The formin Fus1 nucleates a novel actin structure in fission yeast, named the actin fusion focus, which consists of an aster of actin filaments whose barbed ends are focalized at a membrane proximal site and serves to focalize cell wall hydrolase delivery for cell fusion.
Abstract
Cell–cell fusion is essential for fertilization. For fusion of walled cells, the cell wall must be degraded at a precise location but maintained in surrounding regions to protect against lysis. In fission yeast cells, the formin Fus1, which nucleates linear actin filaments, is essential for this process. In this paper, we show that this formin organizes a specific actin structure—the actin fusion focus. Structured illumination microscopy and live-cell imaging of Fus1, actin, and type V myosins revealed an aster of actin filaments whose barbed ends are focalized near the plasma membrane. Focalization requires Fus1 and type V myosins and happens asynchronously always in the M cell first. Type V myosins are essential for fusion and concentrate cell wall hydrolases, but not cell wall synthases, at the fusion focus. Thus, the fusion focus focalizes cell wall dissolution within a broader cell wall synthesis zone to shift from cell growth to cell fusion.
Introduction
Cell–cell fusion is a fundamental process that occurs in many cell types during development and underlies sexual reproduction. Two fundamental principles may be generally valid (Shilagardi et al., 2013): First, fusogenic machineries are required to drive cell fusion upon plasma membrane contact, though their molecular nature has been identified in only few instances (Aguilar et al., 2013). Second, the actin cytoskeleton is essential for cell fusion in many cell types, such as osteoclasts, myoblasts, or yeast cells (Abmayr and Pavlath, 2012). The actin cytoskeleton may promote the juxtaposition of the two plasma membranes through precise cell polarization. This has been best described during myoblast fusion, where Arp2/3 complex–assembled actin structures in the two fusing cells drive cell–cell fusion (Kim et al., 2007; Massarwa et al., 2007; Richardson et al., 2007; Sens et al., 2010). In one of the fusing cells, this structure may generate force for membrane protrusion into the partner cell to permit fusogen engagement (Shilagardi et al., 2013).
A function for the actin cytoskeleton in fusion has also been revealed in the fission yeast Schizosaccharomyces pombe, in which a dedicated formin protein, Fus1, is essential for cell fusion. Cell fusion of haploid yeast cells of opposite mating types occurs after pheromone-mediated sexual differentiation to form a diploid zygote (Merlini et al., 2013). Fus1 is induced upon pheromone signaling, localizes to the fusion site, and is essential for cell fusion: indeed, fus1 mutant cells fail to degrade the cell wall at the site of contact and instead keep elongating. Thus, fusion fails completely when both partners lack fus1 and is inefficient in crosses with wild-type partners (Petersen et al., 1995, 1998b). Like other formins, Fus1 nucleates linear actin filaments and efficiently uses profilin-bound actin (Scott et al., 2011). Accordingly, Cdc3 profilin localizes to the fusion site and is required for fusion (Petersen et al., 1998a). In addition, Cdc8 tropomyosin, which decorates and stabilizes formin-assembled actin structures in mitotic cells (Skoumpla et al., 2007), also localizes to the fusion site and is required for fusion (Kurahashi et al., 2002). Finally, the type V myosin motors Myo51 and Myo52 are involved in cell fusion. Type V myosins transport cargoes toward the barbed end of linear actin filaments: in mitotic cells, Myo52 carries vesicular cargoes along actin cables toward cell poles, whereas Myo51 decorates these same cables as well as the cytokinetic ring (Lo Presti and Martin, 2011; Lo Presti et al., 2012; Wang et al., 2014). During sexual reproduction, both motors localize to the fusion site, and overexpression of the Myo51 cargo-binding domain leads to cell fusion defects (Doyle et al., 2009). In combination, these data suggest the existence, during cell fusion, of a Fus1-nucleated actin structure composed of linear actin filaments. However, investigation of F-actin organization on fixed cells has so far only revealed accumulation at the fusion site of actin patches, which are Arp2/3-nucleated structures at sites of endocytosis (Petersen et al., 1998a; Kurahashi et al., 2002; Kovar et al., 2011).
Precise remodeling of the cell wall is required to allow plasma membrane contact and cell fusion between walled cells, such as yeasts. Indeed, these cells are under strong positive turgor pressure relative to their environment and are protected from lysis by their cell wall. Thus, the local dissolution of the cell wall required for cell–cell fusion must be critically controlled to bring plasma membranes into contact at a precise location, while maintaining cell wall integrity in surrounding regions. Major components of the yeast cell wall are glucan polymers, which are synthetized by transmembrane glucan synthases and hydrolyzed by secreted glucanases (Pérez and Ribas, 2004). In Saccharomyces cerevisiae, deletion of two glucanases was shown to compromise fusion efficiency (Cappellaro et al., 1998). How cell wall dissolution for fusion may be focalized to a specific site is unknown.
Two major ideas have been proposed from work in the budding yeast. A recent study suggested that concentration of glucanases for cell wall dissolution is achieved through restricted diffusion in the cell wall at the site of mating partner contact, rather than by a specialized fusion machinery (Huberman and Murray, 2014). In contrast, earlier work has shown that vesicles are highly aligned and clustered in a small region at the site of fusion, suggesting localized release of fusion components (Gammie et al., 1998). For this, both a fusion-specific transmembrane protein Fus1 (unrelated to its fission yeast formin Fus1 namesake) as well as Spa2, a formin-binding factor, are required (Gammie et al., 1998). Further, the Cdc42-interacting protein Fus2, also necessary for cell wall digestion, displays a focused localization at the fusion site, which relies on both Fus1- and actin-based transport (Paterson et al., 2008; Sheltzer and Rose, 2009; Ydenberg et al., 2012). The precise role of the actin cytoskeleton has not been defined, though the formin Bni1, tropomyosin Tpm1, and type V myosin Myo2 are all required for cell fusion (Liu and Bretscher, 1992; Dorer et al., 1997; Sheltzer and Rose, 2009). The tight localization of fusion factors and vesicles suggests the existence of a specific mechanism to focalize cell wall digestion for fusion.
Here, we show that the formin Fus1 nucleates a novel actin structure in fission yeast, which we named the actin fusion focus. The fusion focus consists of an aster of actin filaments whose barbed ends are focalized at a membrane-proximal site. Fusion focus focalization relies on both the formin Fus1 and type V myosins and occurs asynchronously in the two partner cells. We further show that type V myosins are essential for cell fusion and serve to concentrate in the fusion focus cell wall glucanases within a broader region of cell wall synthases to drive local cell wall dissolution for cell fusion.
Results
The fusion focus: A specific Fus1-dependent actin structure
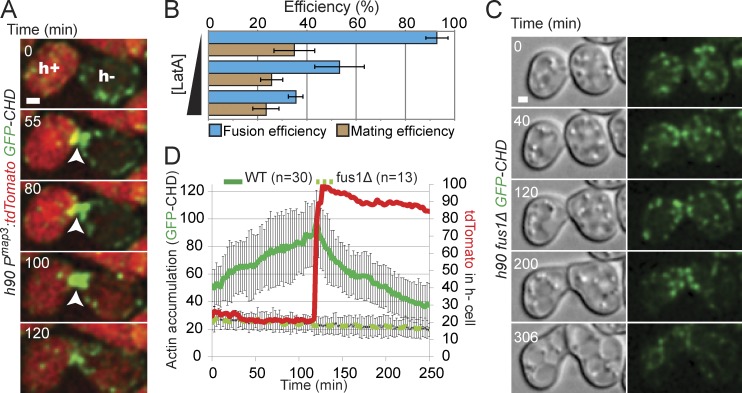
To examine the role of the actin cytoskeleton during cell–cell fusion, we localized F-actin in live cells, using a GFP–calponin homology domain (CHD) reporter construct (Karagiannis et al., 2005; Martin and Chang, 2006). GFP-CHD has been used to study actin structures during mitotic growth, labeling the three actin structures present in these cells: the cytokinetic actin contractile ring nucleated by the formin Cdc12, actin cables assembled by the formin For3, and actin patches, which require Arp2/3 activity (Kovar et al., 2011). Strikingly, during sexual differentiation, we observed an intense accumulation of F-actin at the site of fusion (Fig. 1 A), which appeared distinct from these known actin structures. This structure dynamically formed before cell fusion, which we define as the time of entry in the h− cell of tdTomato driven by an h+ cell-specific promoter (pmap3:tdTomato), and decreased after fusion (Fig. 1, A and D; and Video 1). F-actin accumulation was also observed using LifeAct-GFP in live cells and phalloidin staining on fixed samples (Fig. S1). Disruption of F-actin by treatment with Latrunculin A (LatA), added 4 h after initiation of sexual differentiation upon nitrogen starvation, reduced fusion efficiency in a dose-dependent manner (Fig. 1 B), suggesting that F-actin is essential for cell–cell fusion. Consistent with the molecular function of the pheromone-dependent formin Fus1, F-actin did not accumulate at the site of fusion in fus1Δ pairs, though dynamic actin patches were detected at the shmoo tip of these cells (Fig. 1, C and D; Fig. S1; and Video 2). Similarly, fus1-dependent actin accumulation at the fusion site was previously observed on fixed cells and described as an accumulation of actin patches (Petersen et al., 1998a,b). In contrast, we describe in Figs. 2 and S2 a distinct architecture and composition of this actin structure, which we named the actin fusion focus.
Figure 1.
Fus1-dependent actin accumulation at the prospective fusion site. (A) Homothallic h90 pmap3:tdTomato GFP-CHD strain. Arrowheads show the fusion site where actin gradually accumulates. Fusion between partner cells occurs at 100 min as shown by appearance of the tdTomato signal in the h− cell. (B) LatA treatment reduces fusion efficiency of wild-type homothallic h90 strain. Mating cells were starved in MSL−N for 4 h, to allow pheromone response and shmooing, before addition of increasing concentrations of LatA (0, 50, and 200 µg/µl). Cells were immediately spotted on MSL−N 2% agarose pads (not containing LatA and thus diluting the LatA concentration) and incubated overnight at 25°C before imaging for fusion efficiency quantification. n > 200. (C) Homothallic h90 fus1Δ GFP-CHD strain. Cells grow toward each other but are unable to fuse. Though actin patches are present, no actin focus is detected. (D) Quantification of GFP-CHD intensity at the zone of cell contact and of pmap3-driven tdTomato intensity in the h− partner cell in homothallic h90 wild-type mating pairs expressing both markers (as in A). Individual curves were aligned to fusion time and averaged. GFP-CHD intensity at the zone of cell contact in fus1Δ is also indicated, though no alignment could be performed as a result of fusion failure. Error bars are standard deviations. WT, wild type. Bars, 1 µm.
Figure 2.
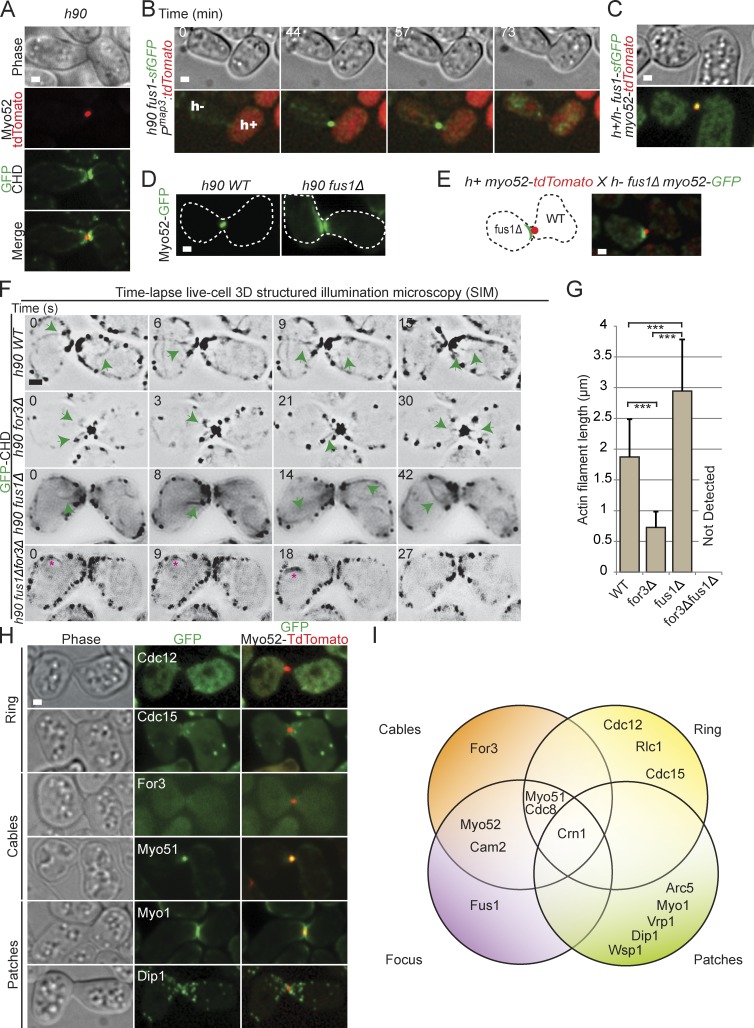
Composition and architecture of the actin fusion focus. (A) Homothallic h90 myo52-tdTomato GFP-CHD strain. Myo52 localizes as an intense dot at the cell–cell contact site, at the edge of the actin density. (B) Time-lapse imaging of homothallic h90 fus1-sfGFP pmap3:tdTomato strain. Entry of tdTomato in the h− cell is used as a marker for fusion. Fus1 is detected as an intense dot at the cell–cell contact site. (C) Homothallic h90 myo52-tdTomato fus1-sfGFP strain. Myo52 and Fus1 colocalize at the fusion site. (D) Homothallic h90 wild-type (left) and fus1Δ (right) strains expressing Myo52-GFP. Myo52 localizes as a crescent at the shmoo tip in the absence of Fus1. Cell outlines are shown with dotted lines. (E) Cross of heterothallic h+ myo52-tdTomato and h90 fus1Δ myo52-GFP. Myo52 forms a crescent in the fus1Δ cell and a dot in the wild-type cell. (F) 3D SIM time-lapse of GFP-CHD in homothallic h90 wild-type (WT), for3Δ, fus1Δ, and fus1Δ for3Δ mating pairs. Inverted images are shown. Green arrows point to actin filaments emanating from the fusion focus. No actin cables were detected in fus1Δ for3Δ double mutant, but some mating pairs showed a perinuclear actin ring (asterisks). (G) Mean length of actin filaments emanating from the fusion focus in strains as in F. Filaments are significantly shorter in for3Δ and longer in fus1Δ than wild-type cells (t test, ***, P < 10−6). This indicates that Fus1-dependent filaments are shorter than For3-dependent filaments and that wild-type cells likely contain both types. n = 30 actin filaments measured in three distinct mating pairs. Error bars are standard deviations. (H) Crosses of heterothallic h+ and h− myo52-tdTomato strains coexpressing Cdc12-3GFP, mEGFP-Cdc15, For3-3GFP, Myo51-3YFP, mGFP-Myo1, or Dip1-GFP. Images shown are time-averaged maximum intensity projection of 15 z stacks over 15 min. (I) Venn diagram summarizing the actin binding proteins that we show to be localized or not at the actin fusion focus. Attribution to cables, ring, or patches is adapted from Kovar et al. (2011). Bars, 1 µm.
Mutation or deletion of actin ring, patch, and cable components did not impair fusion focus formation. Indeed, F-actin accumulated at the fusion site during mating, and cell pairs fused even when actin cables were disrupted by for3 deletion (Fig. S2, A and C). Similarly, cdc12-112 mutants, or even cdc12-112 for3Δ double mutants, accumulated F-actin at the fusion site, mated, fused, and generated complete tetrads at 33°C both in homothallic crosses and in heterothallic crosses with wild-type cells (Fig. S2, D and E), a temperature at which these mutants fail to assemble a cytokinetic ring (Bendezú and Martin, 2011; Zhou et al., 2015). Thus, these other formins are not required for cell fusion. We could not completely disrupt actin patches, as endocytosis is required for earlier mating events, such as pheromone signaling and shmoo polarization (Iwaki et al., 2004). However, deletion of nonessential actin patch components involved in Arp2/3 activation (dip1Δ and vrp1Δ) did not prevent F-actin accumulation at the fusion site and only mildly affected fusion efficiency in heterothallic crosses with wild-type cells (Fig. S2, B and C). Thus, the fusion focus forms largely independently of the actin ring, actin patches, and actin cables.
We probed the polarity of actin filaments in the fusion focus by monitoring the localization of the barbed end–directed type V myosin motor Myo52 and the formin Fus1 (Fig. 2, A and B). Myo52-tdTomato formed a sharp dot at the fusion site (one in each partner cell; Fig. 3), which was located proximal to the shmoo tip and surrounded by F-actin on its cell-internal side (Figs. 2 A and S2 F). Fus1–superfolder GFP (sfGFP) colocalized with Myo52 at the fusion site as a concentrated dot before fusion and, like the fusion focus, disappeared after fusion (Fig. 2, B and C). Deletion of fus1 disrupted Myo52 dot formation but not its localization at the shmoo tip (Fig. 2, D and E). These data suggest that the formin Fus1 and the type V myosin Myo52 mark a site of actin filaments assembly and focalization.
Figure 3.
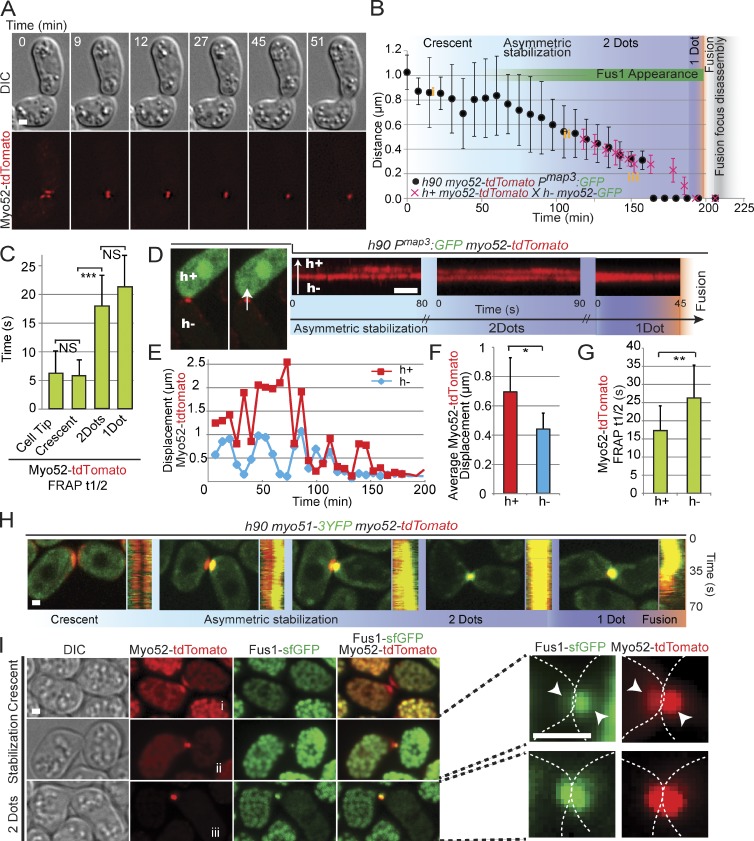
Type V myosin localization and dynamics define multiple steps in the formation of the fusion focus. (A) Time-lapse images of homothallic h90 myo52-tdTomato strain. (B) Distance between Myo52 signals in the two partner cells. The black symbols show mean distances over time in h90 myo52-tdTomato Pmap3:GFP cell pairs (n = 20), aligned to fusion time. The red symbols show mean distances over time in h+ myo52-tdTomato × h− myo52-GFP (n = 20), aligned to the time of fusion focus disassembly. The two curves were then manually aligned to the time of fusion focus disassembly. The distinct phases of Myo52 localization as described in the rest of the figure are indicated; i.e., the crescent phase, the asymmetric dot stabilization phase, the two-dot phase, and the one-dot phase. Note that the one-dot phase simply indicates that two dots cannot be resolved. The length of the asymmetric phase, during which a dot is observed in the h− cell, whereas a crescent or a mobile dot is present in the h+ cell, is variable from pair to pair. The yellow i, ii, and iii refer to the distances between Myo52 structures measured in I and thus provide an indication of the timing of Fus1 appearance. (C) Mean FRAP recovery half-times of Myo52-tdTomato at the cell tip of vegetative growing cells or at indicated steps of cell–cell fusion. Homothallic h90 pairs expressing Myo52-tdTomato and Myo51-3YFP were used for this experiment to precisely monitor the appearance of the stable two-dot phase (see H). Cells with distinct, focalized Myo51 signals were attributed to the two-dot and one-dot categories. Myo52 is less mobile when localized at the fusion focus. t test, ***, P = 1.9 × 10−7; n = 15 for each category. (D) High-temporal resolution imaging of homothallic h90 myo52-tdTomato pmap3:GFP strain. The h+ cell-specific GFP expression allows to mark the two cell types. The left images show a pair with a stable focus only in the h− cell. The right images show kymographs of Myo52-tdTomato at the fusion site in different h− and h+ partner cells at distinct stages of the fusion process: during asymmetric dot stabilization, when a stable dot is present in each cell, and after the two dots have merged into one. In early stages, Myo52-tdTomato is more stable in the h− cell than the h+ cell. The arrow represents the direction of the line drawn to make the kymographs. (E) Instantaneous displacement of Myo52-tdTomato signal over the entire fusion process. The graph shows one representative mating pair. (F) Mean Myo52-tdTomato instantaneous displacement over the entire mating process (n = 8 cells). t test, *, P = 1.8 × 10−2. (G) Mean FRAP recovery half-times of Myo52-tdTomato focus in heterothallic h− (n = 10) or h+ (n = 12) myo52-tdTomato mating cells crossed to cells expressing CHD-GFP. t test, **, P = 8.19 × 10−3 (H) Time-averaged projections and kymographs of h90 myo52-tdTomato myo51-3YFP cells, imaged every second over 70 s. Myo51 strongly colocalizes with Myo52 upon asymmetric dot stabilization. (I) Time-averaged projections over 2 min of h90 myo52-tdTomato fus1-sfGFP mating, imaged every 30 s. Mating pairs at crescent, asymmetric dot stabilization and two-dot stages are shown. i, ii, and iii refer to distance between dots, as highlighted in B. Right images show enlargements of the fusion site. Arrowheads point out the difference in intensity of Myo52-tdTomato and Fus1-sfGFP between both mating partners. Dotted lines show the outline of the mating pair. DIC, differential interference contrast. Bars, 1 µm. Error bars are standard deviations.
Time-lapse live-cell 3D structured illumination microscopy (SIM) indeed revealed long and short actin filaments, of mean length of 1.8 µm, emerging from the fusion focus in wild-type cells (Fig. 2, F and G; Fig. S2 F; and Video 3). In for3Δ cells, we still observed clear actin filaments, of reduced length (∼0.7 µm), emanating from the focus (Fig. 2, F and G; and Video 4). In contrast, in fus1Δ cells, actin cables were significantly longer (∼3 µm) and not focalized (Fig. 2, F and G; and Video 5). In for3Δ fus1Δ double mutants, no actin cables were observed. Similar observations were obtained by scanning confocal microscopy (Videos 7 and 8). This suggests that Fus1 and For3 assemble cables of distinct length and organization, with Fus1 assembling short, highly focalized cables and For3 assembling longer, more broadly distributed cables, as during mitotic growth (Feierbach and Chang, 2001). In both SIM and confocal time-lapse imaging, we also noted that actin patches often appeared to be moving toward the fusion site in fus1+ cells (Videos 3–8). We conclude that the actin fusion focus is an aster of actin filaments, whose barbed ends are focalized at a single membrane-proximal region.
We further probed the actin-binding protein composition of the fusion focus. Like Myo52 and Fus1, the actin cable components tropomyosin Cdc8, calmodulin Cam2, and the second type V myosin Myo51, formed strong dots at the fusion site, consistent with previous description of their localization (Figs. 2 H and S2 G; Kurahashi et al., 2002; Itadani et al., 2006; Doyle et al., 2009). The ubiquitous F-actin binding protein coronin Crn1 also strongly accumulated at the fusion site, decorating the entire actin focus (Fig. S2 H). In contrast, the formin For3, responsible for actin cable formation, was present at the shmoo tip but did not form a tight dot and only very partially colocalized with Myo52 at the fusion site (Fig. 2 H). We also did not detect actin ring markers, such as the formin Cdc12, the FCH (Fes/CIP homology) protein Cdc15, or the myosin light chain Rlc1, at the fusion site (Figs. 2 H and S2 G). Finally, Arp2/3 complex components (Arc5), Arp2/3 activators (Wsp1 and Myo1), and other actin patch components (Dip1 and Cdc15) did not form a tight dot at the fusion site, though they were present at the shmoo tip over a broader area (Figs. 2 H and S2 G). Fig. 2 I provides a summary of the localization of all actin-binding factors investigated.
In summary, the actin fusion focus does not simply represent a local enrichment of actin patches but defines a distinct underlying actin structure. This structure resembles For3-nucleated actin cables in composition but is nucleated by the distinct formin Fus1 and is organized in an aster-like structure with actin filament barbed ends focalized at a membrane-proximal location.
Analysis of Myo52 localization and dynamics reveals multiple steps in fusion focus formation
We studied the formation of the fusion focus by time-lapse microscopy of the entire mating process over several hours, using Myo52 as a marker. In early stages, Myo52 was detected as a pool of dots collectively forming a crescent at the shmoo tips of both partner cells. This crescent then compacted into a single focus in each cell, such that each mating pair showed two dots in close proximity at their contact site (Fig. 3 A; see model in Fig. 6 A). Over time, the distance between the two dots reduced, suggesting progressive degradation of the cell wall between the partner cells (Fig. 3, A and B). The distance between Myo52-tdTomato dots was measured relative to fusion time as defined by entry in the h− cell of GFP driven by an h+ cell-specific promoter (pmap3:GFP). After fusion, the Myo52 focus disassembled within 13.9 ± 4.5 min (n = 20). To measure the distance once the two dots were within the light diffraction limit, we used Myo52 tagged with distinct fluorophores in the two mating partners until focus disassembly. The time between apparent overlap of the two dots to disassembly was 20.7 ± 3.5 min (n = 20). By aligning the two curves on the time of focus disassembly, we conclude that the two Myo52 dots converge into an apparent single dot at the contact between the two cells ∼7 min before fusion pore opening (Fig. 3 B) and are disassembled ∼14 min after fusion.
Figure 6.
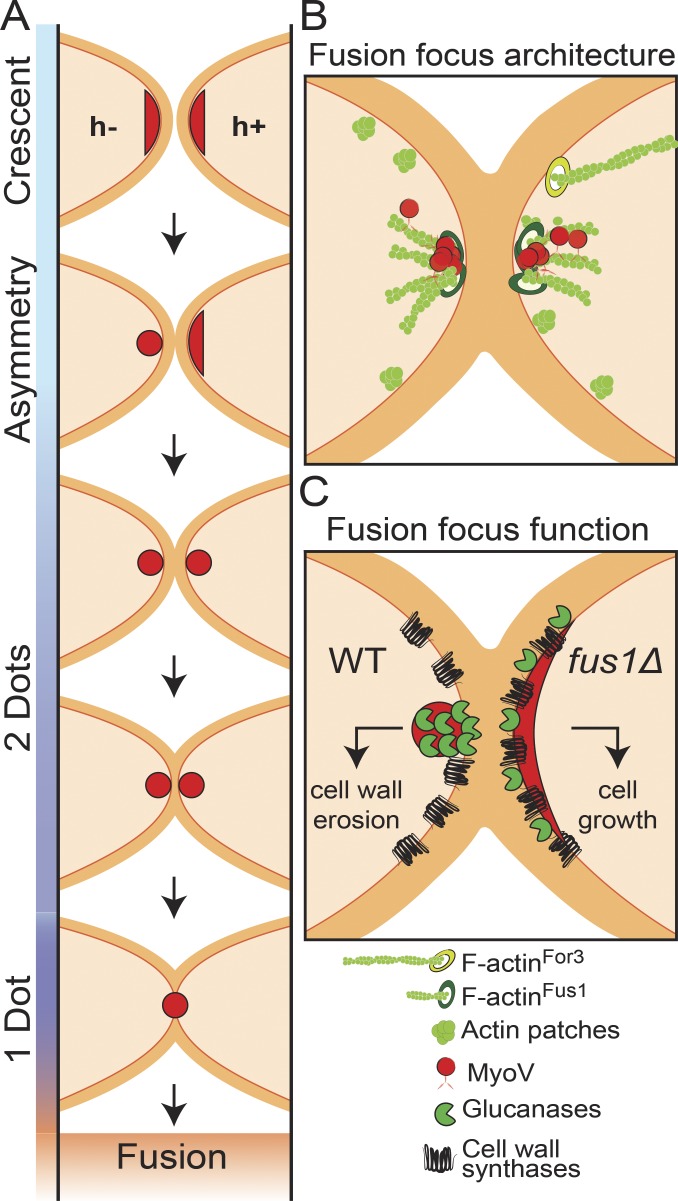
Model for the fusion focus multi-step formation, architecture, and function. (A) Schematic representation of the cell–cell fusion process in fission yeast. Type V myosins first assume a crescent localization, decorating the shmoo tip. Focalization is observed in the h− cell fist and then in the h+ cell. The distance between the two dots then reduces over time, indicating cell wall thinning, until the two structures merge into one and fusion occurs. (B) Illustration of the architecture of the fusion focus. The formin Fus1 nucleates short actin filaments, which are focalized with type V myosins near the plasma membrane. Focalization requires both Fus1 and type V myosins. Longer For3-nucleated cables are also polarized toward the shmoo tip. (C) Model of the function of the fusion focus. For comparison, the wild-type situation is compared with that in fus1Δ cells. In the wild type, glucanases are concentrated at the fusion focus, therefore segregating them from the location of cell wall synthases, which decorate the entire shmoo tip. This geometrical organization permits cell wall thinning and fusion. In absence of Fus1, the localizations of glucanases and cell wall synthases overlap over the shmoo tip, promoting cell growth.
Examination of Myo52-tdTomato or Myo52-GFP dynamics by FRAP in homothallic mating cells revealed that the crescent and dot localizations of Myo52 exhibit distinct dynamic turnover: Myo52-tdTomato was highly dynamic when it localized as a crescent at shmoo tips, displaying a FRAP half-time of ∼5 s. This indicates that half of the Myo52 molecules exchange in the crescent within 5 s. This was indistinguishable from its dynamics at cell tips in vegetative growing cells (Fig. 3 C). In contrast, Myo52 was significantly more stable when it was compacted in a dot during cell fusion, with FRAP half-time of ∼20 s (Fig. 3 C), suggesting Myo52 forms distinct or longer-lived molecular connections in the fusion focus than on actin cables. Consistent with the role of Fus1 in focalizing Myo52, this slower half-time in the dot was dependent on Fus1 (Fig. S3 A). No significant difference was detected in comparing Myo52 dynamics at the fusion focus when a dot was present in each partner cell or when a single unresolved dot was present at the interface of the two partners at the end of the process (Fig. 3 C).
To better understand the formation of the fusion focus, we centered our analysis on the transition between the Myo52 crescent and the focus, using high temporal resolution time-lapse microscopy acquiring images at a 1-s interval. We monitored Myo52-tdTomato localization in homothallic h90 cells in which mating type was visualized by h+ cell-specific GFP expression (pmap3:GFP; Fig. 3 D). Unexpectedly, the transition between the Myo52 crescent and the focus was asymmetric, with one cell forming an apparently stable Myo52 dot sooner than the other (Video 9). This asymmetry was highly predictable: in all cases (n > 50), the h− cell exhibited a stable Myo52 dot before the h+ cell, which exhibited a weaker, more fluctuating Myo52 signal, as shown in kymographs of the fusion site (Fig. 3 D). Myo52 dots were then stabilized in both cells before fusing into a single structure (Fig. 3 D). Measures of Myo52 dynamics further revealed asymmetries between the two mating types: first, the instantaneous displacement of Myo52-tdTomato between consecutive time points at 7.2-min intervals throughout the fusion process was significantly smaller in the h− than the h+ cell (Fig. 3, E and F). Second, FRAP analysis revealed that Myo52 recovery half-time in the fusion focus was significantly higher in h− than h+ cells (Fig. 3 G). Thus, the stabilization of the fusion focus is asymmetric and occurs in the h− cell before the h+ cell (see Fig. 6 A).
Remarkably, the localization of both the second Myosin V Myo51 and the formin Fus1 were also asymmetric between h− and h+ cells. When Myo52-tdTomato localized as a crescent at the beginning of the fusion process, Myo51-3YFP and Fus1-sfGFP signals were very low or undetectable (Fig. 3, H and I; and Fig. S3 B). Myo51 accumulated at the fusion site concomitant with the stabilization of Myo52 into a dot first in the h− cell and later in the h+ cell, with the h− cell type identified as the cell with a stable Myo52 dot (Figs. 3 H and S3 B and Video 9). Myo52 and Myo51 then colocalized perfectly until fusion, though the Myo51-3YFP signal disappeared first during fusion (Fig. 3 H). Like Myo52, Myo51 localization to a dot required Fus1 (Fig. S3 C). Similarly, Fus1 distribution was asymmetric, with only the h− cell, with a strong Myo52 dot showing a clear colocalization with Fus1, whereas the h+ cell with a more dispersed and less intense Myo52 localization showed no or very weak Fus1 signal (Fig. 3 I). This asymmetry was not present in later stages of fusion when Myo52 and Fus1 colocalized in both cells. In conclusion, the localization and dynamic behavior of myosin V and formin Fus1 reveal a stepwise, asymmetric maturation of the cytoskeletal structure that underlies cell fusion.
Type V myosins Myo52 and Myo51 are crucial for cell–cell fusion
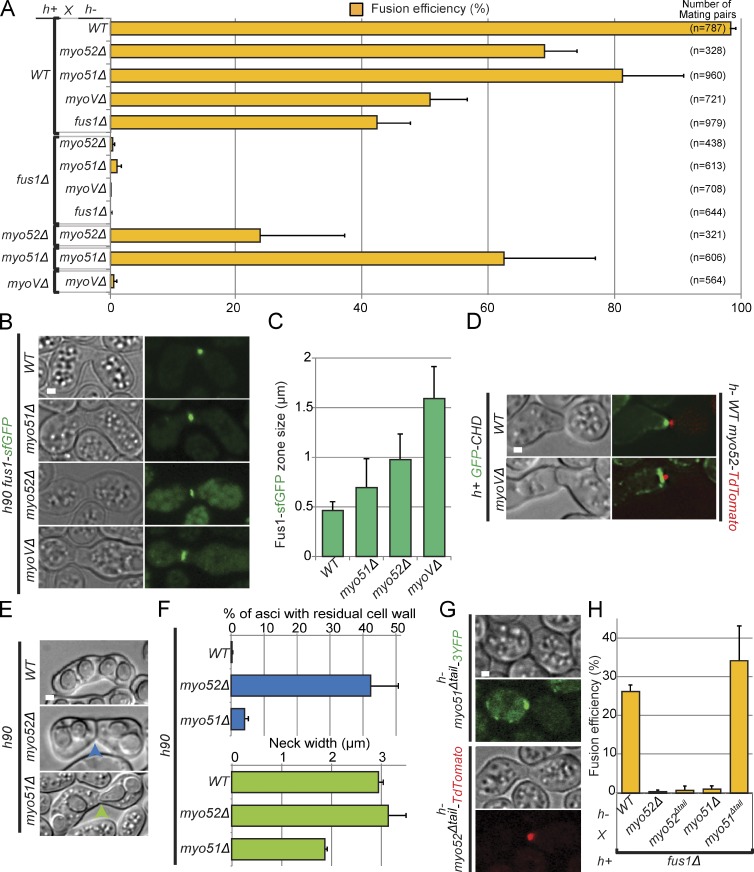
We tested the function of type V myosins during cell fusion. Myosin V deletion strains (myo51Δ and myo52Δ and the double myo51Δmyo52Δ mutant, noted myoVΔ throughout) fused inefficiently with wild-type cells and were fusion defective when crossed to fus1Δ (Fig. 4 A). We note that myoVΔ cells fused more efficiently with h+ than h− wild-type partners (not depicted), as also observed for fus1Δ × wild type (Fig. 4, compare A and H), though the significance of this observation is currently unknown. Homothallic myoVΔ cells were also fusion defective. However, even myoVΔ double mutant displayed efficient mating pair formation (similar to their ability to polarize during mitotic growth; Motegi et al., 2001; Win et al., 2001; Bendezú and Martin, 2011), suggesting the observed fusion defect is not caused by prior cell polarization defects. The stronger phenotype of the myoVΔ double mutant, compared with each single mutant, indicates that Myo51 and Myo52 contribute at least partially overlapping function to cell fusion. Fus1 displayed a broader localization in myosin V mutants, especially in the double mutant in which it localized over the entire surface at the contact zone (Fig. 4, B and C). Similarly, actin accumulation at the fusion site spread along the contact zone in the double myoVΔ mutant (Fig. 4 D). Thus, type V myosins are required for focalization of the formin Fus1 and of actin filaments in the focus. As we have shown in Fig. 2 (D and E) that Fus1 is required for myosin V focalization, we conclude that actin fusion focus formation relies on positive reinforcement between formin and type V myosins.
Figure 4.
Type V myosins Myo52 and Myo51 are essential for cell fusion. (A) Fusion efficiency of indicated heterothallic crosses. myoVΔ (=myo51Δ myo52Δ double mutant) is unable to fuse with either fus1Δ or itself. The total number of mating pairs analyzed (three experiments combined) is indicated on the right. (B) Homothallic h90 wild-type, myo51Δ, myo52Δ, and myoVΔ strains expressing Fus1-sfGFP. Images are time-averaged projections over time 15 min at 1 image/min. Type V myosins are important for Fus1 focalization. (C) Quantifications of Fus1-sfGFP zone size in strains as in B; n = 12. (D) Crosses of h+ wild-type (WT) and myoVΔ strains expressing GFP-CHD to h− myo52-tdTomato. Images are time-averaged projections over 15 min at 1 image/min. Type V myosins are important for actin focalization at the fusion site. (E) Asci derived from homothallic h90 myo52Δ and myo51Δ matings. We observed residual cell wall (blue arrowhead) and narrow necks (green arrowhead) in myo52Δ and myo51Δ, respectively. (F) Percentage of asci with residual cell wall and mean neck width in strains as in E. n > 200. (G) Crosses of h− myo51Δtail-3YFP (top) and h− myo52Δtail-tdTomato (bottom) to h+ wild-type cells. Both truncated motors localize correctly to the fusion focus. (H) Fusion efficiency of indicated heterothallic crosses. Note that a lower fusion efficiency is observed for h+ fus1Δ × h− wild type than for h− fus1Δ × h+ wild type in A. n > 100. Bars, 1 µm. Error bars are standard deviations.
Although Myo51 and Myo52 are together essential to achieve cell fusion, we note that each single mutant displayed a distinct phenotype after fusion. Tetrads derived from homothallic h90 myo52Δ mating reactions often displayed residual undigested cell wall at the fusion site (Fig. 4, E and F), suggesting defective cell wall degradation during cell fusion. In contrast, tetrads derived from h90 myo51Δ mating reactions showed a narrower neck compared with wild type, suggesting a defect in neck expansion after fusion (Fig. 4, E and F). Thus, in addition to their functional overlap for cell–cell fusion, each type V myosin may have distinct roles in postfusion events.
Most type V myosins serve to transport cargoes through interaction with their C-terminal tail. We investigated the effect of deleting the cargo-binding C-terminal tail domain of type V myosins on cell–cell fusion. Both Myo52Δtail-tdTomato and Myo51Δtail-3YFP localized as a dot at the fusion site, indicating that their cargo-binding domain is not essential for fusion focus formation (Fig. 4 G). Interestingly, cell fusion was impaired in myo52Δtail mutants, whereas myo51Δtail mutants remained fusion competent in crosses to fus1Δ partner cells (Fig. 4 H). These results are similar to observations made on the function of type V myosin in actin cable organization during vegetative growth (Lo Presti et al., 2012). They suggest that Myo52 promotes cell fusion by delivering cargoes, whereas Myo51 may play a more structural, C-terminal tail-independent role for fusion focus formation.
Type V myosins deliver cell wall degradation enzymes for cell fusion
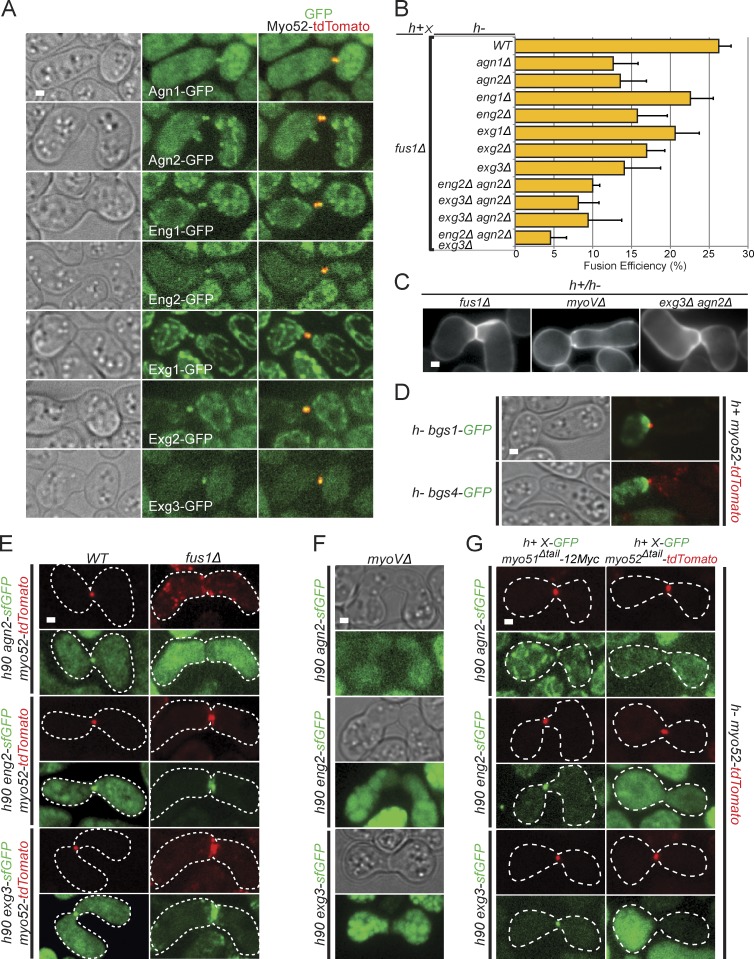
Cell fusion in yeast requires important cell wall remodeling, to allow plasma membrane contact while preserving cell integrity. The progressive shortening of the distance between Myo52 dots during fusion suggests that the cell wall may be progressively eroded. We thus tested whether the fusion focus is required to localize cell wall degrading enzymes. We used GFP tagged alleles of seven distinct glucanases—the endo-α(1,3)-glucanases Agn1 and Agn2 (Dekker et al., 2004, 2007), the endo-β(1,3)-glucanases Eng1 and Eng2 (Martín-Cuadrado et al., 2003, 2008), and the β(1,6)-glucanases Exg1, Exg2, and Exg3 (Dueñas-Santero et al., 2010). Remarkably, all seven colocalized with Myo52-tdTomato at the fusion focus (Fig. 5 A). Deletion of each single one of these, except eng1Δ, led to reduction in fusion efficiency in crosses to fus1Δ (Fig. 5 B). Examination of mating pairs that failed to fuse revealed cell wall at the cell–cell junction, as for fus1Δ and myoVΔ pairs, suggesting the cell wall is not degraded (Fig. 5 C). We also observed a high occurrence of cell lysis, suggesting deregulation of the fusion process (unpublished data). Further combinatorial double and triple deletion of agn2, eng2, and exg3 led to progressive decrease in fusion efficiency, indicating that each glucanase additively contributes to fusion.
Figure 5.
Cell wall degradation enzymes focalize at the fusion focus. (A) Crosses of heterothallic h− and h+ myo52-tdTomato strains expressing GFP-tagged glucanases as indicated. All glucanases localize to the fusion focus. (B) Fusion efficiency of crosses between h+ fus1Δ strains and h− single, double, and triple glucanase deletion strains. n > 100. (C) Calcofluor images of nonfusing pairs in heterothallic crosses of h+ fus1Δ, myoVΔ, and agn2Δ eng2Δ to h− fus1Δ, myoVΔ, and agn2Δ eng2Δ, as indicated. This shows presence of cell wall at the cell–cell contact. (D) Time-averaged projections over 10 s of crosses of heterothallic h− bgs1-GFP (top) and bgs4-GFP (bottom) to h+ myo52-tdTomato, imaged every second. Cell wall synthases localize as a crescent at the fusion site. (E) Homothallic h90 wild type (left) and fus1Δ (right) myo52-tdTomato strains coexpressing sfGFP-tagged versions of Agn2, Eng2, and Exg3. Cell wall glucanases colocalize at fusion site with Myo52 either as a dot in wild type or as a crescent in fusion-deficient fus1Δ cells. Cell outlines are shown with dotted lines. (F) Homothallic h90 myo51Δ myo52Δ (myoVΔ) strains coexpressing sfGFP-tagged versions of Agn2, Eng2, and Exg3. Glucanases are not detected at fusion site. (G) Heterothallic h+ myo51Δtail-12Myc (left) and h+ myo52Δtail-tdTomato (right) strains coexpressing sfGFP-tagged versions of Agn2, Eng2, and Exg3 crossed to h− myo52-tdTomato cells. Cell wall glucanases localize at the fusion site in myo51Δtail mutants but not, or are highly reduced, in myo52Δtail. WT, wild type. Error bars are standard deviations. Cell outlines are shown with dotted lines. Bars, 1 µm.
In contrast to the focalized localization of glucanases, the cell wall β(1,3)-glucan synthases Bgs1 and Bgs4 (Cortés et al., 2002, 2005) decorated the entire shmoo tip (Fig. 5 D). Thus, during cell–cell fusion, cell wall glucanases are focalized at the fusion focus within a broader zone of cell wall synthases.
Finally, we studied the dependency of glucanases on fusion focus formation by tagging one member from each family—Agn2, Eng2, and Exg3—with sfGFP, which we found provides significantly stronger signal for many tagged proteins, likely because of its faster maturation time (Fig. 5 E; Pédelacq et al., 2006). In fus1Δ, the glucanases decorated the entire shmoo tip, similar to Myo52. In myoVΔ, they failed to localize to either shmoo tip or fusion focus, suggesting they may be cargoes for the type V myosins (Fig. 5 F). In agreement with this idea, the glucanases failed to localize to the fusion focus in myo52Δtail mutant cells, which show fusion defect but form a fusion focus. In contrast, they localized correctly in myo51Δtail mutants, which do not exhibit fusion defects (Fig. 5 G). In conclusion, glucanases are cargoes for myosin V Myo52, which concentrates them at the fusion focus for cell wall digestion during cell fusion.
Discussion
Architecture of the fusion focus
We present here a novel actin structure in yeast, the actin fusion focus. The fusion focus is assembled by the formin Fus1, which has long been known to underlie cell–cell fusion (Petersen et al., 1995). Fus1, like other formins, nucleates linear actin filaments (Scott et al., 2011). However, the specific actin structure it organizes was not understood.
The fusion focus is distinct from actin patches, organized by the Arp2/3 actin nucleator. Most actin patch components do not assume a tight localization at the fusion site, and Arp2/3 activators are not strongly involved in cell–cell fusion, consistent with data in the budding yeast that many endocytosis mutants do not have a fusion defect (Brizzio et al., 1998). We note that earlier requirements of endocytosis for pheromone signaling and polarized growth preclude complete inactivation of the Arp2/3 complex and that a function of Arp2/3 and endocytosis in cell–cell fusion can therefore not be excluded. Indeed, the apparent directional movement of actin patches toward the fusion site seen in our high-resolution time-lapse imaging suggests they may for instance contribute to membrane recycling. However, our data demonstrate that, although actin patches normally accumulate in the vicinity of the prospective fusion site (Petersen et al., 1998a), they are superimposed upon a distinct underlying actin structure.
The architecture of the fusion focus is also distinct from that of actin cables and the cytokinetic ring, nucleated by the two other formins For3 and Cdc12. Actin cables are bundles of largely parallel actin filaments (Kamasaki et al., 2005), whereas the cytokinetic ring relies on an antiparallel actin filament organization (Kamasaki et al., 2007). In contrast, in the fusion focus, the focal localization of Fus1 and type V myosins at the edge of an actin cloud suggests an aster-like filament configuration centered round filament barbed ends. Structured illumination and confocal microscopy further support this architecture through observation of actin filaments emanating from the fusion focus. Thus, the actin fusion focus consists of an aster of actin filaments nucleated by the formin Fus1 (Fig. 6 B).
Formin Fus1 and type V myosins are required to form the fusion focus
The convergence of actin filaments in the fusion focus relies on both Fus1 and type V myosins, and these factors are codependent for focalization, suggesting positive reinforcement between Myosin V and formin Fus1. The role of type V myosins in formation of the fusion focus is reminiscent of the function of microtubule minus end-directed motors for spindle pole formation (Heald et al., 1997; Goshima et al., 2005). Here, multimerization of motor proteins is sufficient to form microtubule asters in vitro (Surrey et al., 2001). Minus end–directed motors, such as Dynein, also contribute to focalization by transporting kinetochore fibers along astral microtubules (Maiato et al., 2004). Myo51 and Myo52 may use similar mechanisms, for instance forming multimeric assemblies by interacting with vesicular cargoes and thus contributing to actin filament focalization. These motors may also more directly transport Fus1, thus promoting new filament nucleation in the vicinity of existing barbed ends. In an analogous manner, we had previously shown that Myo52 associates with the formin For3 and contributes to its delivery to cell tips (Lo Presti et al., 2012). Our data showing distinct C-terminal tail requirements and postfusion phenotypes suggest that Myo51 and Myo52 each contribute to actin focalization through distinct mechanism. Distinct contributions of Myo51 and Myo52 were also previously observed in the organization and function of actin cables and the cytokinetic ring (Lo Presti et al., 2012; Wang et al., 2014). The role of type V myosins in focus formation is also reminiscent to that of Myosin X, a distinct myosin that promotes actin filament convergence for filopodia formation (Tokuo et al., 2007).
Fus1 is a key determinant of the fusion focus, though other actin-binding proteins, such as tropomyosin Cdc8, or profilin Cdc3, are likely also required (Petersen et al., 1998a; Kurahashi et al., 2002). Fus1 N-terminal FH3 domain confers localization to the shmoo tip (Petersen et al., 1998b), but it is unclear whether this domain alone promotes focalization. Interestingly, in vitro dissection of Fus1 FH1-FH2 domains revealed a unique set of activities, including actin filament nucleation, barbed end capping, elongation, and also bundling (Scott et al., 2011). What specific features of Fus1 confer its unique ability to organize the fusion focus remains to be tested.
Function of the fusion focus: Focalized delivery of cell wall–degrading enzymes
In walled cells under strong turgor pressure, such as those of fungi, cell–cell fusion requires precise remodeling of the cell wall to allow plasma membrane contact while protecting the cells from lysis: the cell wall has to be digested at precisely opposed locations in the two partner cells while maintained intact, likely reinforced, in surrounding regions. The fusion focus serves to precisely position and focalize the cell wall degradation machinery to permit this remodeling.
Indeed, we show that many, if not all, fission yeast glucanases are enriched at the fusion focus and required for efficient cell fusion. The yeast cell wall mainly consists of polymers of α- and β-glucans (Pérez and Ribas, 2004). The fusion focus–localized glucanases exhibit distinct hydrolytic activities, hydrolyzing the major linkage bonds in the glucan polymers. Focusing on three of these glucanases, Agn2, Eng2, and Exg3, we show they are likely cargoes of Myo52. Indeed, each of them fails to localize to the fusion focus in the myo52Δtail mutant, in which the cargo-binding tail of Myo52 is absent, yet the fusion focus forms correctly. Although many glucanases are either secreted or transmembrane proteins, and thus likely transported to the fusion site in secretory vesicles, one intriguing observation is that Agn2, Eng2, and Exg3 all lack a signal sequence or transmembrane domain (Dekker et al., 2004; Encinar del Dedo et al., 2009; Dueñas-Santero et al., 2010). Yet, in combination, these glucanases significantly contribute to fusion efficiency. How these proteins are delivered to the cell outside is currently unknown. In summary, these data show that the fusion focus concentrates these and other glucanases to a single location to promote cell wall erosion.
In contrast, the cell wall synthases Bgs1 and Bgs4 do not concentrate at the fusion focus but rather decorate the entire shmoo tip throughout the fusion process (Cortés et al., 2002, 2005). Bgs1 was proposed to be a Myo52 cargo (Mulvihill et al., 2006), but its localization in a broad crescent when Myo52 is focalized indicates that transport along actin filaments is not the sole determinant of its localization. Cell wall synthases may be delivered at the fusion focus but spread laterally from the zone of insertion, thus decorating the entire shmoo tip. This is in agreement with the observation that Bgs4 remains well polarized upon acute F-actin disruption (Cortés et al., 2005) or in the absence of directional transport along actin filaments (Bendezú and Martin, 2011), suggesting a long residence time at the polarized zone. Thus, the formation of the fusion focus creates a geometrical difference in the localization of cell wall synthases and hydrolases.
Two pieces of evidence indicate that focalization of the glucanases, rather than their mere localization to the shmoo tip, is required for fusion. First, the progressive thinning of the connecting cell wall, shown through reduction in distance between MyoV signals, occurs only after fusion focus formation. Second, the phenotype of fus1Δ cells shows that an unfocused localization of glucanases to the shmoo tip is not sufficient to promote cell fusion. Instead, fus1Δ cells keep growing toward each other, extending long projections (Petersen et al., 1995). These observations suggest that a change in the geometry of cell wall enzyme delivery distinguishes cell growth from cell fusion (or lysis if deregulated). Though changes in enzyme activities are also possible, this simple model does not need to invoke such changes. We propose that an overlapping distribution of cell wall synthases and hydrolases, as in fus1Δ, balances cell wall stretching and integrity for turgor pressure to drive polarized growth, as proposed for other tip-growing cells (Rojas et al., 2011). In contrast, specific focalization of the hydrolases within a broader distribution of the synthases may promote local cell wall thinning for fusion (Fig. 6 C).
Asymmetry in the cell fusion process
Fusion focus formation occurs asynchronously in the two cell types and is highly stereotypical, with the h− cell always displaying a stable focus before the h+ cell (Fig. 6 A). Thus, this asymmetry is caused by intrinsic differences between the two cell types. This may be a result of the slightly distinct sexual gene expression programs in the two types, though the number of mating type–specific factors identified is small (Mata and Bähler, 2006; Xue-Franzén et al., 2006). This may also be caused by distinct qualities of the two pheromones inducing temporally or spatially distinct responses in their partner cell. Indeed, previous data suggested that high local pheromone concentrations are needed for cell–cell fusion in the budding yeast (Brizzio et al., 1996). In either case, the delayed stabilization of the h+ cell fusion focus always occurs at a site precisely facing the fusion focus of the h− cell, suggestive of strong spatial communication between the two partner cells.
An important question is whether this asymmetry is physiologically important. The normally delayed formation of the fusion focus in the h+ cell does not strictly depend on fusion competency of the h− cell. Indeed, cell fusion happens, though at reduced efficiency, in crosses between fus1Δ and wild-type cells irrespective of which partner is mutant, and is completely blocked only when both partners are mutant (Petersen et al., 1995). We made similar observations in myoVΔ mutants. We note however that fusion consistently occurs more readily in crosses of mutant cells with wild-type h+ cells than h− cells. We postulate that an asymmetric setup promotes precision in positioning of the two fusion machineries, ensuring formation of a single membrane contact site.
Parallels with cell–cell fusion events in other cells
The involvement of actin in cell–cell fusion is not unique to the fission yeast. In the budding yeast S. cerevisiae, although there is no cell fusion-specific formin, the actin cytoskeleton plays a critical role in the clustering of signaling and fusion molecules at the shmoo tip (Ayscough and Drubin, 1998; Bagnat and Simons, 2002). In addition, the formin Bni1 and the tropomyosin Tpm1 are required not only for shmoo polarization but also for cell fusion (Liu and Bretscher, 1992; Dorer et al., 1997); electron microscopy analysis described highly clustered vesicles over a small region (Gammie et al., 1998); and the fusion factor Fus2 focalizes in a polarizome-dependent manner at the fusion site (Paterson et al., 2008). Thus, although recent work proposed that local concentration of glucanases required for fusion stems from restricted diffusion upon cell–cell contact (Huberman and Murray, 2014), all these data are highly suggestive of the existence of a similar actin fusion focus in this organism to focalize the delivery of cell wall glucanases.
In nonwalled cells, fusion-specific actin structures also underlie cell fusion. A possible Formin3-dependent F-actin structure underlies tracheal cell fusion in Drosophila melanogaster (Tanaka et al., 2004), but the best-studied case occurs during myoblast fusion, in which the two fusing cells—a founder cell and a fusion-competent myoblast—organize an Arp2/3-dependent structure at the fusion site before fusion (Kim et al., 2007; Massarwa et al., 2007; Richardson et al., 2007; Sens et al., 2010). This structure is highly asymmetric, forming a fusion focus only in the fusion-competent myoblast, and likely provides force for fusion within an invasive podosome-like structure (Sens et al., 2010; Shilagardi et al., 2013). As podosomes are sites of extracellular matrix degradation (Linder, 2007), it suggests an interesting analogy with the role of the fusion focus in fission yeast. This analogy also raises the question of whether the Fus1-nucleated fusion focus in yeast provides force for fusion. Future work should reveal whether actin fusion foci of distinct molecular composition have evolved to fulfill the same tasks in diverse species.
Materials and methods
Strains, media, and growth conditions
Strains used in this study are listed in Table S1. Homothallic (h90) strains able to switch mating type or 1:1 mixtures of heterothallic h+ × h− cells (also called P × M cells) were used as indicated. Minimal sporulation liquid (MSL) media with or without nitrogen (MSL+N and MSL−N) liquid or agar were used to grow and mate the cells, respectively (Egel et al., 1994). All live-cell imaging was performed on cells placed on MSL−N with 2% electrophoresis-grade agarose pads, covered with a coverslip sealed with VALAP (1:1:1 Vaseline/lanolin/paraffin).
Genes were tagged at their endogenous genomic locus at their 3′ end, yielding C-terminally tagged proteins. This was achieved by PCR amplification of a fragment from a template plasmid with primers carrying 5′ extensions corresponding to the last 80 nucleotides of the ORF and the first 80 nucleotides of the 3′UTR, which was transformed and integrated in the genome by homologous recombination, as previously described (Bähler et al., 1998). For tagging of genes with sfGFP, a pFA6a-sfGFP-kanMX plasmid was used as a template. sfGFP was amplified from pMaM4 (a plasmid provided by M. Knop, University of Heidelberg, Heidelberg, Germany; containing yeast codon-optimized sfGFP), with primers osm2680 (5′-ccTTAATTAActccaagggtgaagagctatttac-3′; PacI site uppercase) and osm2681 (5′-aGGCGCGCCcttataaagctcgtccattccg-3′; AscI site uppercase), digested with AscI and Pac1 and ligated to similarly treated pSM674 (pFA6a-EGFP-kanMX6; described in Bähler et al., 1998). The sfGFP replaced EGFP, resulting in pFA6a-sfGFP-kanMX6 (pSM1538). We then used this vector as template for PCR-based targeted tagging of fus1, agn2, eng2, and exg3 (Bähler et al., 1998).
To yield Pmap3-driven fluorescent reporters, the map3 promoter region was amplified from genomic DNA with primers osm935 (5′-cccCTGCAGaagcatgcacgctgctcac-3′; PstI site uppercase) and osm936 (5′-agaGTCGACggtaaactcaacgtataag-3′; SalI site uppercase), digested with Pst1 and SalI, and ligated to similarly treated pSM242 (pRIP42:GFP; an integrative plasmid containing GFP under control of nmt41 promoter and a ura4+ selection marker), replacing the nmt41 promoter and yielding plasmid pSM793 (pRIP-Pmap3:GFP, ura4+). To generate a red reporter, the tdTomato tandem repeat was amplified from pFA6a-tdTomato-kanMX with primers osm944 (5′-aatGGATCCatggtgagcaagggcgaggaggtc-3′; BamHI site uppercase) and osm945 (5′-ttaCCCGGGcttgtacagctcgtccatgc-3′; XmaI site uppercase), digested with BamHI and XmaI, and ligated to similarly treated pSM793, yielding plasmid pSM1709 (pRIP-Pmap3:tdTomato; ura4+). Plasmids were linearized with NruI and integrated at the map3 promoter in h90 cells.
Mating assays
Mating assays were performed as in Bendezú and Martin (2013). In brief, precultures of cells were grown at 25°C to reach an OD600 of between 0.4 and 1 in MSL+N. Cultures were then diluted to an OD600 of 0.025 in MSL+N (for heterothallic crosses, cells were mixed in equal parts) and grown for 18–20 h to an OD600 of between 0.4 and 1 at 25°C, or 30°C for slow growing mutants (for3Δ and myosin deletion mutants), in MSL+N. Cells were pelleted by centrifugation and washed three times with MSL−N. Cells were then added onto MSL−N + 2% electrophoresis-grade agarose pads and incubated either at 25°C for 1 h before imaging in overnight videos or at 18°C overnight before imaging. For fusion efficiency, the total number of mating pairs and the number of fused mating pairs were quantified using the ObjectJ plugin in ImageJ (National Institutes of Health). Fused mating pairs were identified in differential interference contrast images as asci containing ascospores or mating pairs without residual cell wall between them. The obtained data were used to calculate fusion efficiency = (number of fused mating pairs/total number of mating pairs) × 100 and the mating efficiency = (number of mating pairs × 2/total number of cells) × 100 for each crossing. Fusion efficiencies of h90 wild-type or h+ × h− wild-type matings were identical. We also verified that tagging of Myo52 did not affect fusion efficiency.
Microscopy and image analysis
A DeltaVision epifluorescence system and/or a spinning-disk confocal microscope were used to acquire images. Wide-field microscopy was performed on a DeltaVision platform (Applied Precision) composed of a customized inverted microscope (IX-71; Olympus), a UPlan Apochromat 100×/1.4 NA oil objective, a camera (CoolSNAP HQ2; Photometrics), and a color combined unit illuminator (Insight SSI 7; Social Science Insights). Figures were acquired using softWoRx v4.1.2 software (Applied Precision). Spinning-disk microscopy was performed using an inverted microscope (DMI4000B; Leica) equipped with an HCX Plan Apochromat 100×/1.46 NA oil objective and an UltraVIEW system (PerkinElmer; including a real-time confocal scanning head [CSU22; Yokagawa Electric Corporation], solid-state laser lines, and an electron-multiplying charge-coupled device camera [C9100; Hamamatsu Photonics]). Stacks of z-series confocal sections were acquired at 0.3-µm intervals using Volocity software (PerkinElmer).
The DeltaVision platform was used for quantitative analyses of mating and fusion efficiency and overnight videos, whereas the spinning disk was used for high-temporal resolution images to study the transitions between the fusion steps as well as z-stack maximal projection images (Fig. 2, A and H; Fig. 3, D, H, and I; Fig. 4, C and G; Fig. 5, A and D–G; Fig. S1; Fig. S2, A–H; and Fig. S3 C). The DeltaVision platform was described previously (Bendezú and Martin, 2013). To limit photobleaching, overnight videos were captured by optical axis integration imaging of a 4.6-µm z section, which is essentially a real-time z sweep (Fig. 1, A–C; Fig. 2, B–E; Fig. 3 A; Fig. 4, B and E; and Fig. S3 B). The spinning-disk microscope system was as previously described (Bendezú et al., 2012). For spinning-disk confocal imaging, optical slices were acquired every 0.6 µm, and all panels show maximum projections, unless otherwise indicated. Time projections are sum projections of time-lapse series, made using ImageJ. All imaging, except for cdc12-112ts mutants and control strains (Fig. S2, D and E), was performed at room temperature (∼22°C). cdc12-112ts mutant strains were imaged at 33°C using an objective heater.
Actin phalloidin staining was performed using Alexa Fluor 488–phalloidin (Invitrogen). Mating reactions were performed on MSL−N agar plates for 18–20 h at 25°C. Mating cells were then scratched off the plates and added directly into PM buffer (35 mM K-phosphate buffer 6, pH 6.8, and 0.5 mM MgSO4) with 4% formaldehyde for 30 min. The cells were then washed three times with PM buffer and span down at 1,000 rpm for 3 min, permeabilized in PM buffer with 0.5% Triton X-100 for 30 s, and washed again three times before adding 10 µl of Alexa Fluor 488–phalloidin to 5 µl of concentrated sample. Calcofluor (Sigma-Aldrich) was added at a final concentration of 5 µg/ml from a 200× stock solution (1 mg/ml; Fig. 5 C).
FRAP was performed with the photokinesis unit of the spinning-disk system (Bendezú and Martin, 2011). A circular 0.9-µm zone was photobleached using maximal laser power at the shmoo tip or the cell tip for cells during mitotic growth. In cells with a fusion focus, the 0.9-µm circular zone was centered over the fusion focus and photobleached the entire structure. Initial FRAP experiments (Figs. 3 C and S3 A) were conducted in h90 homothallic crosses, photobleaching, and recording recovery of the fluorescent Myo52 signal in both partner cells. To probe the specific dynamics in each mating type (Fig. 3 G), heterothallic crosses with GFP-CHD–tagged cells were used. Images were recorded before photobleaching and immediately after, every second for 90 s. Kymographs at the fusion site (Fig. 3, D and H) were constructed in ImageJ v1.46 by drawing a 3-pixel-wide (0.39 µm) line connecting the myosins dots in both partner cells. FRAP measurements were performed as previously published (Bendezú et al., 2012). In brief, for FRAP analysis, the mean fluorescence intensities were measures over time in three regions: (1) the photobleached region, (2) the background outside the cell, and (3) another nonbleached cell. The background fluorescence was subtracted from the fluorescence intensities of the photobleached and the nonbleached cell. The loss of signal as a result of imaging was corrected by dividing the adjusted bleached regions intensity by the adjusted intensity of the nonbleached cell. All values were normalized so that the prephotobleaching value equals 1.
GFP-CHD intensity at the fusion focus (Fig. 1 D) was measured in ImageJ within a manually drawn box of ∼40 × 40 pixels at the cell–cell contact zone over time. Background fluorescence outside the cell was measured within a box of same dimensions and was then subtracted from the GFP-CHD fluorescence measurements at the fusion site. Fluorescence was then normalized to the maximum intensity signal. Fusion time was defined by the sudden increase in the intensity of tdTomato in the h− partner cell, which was measured within a box of 50 × 50 pixels positioned in the center of the h+ partner cell. Background signal was subtracted similarly as for GFP-CHD and then normalized to the maximum intensity signal. Fluorescence signals were then aligned to fusion time and averaged. The distance between Myo52 signals was measured on time-lapse videos of h− myo52-GFP crossed to h+ myo52-tdTomato (Fig. 3 B), using the ImageJ line measure tool to measure the distance between the highest intensity pixel of the Myo52-tdTomato and Myo52-GFP signals.
The displacement of Myo52 signal (Fig. 3, E and F) was measured by following the x and y coordinates of the highest intensity pixel of the Myo52-tdTomato signal in each mating partner over time. The homothallic strain used, also expressed pmap3:GFP as a marker for the mating type. Time interval of the video is 7.2 min. During the Myo52 crescent phase, we recorded the coordinates of the maximum fluorescence intensity. Displacement was then calculated as
For Fig. 3 F, the mean instantaneous displacement over the course of fusion was calculated for each cell. The graph shows n mean over eight cells.
Fluorescence intensities of Myo52-tdTomato and Myo51-3YFP signals in Fig. S3 B were measured in ImageJ using a manually drawn box of 40 × 30 pixels surrounding the contact zone of each mating partner. Background fluorescence for YFP and tomato were measured over time and subtracted from the original measurements. The fluorescence signal was normalized to the maximum intensity signal.
Structured illumination images (Fig. 2 F and Videos 3 and 4) were acquired using a Nikon SIM setup (Eclipse T1 microscope fitted with a super-resolution Apochromat total internal reflection fluorescence 100×/ 1.49 NA objective and an electron-multiplying charge-coupled device camera (IXON3; Andor Technology). Imaging was performed at 3.4-s interval in 3D SIM acquisition mode (15 image per plane; five phases of three rotations) with an 80-ms exposure time using a 488-nm coherent sapphire laser at 1.30 mW (measured in the back focal plane of the objective). Image reconstruction was performed using the NIS-Elements software (Nikon; based on Gustafsson et al., 2008); reconstruction parameters were as follows: contrast 0.70; apodization 1.00; and Widh3DFilter 0.20.
Laser-scanning confocal microscopy (Videos 5 and 6) was performed on a microscope (LSM 710; Carl Zeiss) with external-port GaAsP detectors. Pinhole was reduced to 0.5 arbitrary units, and the 488-nm argon laser line was set to 0.15 mW (in the backfocal plane of the objective) to easily observe actin filaments.
Figures were assembled with Photoshop CS5 (Adobe) and Illustrator CS5 (Adobe). All error bars are standard deviations of the number of indicated samples (cells or actin cables) analyzed, except for Fig. 1 B, Fig. 4 (A, F, and H), Fig. 5 B, and Fig. S2 E, in which the error bars are standard deviations of three independent experiments. All experiments were performed a minimum of three independent times except for Fig. 2 (F and G), Fig. 3 C, Fig. S2 C, and Fig. S3 A, which were performed two independent times.
Online supplemental material
Fig. S1 (related to Fig. 1) shows Fus1-dependent actin accumulation at the prospective fusion site. Fig. S2 (related to Fig. 2) shows fusion focus formation is independent of formins For3 and Cdc12 and of actin patch components. Fig. S3 (related to Fig. 4) shows type V myosin localization and dynamics define multiple steps in the formation of the fusion focus. Table S1 shows strains used in this study. Video 1 (related to Fig. 1) shows an actin fusion focus forms before cell fusion. Video 2 (related to Fig. 1) shows absence of actin fusion focus in fus1Δ. Video 3 (related to Fig. 2) shows the actin fusion focus visualized by 3D SIM. Video 4 (related to Fig. 2) shows the actin fusion focus visualized by 3D SIM in for3Δ. Video 5 (related to Fig. 2) shows actin at the zone of cell–cell contact visualized by 3D SIM in fus1Δ. Video 6 (related to Fig. 2) shows actin at the zone of cell–cell contact visualized by 3D SIM in fus1Δ for3Δ. Video 7 (related to Fig. 2) shows the actin fusion focus visualized by scanning confocal microscopy in wild type and for3Δ. Video 8 (related to Fig. 2) shows actin at the zone of cell–cell contact visualized by scanning confocal microscopy in fus1Δ and fus1Δ for3Δ. Video 9 (related to Fig. 3) shows asymmetric maturation of the fusion focus. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201411124/DC1. Additional data are available in the JCB DataViewer at http://dx.doi.org/10.1083/jcb.201411124.dv.
Supplementary Material
Acknowledgments
We thank Fred Chang, Kathy Gould, Michael Knop, David Kovar, Olaf Nielsen, Thomas Pollard, Yolanda Sanchez, Chikashi Shimoda, Vladimir Sirotkin, and Carlos R. Vàzquez de Aldana for strains and reagents; the Martin laboratory for discussion; and Richard Benton, Serge Pelet, and members of the Martin and Pelet laboratories for comments on the manuscript.
This work was supported by a European Research Council Starting grant (260493) and a Swiss National Science Foundation (SNF) research grant (31003A_138177) to S.G. Martin. The SIM microscope was funded via an SNF R’equip grant.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- CHD
- calponin homology domain
- LatA
- Latrunculin A
- MSL
- minimal sporulation liquid
- sfGFP
- superfolder GFP
- SIM
- structured illumination microscopy
References
- Abmayr S.M., and Pavlath G.K.. 2012. Myoblast fusion: lessons from flies and mice. Development. 139:641–656 10.1242/dev.068353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar P.S., Baylies M.K., Fleissner A., Helming L., Inoue N., Podbilewicz B., Wang H., and Wong M.. 2013. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29:427–437 10.1016/j.tig.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K.R., and Drubin D.G.. 1998. A role for the yeast actin cytoskeleton in pheromone receptor clustering and signalling. Curr. Biol. 8:927–930 10.1016/S0960-9822(07)00374-0 [DOI] [PubMed] [Google Scholar]
- Bagnat M., and Simons K.. 2002. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA. 99:14183–14188 10.1073/pnas.172517799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J., Wu J.Q., Longtine M.S., Shah N.G., McKenzie A. III, Steever A.B., Wach A., Philippsen P., and Pringle J.R.. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 14:943–951 [DOI] [PubMed] [Google Scholar]
- Bendezú F.O., and Martin S.G.. 2011. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol. Biol. Cell. 22:44–53 10.1091/mbc.E10-08-0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú F.O., and Martin S.G.. 2013. Cdc42 explores the cell periphery for mate selection in fission yeast. Curr. Biol. 23:42–47 10.1016/j.cub.2012.10.042 [DOI] [PubMed] [Google Scholar]
- Bendezú F.O., Vincenzetti V., and Martin S.G.. 2012. Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. PLoS ONE. 7:e40248 10.1371/journal.pone.0040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzio V., Gammie A.E., Nijbroek G., Michaelis S., and Rose M.D.. 1996. Cell fusion during yeast mating requires high levels of a-factor mating pheromone. J. Cell Biol. 135:1727–1739 10.1083/jcb.135.6.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzio V., Gammie A.E., and Rose M.D.. 1998. Rvs161p interacts with Fus2p to promote cell fusion in Saccharomyces cerevisiae. J. Cell Biol. 141:567–584 10.1083/jcb.141.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellaro C., Mrsa V., and Tanner W.. 1998. New potential cell wall glucanases of Saccharomyces cerevisiae and their involvement in mating. J. Bacteriol. 180:5030–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés J.C., Ishiguro J., Durán A., and Ribas J.C.. 2002. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 115:4081–4096 10.1242/jcs.00085 [DOI] [PubMed] [Google Scholar]
- Cortés J.C., Carnero E., Ishiguro J., Sánchez Y., Durán A., and Ribas J.C.. 2005. The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 118:157–174 10.1242/jcs.01585 [DOI] [PubMed] [Google Scholar]
- Dekker N., Speijer D., Grün C.H., van den Berg M., de Haan A., and Hochstenbach F.. 2004. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell. 15:3903–3914 10.1091/mbc.E04-04-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N., van Rijssel J., Distel B., and Hochstenbach F.. 2007. Role of the alpha-glucanase Agn2p in ascus-wall endolysis following sporulation in fission yeast. Yeast. 24:279–288 10.1002/yea.1464 [DOI] [PubMed] [Google Scholar]
- Dorer R., Boone C., Kimbrough T., Kim J., and Hartwell L.H.. 1997. Genetic analysis of default mating behavior in Saccharomyces cerevisiae. Genetics. 146:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A., Martín-García R., Coulton A.T., Bagley S., and Mulvihill D.P.. 2009. Fission yeast Myo51 is a meiotic spindle pole body component with discrete roles during cell fusion and spore formation. J. Cell Sci. 122:4330–4340 10.1242/jcs.055202 [DOI] [PubMed] [Google Scholar]
- Dueñas-Santero E., Martín-Cuadrado A.B., Fontaine T., Latgé J.P., del Rey F., and Vázquez de Aldana C.. 2010. Characterization of glycoside hydrolase family 5 proteins in Schizosaccharomyces pombe. Eukaryot. Cell. 9:1650–1660 10.1128/EC.00187-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R., Willer M., Kjaerulff S., Davey J., and Nielsen O.. 1994. Assessment of pheromone production and response in fission yeast by a halo test of induced sporulation. Yeast. 10:1347–1354 10.1002/yea.320101012 [DOI] [PubMed] [Google Scholar]
- Encinar del Dedo J., Dueñas E., Arnáiz Y., del Rey F., and Vázquez de Aldana C.R.. 2009. β-glucanase Eng2 is required for ascus wall endolysis after sporulation in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell. 8:1278–1286 10.1128/EC.00148-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B., and Chang F.. 2001. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 11:1656–1665 10.1016/S0960-9822(01)00525-5 [DOI] [PubMed] [Google Scholar]
- Gammie A.E., Brizzio V., and Rose M.D.. 1998. Distinct morphological phenotypes of cell fusion mutants. Mol. Biol. Cell. 9:1395–1410 10.1091/mbc.9.6.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Nédélec F., and Vale R.D.. 2005. Mechanisms for focusing mitotic spindle poles by minus end–directed motor proteins. J. Cell Biol. 171:229–240 10.1083/jcb.200505107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M.G., Shao L., Carlton P.M., Wang C.J., Golubovskaya I.N., Cande W.Z., Agard D.A., and Sedat J.W.. 2008. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94:4957–4970 10.1529/biophysj.107.120345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., and Hyman A.. 1997. Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol. 138:615–628 10.1083/jcb.138.3.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman L.B., and Murray A.W.. 2014. A model for cell wall dissolution in mating yeast cells: polarized secretion and restricted diffusion of cell wall remodeling enzymes induces local dissolution. PLoS ONE. 9:e109780 10.1371/journal.pone.0109780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itadani A., Nakamura T., and Shimoda C.. 2006. Localization of type I myosin and F-actin to the leading edge region of the forespore membrane in Schizosaccharomyces pombe. Cell Struct. Funct. 31:181–195 10.1247/csf.06027 [DOI] [PubMed] [Google Scholar]
- Iwaki T., Tanaka N., Takagi H., Giga-Hama Y., and Takegawa K.. 2004. Characterization of end4+, a gene required for endocytosis in Schizosaccharomyces pombe. Yeast. 21:867–881 10.1002/yea.1134 [DOI] [PubMed] [Google Scholar]
- Kamasaki T., Arai R., Osumi M., and Mabuchi I.. 2005. Directionality of F-actin cables changes during the fission yeast cell cycle. Nat. Cell Biol. 7:916–917 10.1038/ncb1295 [DOI] [PubMed] [Google Scholar]
- Kamasaki T., Osumi M., and Mabuchi I.. 2007. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J. Cell Biol. 178:765–771 10.1083/jcb.200612018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis J., Bimbó A., Rajagopalan S., Liu J., and Balasubramanian M.K.. 2005. The nuclear kinase Lsk1p positively regulates the septation initiation network and promotes the successful completion of cytokinesis in response to perturbation of the actomyosin ring in Schizosaccharomyces pombe. Mol. Biol. Cell. 16:358–371 10.1091/mbc.E04-06-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Shilagardi K., Zhang S., Hong S.N., Sens K.L., Bo J., Gonzalez G.A., and Chen E.H.. 2007. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev. Cell. 12:571–586 10.1016/j.devcel.2007.02.019 [DOI] [PubMed] [Google Scholar]
- Kovar D.R., Sirotkin V., and Lord M.. 2011. Three’s company: the fission yeast actin cytoskeleton. Trends Cell Biol. 21:177–187 10.1016/j.tcb.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi H., Imai Y., and Yamamoto M.. 2002. Tropomyosin is required for the cell fusion process during conjugation in fission yeast. Genes Cells. 7:375–384 10.1046/j.1365-2443.2002.00526.x [DOI] [PubMed] [Google Scholar]
- Linder S.2007. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17:107–117 10.1016/j.tcb.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Liu H., and Bretscher A.. 1992. Characterization of TPM1 disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J. Cell Biol. 118:285–299 10.1083/jcb.118.2.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Presti L., and Martin S.G.. 2011. Shaping fission yeast cells by rerouting actin-based transport on microtubules. Curr. Biol. 21:2064–2069 10.1016/j.cub.2011.10.033 [DOI] [PubMed] [Google Scholar]
- Lo Presti L., Chang F., and Martin S.G.. 2012. Myosin Vs organize actin cables in fission yeast. Mol. Biol. Cell. 23:4579–4591 10.1091/mbc.E12-07-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H., Rieder C.L., and Khodjakov A.. 2004. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J. Cell Biol. 167:831–840 10.1083/jcb.200407090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.G., and Chang F.. 2006. Dynamics of the formin for3p in actin cable assembly. Curr. Biol. 16:1161–1170 10.1016/j.cub.2006.04.040 [DOI] [PubMed] [Google Scholar]
- Martín-Cuadrado A.B., Dueñas E., Sipiczki M., Vázquez de Aldana C.R., and del Rey F.. 2003. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 116:1689–1698 10.1242/jcs.00377 [DOI] [PubMed] [Google Scholar]
- Martín-Cuadrado A.B., Fontaine T., Esteban P.F., del Dedo J.E., de Medina-Redondo M., del Rey F., Latgé J.P., and de Aldana C.R.. 2008. Characterization of the endo-beta-1,3-glucanase activity of S. cerevisiae Eng2 and other members of the GH81 family. Fungal Genet. Biol. 45:542–553 10.1016/j.fgb.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Massarwa R., Carmon S., Shilo B.Z., and Schejter E.D.. 2007. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev. Cell. 12:557–569 10.1016/j.devcel.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Mata J., and Bähler J.. 2006. Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast. Proc. Natl. Acad. Sci. USA. 103:15517–15522 10.1073/pnas.0603403103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L., Dudin O., and Martin S.G.. 2013. Mate and fuse: how yeast cells do it. Open Biol. 3:130008 10.1098/rsob.130008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F., Arai R., and Mabuchi I.. 2001. Identification of two type V myosins in fission yeast, one of which functions in polarized cell growth and moves rapidly in the cell. Mol. Biol. Cell. 12:1367–1380 10.1091/mbc.12.5.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill D.P., Edwards S.R., and Hyams J.S.. 2006. A critical role for the type V myosin, Myo52, in septum deposition and cell fission during cytokinesis in Schizosaccharomyces pombe. Cell Motil. Cytoskeleton. 63:149–161 10.1002/cm.20113 [DOI] [PubMed] [Google Scholar]
- Paterson J.M., Ydenberg C.A., and Rose M.D.. 2008. Dynamic localization of yeast Fus2p to an expanding ring at the cell fusion junction during mating. J. Cell Biol. 181:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pédelacq J.D., Cabantous S., Tran T., Terwilliger T.C., and Waldo G.S.. 2006. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24:79–88 10.1038/nbt1172 [DOI] [PubMed] [Google Scholar]
- Pérez P., and Ribas J.C.. 2004. Cell wall analysis. Methods. 33:245–251 10.1016/j.ymeth.2003.11.020 [DOI] [PubMed] [Google Scholar]
- Petersen J., Weilguny D., Egel R., and Nielsen O.. 1995. Characterization of fus1 of Schizosaccharomyces pombe: a developmentally controlled function needed for conjugation. Mol. Cell. Biol. 15:3697–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J., Nielsen O., Egel R., and Hagan I.M.. 1998a. F-actin distribution and function during sexual differentiation in Schizosaccharomyces pombe. J. Cell Sci. 111:867–876. [DOI] [PubMed] [Google Scholar]
- Petersen J., Nielsen O., Egel R., and Hagan I.M.. 1998b. FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J. Cell Biol. 141:1217–1228 10.1083/jcb.141.5.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B.E., Beckett K., Nowak S.J., and Baylies M.K.. 2007. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 134:4357–4367 10.1242/dev.010678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E.R., Hotton S., and Dumais J.. 2011. Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys. J. 101:1844–1853 10.1016/j.bpj.2011.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B.J., Neidt E.M., and Kovar D.R.. 2011. The functionally distinct fission yeast formins have specific actin-assembly properties. Mol. Biol. Cell. 22:3826–3839 10.1091/mbc.E11-06-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens K.L., Zhang S., Jin P., Duan R., Zhang G., Luo F., Parachini L., and Chen E.H.. 2010. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J. Cell Biol. 191:1013–1027 10.1083/jcb.201006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer J.M., and Rose M.D.. 2009. The class V myosin Myo2p is required for Fus2p transport and actin polarization during the yeast mating response. Mol. Biol. Cell. 20:2909–2919 10.1091/mbc.E08-09-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilagardi K., Li S., Luo F., Marikar F., Duan R., Jin P., Kim J.H., Murnen K., and Chen E.H.. 2013. Actin-propelled invasive membrane protrusions promote fusogenic protein engagement during cell-cell fusion. Science. 340:359–363 10.1126/science.1234781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoumpla K., Coulton A.T., Lehman W., Geeves M.A., and Mulvihill D.P.. 2007. Acetylation regulates tropomyosin function in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 120:1635–1645 10.1242/jcs.001115 [DOI] [PubMed] [Google Scholar]
- Surrey T., Nedelec F., Leibler S., and Karsenti E.. 2001. Physical properties determining self-organization of motors and microtubules. Science. 292:1167–1171 10.1126/science.1059758 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Takasu E., Aigaki T., Kato K., Hayashi S., and Nose A.. 2004. Formin3 is required for assembly of the F-actin structure that mediates tracheal fusion in Drosophila. Dev. Biol. 274:413–425 10.1016/j.ydbio.2004.07.035 [DOI] [PubMed] [Google Scholar]
- Tokuo H., Mabuchi K., and Ikebe M.. 2007. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J. Cell Biol. 179:229–238 10.1083/jcb.200703178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Lo Presti L., Zhu Y.H., Kang M., Wu Z., Martin S.G., and Wu J.Q.. 2014. The novel proteins Rng8 and Rng9 regulate the myosin-V Myo51 during fission yeast cytokinesis. J. Cell Biol. 205:357–375 10.1083/jcb.201308146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win T.Z., Gachet Y., Mulvihill D.P., May K.M., and Hyams J.S.. 2001. Two type V myosins with non-overlapping functions in the fission yeast Schizosaccharomyces pombe: Myo52 is concerned with growth polarity and cytokinesis, Myo51 is a component of the cytokinetic actin ring. J. Cell Sci. 114:69–79. [DOI] [PubMed] [Google Scholar]
- Xue-Franzén Y., Kjaerulff S., Holmberg C., Wright A., and Nielsen O.. 2006. Genomewide identification of pheromone-targeted transcription in fission yeast. BMC Genomics. 7:303 10.1186/1471-2164-7-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ydenberg C.A., Stein R.A., and Rose M.D.. 2012. Cdc42p and Fus2p act together late in yeast cell fusion. Mol. Biol. Cell. 23:1208–1218 10.1091/mbc.E11-08-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Munteanu E.L., He J., Ursell T., Bathe M., Huang K.C., and Chang F.. 2015. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol. Biol. Cell. 26:78–90 10.1091/mbc.E14-10-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.