Abstract
Avian feathers have robust growth and regeneration capability. To evaluate the contribution of signaling molecules and pathways in these processes, we profiled gene expression in the feather follicle using an absolute quantification approach. We identified hundreds of genes that mark specific components of the feather follicle: the dermal papillae (DP) which controls feather regeneration and axis formation, the pulp mesenchyme (Pp) which is derived from DP cells and nourishes the feather follicle, and the ramogenic zone epithelium (Erz) where a feather starts to branch. The feather DP is enriched in BMP/TGF-β signaling molecules and inhibitors for Wnt signaling including Dkk2/Frzb. Wnt ligands are mainly expressed in the feather epithelium and pulp. We find that while Wnt signaling is required for the maintenance of DP marker gene expression and feather regeneration, excessive Wnt signaling delays regeneration and reduces pulp formation. Manipulating Dkk2/Frzb expression by lentiviral-mediated overexpression, shRNA-knockdown, or by antibody neutralization resulted in dual feather axes formation. Our results suggest that the Wnt signaling in the proximal feather follicle is fine-tuned to accommodate feather regeneration and axis formation.
Keywords: Feather follicle, Regeneration, Axis formation, Dermal papillae, Dkk2/Dkk3/Frzb
Introduction
Avian feathers serve as a useful model for developmental studies (reviewed in Lin et al., 2013, 2006; Yu et al., 2004). Major signaling pathways are involved in embryonic feather bud development, including Wnt/β-catenin, BMP/Tgf-β, FGF, Shh, Notch/Delta, EGF, Eda/Edar etc. (reviewed in Lin et al., 2006). In particular, various Wnt ligands are involved, such as Wnt1, Wnt3a, Wnt5a, Wnt6, Wnt7a, Wnt11, and Wnt14 (Widelitz et al., 1999; Chodankar et al., 2003; Chang et al., 2004). A similar set of signaling molecules and pathways are also involved in adult feather growth and regeneration (Yu et al., 2002; Yue et al., 2006, 2012). Previous studies have characterized the expression of Wnt ligands including Wnt3a, Wnt5a, Wnt6 and Wnt8c (Chodankar et al., 2003; Yue et al., 2006). Quantifying gene expression at the whole genome scale during these processes will help evaluate the contribution of each molecule at each developmental stage. Methods based on next generation sequencing technology provide such an opportunity, as compared with the more traditional hybridization-based microarray technology (Saha et al., 2002).
Similar to the mammalian hair follicle, the feather follicle is a “professional” regenerative organ that undergoes physiological renewal and regeneration after wounding or plucking (Lucas and Stettenheim, 1972; Lin et al., 2013; Yu et al., 2004). We have shown previously there are slow-cycling epithelial stem cells in the feather follicle that contribute to its episodic renewal (Yue et al., 2005). Furthermore, classical surgery experiments have established the critical role of the DP in feather regeneration (Lillie and Wang, 1941, 1944). It was shown that the DP controls feather shape, size, and axis determination (Lillie and Wang, 1941, 1943; Yue et al., 2006). However, little is known about the “molecular encoding” of the feather DP.
Molecular expression in the hair DP has been investigated in detail (Driskell et al., 2011, 2009; Rendl et al., 2005). Wnt3a, but not Shh signaling has been shown to maintain DP cell properties, i. e. hair reconstitution capability and marker gene expression (Kishimoto et al., 2000). DP-specific knockout of β-catenin disrupts hair regeneration (Enshell-Seijffers et al., 2010). In addition, BMP signaling plays an important role in maintaining DP cell properties (Rendl et al., 2008). In the DP niche, Sox2 may regulate the strength of BMP signaling and hair growth (Clavel et al., 2012). Moreover, TGF-β signaling may also modulate BMP signaling and contribute to hair regeneration (Oshimori and Fuchs, 2012).
Here we use the feather follicle as a model to analyze the regulatory logic of tissue regeneration. We started by using an unbiased transcriptional profiling of the feather DP. Interestingly, we noticed high levels of Dkk2/Dkk3/Frzb, which presumably encode Wnt signaling inhibitors. Functional analysis of the specific roles of these molecules revealed intriguing regulatory modes, i.e. both overexpression and knockdown of Dkk2/Frzb will lead to delayed feather regeneration and perturbation of feather axis formation. Our results have thus established novel concepts regarding the molecular mechanism of feather regeneration.
Results
A whole-genome survey of gene expression in the feather DP
The structure of the feather follicle has been described previously (Lin et al., 2013; Yu et al., 2004 and Fig. S1A). After plucking induced wounding, the follicle wall and DP (with the covering papillae ectoderm) still remain (Fig. S1B). This structure will always regenerate. However, if the DP is surgically removed, this “empty follicle” cannot regenerate, unless a DP is re-supplied (Lillie and Wang, 1944; Fig. S1 C). Feather regeneration is a rather quick process. After a short period of wound healing and remodeling (day 2), the follicle structure is re-established by day-4 (Fig. S1D–F).
To investigate the “molecular encoding” of the feather DP, we took an unbiased whole-genome profiling approach based on next generation sequencing technology. Compared to microarray, sequencing technology provides absolute quantification of gene expression and is more accurate (Saha et al., 2002). We isolated the DP, pulp mesenchyme (Pp) and ramogenic zone feather epithelium (Erz) in growth phase follicles by microsurgery (Fig. 1A and B; and S2). The Pp is a distinct component in the feather follicle that is derived from DP cells and supports the actively growing feather epithelium (the hair follicle does not have a similar component) (Yue et al., 2012). Erz is included as a control because it is an epithelial component and should have very different molecular expression profile compared to the other mesenchymal components. Only high quality RNAs were used for analysis, which were monitored by an Agilent Bioanalyzer (Fig. S3).
Fig.1.
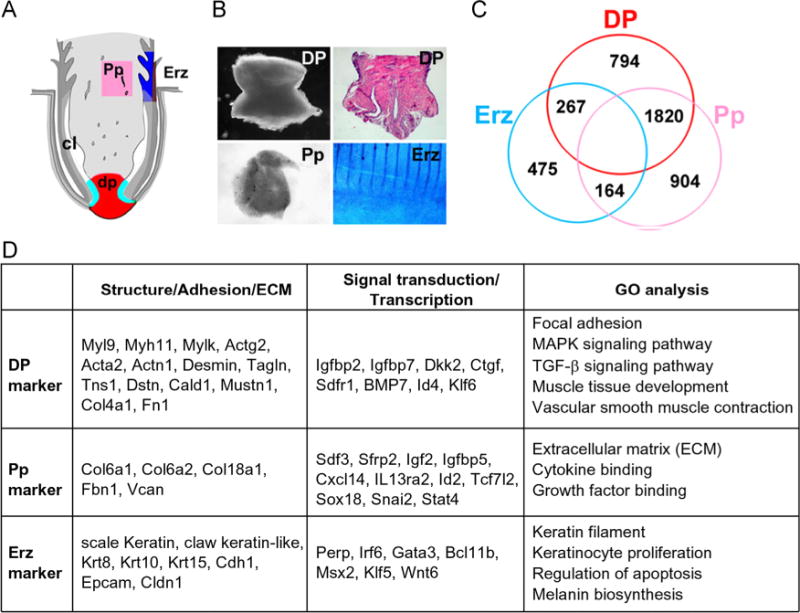
Gene expression profiling in the feather follicle. (A) Diagram and (B) examples showing the feather structure and the dissection process. H&E showing the structure of the DP. Erz was illustrated based upon DAPI staining. The stripes in the Erz sample are feather branches. dp, dermal papilla; Pp, pulp; Erz, ramogenic zone feather epithelium. (C) Venn diagram showing differentially expressed genes among DP, Pp and Erz. (D) Lists of highly expressed genes in each compartment that could serve as “markers”. The gene abbreviations are according to the NCBI listings. Gene ontology (GO) analysis results are also shown.
In about 19,000 chicken genes in our database used for analysis, about 45% are expressed in the feather DP. A detailed description of the data processing and analysis procedure is in Supplemental data. Using the criteria of 2-fold difference and a false discovery rate (FDR) < 0.001, 794 genes are considered DP specific when compared with Erz and Pp (Fig. 1C). Similarly, 475 genes are Erz specific, and 904 genes are Pp specific. A full list of the differentially expressed genes, and the results of pathway enrichment/gene ontology (GO) analysis are shown in Supplemental Table 1. Potential “marker genes” for each compartment, together with the GO analysis results are also shown (Fig. 1D).
Previously there are very few molecules known to be expressed in the feather DP, mainly extracellular matrix proteins or cell adhesion molecules such as Fibronectin, Tenascin, Laminin, and Ncam (neural cell adhesion molecules) (Lin et al., 2006; Yue et al., 2012). Their expressions were confirmed by gene profiling analysis and by immunohistochem-istry (Fig. 2A). A significant molecular feature of the feather DP is enrichment in muscle-related genes. These include Actg2 (smooth muscle actin, gamma 2), Acta2 (smooth muscle actin, alpha 2), Desmin, Myh11 (myosin heavy chain 11), Myl4 (myosin light chain 4), Myl9 (myosin light chain 9), Mylk (myosin light chain kinase), etc. These genes are expressed at comparable or higher levels than β-actin. Sox2 is not expressed, but Ncam is present in the DP. The developmental origin of the feather DP has not been clarified yet, however, gene expression profiling suggests a close relationship with muscle cells. GO analysis also revealed features such as “muscle tissue development”, “vascular smooth muscle contraction”. The specific expression patterns of some genes listed were confirmed by immunohistochemistry (Fig. 2A).
Fig. 2.
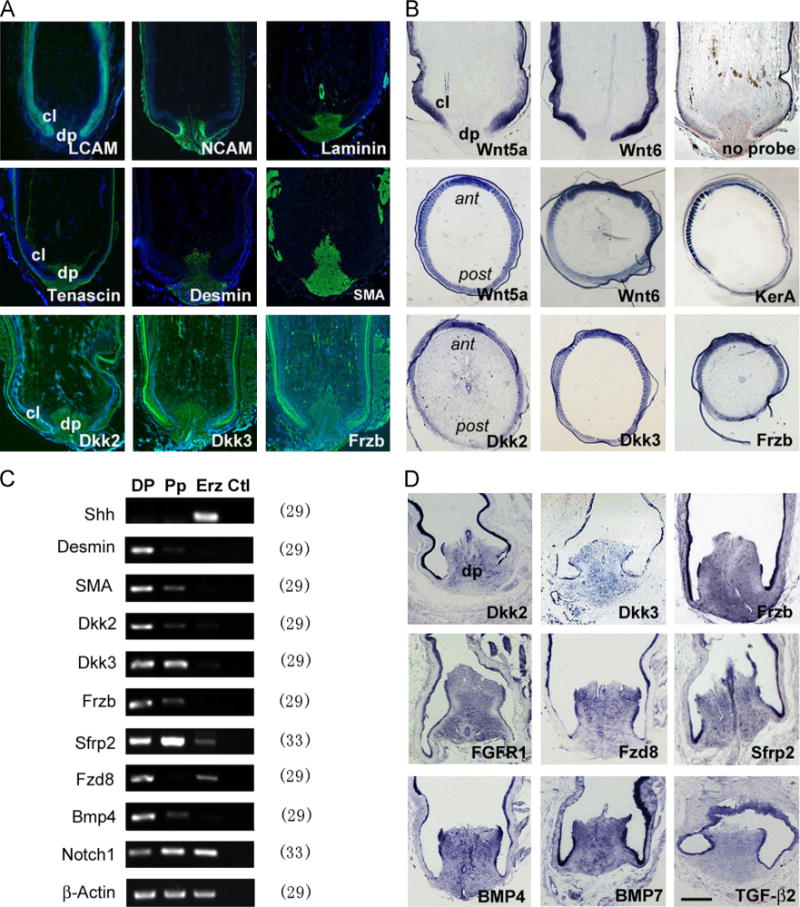
Gene expression analysis in the feather follicle. (A) Marker gene expression shown by immunofluorescence (green). LCAM marks the feather epithelium. NCAM marks the DP/dermal sheath and weakly the feather branching epithelium. Laminin marks the DP and vessel walls. Tenascin marks the DP/dermal sheath. Desmin is more DP specific, and SMA marks the DP and vessel walls. Dkk2/Dkk3/Frzb is enriched in the DP, presents in the pulp but less in the epithelium. Some unspecific staining is found in the keratinized feather sheath. (B) Expression of Wnt ligands and inhibitors in the feather follicles shown by in situ hybridization. Notice Wnt5a and Wnt6 appear primarily in the epithelium. A gradient distribution pattern is detected for Wnt5a/Wnt6, Dkk2/Dkk3/Frzb and feather keratin A in the Erz region. A control staining with no probe is also shown. (C) Semi-quantitative RT-PCR and (D) in situ hybridization analysis of gene expression in the feather follicles. No template reactions are used as control for PCR analysis, and equal amount of RNAs are monitored by β-Actin gene expression. The number after each gene indicates PCR cycles. dp, dermal papilla; cl, collar; ant, anterior where the rachis locates; post, posterior as opposite to the rachis position. Bar = 1 mm in A and B, and 0.5 mm in D (shown in D).
Due to the critical roles of the DP in feather formation, we paid special attention to molecules involved in major signaling pathways. A list of genes with their expression levels are shown in Table 1. Dkk2/Dkk3/Frzb and Sfrp1/Sfrp2 are expressed at high levels in the DP, which presumably encode inhibitors of the Wnt signaling pathway. The expression levels of Wnt ligands, mainly Wnt5a/Wnt5b/Wnt6, are very low in the DP, but high in the feather epithelium and pulp. The receptors including Fzd1/Fzd2/Fzd7/Fzd8 are expressed in the DP, suggesting active Wnt signaling in this tissue. We previously suggested a Wnt3a signaling gradient in the epithelium controls feather A–P axis orientation (Yue et al., 2006). Here we found Wnt3a is actually expressed at a low level in the feather Erz. A clear gradient distribution is found for Wnt5a/Wnt6, Dkk2/Dkk3/Frzb and a structural protein, feather keratin A in the Erz region (Fig. 2B). We further cloned or synthesized the full ORFs of the chicken Dkk2, Dkk3, Frzb genes, expressed in a prokaryotic system and purified the encoded proteins. We made and affinity purified polyclonal antiserum for each of these proteins (Fig. S4). These antisera confirmed the specific expression patterns of these proteins by immunohistochemistry (Fig. 2A).
Table 1.
Expression of major signaling pathway members in the feather follicle.
| Gene | DP | Pp | Erz | |
|---|---|---|---|---|
| Reference genes | β-Actin | 1340 | 2406 | 660 |
| Gapdh | 3914 | 2720 | 2600 | |
| Wnt signaling pathway | Wnt5a | 8 | 90 | 27 |
| Wnt5b | 1 | 15 | 19 | |
| Wnt6 | 0 | 12 | 30 | |
| Wnt11 | 1 | 15 | 0 | |
| Wnt4 | 4 | 2 | 4 | |
| Wnt3a | 0 | 0 | 1 | |
| Fzd8 | 36 | 3 | 16 | |
| Fzd7 | 24 | 8 | 10 | |
| Fzd2 | 16 | 21 | 1 | |
| Fzd1 | 20 | 19 | 4 | |
| Fzd6 | 3 | 33 | 3 | |
| Fzd9 | 6 | 6 | 30 | |
| Frzb | 785 | 630 | 4 | |
| Dkk2 | 230 | 27 | 3 | |
| Dkk3 | 190 | 173 | 10 | |
| Sfrp1 | 158 | 107 | 1 | |
| Sfrp2 | 95 | 346 | 0 | |
| TGF-β superfamily signaling Pathway | Ltbp1 | 485 | 1108 | 5 |
| Decorin | 285 | 58 | 14 | |
| Chordin-like1 | 110 | 2 | 2 | |
| Tgf-b3 | 48 | 96 | 43 | |
| Tgf-b2 | 12 | 2 | 4 | |
| Inhba | 7 | 36 | 0 | |
| Admp | 5 | 3 | 110 | |
| Bmp4 | 256 | 144 | 1 | |
| Bmp7 | 111 | 28 | 17 | |
| Bmp2 | 2 | 5 | 1 | |
| Noggin2 | 46 | 18 | 0 | |
| Other signaling molecules | Fgfr1 | 305 | 215 | 25 |
| Fgfr2 | 42 | 28 | 33 | |
| Fgf7 | 14 | 2 | 0 | |
| Fgf12 | 8 | 8 | 3 | |
| Spry2 | 19 | 25 | 5 | |
| Notch1 | 115 | 245 | 262 | |
| Jagged1 | 40 | 24 | 30 | |
| Serrate2 | 67 | 42 | 69 |
Values are tags-per-million (TPM) counts from gene expression profiling. DP, dermal papilla; Pp, pulp; Erz, ramogenic zone feather epithelium.
Previous work suggested BMP signaling is involved in feather formation (Yu et al., 2002). GO analysis revealed TGF-β signaling is a distinct feature of DP gene expression (Fig. 1D). Here we found BMP4, BMP7, Tgfβ2, Tgfβ3, Noggin2, Chordin-like 1, Decorin, but not BMP2 or Noggin are expressed at high levels in the DP (Table 1). Other signaling molecules expressed include FGFR1, PDGF, CTGF and Notch1/Jagged 1/Serrate2. Interestingly, members of the Shh signaling pathway are absent, consistent with their later role in feather epithelial branching (Yu et al., 2002; Harris et al., 2002, 2005). We confirmed the expression of some molecules by RT-PCR (Fig. 2C) and in situ hybridization (Fig. 2D). The results also confirmed the specific expression patterns of several marker genes. For example, Shh gene is only expressed in the Erz but not DP or Pp, and SMA/Desmin is highly enriched in the DP but not Erz or Pp. In summary, we find many signaling molecules or regulators of major signaling pathways are expressed in the feather DP, consistent with its critical role in feather growth and regeneration.
cDkk2/cFrzb antagonizes Wnt signaling
The gene expression profile of the feather DP provides interesting insights into the functional role of this structure. In early embryonic development, the organizer (or the node) controls body axis formation and elongation, which expresses high levels of FGF/TGF-β signaling molecules and regulators, and inhibitors of Wnt signaling such as Dkk1 (reviewed in Niehrs, 2001; De Robertis, 2006). In the hair follicle, the critical role of BMP signaling in DP function is well-documented (Rendl et al., 2008; Clavel et al., 2012). Wnt signaling is required for DP function (Kishimoto et al., 2000; Enshell-Seijffers et al., 2010), and inhibitors such as Wif1 and Sfrp1/Sfrp2 are also expressed in the hair DP (Rendl et al., 2005). Dkk1 overexpression blocks hair follicle morphogenesis; however, its expression level in the hair DP is very low (Andl et al., 2002). Therefore, the function of these inhibitors in the hair follicle has not been investigated in detail.
To examine whether Dkk2/Dkk3/Frzb are truly inhibitors for Wnt signaling, we tested their functions both in vitro and in vivo. Previous work suggested that Xenopus Dkk2 (xDkk2) could activate or inhibit Wnt signaling depending on the specific context, while xDkk3 did not regulate Wnt signaling (Wu et al., 2000). In a Wnt responsive reporter assay in HEK 293 T cells, we found cDkk2 is a potent inhibitor for Wnt signaling, even stronger than the positive control used here, hDkk1 (Fig. 3A). cFrzb is a weaker but consistent inhibitor, whereas cDkk3 does not significantly interfere with Wnt signal transduction. In Xenopus embryos, mRNAs of chicken Dkk2, Dkk3 or Frzb were injected into all four blastomeres at 4-cell stage. cDkk3 injected embryos were largely normal with very few (3/33) showing weak anteriorization, an indication of Wnt inhibition. In contrast, all cFrzb injected embryos (52/52) were modestly anteriorized. Even stronger anteriorization effect was observed after cDkk2 injection, in which 7 out of 35 injected embryos had enlarged head and shortened trunk/tail structures (Fig. 3B). A summary of the tests in Xenopus embryos is shown in Fig. 3C. These results suggest that cDkk2 is a potent Wnt inhibitor, cFrzb is a mild but consistent Wnt inhibitor, whereas cDkk3 shows almost no activity in this regard.
Fig. 3.
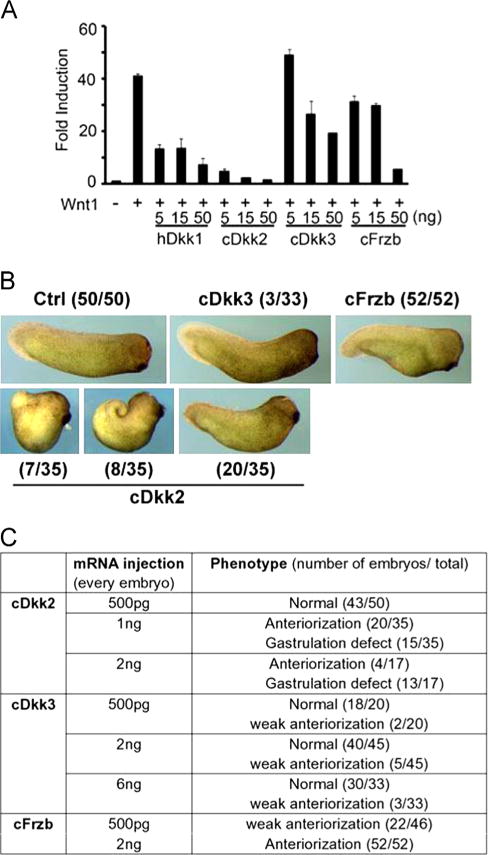
Dkk2/Frzb antagonizes Wnt signaling. (A) Wnt reporter assay in HEK 293T cells. Super-TOPFLASH, a Wnt responsive reporter was co-transfected into HEK293T cells together with Wnt1 and other plasmids as indicated. hDkk1 (human Dkk1) was used as a positive control. Fold induction of Wnt reporter activity is shown. The numbers for each gene indicates the amount of DNA transfected; total amount of DNA transfected in each well was 150 ng, adjusted with pCS2+ plasmid. (B) Anteriorization of Xenopus embryos by injected mRNAs as indicated. mRNAs of chicken Dkk2 (1 ng per embryo), Dkk3 (6 ng per embryo) or Frzb (2 ng per embryo) were injected at 4-cell stage. The numbers of embryos with indicated phenotypes are also shown. (3/33) stands for 3 out of 33 injected embryos showed indicated phenotype. Control animals were injected with 250 pg mRNA of preprolectin gene. (C) Summary of the Xenopus injection experiment.
Overexpression of Dkk2/Frzb but not Dkk3 disrupts feather regeneration
To directly examine the roles of Dkk2/Dkk3/Frzb in feather regeneration, we developed methods based on lentiviral delivery that could either overexpress or knockdown gene expression. Previously, gene overexpression in the feather follicle was achieved by RCAS-mediated gene delivery, which led to widespread and long-term expression (Yu et al., 2002; Yue et al., 2006). However, this method does not permit RNAi-mediated gene knockdown. Recently, methods based on siRNA – (Harpavat and Cepko, 2006) or microRNA – (Das et al., 2006; Smith et al., 2009) mediated gene knockdown were developed. Here we show that lentiviral-mediated gene delivery is efficient in the feather follicle. Moreover, while RCAS infection is usually limited to actively replicating cells, lentivirus has the advantage of infecting both dividing and non-dividing cells. This property is of particular significance, because DP cells usually replicate infrequently. Using a lentivirus carrying a GFP reporter, we found extensive viral gene expression at day 4 post-infection (Figs. 4M and N and S5). In our study, we also found extensive yet distinct phenotypes in feather formation by lentiviral-mediated Dkk2/Frzb/Shh/Lfringe/Notch1 gene overexpression, further confirmed the effectiveness of this method (see below and data not shown).
Fig. 4.
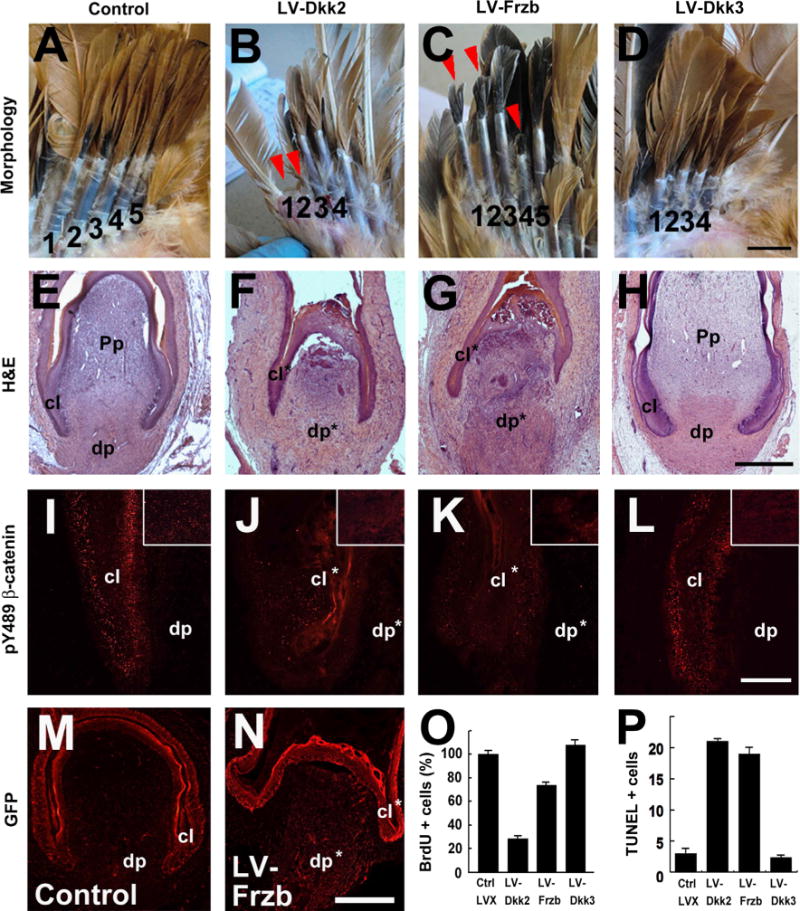
Overexpression of Dkk2/Frzb, but not Dkk3, disrupts feather regeneration. (A–D) Representative samples of lentivirus (LV) mediated gene overexpression in the feather follicle. A control virus carrying GFP only was also shown. Four to five wing flight feather follicles in their resting phase were plucked to induce regeneration and infected with the virus, and photographed 3 weeks afterwards. Delayed feather regeneration was indicated by arrow heads. (E–H) H&E analysis of virus-infected feather follicles 4 days post-infection. (I–L) pY489 β-catenin antibody staining (red spots) showing the reduced Wnt signaling after Dkk2/Frzb overexpression. The epithelia in the collar regions are shown, and the DP regions are shown in inserts. (M and N) Anti-GFP immunostaining (red) showing lentiviral-mediated GFP expression in the feather follicles. (O and P) Quantification of BrdU and TUNEL staining results in the feather follicles. The dp, Pp and collar (cl) are labeled; * indicates the disrupted structures. Bar = 1 cm (A–D, shown in D), 1 mm (E–H, shown in H; M and N, shown in N), and 200 μm (I–L, shown in L).
We found overexpression of Dkk2 or Frzb led to defective feather regeneration, whereas Dkk3 overexpression did not have an obvious phenotype (Fig. 4A–H). Both Dkk2 (13/20) and Frzb (8/15) delayed regeneration in about half the cases, whereas an empty pLVX virus (control) produced no abnormality (n = 15). Based on their growth rates, virus perturbation delayed feather regeneration for about 1–2 weeks. The impacts of virus-mediated gene perturbation on Wnt signaling were monitored by staining a phosphorylated form of β-catenin (Rhee et al., 2007; Song et al., 2009; Livnat et al., 2010). Compared to control or Dkk3 over-expression, Dkk2/Frzb overexpression reduced pY489-β-catenin both in the collar epithelium and the DP (Fig. 4I–L). Dkk2/Frzb-induced regeneration defects were further characterized by reduced cell proliferation in the feather follicles, as shown by BrdU incorporation assay (Fig. 4O; Fig. S6), and increased cell apoptosis, as shown by TUNEL staining (Fig. 4P; Fig. S7).
We examined marker gene expression after perturbation. In normal regeneration, Desmin/Laminin/SMA marked the DP, while Tenascin showed widespread expression in the follicle (Fig. 5A–D). However, overexpression of Dkk2 or Frzb led to reduced expression of DP markers including Desmin/Laminin/SMA, whereas Tenascin maintained its widespread pattern (Fig. 5E–L). In Masson staining, the dermal sheath appeared blue due to collagen fibers, but the DP appeared red due to its muscle property (Fig. 5M). Dkk2/Frzb overexpression reduced the DP property (Fig. 5N–O). This is not due to an oblique section plane, because we collected every other sections of the follicle and did not find a normally stained sample. Eventually, all feather follicles recovered and new feather growth resumed, possibly because not all cells were infected, and the virus might be silenced after certain period of time. In addition, the virus was not spreading. In summary, Dkk2 or Frzb overexpression delays feather regeneration, and reduces the DP properties (Fig. 5P).
Fig. 5.
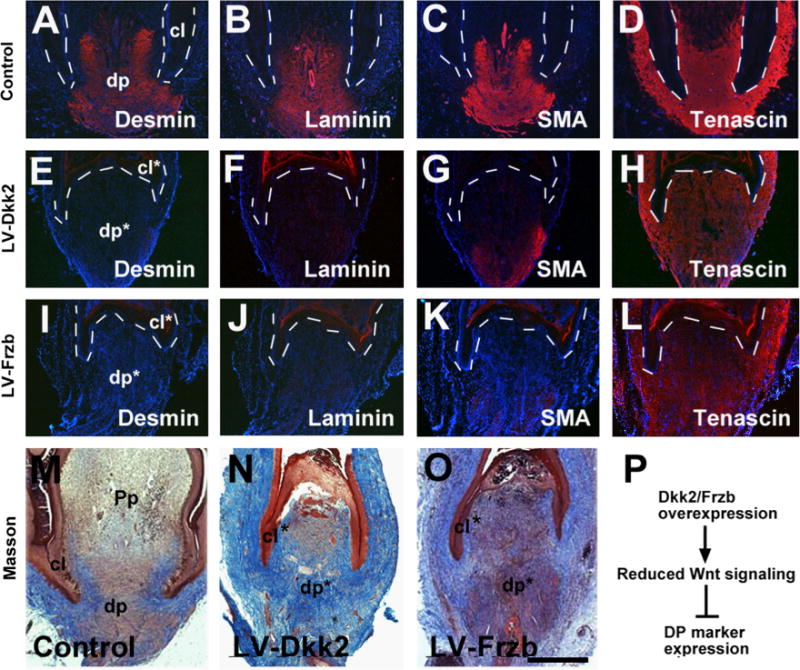
Overexpression of Dkk2/Frzb, but not Dkk3, reduces DP marker gene expression. (A–L) DP marker gene expression showing reduced Desmin/Laminin/SMA, but not Tenascin in LV-Dkk2 and LV-Frzb transduced feathers. An empty pLVX virus was used as control. Samples were collected at day 4 post-infection. For a comparison of staining intensity, equal exposure time was used when taken pictures. (M–O) Masson staining showing altered DP characteristics in Dkk2/Frzb overexpressed follicles. (P) Summary of the events after Dkk2/Frzb overexpression. Dashed lines indicate the epithelial–mesenchymal borders. The dp and collar (cl) are labeled; *indicates the disrupted structures. Bar = 1 mm (shown in O).
RNAi-mediated knockdown of Dkk2/Frzb but not Dkk3 disrupts feather regeneration
To specifically knockdown gene expression, we designed shRNA for Dkk2, Dkk3 or Frzb and used lentiviral vectors to deliver these constructs into the regenerating feather follicle. The effectiveness of shRNA was confirmed in DF-1 cells in vitro. Compared with a random control construct, RNAi-Dkk2 or RNAi-Dkk3 reduced endogenous gene expression to less than 20%, while RNAi-Frzb had an efficiency of 50% knockdown (Fig. 6A). The in vivo impacts of RNAi knockdown were also confirmed (Fig. S8). Interestingly, RNAi knockdown of Dkk2 or Frzb but not Dkk3 led to delayed feather regeneration, which was characterized by significantly reduced epithelial and mesenchymal cell mass (Figs. 6B–E, and S8). In 15 feather follicles examined for each gene, about 70% (10/15) showed obvious defects when analyzed at day 4 post-infection. A control virus targeting a random sequence did not produce visible effect (n = 10). The impacts of RNAi-mediated gene knockdown were also monitored by pY489 β-catenin staining. Compared to control or Dkk3 knockdown, Dkk2/Frzb knockdown significantly increased pY489 β-catenin (Fig. 6F–I), suggesting increased Wnt signaling. Virus expression was confirmed by GFP staining (Figs. 6J–K; S5). Again cell proliferation was decreased in the feather follicles after Dkk2/Frzb knockdown (Figs. 6L; S6), but no change in apoptosis was found (Figs. 6M; S7).
Fig. 6.
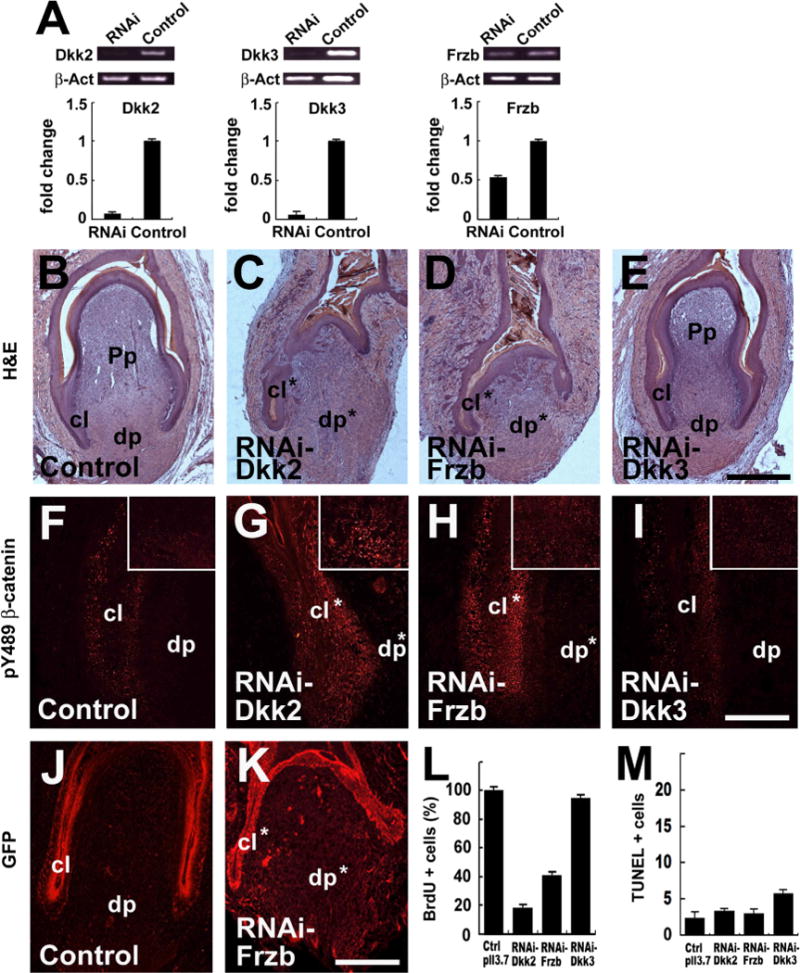
RNAi knockdown of Dkk2/Frzb, but not Dkk3, disrupts feather regeneration. (A) Efficiency of shRNA tested in DF-1 cells. Semi-quantitative RT-PCR and qPCR results were shown. A construct targeting a random sequence was used as control. Endogenous mRNA levels were quantified. (B–E) Representative samples of lentiviral-mediated shRNA knockdown in the feather follicles 4 days post-infection. A shRNA virus targeting a random sequence was used as control. (F–I) pY489 β-catenin antibody staining (red spots) showing the increased Wnt signaling after Dkk2/Frzb knockdown. The epithelia in the collar regions are shown, and the DP regions are shown in inserts. (J–K) Virus expression monitored by GFP staining (red). (L and M) Quantification of cell proliferation (BrdU staining) and apoptosis (TUNEL) in the feather follicles. The dp, Pp and collar (cl) are labeled; *indicates the disrupted structures. Bar = 1 mm (B–E, shown in E; J and K, shown in K), and 200 μm (F–I, shown in I).
We further analyzed marker gene expression in the DP. In Dkk2 or Frzb knockdown samples, similar expression levels of DP markers were found, including Desmin/Laminin/SMA and Tenascin (Fig. 7A–L). Masson staining confirmed the retained DP property, but no pulp was formed (Fig. 7M–O). These results differ significantly from Dkk2/Frzb overexpression. In summary, Dkk2/Frzb knockdown leads to delayed feather regeneration and reduced pulp formation (Fig. 7P).
Fig. 7.
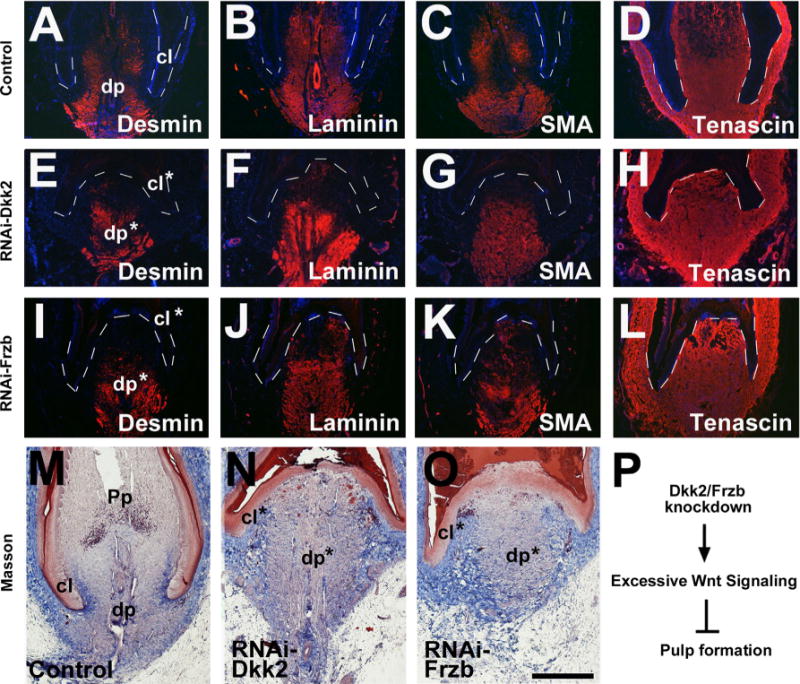
RNAi knockdown of Dkk2/Frzb, but not Dkk3, maintains DP properties but reduces pulp formation. (A–L) RNAi-Dkk2/Frzb maintains DP marker gene expression. A virus targeting a random sequence was used as control. Samples were collected at day 4 post-infection. For a comparison of staining intensity, equal exposure time was used when taken pictures. (M–O) Masson staining showing Dkk2/Frzb knockdown retains DP characteristics but reduces pulp formation. (P) Summary of the events after Dkk2/Frzb knockdown. Dashed lines indicate the epithelial–mesenchymal borders. The dp and collar (cl) are labeled; * indicates the disrupted structures. Bar = 1 mm (shown in O).
Dkk2/Frzb regulates feather axis formation
Previous work suggested that a Wnt signaling gradient in the feather follicle helps set up the overall axis of the feather (Yue et al., 2006). However, here we found that Wnt ligands are absent or only weakly expressed in the feather DP. This raises the question of how the DP controls feather axis at the molecular level. In early embryonic development, xDkk1 is the head-inducer and controls body axis formation (Glinka et al., 1998; Niehrs, 2001; De Robertis, 2006). We tested whether manipulation of Dkk2/Dkk3/Frzb expression in the feather follicle could also alter feather axis formation. To this end, the most dramatic phenotype obtained is by micro-bead coated antibody delivery into the regenerating feather follicle. In 20% of the cases (3/15), we observed the formation of feathers with two-axes from the same follicle (Fig. 8A). The final feather form is two-vanes joined together at the base (Fig. 8B–C), similar to those obtained by DP bisection (Lillie and Wang, 1943). Control serum or antibodies to each individual antigens produced morphologically normal feathers (Figs. 8D and S9). Viral infection mainly led to delayed feather regeneration. In some cases (20%; 3 out of 15), overexpression or RNAi knockdown of Dkk2/Frzb produced additional axis in the feather, confirming their roles in regulating feather axis formation (Fig. 8E–H). An empty control virus or LV-Dkk3 transduced follicles remained normal (n = 10 for each). The incidence of phenotype by these manipulations was low (about 20%), and no bilateral to radial transition of feather morphology was observed, possibly because lentivrial infection is more homogenous, and only patches of infection would lead to a perturbation of the overall Wnt signaling gradient hence a clear phenotype.
Fig. 8.
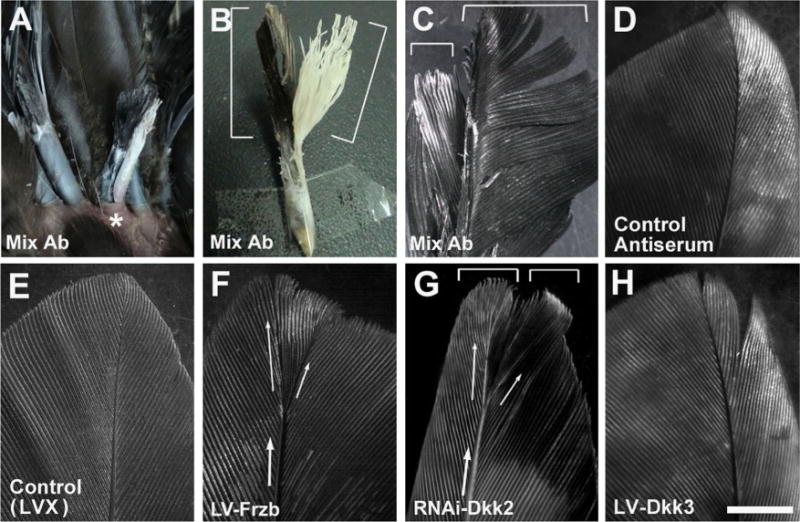
Dkk2/Frzb regulates feather axis formation. (A–D) Micro-bead mediated antibody delivery into the feather follicle induces two feathers from a single follicle (marked by*). Two feather vanes were formed in 20% (3/15) of the cases. Control antiserum delivery caused no abnormality in the feather (n = 5). (E–H) Lentiviral-mediated overexpression of Frzb or RNAi knockdown of Dkk2 produced two feather axes, and to a less extent, two feather vanes in 20% of the cases (3/15). No abnormality was produced by an empty viral vector pLVX transduction in the control feather follicles (n = 10), or LV-Dkk3 transduced feather follicles (n = 10). Bar = 1 cm (shown in H).
Discussion
Comparison of molecular encodings in the feather and hair DP
Avian feather and mammalian hair are two prominent skin appendages in nature that undergo constant physiological renewal and have robust regeneration capability. They share many similarities, such as a follicular structure, slow-cycling epithelial stem cells, and an inductive DP (Paus and Cotsarelis, 1999; Lin et al., 2013; Yu et al., 2004). There are also distinctions between their growth cycle and regeneration. For example, after removal of the hair bulb together with the DP, the hair follicle still regenerates (Oliver, 1966a, 1966b; Jahoda et al., 1992, 1996; Waters et al., 2011). This is not the case for the feather follicle, as removal of the DP renders the follicle unable to regenerate (Lillie and Wang, 1941, 1944).
One notable molecular feature of the feather DP is the high levels of Wnt signaling inhibitors, including Frzb, Dkk2, Dkk3, Sfrp1 and Sfrp2. Only Wnt5a is weakly expressed in the feather DP, otherwise there is an obvious lack of Wnt ligands. High levels of Wnt ligands are found in the epithelium and mesenchymal pulp, including Wnt5a, Wnt5b, Wnt6 and Wnt11. This intriguing two-compartment distribution pattern may suggest a possible interactive mode (Fig. 9A). We also noticed many TGF-β super-family members and regulators are expressed at high levels in the feather DP, including Tgf-β2/3, Bmp4/7, Tgf-βR1, BmpR2, Activin-R1, Noggin2, chrodin-like 1, decorin, Ltbp1, etc. FGF ligands (Fgf7, Fgf12), receptors (Fgfr1, Fgfr2) and modulators (Spry2) are present. Consistently, our recent work suggested FGF signaling is important for feather DP maintenance and regulates the feather proximal–distal patterning (Yue et al., 2012). Interestingly, we noticed an absence of Shh signaling pathway members in the feather DP. We and others have shown previously Shh is expressed in the ramogenic zone epithelium and regulates feather branching morphogenesis (Yu et al., 2002; Harris et al., 2002, 2005). Given the critical role of Shh signaling in hair DP function (Woo et al., 2012), the absence of Shh signaling in the feather DP may suggest an important distinction. Together, our profiling data provide a rich resource for future investigations on feather growth, morphogenesis, and regeneration. They also offer the opportunity to compare with the hair follicle, an evolutionarily related skin appendage.
Fig. 9.
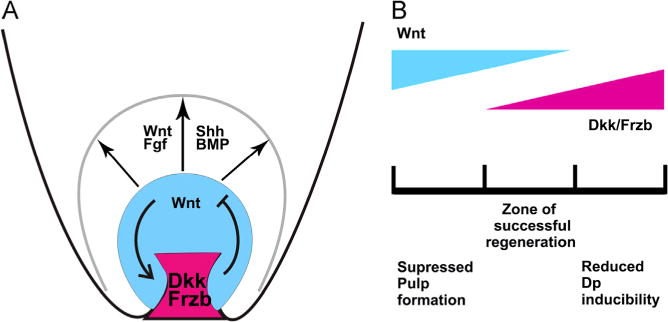
Wnt/Dkk regulates feather growth and regeneration. (A) The feather DP expresses high levels of Wnt inhibitors including Dkk2 and Frzb. These inhibitors interact with the mostly epithelial/pulp Wnt signaling, and regulate feather regeneration and axis formation. Other signaling molecules are also involved in various stages of feather growth and regeneration, including FGF/BMP./Shh etc. (B) The Wnt signaling must be properly balanced to promote successful feather regeneration. When Dkk2/Frzb is overexpressed (reduced Wnt signaling), there is less DP property; when Dkk2/Frzb is knockdown (excessive Wnt signaling), pulp formation is reduced. Both will lead to delayed feather regeneration.
The complex role of Wnt signaling in feather regeneration
Wnt signaling is required at various stages during tissue regeneration, such as maturation of the wound epidermis, formation of the blastema, and regenerative outgrowth (Stoick-Cooper et al., 2007a, 2007b). Here we find that Wnt signaling is important for feather regeneration. Overexpressing inhibitors of Wnt signaling such as Dkk2 or Frzb delays feather regeneration. By reducing Wnt signaling, the expression levels of many DP marker genes are reduced, such as Desmin/Laminin/SMA. Therefore, Wnt signaling is important for the maintenance of the molecular properties of the feather DP. On the other hand, if Dkk2/Frzb is knockdown, feather DP properties are maintained but pulp formation is delayed. Again feather regeneration is delayed. It seems proliferation and/or initial differentiation of the DP cells, hence the formation of the pulp, requires a reduced Wnt signaling. These results suggest that Wnt signaling controls many aspects of feather regeneration, and an appropriately fine-tuned Wnt signaling both in time and space is required for successful regeneration (Fig. 9B).
Wnt signaling triggers complex downstream events, often categorized into canonical and non-canonical pathways. Among the Wnt ligands expressed at high levels in the feather follicles, some are involved in non-canonical pathways such as Wnt5a/Wnt11. The details of these downstream signaling events will need further clarification. In this study, we manipulated the expression levels of Dkk2/Frzb by various methods, including overexpression and knockdown. The impacts on canonical Wnt signaling were monitored by activation of β-catenin. Overexpression of Dkk2/Frzb reduced pY489 β-catenin, whereas knockdown of Dkk2/Frzb increased this particular active form. These results are consistent with their expected roles in Wnt signal transduction.
Epithelial–mesenchymal interactions are important for embryonic development, and similarly for regeneration. Our model thus provides a mechanism for such interactions in feather regeneration: Wnt ligands in the epithelium/pulp maintain the DP property, and Dkk/Frzb in the DP controls appropriate level of Wnt signaling. After wound plucking, the expression levels of Wnt ligands are reduced. The re-acquisition and accumulation of Wnt ligands seem to require the wound healing process and FGF signaling. Our previous work suggested FGF signaling expands, while spry diminishes the feather epithelium (Yue et al., 2012). FGF ligands and Fgfr1/Fgfr2 are present in the feather DP and therefore may contribute to the re-acquisition of Wnt ligands.
Dkk2/Frzb in feather axis formation
In previous work, we showed that a Wnt signaling gradient controls feather axis and topological arrangement of the branching feather epithelium (Yue et al., 2006). By RCAS-mediated overexpres-sion of Wnt3a or Dkk1, the feather phenotypes mostly changed from bilateral symmetry to radial symmetry. The more dramatic pheno-types such as two feather axes/vanes were not observed. Here we show that by manipulating Dkk2/Frzb expression, either through lentiviral-mediated overexpression/RNAi knockdown, or antibody neutralization, two axes/vanes can be produced. These results suggest that manipulating Dkk2/Frzb expression is a more efficient way to control the formation of feather axis. Alternatively, the different methods used to manipulate gene expression in the feather follicles may cause a difference. However, due to the inherited technical difficulties (an early analysis during development would be destructive), and the biological complexity in the system (apparently BMP/noggin signaling is also involved in feather axis formation, among other possible factors) (Yu et al., 2002), a clear description of the axis formation process at the molecular and cellular level is not achieved at this moment.
In summary, we show that the feather DP expresses high levels of Dkk2/Frzb that are inhibitors for Wnt signaling, whereas Wnt ligands are mainly expressed in the feather epithelium and pulp. This two-compartment distribution pattern suggests a feedback interaction. Other signaling pathways may also involve in various stages of feather growth and regeneration, including FGF/BMP/Shh (Fig. 9A). These results provide new insights into the regulatory logic of tissue regeneration.
Materials and methods
Experimental animals
Three to 6 months old chickens were bought from a local farm, and housed in Fuzhou University Animal Facility Center. Adult Xenopus laevis frogs were obtained from Nasco. All operations and procedures were according to the Institutional Guidelines of Fuzhou University Ethics Board.
Microdissection
For whole-genome expression analysis, only wing flight feathers in their growth phase were used, which was determined by the overall feather length (usually feathers grow to their half-length were used). The chickens were sacrificed before sample collection. For dissection, the DPs were excised from the follicles directly after plucking the feathers or dissected in vitro (Fig. S2). Erz and Pp were separated using the open-prep procedure described previously (Yue et al., 2006). To collect the required 5 μg total RNAs for analysis, 10 DPs were collected from the wing flight feather follicles from two birds. Pp and Erz samples were from two follicles in their growth phase.
Whole-genome expression profiling and data analysis
Detailed descriptions of the process and data analysis were in Supplemental data. Briefly, samples were collected, and total RNAs were isolated using Trizol reagent (Invitrogen). Total RNAs were quality monitored by Agilent 2100 analysis and sequenced using the Illumina Genome Analyzer at the Beijing Genome Institute (BGI), Shenzhen, China. The sequencing results were annotated according to a reference chicken gene database provided by BGI. Raw data and processed data were deposited in the NCBI database under accession #GSE 42017.
Antibody production, purification and in vivo perturbation
We cloned the full ORF for cDkk2, and synthesized the full ORF for cDkk3 and cFrzb (Sangon Biotech, Shanghai). To reduce the high GC content in cDkk3/cFrzb, we modified the sequences but kept the amino acid coding. These genes were subcloned into pET-32a, a bacterial expression vector, and His-tag fusion proteins were produced. We purified the proteins by a Ni column (Genescript) following the manufacturer’s instruction, and produced antibodies in mice. We further affinity purified the antibodies using an antigen-coupled column (Genescript). These antibodies were used for immunohistochemistry. For feather follicle perturbation, 50 μl antiserum for each antigen, or their mix, or a control antiserum immunized with BSA, were mixed with equal volume of DEAE-Sepharose beads (BBI) in PBS and injected into a plucked wing flight feather follicle. The regenerated feathers were photographed when growing, and collected after finished the growth cycle.
Histology, immunostaining and in situ hybridization
H&E staining, immunostaining and in situ hybridizations were processed as described (Yue et al., 2006). For immunostaining, the feather follicles were collected at day 4 post-infection. Eight um cryosections were used. We used antibodies for LCAM, NCAM, Laminin, Desmin, SMA, Tenascin C, pY-489 P-catenin, BrdU (Developmental Study Hybridoma Bank), GFP (Santa Cruz). For a comparison of staining intensity in each group, same exposure time was used. For Masson staining, a kit was purchased from ZSBio Co (Beijing), and instructions were followed. RNA probes used in this study: Wnt5a (nt 382–1208; NM_204887.1), Wnt6 (nt 191–507; NM_001007594.2), Dkk2 (nt 961–1260; XM_420494.2), Dkk3 (nt 821–1050; NM_205125.1), Frzb (nt 521–771; NM_204772.2), Fzd8 (nt 800–1138; XM_418566.2), FGFR1 (nt 511–760; NM_205510.1), Sfrp2 (nt 651–925; NM_204773.1), TGF-β2 (nt 1146–1375; XM_003640970.2), BMP4 (nt 486–755; NM_205237.3), BMP7 (nt 1212–1480; XM_417496.2).
BrdU staining
For BrdU staining, animals were i.p. injected with 50 mg/kg BrdU (Sigma) and samples collected 1 h later. Samples were fixed in 4% PFA in PBS at 4 °C overnight. Eight um paraffin sections were collected, proceeded for BrdU staining and developed using an AEC substrate. Sections were counterstained with hematoxylin and photographed. Quantification of BrdU staining results were performed by counting the positive cells in each follicle in three sections of the corresponding samples, and normalized to that of a control pLVX virus-infected follicle.
TUNEL staining
For TUNEL staining, a commercial kit from Beyotimes was used and instructions followed. Briefly, paraffin sections were hydrolyzed and digested with 20 μg/ml proteinase K at 37 °C for 15 min. After 3 × PBS wash, TdT enzyme and FITC-dUTP reaction buffer was applied to the slide and incubated for 60 min at 37 °C. After 3 × additional wash with PBS, slides were counterstained with DAPI, mounted and photographed under a Leica fluorescence microscope. Quantification of the staining results was performed by counting positive cells per follicle by three independent investigators.
RT-PCR and qRT-PCR analysis
Total RNAs were isolated using Trizol reagent (Invitrogen) and reverse transcribed using RevertAid first strand cDNA synthesis kit (Fermentas). PCR was performed using a pre-mix from CWBIO, Beijing. The conditions used were: 5 min at 95 °C, 29–33 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, followed by 72 °C for 7 min. qPCR was performed in triplicate using SYBR green pre-mix (CWBIO) and a LightCycler480 real-time PCR machine (Roche). Data were quantified using the delta-delta CT method. Each pair of primers was independently tested, with the correct size and single band in electrophoresis. Primer sequences available upon request.
Virus production and infection
Lentivirus were produced and harvested in 293 T cells using the standard protocol. The vector used for overexpression is pLVX-ZsGreen (a gift from Dr. Jun Xu, Tongji University, Shanghai, China), and for RNAi knockdown is pLL3.7. Sequences targeted in shRNA: Dkk2 (ggtgaactccatcaagtcc), Dkk3 (gccacttcaagaggagaaa), Frzb (gctacccagaagacctatc), and a random control sequence (agatacgacagaggacact). These sequences were designed according to the Broad Institute website instruction http://www.broadinstitute.org/rnai/public/, and blasted to ensure they do not have significant sequence homology with other genes. To test the efficiency of shRNA knockdown, these constructs were transfected into DF-1 cells (ATCC). Cells were collected 48 h later, and total RNAs extracted. Endogenous gene expression levels were quantified and compared with the control. Virus transfec-tion of regenerative feathers and sample processing were performed as described (Yu et al., 2002). Briefly, plucked feather follicles were washed with PBS, and virus supernatant injected immediately. Total injection volume is 80–120 μl per follicle. To reduce variation in the experiment and avoid bleeding after plucking, only flight feathers in their resting phase were used.
Wnt reporter assay
Wnt responsive Super-TOPFLASH luciferase reporter assays in HEK293T cells were performed in 96-well plates in triplicate as described previously (Wang et al., 2010). Briefly, HEK293T cells reached 50–60% confluence at the time of transfection. Total DNA transfected per each well was 150 ng with VigoFect reagent (Vigorous), using pCS2+ to adjust the DNA amount. Wnt1 was used at 15 ng/well; 10 ng TOPFLASH and 1 ng Renilla luciferase plasmids were co-transfected. Fourty-eight hours later, the cells were lysed and luciferase activity determined using Dual-luciferase assay kit (Promega). TOPflash luciferase activity was normalized to that of Renilla.
Xenopus embryo injection
cDkk2, cDkk3 and cFrzb were subcloned into pCS2+ vector using PCR and verified by sequencing. The plasmids were linearized with NotI and transcribed with SP6 RNA polymerase according to the manufacturer’s instructions (MEGAscript kit, Ambion). In vitro synthesized mRNAs were injected into 4-cell stage embryos at the equatorial region. For control animals, mRNA of preprolactin gene was injected.
Supplementary Material
Acknowledgments
We thank Drs. Colin A.B. Jahoda (Durham University), Anming Meng (Tsinghua University), Shengcai Lin (Xiamen University) for helpful input, Guoping Fan (UCLA), Jun Xu (Tongji University, Shanghai) for providing lentivirus or package plasmids. This work is supported by startup funds from Fuzhou University (to Z. Yue, G. Zhou, and X. Lin), Natural Science Foundation of China to Z. Yue (NSFC31071285, 31371472), and Natural Science Foundation of Fujian Province to G. Zhou (2013J05051). Z. Yue is a Minjiang Scholar honored by Fuzhou University.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2014.01.010.
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. Wnt signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz RB. Distinct Wnt members regulates the hierachical morphogenesis of skin regions (spinal tract) and individual feathers. Mech Dev. 2004;121:157–171. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodankar R, Chang CH, Yue Z, Suksaweang S, Burrus L, Chuong CM, Widelitz RB. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway. J Invest Dermatol. 2003;120:19–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel C, Grisanti L, Zemla R, Rezza A, Barros R, Sennett R, Mazloom AR, Chung CY, Cai X, Cai CL, Pevny L, Nicolis S, Ma’ayan A, Rendl M. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012;23:981–994. doi: 10.1016/j.devcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RM, Van Hateren NJ, Howell GR, Farrell ER, Bangs FK, Porteous VC, Manning EM, McGrew MJ, Ohyama K, Sacco MA, Halley PA, Sang HM, Storey KG, Placzek M, Tickle C, Nair VK, Wilson SA. A robust system for RNA interference in the chicken using a modified microRNA operon. Dev Biol. 2006;294:554–563. doi: 10.1016/j.ydbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-Catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Harpavat S, Cepko CL. RCAS-RNAi: a loss-of-function method for the developing chicken retina. BMC Dev Biol. 2006;6:2. doi: 10.1186/1471-213X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh–Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator–inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci USA. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Mauger A, Bard S, Sengel P. Cellular and extracellular involvement in the regeneration of the rat lower vibrissa follicle. Development. 1992;114:887–897. doi: 10.1242/dev.114.4.887. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Oliver RF, Reynolds AJ, Forrester JC, Horne KA. Human hair follicle regeneration following amputation and grafting into the nude mouse. J Invest Dermatol. 1996;107:804–807. doi: 10.1111/1523-1747.ep12330565. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the feather. V Experimental morphogenesis Physiol Zool. 1941;14:103–133. [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the feather. VI. The production and analysis of feather-chimerae in fowl. Physiol Zool. 1943;16:1–21. [Google Scholar]
- Lillie FR, Wang H. Physiology of development of the feather. VII. An experimental study of induction. Physiol Zool. 1944;17:1–31. [Google Scholar]
- Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr Opin Cell Biol. 2006;18:730–741. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Wideliz RB, Yue Z, Li A, Wu X, Jiang TX, Wu P, Chuong CM. Feather regeneration as a model for organogenesis. Dev Growth Differ. 2013;55:139–148. doi: 10.1111/dgd.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnat I, Finkelshtein D, Ghosh I, Arai H, Reiner O. PAF-AH catalytic subunits modulate the wnt pathway in developing GABAergic neurons. Front Cell Neurosci. 2010;4:19. doi: 10.3389/fncel.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR, editors. Avain Anatomy-integument. Agriculture Handbook 362: Agriculture Research Services. US Department of Agriculture; Washington DC: 1972. [Google Scholar]
- Niehrs C. The Spemann organizer and embryonic head induction. EMBO J. 2001;20:631–637. doi: 10.1093/emboj/20.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver RF. Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J Embryol Exp Morphol. 1966a;15:331–347. [PubMed] [Google Scholar]
- Oliver RF. Regeneration of dermal papillae in rat vibrissae. J Invest Dermatol. 1966b;47:496–497. [PubMed] [Google Scholar]
- Oshimori N, Fuchs E. Paracrine TGF-b signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal—epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound b-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol. 2007;9:883–892. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- Saha S, Sparks AB, Rago C, Akmaev V, Wang CJ, Vogelstein B, Kinzler KW, Velculescu VE. Using the transcriptome to annotate the genome. Nat Biotechnol. 2002;20:508–512. doi: 10.1038/nbt0502-508. [DOI] [PubMed] [Google Scholar]
- Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- Song L, Li Y, Wang K, Wang YZ, Molotkov A, Gao L, Zhao T, Yamagami T, Wang Y, Gan Q, Pleasure DE, Zhou CJ. Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development. 2009;136:3161–3171. doi: 10.1242/dev.037440. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007a;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distict Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007b;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fu Y, Gao L, Zhu G, Liang J, Gao C, Huang B, Fenger U, Niehrs C, Chen YG, Wu W. Xenopus skip modulates Wnt/beta-catenin signaling and functions in neural crest induction. J Biol Chem. 2010;285:10890–10901. doi: 10.1074/jbc.M109.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JM, Lindo JE, Arkell RM, Cowin AJ. Regeneration of hair follicles is modulated by flightless I (Flii) in a rodent vibrissa model. J Invest Dermatol. 2011;131:838–847. doi: 10.1038/jid.2010.393. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Chen CW, Stott NS, Chuong CM. Wnt-7a in feather morphogenesis: involvement of anteror–posterior asymmetry and proximal–distal elongation demonstrated with an in vitro reconstitution model. Development. 1999;126:2577–2587. doi: 10.1242/dev.126.12.2577. [DOI] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signaling. Curr Biol. 2000;10:1611–1614. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–313. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. The developmental biology of feather follicles. Int J Dev Biol. 2004;48:181–192. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci USA. 2006;103:951–955. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Wu P, Widelitz RB, Chuong CM. Sprouty/FGF signaling regulates the proximal–distal feather morphology and the size of dermal papillae. Dev Biol. 2012;372:45–54. doi: 10.1016/j.ydbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


