Abstract
Objective
RhoB is a small GTPase localized at the plasma membrane and endosomes that participates in the regulation of endocytic trafficking of the epidermal growth factor (EGF) receptor and the nonreceptor kinases Src and Akt. This study was performed to determine whether RhoB plays a critical role in trafficking and signaling by the platelet-derived growth factor receptor-β (PDGFR-β) in vascular smooth muscle cells.
Methods and Results
Cells derived from RhoB knockout mice failed to proliferate in response to PDGF, and downstream signaling was compromised as reflected by reduced phosphorylation of the effector kinases Akt and ERK1/2. In normal cells, PDGF stimulated trafficking of PDGFR-β into a perinuclear late endosomal compartment and triggered entry of Src, Akt, extracellular signal-regulated kinase (ERK) into the cell nucleus. In contrast, PDGF treatment of RhoB null cells resulted in neither PDGFR-β trafficking to late endosomes nor nuclear localization of Src, Akt, or ERK. In support of an essential function in these processes, restoring expression of RhoB in null cells rescued these defects and restored cell proliferation in response to PDGF.
Conclusion
ur findings establish RhoB as a critical regulator of PDGFR-β trafficking and signaling in vascular smooth muscle cells.
Keywords: RhoB, patelet-derived growth factor receptor, endocytic trafficking, vascular smooth muscle cells
Platelet-derived growth factor (PDGF) is a potent mitogen, chemoattractant, and survival factor for vascular smooth muscle cells (SMCs). The biological effects of PDGF are initiated through 2 related receptor tyrosine kinases, receptor-α (PDGFR-α) and receptor-β (PDGFR-β). Both PDGFR-α and PDGFR-β are expressed in vascular SMCs, but PDGFR-β expression is higher and PDGFR-β has been implicated in vascular remodeling.1,2 PDGFR-β triggers cellular proliferation, migration, and survival3 but also contributes to atherosclerosis4,5 and malignant neoplasia.6 Ligand-induced dimerization of the PDGF receptor leads to autophosphorylation of the receptor and the subsequent binding and phosphorylation of downstream signaling proteins.3,7 Activated PDGF receptors are known to bind a variety of SH2-domain containing signal transduction molecules at specific tyrosine residues, such as phospholipase C-γ (PLCγ), phosphatidylinositol 3′ kinase (PI3K), GTPase activating protein for Ras (RasGAP), the tyrosine phosphatase SHP-2, the Src family of tyrosine kinases, as well as signal transducers and activators of transcription (Stats), and adaptor molecules such as Grb2, Shc, Nck, Grb7, and Crk. Activation of these signaling proteins leads to the induction of highly specific signal relay cascades. Downstream mediators of the PDGFR-β include Akt, ERK, and small G proteins including rho and rac-1, which mediate various cellular responses such as cell cycle progression, actin reorganization, migration, and survival.3,8,9
Endocytic trafficking of EGF receptor has been well studied, but less is known about PDGF receptor trafficking and endosomal signaling, and it is not completely clear how endocytosis and trafficking of the PDGF receptor are regulated. As a receptor tyrosine kinase (RTK), one of the earliest responses of the PDGF receptor is to stimulate its own internalization. Internalized receptors can recycle back to the cell surface10,11 or be sorted to the lysosome for degradation.12–14 It has been reported that the SH2 domain–containing proteins that bind to receptor cytoplasmic domains may play a role in the trafficking of PDGF receptor.14,15 Additionally, the PI3K binding sites present on the PDGF receptor are required for the correct trafficking and assembly of the receptor into juxtanuclear vesicular structures after PDGF stimulation.15 However, continued investigation of the importance of the PI3K binding sites revealed that they are not required for internalization of PDGF receptor, but are required to divert the PDGF receptor to a degradation pathway.16 Emerging evidence has suggested that endocytosis of RTKs is not only a mechanism for deactivation and degradation, but also a positive signal for downstream biological responses. Therefore, the endosome can serve as an assembly site for the formation of progrowth and survival signaling complexes after activation of the EGF receptor or PDGF receptor.17,18
The Rho family of small GTPases plays a pivotal role in the dynamic regulation of the actin cytoskeleton. Recent studies also point to multiple functions for these signaling proteins in endocytic trafficking pathways. RhoB localizes both to the plasma membrane and the membrane of multivesicular late endosomes (MVBs).19–21 It has been demonstrated that through binding and activating of protein kinase C-related protein kinase (PRK1), RhoB regulates the kinetics of EGF receptor traffic from endosomes to a prelysosomal compartment.21,22 In addition, RhoB and PRK1 cause recruitment of the PI3K effector PDK1 to endosomes.23 Further study of the role of RhoB in the endocytic pathway has revealed that activation of RhoB inhibits the transfer of the receptor from the late endosomal compartment to the lysosome.24 The importance of RhoB in endocytic-trafficking has been extended to include the trafficking of other important cellular factors, such as Akt and Src.25,26 RhoB is an important determinant of Akt stability and trafficking to the nucleus in endothelial cells, and regulates endothelial cell survival during vascular development.25 In the case of Src protein, RhoB is associated with Src in perinuclear recycling compartments, and regulates both catalytic activation of Src and translocation of active kinase to peripheral membrane structures.26 Recent work has revealed that RhoB regulates endosome transport by recruiting and activating the Diaphanous-related formin, Dia1, which interacts with other endosomal proteins (such as actin) and promotes actin assembly on endosomes.27 Thus, evidence to date strongly implicates RhoB as a mediator of vesicle trafficking that may contribute to the regulation of RTKs and their downstream signaling cascade. Whether RhoB plays a role in the trafficking and signaling of the PDGF receptor is not known.
By using a RhoB knockout mouse model, we addressed the role of RhoB in PDGF signaling and PDGFR-β trafficking in vascular SMCs. Our results suggest that RhoB may play a critical role in the physiological function of vascular SMCs by regulating PDGFR-β trafficking processes.
Methods
Isolation and Culture of Vascular SMCs
The generation and characterization of RhoB heterozygous (+/−) and RhoB nullizygous (−/−) mice, which are fertile and lack apparent developmental defects, have been described previously.28 RhoB +/− and −/− mice were euthanized and the aorta removed as a source for vascular SMCs. Cells were isolated and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified 5% CO2 atmosphere. For all experiments in this study, cells of only 3 to 5 passages were used. For quantification, cells were seeded into 24-well culture plates at a density of 105 cells per well, transferred to serum-fee medium (SFM) for 16 hours, treated with or without recombinant PDGF-BB (Cell Signaling Technology) as noted, and then harvested by trypsinization and counted using a hemocytometer at the indicated time points.
Western Blot Analysis
Cells were harvested by scraping and washing twice in PBS, then lysed in RIPA buffer containing protease and phosphatase inhibitors. Equal protein for each sample (typically 30 µg/lane) was separated by SDS-PAGE and blotted to Immobilon-NC membrane (Millipore). Blots were incubated with primary antibodies as recommended by the vendor and were detected with HRP conjugated secondary antibodies using the ECL reagents from PIERCE according to the manufacturer’s instructions. Primary antibodies to the following antigens were used: RhoB (Bethyl Laboratories); PDGFR-β and actin (Santa Cruz Biotechnology); phospho-y751-PDGFRβ, Akt, phospho-s473-Akt, ERK, phospho-ERK, Src, and phospho-y416-Src (Cell Signaling Technology). The phospho-PDGFR antibody used recognizes a phosphorylated tyrosine residing in the docking site for PI3K.29 Blots were stripped and with actin antibody to show equal protein loading.
Retrovirus Production and Infection
Ectopic expression of wild-type RhoB (RhoB-WT) was restored in RhoB null cells by infection with recombinant retroviruses described previously.30 Briefly, a hemagglutinin antigen epitope (HA)-tagged RhoB-WT cDNA was subcloned into the replication-incompetent retroviral vector MSCVpac which includes a puromycin selection marker. Recombinant MSCVpac viruses were packaged by standard methods in Phoenix cells. After infection, cells were maintained in supplemented DMEM containing 1 to 2 µg/mL puromycin to select for drug-resistant cell populations.
Immunofluorescence Studies
Cells were washed twice in cold phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, washed with PBS, and permeablized with PBS/0.1% Triton X-100. After blocking with PBS plus 10% goat serum, cells were incubated with primary antibodies overnight. In addition to the antibodies noted above, anti-LAMP-1 and anti-Rab11 (BD Transduction Laboratories) were used to detect late endosome/lysosomes and recycling endosomes, anti-EEA-1 (Upstate Biotechnology) was used to detect early endosomes, Nonconjugated antibody was detected by 1-hour incubation with species-specific fluorescein isothiocyanate (FITC) or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). To visualize nuclei, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) in the mounting medium, before examination by fluorescence microscopy. Fluorescent signal was analyzed using a Zeiss Axiovert 220 mol/L microscope powered by Axiovision 4.0 software with multi-channel/Z-stack acquisition and 3D–deconvolution modules.
Results
Loss of RhoB Ablates PDGF-Induced Proliferation of Vascular SMCs
Initially, we asked whether PDGF would elicit a different proliferative response in aortic vascular SMCs isolated from RhoB +/− and −/− mice. Based on earlier evidence of haplosufficiency of RhoB we used vascular SMCs from RhoB +/− animals to control for the presence of the neomycin resistance cassette used in the generation of the knockout RhoB mice.28 In +/− cells, addition of 8 ng/mL PDGF stimulated a ≈50% increase in cell growth within 48 hours, compared with −/− cells where only a ≈10% increase in cell growth occurred (Figure 1). Moreover, by 72 hours after PDGF addition +/− cells had continued to proliferate to confluence whereas −/− cells did not reach confluence in the experiment (data not shown). To examine whether RhoB is specifically required for PDGF-induced growth of vascular SMCs, we performed additional experiments to test the effects of various growth factors such as EGF, endothelin I, and angiotensin II on the growth of vascular SMCs. None of these growth factors exhibited the growth stimulatory effects on both RhoB +/− and −/− cells under serum free culture condition within 72 hours (data not shown). We concluded that RhoB was crucial for PDGF-induced proliferation of vascular SMCs.
Figure 1.
Mouse RhoB +/− and −/− vascular SMCs, grown in primary culture, seeded into multi-well plates, were incubated with or without PDGF-BB, and cell counts were measured at 0, 24, 48 hours (n=3). The cell counts were normalized to the Day 0 count taken before stimulation.
RhoB Is Crucial for PDGFR-β Phosphorylation and Localization in Vascular SMCs
RhoB is required for the control of endocytic trafficking and has been linked to the intracellular trafficking of EGFR, Akt, and Src.24–26 To explore whether RhoB regulates PDGFR signaling and trafficking in vascular SMCs, we first examined the protein level and the phosphorylation status of the PDGFR-β. Both +/− and −/− cells were incubated with 8 ng/mL PDGF for the indicated times before cells were harvested and lysed for Western analyses. The results showed no obvious difference in the protein levels of PDGFR-β between +/− cells and −/− cells after a 0- to 4-hour treatment with PDGF, however more PDGFR-β protein was observed in +/− cells as compared with −/− cells after stimulation with PDGF for 24 hours (Figure 2A). Interestingly, there appeared to be relatively higher phosphorylated PDGFR-β protein levels in +/− cells after PDGF treatment, particularly at 30 minutes after stimulation (Figure 2A). Next, we compared PDGFR-β subcellular localization in +/− and −/− cells by immunofluorescence staining. We observed that before PDGF stimulation, PDGFR-β distributes throughout the cell and that there is a very low level of phospho-PDGFR-β in both +/− and −/− cells. After treatment with 8 ng/mL PDGF for 4 hours, the punctate staining of the PDGFR-β was observed in the cytoplasm and predominantly in the perinuclear region of the +/− cells, whereas in −/− cells this punctate staining pattern was not observed (Figure 2B). Similarly, phosphorylated PDGFR-β also showed a punctate perinuclear pattern of staining in +/− cells after PDGF treat for 30 minutes, but not in −/− cells (Figure 2C). These observations indicate that RhoB is essential for PDGFR-β phosphorylation and localization in vascular SMCs.
Figure 2.
A, Immunoblot analysis of the levels of total and phosphorylated PDGFR-β in mouse RhoB +/− or −/− SMCs. The cells were incubated with serum-free medium and PDGF-BB (8 ng/mL) for the indicated time. Quantitation analysis is shown for phosphorylated PDGFR-β. B, Immunofluorescence staining of PDGFR-β in mouse RhoB +/− or −/− SMCs stimulated with PDGF-BB for 4 hours. Images were taken at the same magnification and exposure to demonstrate the PDGFR protein in the cells. These results represent 3 separate experiments. C, Immunofluorescence staining of phospho-PDGFR-β in SMCs after PDGF-BB stimulation for 30 minutes as above but using a Cy3-conjugated antibody. Scale bars=50 µm.
RhoB Is Required for PDGF-Induced Activation of Akt and ERK in Vascular SMCs
RhoB has been shown to regulate Akt trafficking and phosphorylation in vascular endothelial cells.25 It is well established that PDGF-stimulates vascular smooth muscle proliferation through the phosphoinositide-3-kinase (PI3K)/Akt pathway as well as the Ras/Raf/MEK/ERK pathway. To study whether loss of RhoB affects the operation of these signaling pathways, we first examined the levels and phosphorylation status of Akt in +/− and −/− vascular SMCs by Western blotting. After PDGF treatment we observed higher levels of both total and phosphorylated Akt protein in +/− versus −/− cells (Figure 3A). PDGF-induced Akt phosphorylation reached a peak at 30 minutes and decreased after 4 hours in both +/− and −/− cells. Immunostaining results also suggested a reduction of phospho-Akt in −/− cells with or without PDGF stimulation when compared with +/− cells (Figure 3B). Immunostaining of phospho-Akt was more obvious and abundant in the nuclei of the +/− cells after PDGF stimulation for 4 hours (Figure 3B). This finding suggests that RhoB regulates the trafficking of Akt protein from the cytoplasm into the nucleus following PDGF stimulation. We next compared the phosphorylation and localization status of ERK protein. Western analysis revealed a relative attenuation of ERK phosphorylation in −/− cells (Figure 3A). Additionally, similar to phosphorylated Akt, phosphorylated-ERK was observed predominantly in the perinuclear region of +/− cells (Figure 3C). After PDGF stimulation for 24 hours, phospho-ERK localized to the nucleus in +/− cells whereas this pattern of localization was not observed in −/− cells (Figure 3C). Taken together, these results indicate that RhoB plays a crucial role in PDGF-induced phosphorylation and trafficking of Akt and ERK.
Figure 3.
A, Immunoblot analysis of total and active Akt and ERK in RhoB +/− or −/− SMCs. The cells were incubated with PDGF-BB for the indicated times. Quantitation analysis is shown for phosphorylated Akt and ERK. B, Immunofluorescence staining of phosphorylated Akt (Ser473) in mouse RhoB +/− or −/− SMCs stimulated with PDGF-BB for 4 hours. Images were taken at the same magnification for the same period of exposure. C, Immunofluorescence staining of phosphorylated ERK in mouse RhoB +/− or −/− SMCs stimulated with PDGF-BB for 24 hours as in panel B. Scale bars=50 µm.
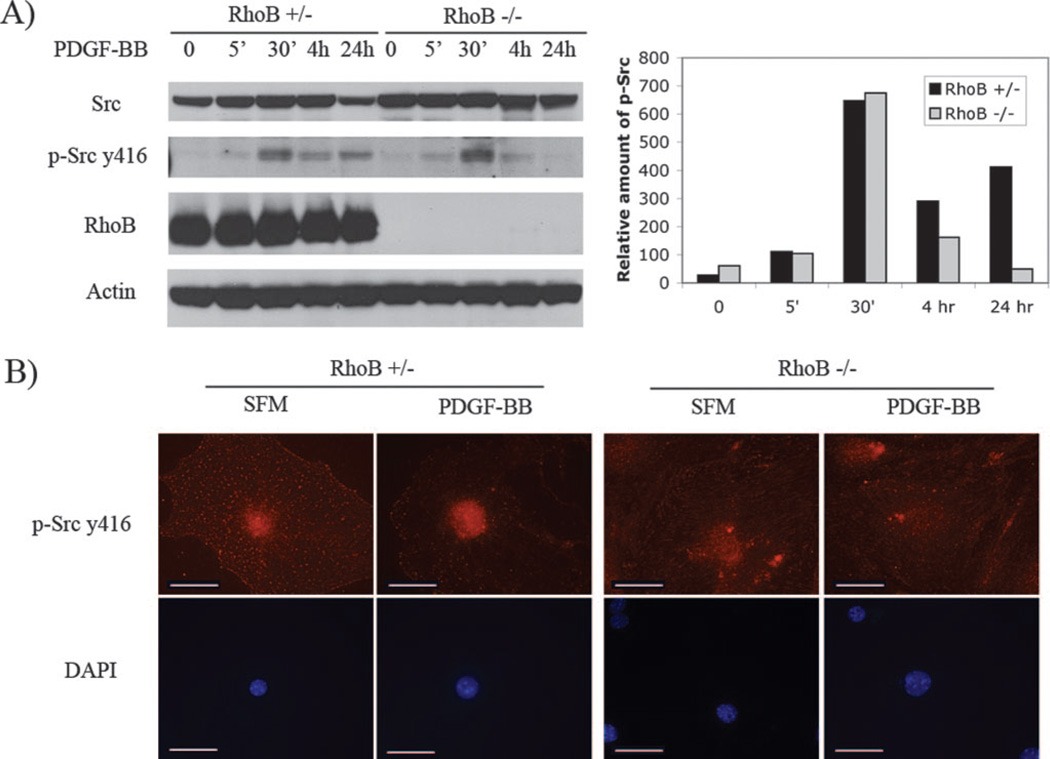
RhoB Mediates the PDGF-Induced Src Activation and Trafficking
Src has been implicated in PDGF signaling that leads to DNA synthesis, actin cytoskeleton rearrangement, and receptor endocytosis.31 RhoB regulates both catalytic activation of Src and translocation of active kinase to peripheral membrane structures in fibroblasts.26 Whether RhoB also plays this role in vascular SMCs is not known. We tested the activation of Src by Western blotting using a phospho-Src specific antibody, which recognizes the active form of this kinase. Incubation of cells with PDGF resulted in a rapid increase in phosphorylation of the tyrosine 416 residue of Src in both RhoB +/− and −/− vascular SMCs. This phosphorylation status of Src was still observed in +/− cells after 24 hours of PDGF stimulation, but not in −/− cells (Figure 4A). We next examined the localization of active Src in vascular SMCs. We observed a punctate staining pattern of active Src dispersed throughout the cytoplasm, including in the perinuclear region of +/− cells in serum starved cultures (Figure 4B). Four hours after PDGF stimulation, phospho-Src was preferentially concentrated in the nuclear area of +/− cells (Figure 4B). This nuclear accumulation of active Src was not observed in the −/− cells. Taken together the observations indicated that RhoB is required for PDGF-induced trafficking of Src kinase from cytoplasm to nucleus.
Figure 4.
A, Immunoblot analysis of total and active Src in RhoB +/− or −/− SMCs. The cells were incubated with SFM plus PDGF-BB for the indicated times and cell lysates were analyzed by Western blotting using the antibodies to detect total or phosphorylation/activation Src status. Quantitation analysis is shown for phosphorylated Src. B, Immunofluorescence staining of phosphorylated Src (y416) in mouse RhoB +/− or −/− SMCs stimulated with PDGF-BB for 4 hours. Images were taken at the same magnification and exposure. Scale bars=50 µm.
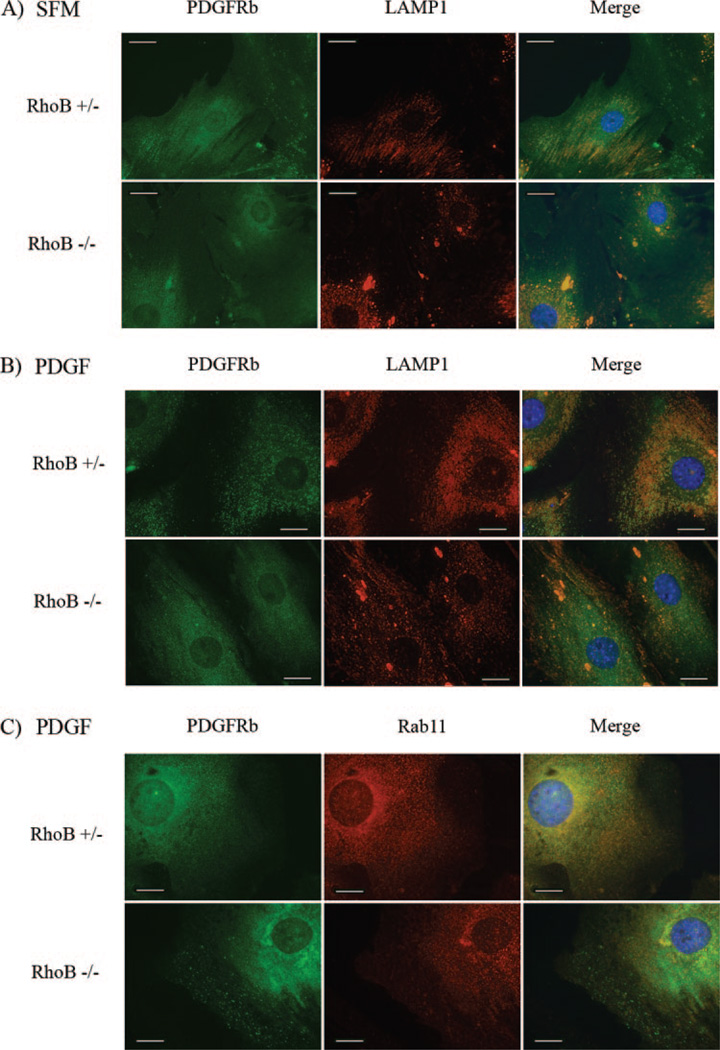
Loss of RhoB Alters PDGFR-β Trafficking in Vascular SMCs
There is little known about the regulation of PDGF receptor trafficking and endosomal signaling. We have observed that the loss of RhoB results in a change in PDGFR-β localization. To determine whether PDGFR-β localization is associated with the endosomes, and which step of PDGFR-β endocytic trafficking might be affected by RhoB, we performed immunostaining of PDGFR and endosomal markers. First, we investigated the colocalization of PDGFR and EEA-1, an early endosome marker. Immunofluorescence results indicated that the colocalization of PDGFR-β on EEA-1 associated early endosomes was not significantly different between RhoB +/− and −/− vascular SMCs (supplemental Figure I). By coimmunostaining with LAMP-1, a marker of the late endosomal/lysosomal compartment, we are able to examine the association of PDGFR-β with later endosomes. The results demonstrate a lack of colocalization of PDGFR-β with LAMP-1 in −/− cells at 4 hours, whereas these proteins colocalized to punctuate structures in the perinuclear area of +/− cells (Figure 5B). However, we did observe colocalization of the PDGFR and LAMP-1 in large aggregate structures within the −/− cells at 0 and 4 hours, suggesting altered PDGFR trafficking or processing in the absence of RhoB. Furthermore, we used Rab11, a marker of recycling endosomes, to perform coimmunostaining with the PDGFR-β. The results demonstrate a decrease in the colocalization of PDGFR-β and Rab11 in RhoB −/− SMCs, indicating that loss of RhoB interrupts receptor trafficking through recycling endosomes (Figure 5C). Thus, our results argued that the loss of RhoB and associated alteration of receptor trafficking leads to a reduction in the efficiency of nuclear accumulation of phospho-Akt and phospho-ERK, thereby, attenuating PDGF/PDGFR signaling.
Figure 5.
Cells were incubated with fresh SFM (A) or SFM plus PDGF-BB for 4 hours (B) and 8 hours (C). Immunofluorescence staining was performed on fixed permeabilized cells using an anti-LAMP1 antibody detected with goat anti-rat CY3 (A and B) or an anti-Rab11 antibody detected with goat anti-mouse CY3 (C), as well as, an anti-PDGFR-β antibody detected with goat anti-rabbit FITC. Images were taken at the same magnification and exposure. Scale bars=20 µm.
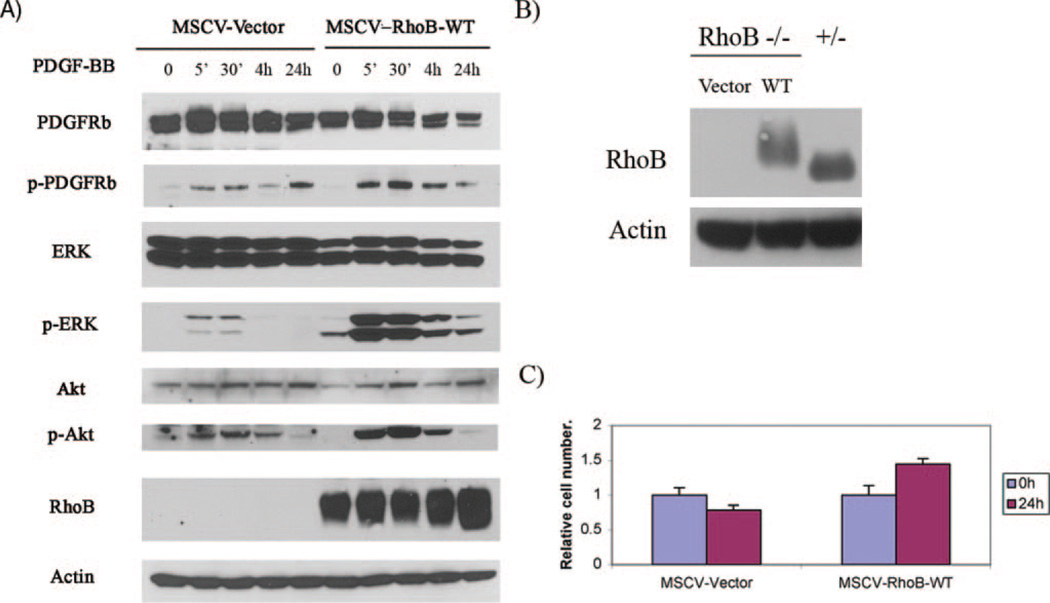
Restoring Expression of RhoB in Null Vascular SMCs Rescues Cell Growth
To rule out the possibility that nonspecific compensating alternations are responsible for cell growth changes in our cell models, we introduced RhoB back into null cells to attempt to restore PDGF-stimulated cell growth. Briefly, retroviral vectors were used to introduce an epitope-tagged wild-type RhoB protein (RhoB-WT) to RhoB-null cells. The restored expression of RhoB-WT was confirmed by Western blotting using a RhoB polyclonal antibody (Figure 6A and 6B), and the restored RhoB expression level of the infected RhoB null cells was comparable to the RhoB+/−, control cells (Figure 6B). Consistent with expectations, RhoB-WT reversed the phosphorylation status of PDGFRβ and downstream signaling proteins Akt and ERK in response to PDGF-BB stimulation (Figure 6A). Furthermore the ectopic expression of RhoB also increased PDGF-induced cell proliferation when compared with vector control −/− cells (Figure 6C). Taken together, these observations confirmed that the poor survival and growth of null cells was indeed directly due to the absence of RhoB.
Figure 6.
A, Immunoblot analysis of total and active PDGFR-β, Akt, and ERK in extracts prepared from the RhoB null cells infected with either empty vector or RhoB-WT in MSCVpac, and then treated with PDGF-BB for the indicated times. Confirmation of RhoB protein expression in infected RhoB null SMCs is shown. B, Immunoblot analysis of RhoB expression level in cell extracts from the RhoB −/− cells infected with empty MSCVpac vector or MSCVpac-RhoB-WT, and the RhoB +/− cells by using the anti-RhoB antibody. Note: the HA-tagged RhoB-WT has a slightly higher molecular weight than endogenous RhoB. C, Cell growth of infected RhoB null cells after PDGF-BB stimulation. Cells infected with either vector or RhoB-WT were selected and cultured in low serum (1%). Cell number was determined at indicated times after PDGF-BB stimulation (n=3). Cell counts were normalized to the Day 0 count taken before stimulation.
Discussion
In response to ligand-induced receptor dimerization, PDGFR autophosphorylation occurs, and the receptor associates with a variety of SH2-domain containing signal transduction molecules that mediate cell growth, chemotaxis, actin reorganization, and antiapoptosis.3,8 The ligand-receptor complexes cluster into clathrin-coated pits that internalize into early endosomes, which recycle back to the cell surface or traffic to lysosomes for degradation.11–14 Internalization of signaling receptors was initially thought to result only in termination of the signaling pathway or in downregulation of the receptor itself via degradation. However, accumulating evidence suggests that signaling takes place not only from the cell surface but also from endosomes and that compartmentalized signaling is physiologically important.18,32 Although there is considerable information on PDGF receptor signaling pathways, the regulation of PDGF receptor trafficking and the mechanistic relationship between PDGF receptor trafficking and endosomal signaling are not well understood. In this study, by using a knockout mouse model system, we found that RhoB is involved in the regulation of PDGFR-β trafficking and signaling through the Akt and Src pathways, which are known to be critical for PDGF-induced proliferation of vascular SMCs.
In studies of the EGFR endocytic pathway, it has been found that RhoB does not affect receptor sorting into early and late endosomes but that it inhibits subsequent transfer of the receptor through multivesicle bodies to the lysosomal compartment.24 Other work has shown that RhoB forms a ternary complex with the Rho effector kinase (PRK) and the Akt regulatory kinase (PDK1) in endosomes, and that this binding is necessary for RhoB to regulate EGFR trafficking.23 In our study, there was no difference in localization of PDGFR-β associated with early (EEA-containing) endosomes in RhoB +/− and −/− vascular SMCs, indicating that early endosomal trafficking of PDGFR-β is not interrupted by loss of RhoB. However, we found that RhoB is required for the association of the PDGFR-β with recycling endosomes (Rab11 containing), and late (LAMP-1 containing) endosomes that assumed a perinuclear localization in vascular SMCs. Our observations are consistent with observations in other cells that PDGF receptors internalize and concentrate in a PI3K–dependent manner to a perinuclear region after PDGF stimulation.15
Receptor-mediated endocytosis coordinates transport of signaling proteins destined for the nucleus through endosomal compartments in the cytosol. For example, EGFR and Stat3 are routed in this manner to the nucleus where they regulate expression of genes such as cyclin D1 that control cell division.33,34 We found that RhoB is important for late endosomal trafficking of PDGFR-β and its ability to effectively activate the ERK and Akt signaling pathways that are known to be critical mediators of PDGF signaling. RhoB was also important for efficient nuclear trafficking of activated Akt stimulated by PDGF. Further, we found that in response to PDGF stimulation RhoB mediated the nuclear accumulation of active Src. Our findings in vascular SMCs parallel those made in sprouting vascular endothelial cells, where RhoB has also been demonstrated to be crucial for Akt activation, Akt nuclear localization, and cell survival.25 Together these findings define an important positive function for RhoB in survival and proliferation of vascular cells, contrasting with the negative functions in growth and survival documented in epithelial cells and cancer.35 From our work and reports from other studies, we suggest that the EGFR and PDGFR require endosomal trafficking for complete propagation of the signaling cascades they engage, and that RhoB is necessary for the correct endosomal compartmentalization of these receptors. To our knowledge this is the first evidence that RhoB is important for vascular SMC survival and proliferation through precise PDGFR-β trafficking and downstream signaling (supplemental Figure II). The findings of this study may have implications for atherosclerosis or other cardiovascular diseases where PDGF-stimulated vascular SMCs are thought to play a role. Additionally, given the ability of farnesyl transferase inhibitors to alter the function of RhoB,25,35 our findings support the possibility of applying farnesyl transferase inhibitors for limiting vascular cell proliferation in these or other disease states.36,37
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the W. W. Smith Charitable Trust (to L.L.K.), the National Cancer Institute (CA100123 and CA82222 to G.C.P.), and the Lankenau Hospital Foundation.
Footnotes
Disclosures
None.
References
- 1.Kitami Y, Inui H, Uno S, Inagami T. Molecular structure and transcriptional regulation of the gene for the platelet-derived growth factor alpha receptor in cultured vascular smooth muscle cells. J Clin Invest. 1995;96:558–567. doi: 10.1172/JCI118068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sano H, Yokode M, Takakura N, Takemura G, Doi T, Kataoka H, Sudo T, Nishikawa S, Fujiwara H, Nishikawa SI, Kita T. Study on PDGF receptor beta pathway in glomerular formation in neonate mice. Ann N Y Acad Sci. 2001;947:303–305. doi: 10.1111/j.1749-6632.2001.tb03951.x. [DOI] [PubMed] [Google Scholar]
- 3.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 4.Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, Nishikawa S, Nishikawa SI, Kita T. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation. 2001;103:2955–2960. doi: 10.1161/01.cir.103.24.2955. [DOI] [PubMed] [Google Scholar]
- 5.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 6.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 8.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkranz S, Kazlauskas A. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors. 1999;16:201–216. doi: 10.3109/08977199909002130. [DOI] [PubMed] [Google Scholar]
- 10.Marshall S, Green A, Olefsky JM. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981;256:11464–11470. [PubMed] [Google Scholar]
- 11.Nilsson J, Thyberg J, Heldin CH, Westermark B, Wasteson A. Surface binding and internalization of platelet-derived growth factor in human fibroblasts. Proc Natl Acad Sci USA. 1983;80:5592–5596. doi: 10.1073/pnas.80.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld ME, Bowen-Pope DF, Ross R. Platelet-derived growth factor: morphologic and biochemical studies of binding, internalization, and degradation. J Cell Physiol. 1984;121:263–274. doi: 10.1002/jcp.1041210202. [DOI] [PubMed] [Google Scholar]
- 13.Sorkin A, Westermark B, Heldin CH, Claesson-Welsh L. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J Cell Biol. 1991;112:469–478. doi: 10.1083/jcb.112.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapeller R, Chakrabarti R, Cantley L, Fay F, Corvera S. Internalization of activated platelet-derived growth factor receptor-phosphati-dylinositol-3′ kinase complexes: potential interactions with the microtubule cytoskeleton. Mol Cell Biol. 1993;13:6052–6063. doi: 10.1128/mcb.13.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joly M, Kazlauskas A, Fay FS, Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding-sites. Science. 1994;263:684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- 16.Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- 19.Adamson P, Paterson HF, Hall A. Intracellular localization of the p21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson D, Paterson HF, Adamson P, Hall A, Monaghan P. Ultra-structural localization of ras-related proteins using epitope-tagged plasmids. J Histochem Cytochem. 1995;43:471–480. doi: 10.1177/43.5.7537292. [DOI] [PubMed] [Google Scholar]
- 21.Mellor J, Flynn P, Nobes CD, Hall A, Parker PJ. PRK1 is targeted to endosomes by the small GTPase, RhoB. J Biol Chem. 1998;273:4811–4814. doi: 10.1074/jbc.273.9.4811. [DOI] [PubMed] [Google Scholar]
- 22.Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase RhoB. Curr Biol. 1999;9:955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- 23.Flynn P, Mellor H, Casamassima A, Parker PJ. Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide-dependent protein kinase. J Biol Chem. 2000;275:11064–11070. doi: 10.1074/jbc.275.15.11064. [DOI] [PubMed] [Google Scholar]
- 24.Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J Cell Sci. 2004;117:3221–3231. doi: 10.1242/jcs.01193. [DOI] [PubMed] [Google Scholar]
- 25.Adini I, Rabinowitz I, Sun JF, Prendergast GC, Benjamin LE. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev. 2003:17. doi: 10.1101/gad.1134603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandilands E, Cans C, Fincham VJ, Mellor H, Prendergast GC, Brunton VG, Superti-Furga G, Frame MC. RhoB and actin polymerisation co-ordinate Src activation with endosome-mediated delivery to the membrane. Devel Cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci. 2005;118:2661–2670. doi: 10.1242/jcs.02384. [DOI] [PubMed] [Google Scholar]
- 28.Liu A-X, Rane N, Liu J-P, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panayotou G, Bax B, Gout I, Federwisch M, Wroblowski B, Dhand R, Fry MJ, Blundell TL, Wollmer A, Waterfield MD. Interaction of the p85 subunit of PI 3-kinase and its N-terminal SH2 domain with a PDGF receptor phosphorylation site: structural features and analysis of conformational changes. Embo J. 1992;11:4261–4272. doi: 10.1002/j.1460-2075.1992.tb05524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng P-Y, Rane N, Du W, Chintapalli J, Prendergast GC. Role of RhoB and PRK in the suppression of epithelial cell transformation by farnesyl-transferase inhibitors. Oncogene. 2003;22:1124–1134. doi: 10.1038/sj.onc.1206181. [DOI] [PubMed] [Google Scholar]
- 31.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 32.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 33.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 34.Bild AH, Turkson J, Jove R. Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO J. 2002;21:3255–3263. doi: 10.1093/emboj/cdf351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang M, Prendergast GC. RhoB in cancer suppression. Histol Histopathol. 2006;21:213–218. doi: 10.14670/HH-21.213. [DOI] [PubMed] [Google Scholar]
- 36.Mattingly RR, Gibbs RA, Menard RE, Reiners JJ., Jr. Potent suppression of proliferation of a10 vascular smooth muscle cells by combined treatment with lovastatin and 3-allylfarnesol, an inhibitor of protein far-nesyltransferase. J Pharmacol Exp Ther. 2002;303:74–81. doi: 10.1124/jpet.102.036061. [DOI] [PubMed] [Google Scholar]
- 37.Work LM, McPhaden AR, Pyne NJ, Pyne S, Wadsworth RM, Wainwright CL. Short-term local delivery of an inhibitor of Ras farnesyltransferase prevents neointima formation in vivo after porcine coronary balloon angioplasty. Circulation. 2001;104:1538–1543. doi: 10.1161/hc3801.095661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.