910269199. [DOI] [PubMed] [Google Scholar]
- 74.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1(9):1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106(29):12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22(5):655–661. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Chuang HC, Lan JL, Chen DY, Yang CY, Chen YM, Li JP, Huang CY, Liu PE, Wang X, Tan TH. The kinase GLK controls autoimmunity and NF-kappaB signaling by activating the kinase PKC-theta in T cells. Nat Immunol. 2011;12(11):1113–1118. doi: 10.1038/ni.2121. [DOI] [PubMed] [Google Scholar]
- 78.Baban B, Chandler P, McCool D, Marshall B, Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61(2):67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Chang MY, Smith C, Duhadaway JB, Pyle JR, Boulden J, Peralta Soler A, Muller AJ, Laury-Kleintop LD, Prendergast GC. Cardiac and gastrointestinal liabilities modulatory enzyme indoleamine 2,3-dioxygenase. Cancer Biol Ther. 2011;12(12):1050–1058. doi: 10.4161/cbt.12.12.18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 81.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 82.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58(1):153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuasa HJ, Ball HJ, Austin CJ, Hunt NH. 1-l-methyltryptophan is a more effective inhibitor of vertebrate IDO2 enzymes than 1-d-methyltryptophan. Comp Biochem Physiol B Biochem Mol Biol. 2010 doi: 10.1016/j.cbpb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Qian F, Liao J, Villella J, Edwards R, Kalinski P, Lele S, Shrikant P, Odunsi K. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merlo LM, Pigott E, Duhadaway JB, Grabler S, Metz R, Prendergast GC, Mandik-Nayak L. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J Immunol. 2014;92(5):2082–2090. doi: 10.4049/jimmunol.1303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9(6):445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 87.Prendergast GC, Metz R. A perspective on new immune adjuvant principles: reprogramming inflammatory states to permit clearance of cancer cells and other age-associated cellular pathologies. Oncoimmunology. 2012;1(6):924–929. doi: 10.4161/onci.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71(16):5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 89.Xie DL, Wu J, Lou YL, Zhong XP. Tumor suppressor TSC1 is critical for T-cell anergy. Proc Natl Acad Sci USA. 2012;109(35):14152–14157. doi: 10.1073/pnas.1119744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soliman HH, Antonia S, Sullivan D, Vahanian N, Link C. Overcoming tumor antigen anergy in human malignancies using the novel indeolamine 2,3-dioxygenase (IDO) enzyme inhibitor, 1-methyl-D-tryptophan (1MT) J Clin Oncol. 2009;27:15s. [Google Scholar]
- 91.Malachowski WP, Metz R, Prendergast GC, Muller AJ. A new cancer immunosuppression target: indoleamine 2,3-dioxygenase (IDO). A review of the IDO mechanism, inhibition, and therapeutic applications. Drugs Fut. 2005;30:897–913. [Google Scholar]
- 92.Sugimoto H, Oda SI, Otsuki T, Hino T, Yoshida T, Shiro Y. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc Natl Acad Sci USA. 2006;103:2311–2316. doi: 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115(17):3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 94.Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, Combs AP, Scherle PA, Vaddi K, Fridman JS. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010 doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 95.Sorensen RB, Berge-Hansen L, Junker N, Hansen CA, Hadrup SR, Schumacher TN, Svane IM, Becker JC, thor Straten P, Andersen MH. The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS ONE. 2009;4(9):e6910. doi: 10.1371/journal.pone.0006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, Thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117(7):2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sorensen RB, Kollgaard T, Andersen RS, van den Berg JH, Svane IM, Straten P, Andersen MH. Spontaneous cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res. 2011;71(6):2038–2044. doi: 10.1158/0008-5472.CAN-10-3403. [DOI] [PubMed] [Google Scholar]
- 98.Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, Zeyher C, Gouttefangeas C, Thomsen BM, Holm B, Thor Straten P, Mellemgaard A, Andersen MH, Svane IM. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res. 2014;20(1):221–232. doi: 10.1158/1078-0432.CCR-13-1560. [DOI] [PubMed] [Google Scholar]
- 99.Sayama S, Yoshida R, Oku T, Imanishi J, Kishida T, Hayaishi O. Inhibition of interferon-mediated induction of indoleamine 2,3-dioxygenase in mouse lung by inhibitors of prostaglandin biosynthesis. Proc Natl Acad Sci USA. 1981;78(12):7327–7330. doi: 10.1073/pnas.78.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177(4):2391–2402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- 101.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. 2009;32(1):22–28. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 102.Muller AJ, DuHadaway JB, Jaller D, Curtis P, Metz R, Prendergast GC. Immunotherapeutic suppression of indoleamine 2,3-dioxygenase and tumor growth with ethyl pyruvate. Cancer Res. 2010;70(5):1845–1853. doi: 10.1158/0008-5472.CAN-09-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, Besmer P, Guo T, Antonescu CR, Taguchi T, Yuan J, Wolchok JD, Allison JP, Dematteo RP. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]