Abstract
Rationale: Interferon-γ release assays are used to diagnose tuberculosis infection. In developed countries, high rates of reversion following conversion have been described.
Objectives: To assess QuantiFERON TB Gold In-Tube test (QFT) conversion and reversion dynamics in a tuberculosis-endemic setting.
Methods: Adolescents aged 12–18 years residing near Cape Town were recruited. Tuberculin skin tests (TSTs) and QFTs were performed at baseline and after 2 years of follow up. Half of the participants had TST and QFT performed at additional time points. Participants were observed for incident tuberculosis disease for up to 5 years.
Measurements and Main Results: Among 5,357 participants, 2,751 (51.4%) and 2,987 (55.8%) had positive QFT and TST results, respectively, at baseline. Annualized QFT and TST conversion risks were 14.0 and 13.0%, respectively, and reversion risks were 5.1 and 4.1%, respectively. Concordance was excellent for conversions (κ = 0.74), but poor for reversions (κ = 0.12). Among recent QFT converters, the magnitude of the QFT value was strongly inversely associated with risk of reversion (P < 0.0001). When longitudinal QFT data were analyzed in a cross-sectional manner, the annual risk of infection was 7.3%, whereas inclusion of reversions in the analysis showed that the actual risk of infection was 14.0%. Incident tuberculosis was 8-fold higher among QFT reverters than in participants with all negative QFT results (1.47 vs. 0.18 cases/100 person-years, P = 0.011).
Conclusions: In this tuberculosis-endemic setting, annual risk of infection was extremely high, whereas QFT and TST conversion concordance was higher and QFT reversion rates were lower than reported in low-burden settings.
Keywords: tuberculosis, epidemiology, interferon-γ release assays, tuberculin skin tests, adolescents
At a Glance Commentary
Scientific Knowledge on the Subject
Interferon-γ release assays (IGRAs) are increasingly used in place of tuberculin skin tests for diagnosis and serial screening for latent Mycobacterium tuberculosis infection. In recent studies done in low-burden settings, researchers have reported high rates of IGRA reversion following conversion, suggestive of frequent false-positive results.
What This Study Adds to the Field
In this large, prospective cohort study among adolescents living in a South African community with a high tuberculosis burden, we found good concordance of IGRA conversions with tuberculin skin test conversions, and we also identified lower rates of reversion. However, the annual risk of infection was much higher than previously estimated in cross-sectional studies, owing to IGRA reversions. IGRAs appear to perform better as a marker of M. tuberculosis infection in high-burden settings.
Interferon-γ release assays (IGRAs) are increasingly replacing tuberculin skin tests (TSTs) for Mycobacterium tuberculosis infection in many settings, from screening of healthcare workers, to investigating tuberculosis contacts, to estimating infection rates in epidemiologic surveys (1–4). Additionally, IGRA conversion as a marker of infection is being used as a primary endpoint in a Phase II tuberculosis vaccine trial (5). Compared with TSTs, IGRAs have the advantage of requiring only a single encounter to perform the test and lack cross-reactivity with bacillus Calmette-Guérin (BCG) antigens.
However, there are increasing concerns about intraindividual variability in IGRA results and the specificity of the currently recommended threshold value (6–11). Rates of serial conversion from negative to positive IGRAs among healthcare workers in North America have recently been reported at nearly an order of magnitude higher than historical or concurrent TST conversion rates (12, 13). These were accompanied by high rates (>60%) of reversion upon subsequent testing. Researchers who have studied healthcare workers and household contacts in India have found modestly higher rates of QuantiFERON TB Gold In-Tube (QFT) (Cellestis, Chadstone, VIC, Australia) conversion among healthcare workers, using the recommended cutoff value (8, 14). Few serial data exist on QFT and TST conversions and reversions in high-burden community settings, and no data have been published on the predictive value of IGRA reversions on subsequent tuberculosis (TB) incidence. Additionally, there has been conflicting evidence on the impact of TST in boosting QFT responses; the duration of boosting is not well known, as most studies have included a short period of follow-up (6, 15–20).
We examined the dynamics of QFT and TST conversion and reversion in the context of a large, prospective, observational cohort of adolescents living in a South African community with high TB burden. Additionally, we estimated the incidence of TB disease following QFT reversion to assess the clinical significance of this phenomenon.
Methods
Study Setting
This study was conducted at schools in Worcester, South Africa, approximately 100 km from Cape Town. The population in the year of study commencement (2005) was 146,101. The TB notification rate in 2006 was approximately 1,400 cases per 100,000 population.
Study Population
From May 2005 through April 2007, students between the ages of 12 and 18 years were recruited from 11 local schools (2). We analyzed a subset (>84%) of the full study population (6,363 individuals) who had both QFT and TST data available. Data on HIV status, BCG vaccination, and exposure risks were not available for review.
Study Procedures
Demographic data were collected on all participants at study entry. Study participants had QFT and TST performed by experienced study staff upon study entry. Blood was drawn from all participants for testing by QFT, performed according to the manufacturer’s protocol. TST was performed by intradermal injection of 2 tuberculin units (RT23; Statens Serum Institut, Copenhagen, Demark), followed by measurement of induration size 48–96 hours later using a ruler or caliper. TSTs were not performed in participants with a history of active TB. In 6 of the 11 schools, representing approximately half (n = 3,236) of the study population, participants underwent intensive QFT and TST surveillance. For these participants, visits were performed every 3 months to assess for incident TB disease; QFTs were performed every 6 months; and TSTs were repeated yearly. Additionally, in the intensive surveillance arm, participants with a TST of less than 5-mm induration at baseline had a repeat TST at 90 days. All participants had QFTs and TSTs performed 2 years after enrollment. A subset of participants with negative QFTs at baseline were followed for incident TB for up to 3 years after the last QFT (5 years in total) at biannual study visits and through passive surveillance of health facility records (1).
At follow-up visits, participants with new symptoms or a new household contact compared with baseline, or with TST or QFT conversion, were investigated for active TB. In addition, passive surveillance was conducted using TB clinic and hospital admission registers in the area for any TB cases diagnosed between visits. Investigation for TB involved collection of two sputum samples for smear examination on separate occasions. For participants with at least one positive smear, a culture was performed; a chest X-ray was done; and an HIV test was offered.
Following South African guidelines, participants with positive TSTs or QFTs were not provided isoniazid, which is recommended only for individuals who are HIV-infected or younger than 5 years of age (21), owing to reinfection rates and concern about lack of sustained benefit.
Outcome Definitions
We used a TST cutoff of greater than or equal to 5 mm to define a positive test, based on the observed distribution of TST indurations, as described previously (3). We defined a positive QFT as greater than or equal to 0.35 IU/ml, as per the manufacturer’s instructions. We excluded indeterminate results, which were rare (0.2%). Conversion by QFT or TST was defined as having a positive test that followed a negative test, and reversion was defined as having a negative test that followed a positive test. TB disease cases were defined by having two or more positive sputum smears, or one positive sputum culture for M. tuberculosis, or a physician’s diagnosis based upon chest X-ray results and clinical symptoms.
Analysis
We compared correlations of paired, cross-sectional TST and QFT results by Cohen’s κ for dichotomized results and Spearman’s rank correlation coefficient. We also compared dichotomous conversion and reversion events by Cohen’s κ. To test for unimodality and multimodality in TST and QFT data, we used Silverman’s test, which is based on nonparametric kernel estimation (22). The P value is given by the proportion of bootstrap samples in which the kernel density estimator, using a minimum bandwidth for k modes in the full sample, has more than k modes. We calculated the annual risk of infection by converting the QFT conversion proportion between 0 and 720 days to an annualized risk. For comparison, risk was calculated separately by treating the QFT results as repeated, independent cross-sectional surveys 2 years apart. We compared QFT reversion probability following conversion, according to quartile of QFT result, using a χ2 test for trend in proportions. To evaluate for possible boosting of QFT by TST administration, which was performed at days 0 and 360, we compared QFT conversion rates between Days 0 and 180 or between Days 360 and 540 with those between Days 180 and 360 or Days 540 and 720, using a two-sample test for equality of proportions.
We calculated TB disease incidence in cases per 100 person-years for the following groups: (1) negative QFTs at all time points (“persistent QFT-negative”), (2) positive QFTs at all time points (“persistent QFT-positive”), (3) after QFT conversion, and (4) after QFT reversion. Not all participants fell into one of these groups (e.g., incident TB diagnosed before QFT conversion or missing one test result). We calculated 95% exact Poisson confidence intervals (CIs) around these estimates and compared TB disease incidence rates by using a two-sample Poisson test. Analyses were performed using R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical Approval
This study was approved by the Human Research Ethics Committee of the University of Cape Town (Reference 045/2005). Written informed consent was obtained from participants aged 18 years and older; among participants under the age of 18, written informed consent was obtained from their parents, and assent was obtained from these participants.
Results
Baseline Results and Agreement
We first examined concordance of baseline TST and QFT. At enrollment, 5,357 participants (median age: 15 yr, interquartile range: 14–16) had results for TSTs and QFTs. Among this group, 2,987 (55.8%) had positive TSTs and 2,751 (51.4%) had positive QFTs (Table 1). Indeterminate results occurred in only 13 (0.2%) QFT tests. Correlation between dichotomous classification of results by QFT and TST was good (κ = 0.70; 95% CI: 0.68–0.72), as previously noted (3), and rank ordering of quantitative results was also good (ρ = 0.72). Among the 1,146 participants in the intensive surveillance group who had a negative TST at baseline, 196 (17.1%) had a positive response when TST was repeated at 90 days. When considering TST positivity to be defined by a positive result on either the day 0 or day 90 TST, the TST positivity was higher (60.6%) and correlation with QFT was slightly lower (κ = 0.68; 95% CI: 0.66–0.70). Among those participants who were TST-positive only after boosting at day 90, 26% had a negative TST result when the test was repeated at day 360.
Table 1.
Comparison of Baseline Results of Tuberculin Skin Test and QuantiFERON
| Baseline Results | TST-Negative [n (%)] | TST-Positive [n (%)] | Total [n (%)] |
|---|---|---|---|
| QFT-negative | 2,079 (39) | 517 (10) | 2,596 (48) |
| QFT-positive | 291 (5) | 2,470 (46) | 2,761 (52) |
| Total | 2,370 (44) | 2,987 (56) | 5,357 |
| Cohen’s κ | 0.70 | ||
| Spearman’s ρ | 0.72 |
Definition of abbreviations: QFT =QuantiFERON TB Gold In-Tube test; TST = tuberculin skin test.
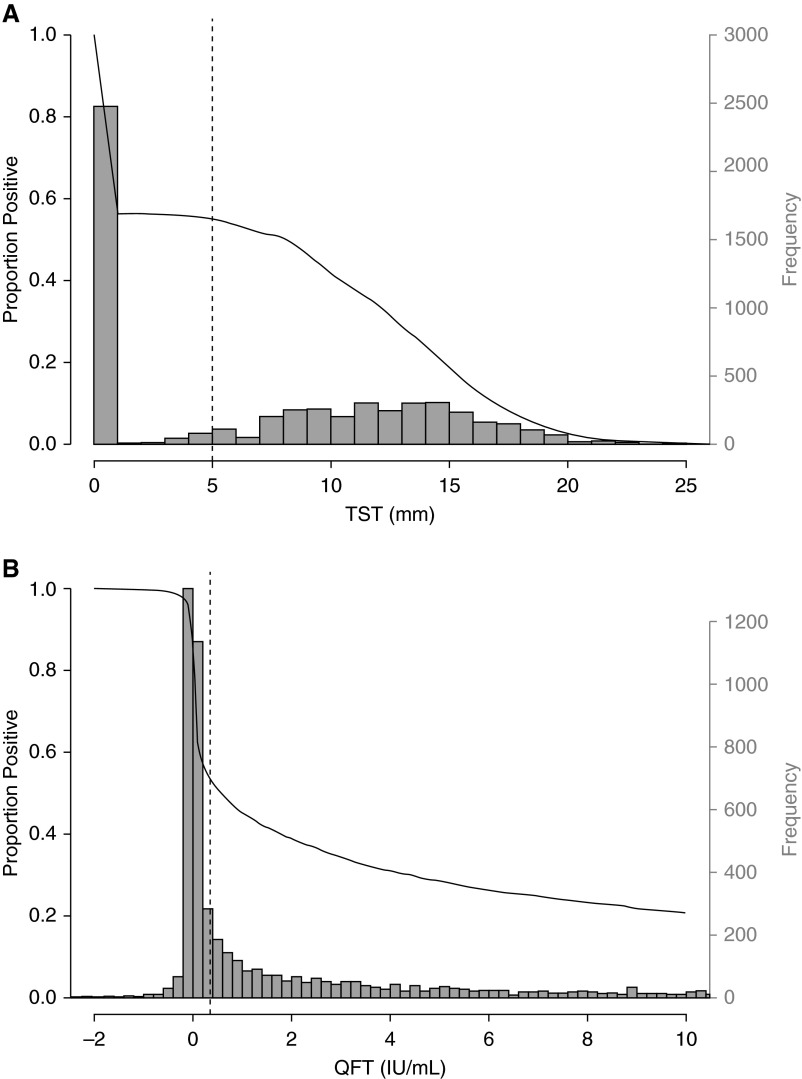
To evaluate the classification of test results from continuous into dichotomous variables, we first assessed modality in the TST and QFT data using kernel density testing. Unimodality was rejected for TST (P < 0.0001), but not for QFT (P = 0.10). More than two modes were not demonstrated in the TST data (P = 0.16). TST positivity was fairly robust around the threshold of 5 mm, used for this study. By contrast, for QFT, positivity was highly sensitive to changes in the threshold around the manufacturer’s recommended cutoff of 0.35 IU/ml (Figure 1).
Figure 1.
Distribution of baseline tuberculin skin test (TST) results (A) and QuantiFERON TB Gold In-Tube test (QFT) results (B) (histograms, right vertical axis) and proportion with a positive test result (black line, left vertical axis) as threshold varies. Vertical dashed lines indicate the positivity criteria used in this study (TST ≥ 5 mm; QFT ≥ 0.35 IU/ml).
Conversions and Reversions
We next examined conversion and reversion risks and their concordance between tests. Comparing days 0 and 720, to avoid a potential boosting effect of recent TST administration on QFT conversion, 26.1% (572 of 2,190) of participants had QFT conversion and 24.4% (441 of 1,809) had TST conversion, with annual risks of conversion of 14.0 and 13.0%, respectively (Table 2). QFT and TST conversion risks were similar in the intensive and nonintensive surveillance cohorts (QFT, 14.5% vs. 13.6%; TST, 12.3% vs. 13.6%). The cumulative reversion incidence rates for QFT and TST were 9.9% (259 of 2,614) and 8.1% (208 of 2,583), respectively (annualized risks of 5.1 and 4.1%, respectively). The annual risk of infection estimated by treating the QFT data as repeated cross-sectional surveys, which ignores reversions, was 7.3% (95% CI: 5.2–9.2%).
Table 2.
Annual Risk of QuantiFERON and Tuberculin Skin Test Conversion and Reversion Risks by Study Surveillance Group
| QFT | TST | Cohen’s κ* | |
|---|---|---|---|
| Nonintensive group | |||
| Conversion risk | 13.6% | 13.6% | 0.72 |
| Reversion risk | 4.3% | 4.5% | 0.14 |
| Intensive group† | |||
| Conversion risk | 14.5% | 12.3% | 0.76 |
| Reversion risk | 5.7% | 3.8% | 0.12 |
| Combined | |||
| Conversion risk | 14.0% | 13.0% | 0.74 |
| Reversion risk | 5.1% | 4.1% | 0.12 |
Definition of abbreviations: QFT = QuantiFERON TB Gold In-Tube test; TST = tuberculin skin test.
Cohen’s κ was calculated by comparing Day 720 QFT and TST results among participants with concordant results at baseline.
Conversion and reversion risks in the intensive surveillance group were calculated using a two-step TST, with negative results at Days 0 and 90 considered negative. When considering Day 0 results only, the TST conversion and reversion risks were 17.9 and 2.8%, respectively, and the respective κ values were lower at 0.69 and 0.08.
Among participants with negative TSTs and QFTs at baseline, concordance of conversion events, by Day 720 TST and QFT, was excellent (κ = 0.74, 95% CI: 0.70–0.78). When we used alternative definitions for QFT conversion (≥0.70 IU/ml; ≥0.35 plus 30% increase from baseline) or TST (10 mm increase; ≥10 mm plus ≥6 mm increase), all results showed slightly decreased concordance (data not shown).
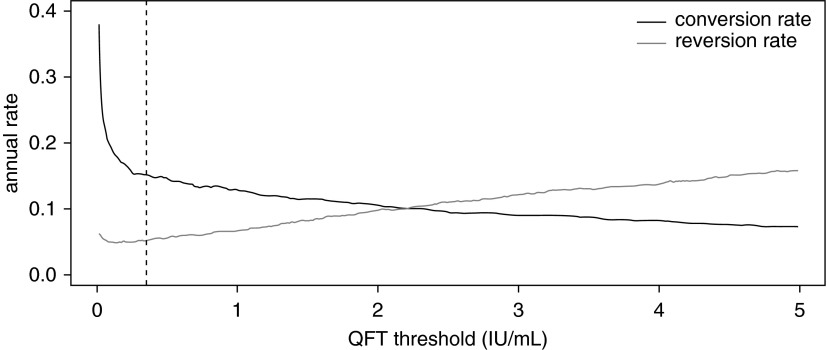
Among participants with positive TSTs and QFTs at baseline, concordance of reversion events at Day 720 was poor (κ = 0.12; 95% CI: 0.06–0.19). There were 55 participants who had negative QFTs and TSTs at Day 0 and converted both tests to positive by Day 360. Among these participants, 9.1% (5 of 55) had reverted QFTs and 1.9% (1 of 54) had reverted TSTs on Day 720. Increasing the QFT cutoff criteria resulted in a moderate decrease in conversion rates, with a slightly greater increase in reversion rates (Figure 2).
Figure 2.
Annual QuantiFERON TB Gold In-Tube test (QFT) conversion and reversion rates according to QFT threshold. Vertical dashed line reflects the manufacturer’s recommended threshold (≥0.35 IU/ml). At values of less than 0.35 IU/ml, conversions decreased precipitously as QFT threshold increased; however, this sensitivity leveled off at values of more than 0.35 IU/ml. By contrast, reversion rates gradually increased with increasing QFT threshold.
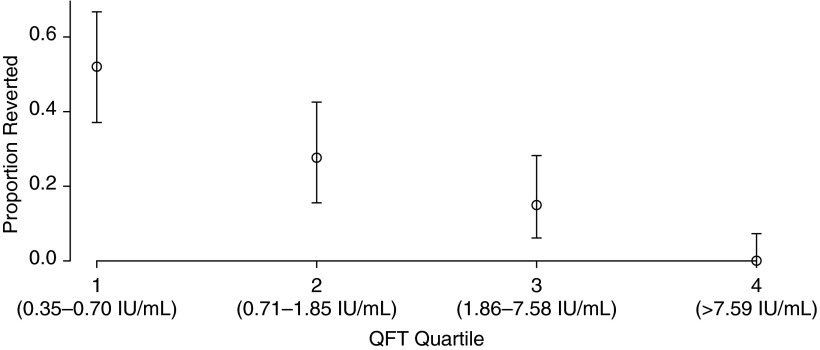
Overall, 23.7% of participants with converted QFTs between Days 0 and 360 had reverted by Day 720. The risk of reversion following conversion was strongly inversely associated with the quantitative value of the conversion QFT (Figure 3). Fifty-two percent of participants in the lowest quartile (QFT, 0.35–0.70 IU/ml) reverted, compared with 27.7% of participants in the second quartile (QFT, 0.71–1.85 IU/ml), 14.9% in the third quartile (QFT, 1.86–7.58 IU/ml), and 0.0% of those in the highest quartile (QFT, >7.59 IU/ml) (P < 0.0001).
Figure 3.
Probability of reversion at 720 days among participants with QuantiFERON TB Gold In-Tube test (QFT) conversion at 360 days, according to QFT quartile at 360 days. The probability of reversion decreased from 52% in the lowest QFT quartile to 0% in the highest quartile (P < 0.0001).
Boosting of QFT by TST in M. tuberculosis–infected Participants
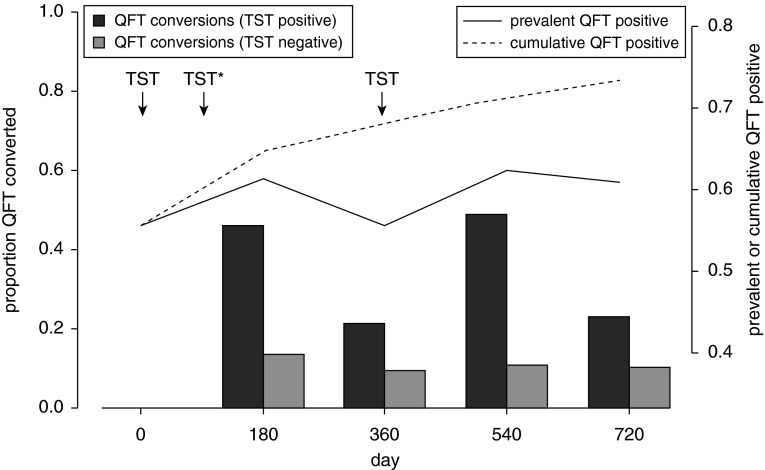
Among participants in the intensive group, who had annual TSTs and biannual QFTs (performed on the same day), we compared QFT conversion rates 180 days after TST (i.e., between Days 0 and 180 and between Days 360 and 540) with those 360 days after TST (i.e., between Days 180 and 360 and between Days 540 and 720). We hypothesized that QFT positivity would be higher at 180 days compared with 360 days after TST administration if TST boosted QFT response. Among participants with a baseline positive TST, QFT conversion rates were significantly increased 180 days after TST (47.5%) compared with the interval between 180 and 360 days after QFT (22.1%) (P < 0.0001) (Figure 4). By contrast, there was no difference in QFT conversion between 0 and 180 days (12.2%), compared with between 180 and 360 days (9.9%), following TST among those with baseline negative TSTs (P = 0.053). This is despite the fact that those with baseline negative TSTs received an additional TST at day 90. Because of the high rates of QFT conversions, followed by reversions, among participants with a baseline positive TST, the total cross-sectional prevalence of positive QFT was higher at Days 180 and 540 compared with Days 0, 360, and 720 (Figure 4). Quantitative QFT values were higher 180 days after TST, compared with 360 days after TST, in participants with negative (P = 0.0002 by Wilcoxon signed-rank test) or positive (P < 10−10) TSTs at baseline, and the difference was greater among those with a positive TST at baseline.
Figure 4.
QuantiFERON TB Gold In-Tube test (QFT) conversions by time and baseline tuberculin skin test (TST) status (left y-axis), along with the cross-sectional and cumulative prevalence of positive QFT (right y-axis), among participants with data available for all five time points. TSTs (arrows) were administered at baseline, Day 90, and Day 180; only participants with negative baseline TST had a TST performed at Day 90, as indicated by the asterisk. QFT conversions were much higher in the 180 days following TST conversion (Days 0 and 540) but only in the TST-positive group. The high rates of reversions led to divergence of the cross-sectional prevalence and cumulative positivity of QFT.
Incident Tuberculosis
Ninety-six cases of TB disease were diagnosed during the total study period, for an overall rate of 0.71 per 100 person-years. TB incidence was lower in those with all negative QFT tests (0.18 cases/100 person-years) than in those with all positive QFT tests (1.05 cases/100 person-years) (P < 0.0001) (Table 3). Compared with participants with all negative QFTs, TB incidence was higher following conversion (1.39 cases/100 person-years) (P < 0.0001). However, TB incidence following conversion was not significantly higher than among those with all positive (nonconversion) QFTs throughout the study period (P = 0.359). TB incidence was more than 8-fold higher among participants with reverted QFTs (1.47 cases/100 person-years) than among those with persistently negative QFTs (P = 0.011), though the numbers of cases were small in both groups. Incident TB rates were similar among participants with all negative TSTs (0.09 cases/100 person-years), all negative QFTs (0.18 cases/100 person-years), and all negative results by both tests (0.07 cases/100 person-years). Baseline TST positivity (0.89 cases/100 person-years) and TST conversion (1.07 cases/100 person-years) were associated with similar incidence-to-baseline QFT positivity and conversion.
Table 3.
Tuberculosis Incidence among Participants According to QuantiFERON Status
| Events (n) | Observation Time (Person-Years) | Rate/100 Person-Years | 95% CI | |
|---|---|---|---|---|
| QFT | ||||
| All negative QFT | 7 | 3,994.6 | 0.18 | 0.07–0.36 |
| All positive QFT | 46 | 4,371.7 | 1.05 | 0.77–1.40 |
| Baseline QFT positive | 57 | 5,854.7 | 0.97 | 0.74–1.26 |
| QFT conversion | 17 | 1,223.1 | 1.39 | 0.81–2.22 |
| QFT reversion | 3 | 203.9 | 1.47 | 0.30–4.30 |
| TST | ||||
| All negative TST | 3 | 3,502.5 | 0.09 | 0.01–0.25 |
| Baseline positive TST* | 58 | 6,519.1 | 0.89 | 0.67–1.15 |
| TST conversion | 7 | 654.3 | 1.07 | 0.43–2.22 |
| TST reversion | 0 | 235.7 | 0.00 | 0.00–1.58 |
| Both | ||||
| All negative QFT/TST | 2 | 2,827.2 | 0.07 | 0.01–0.26 |
| Baseline QFT/TST positive | 47 | 4,642.3 | 1.01 | 0.74–1.35 |
| QFT/TST conversion | 7 | 525.9 | 1.33 | 0.53–2.76 |
Definition of abbreviations: CI = confidence interval; QFT = QuantiFERON-TB Gold In-Tube test; TST = tuberculin skin test.
Participants who developed active tuberculosis did not have TST subsequently performed, and therefore an unbiased all-positive TST group could not be assessed.
Discussion
Although there have been increasing concerns among healthcare workers in low-burden countries about the specificity of repeated QFT testing, there have been few longitudinal data reported from high-burden communities and no data on the clinical implications of QFT reversions. In this large, prospective adolescent cohort, we found extremely high rates of QFT and TST conversions—more than twice estimates from cross-sectional surveys of other communities in the province (23)—with good concordance for conversion between the tests. However, QFT reversion occurred in 23.7% of those who converted between Days 0 and 360, and the magnitude of the QFT value at conversion was a strong negative predictor of the risk of reversion. It is apparent that, in assessments that rely solely on cross-sectional prevalence surveys, the QFT reversion phenomenon obscures the true rate of TB transmission among adolescents.
These results add to a growing literature on the nuances of interpreting IGRAs, particularly when used in serial surveillance. Whereas early cross-sectional studies suggested excellent specificity in individuals at low risk for TB exposure (24), more recent work has raised concerns about their specificity in serial screening for incident infections. In particular, high rates of reversions have been observed following conversions in studies among healthcare workers in low-burden countries (8, 12, 13, 25). Compared with these studies, we found (1) much higher rates of QFT and TST conversion, (2) lower rates of reversion following a QFT conversion event (23.7% vs. >60% [13, 25, 26]), and (3) better concordance of TSTs and QFTs for conversions (25). These findings are consistent with the idea that the predictive value of QFT conversion for incident infection should be higher in high-burden settings, owing to the higher underlying force of infection. The relative predictive value of a single positive test result, and the predictive value of QFT conversion for incident disease, in high- versus low-burden settings remain open questions (27, 28).
Additionally, we found that QFT conversion magnitude strongly and negatively predicted the risk of subsequent QFT reversion. This finding is consistent with earlier, smaller studies in which researchers found higher reversion rates among individuals with lower baseline QFTs (<3.0 IU/ml) (14). Similarly, reversion trends according to quantitative result have previously been observed with TSTs (29, 30). Of note, the QFT reversion risk was much higher (23.7%) 12 months after conversion than it was in those with baseline positive QFTs (4.5%), many of whom likely converted in the more distant past.
Despite mounting data on high rates of IGRA reversions, the clinical implications of reversion events are unclear. In the high-burden community we studied, we found TB incidence among QFT reverters to be no different than among those whose QFTs remained positive and severalfold higher than among those with persistent negative QFT results. Although the number of reverters followed was modest (n = 413), these disease incidence results suggest that transient QFT conversion is not simply the consequence of variability around the QFT assay threshold, nor does it invariably represent TB exposure with aborted infection. However, it is also possible that this group was at a higher risk of repeated exposure. The number of cases in this group was small (n = 3), and further studies are needed to verify this finding.
Additionally, sustained QFT conversion is increasingly being used as a marker in epidemiologic studies and as a TB vaccine trial endpoint, based upon the understanding that QFT conversion is a marker of incident infection (www.clinicaltrials.gov identifier: NCT02075203) (5). The findings of the present study are consistent with earlier studies in that QFT conversion is strongly associated with increased risk of incident TB, reaffirming the relevance of this endpoint (4). However, the substantial rate of QFT reversions suggests that the frequency of sampling may be extremely important in assessing the endpoint of TB infection.
These findings again evoke the question of what an IGRA result indicates about an individual’s infection status, particularly given the lack of concordance between QFT and TST reversions. It has been hypothesized that interferon-γ responsiveness may increase transiently in the setting of an exposure that does not lead to a persistent infection (31, 32), but there are numerous other potential sources of variability between test results in the same individual, such as manufacturing issues (33), specimen processing and analytic variability (34–37), and host immunological variability over time. These sources of variability have been reviewed in detail elsewhere (4). Efforts were made in this study to standardize the protocol and minimize sources of variability in specimen processing and analysis. It remains clear that QFT is more accurate as a marker of M. tuberculosis infection than as a predictive tool for incident TB disease, given the low incidence of TB among infected individuals, which results in a low positive predictive value for infection-based diagnostics.
The bimodal distribution of TSTs in this study, and the infrequency of TST values between 0 and 5 mm, supported the use of a 5-mm threshold. Increasing the threshold to 10 mm had a modest effect on positivity and conversion rates (while reducing concordance with QFT) (3). By contrast, there were no inherent thresholds in the distribution of baseline QFT results, and small changes in the threshold had a substantial impact on positivity. These findings are consistent with other studies in which investigators have questioned the robustness of the manufacturer’s recommended cutoff of greater than or equal to 0.35 IU/ml (14). Larger studies or meta-analyses are needed to determine an optimal cutoff for QFT as a predictor of incident TB.
We also found evidence of boosting of QFT responses by recent tuberculin administration. We observed significantly higher QFT conversion rates at 180 days after TST compared with 180 to 360 days after TST, but this finding was true only for participants with a baseline positive TST. Because cohorts were enrolled throughout the year and the effect occurred only in the TST positive group, we do not believe that seasonality of infection explains these results. Boosting of IGRA response has been described following administration of TST (6, 15–20, 25); however, the duration of effect of boosting is unknown, as most studies had a short duration of follow-up. The findings of this study suggest that the effect of QFT boosting by TST, evident at 180 days, wanes substantially by 360 days. This finding may be important in informing the planning of vaccine trials in which TST and QFT are both performed. We note that the main results of this study pertaining to conversion and reversion rates, concordance, and TB incidence, were based on the Days 360 and 720 tests and should therefore be unaffected by boosting.
Although the QFT results are subject to these potential sources of variability, we also found extremely high rates of TST conversions (13.0%). To minimize the impact of boosting on conversion estimates, a two-stage TST procedure was utilized, and participants were excluded from conversion calculations if the TST was positive at Day 0 or Day 90. Most studies done in South Africa have estimated the annual risk of infection from cross-sectional surveys using latent TB infection prevalence by age, without accounting for TST reversions (35, 38, 39). In earlier studies, researchers observed that this could lead to substantial underestimation of the annual risk of infection (40, 41). In a recent study in another community near Cape Town, investigators estimated an annual risk of infection of 7.9% among 15-year-olds using this approach (39), which is compatible with the estimate of 7.3% in this study by treating the data as cross-sectional. We believe cross-sectional studies are underestimating true rates of infection.
The results of this study should be interpreted within the context of the limitations of the available data. First, data on the HIV status of cohort participants were not available, except for those who developed active TB (among whom only 1 of 96 participants was known to have HIV infection). Undiagnosed HIV infection could have impacted the sensitivity of the TST and QFT results or the relationship between TST and QFT conversions or reversions and subsequent TB risk, but, given the overall low prevalence among cases, it is unlikely to have been a major factor in this study. Second, we did not have comprehensive data for participants regarding household or classroom exposures, which would be useful as an indicator of infection risk. Next, even in this large cohort, we were unable to compare TB incidence among participants with a “transient” versus a “durable” QFT conversion, because the number of disease events was too small. Additionally, data on prior BCG vaccine exposure were incomplete and precluded analysis. BCG vaccination was introduced in South Africa for administration at birth two decades prior to this birth cohort, and coverage is estimated at 98% for the Western Cape province (42). Finally, these results were derived from an age-restricted population in a single community and may not be generalizable to other high-burden settings. By adolescence in South Africa, more than half of individuals are infected with M. tuberculosis, making unbiased measurements of infection in older age groups difficult. Nevertheless, this age group may have higher social contact rates and infection risks than other groups (43).
Overall, the findings of this study suggest that QFT performed reasonably well in a South African community with a high TB burden in that (1) concordance of conversions by QFT and TST was good; (2) reversion rates were comparable to those of TST and lower than those observed in low-burden settings; and (3) conversions were strongly predictive of incident TB. These first two findings appear to be in contrast to those of recent studies among healthcare workers in low-burden settings, which is likely due to the differences in predictive value between these high- and low-risk populations. The latter results have led to some skepticism about the validity of QFT as a marker of incident infections in low-burden countries. By contrast, these longitudinal data from a TB-endemic country suggest not only that QFT performs well as a test of M. tuberculosis infection but also that QFT conversion rates are consistent with an annual risk of infection much greater than previously suspected.
Footnotes
Supported by Aeras and the Gates Grand Challenge 6 and Gates Grand Challenge 12 grants for the QuantiFERON testing. The funders had no role in data collection and analysis, the decision to publish, or the preparation of the manuscript. The funders (Aeras) were involved in the study design and the design of the data collection forms. J.R.A. is supported by a grant from the National Institute of Allergy and Infectious Diseases (K01 AI104411).
Author Contributions: J.R.A., M.H., R.W., and T.J.S. conceived of the experiments; J.R.A., M.C., and T.R.H. performed analyses; J.R.A., M.H., H.M., W.A.H., M.C., T.R.H., R.W., and T.J.S. interpreted analytic results; and J.R.A., M.H., H.M., W.A.H., M.C., T.R.H., R.W., and T.J.S. contributed to the writing of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201409-1704OC on January 7, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Machingaidze S, Verver S, Mulenga H, Abrahams DA, Hatherill M, Hanekom W, Hussey GD, Mahomed H. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am J Respir Crit Care Med. 2012;186:1051–1056. doi: 10.1164/rccm.201206-1134OC. [DOI] [PubMed] [Google Scholar]
- 2.Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, Ehrlich R, Hanekom WA, Hussey GD. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6:e17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams DA, Ehrlich R, Hanekom WA, Hussey GD SATVI Adolescent Study Team. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis. 2011;15:331–336. [PubMed] [Google Scholar]
- 4.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ClinicalTrials.govA randomized, placebo controlled, partially blinded phase II study to evaluate safety, immunogenicity, and prevention of infection with Mycobacterium tuberculosis of AERAS-404 and BCG revaccination in healthy adolescents (040-404) [accessed 2014 Apr 30]. Available from: http://clinicaltrials.gov/ct2/show/NCT02075203
- 6.van Zyl-Smit RN, Pai M, Peprah K, Meldau R, Kieck J, Juritz J, Badri M, Zumla A, Sechi LA, Bateman ED, et al. Within-subject variability and boosting of T-cell interferon-γ responses after tuberculin skin testing. Am J Respir Crit Care Med. 2009;180:49–58. doi: 10.1164/rccm.200811-1704OC. [DOI] [PubMed] [Google Scholar]
- 7.Veerapathran A, Joshi R, Goswami K, Dogra S, Moodie EE, Reddy MV, Kalantri S, Schwartzman K, Behr MA, Menzies D, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One. 2008;3:e1850. doi: 10.1371/journal.pone.0001850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Kalantri S, Reingold AL, Colford JM, Jr, Riley LW, Menzies D. Serial testing of health care workers for tuberculosis using interferon-γ assay. Am J Respir Crit Care Med. 2006;174:349–355. doi: 10.1164/rccm.200604-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwerling A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, Schwartzman K, Benedetti A, Dendukuri N, Menzies D, Pai M. TB screening in Canadian health care workers using interferon-γ release assays. PLoS One. 2012;7:e43014. doi: 10.1371/journal.pone.0043014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaur RL, Pai M, Banaei N. Impact of blood volume, tube shaking, and incubation time on reproducibility of QuantiFERON-TB Gold In-Tube assay. J Clin Microbiol. 2013;51:3521–3526. doi: 10.1128/JCM.01627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB Gold In-Tube assay in clinical practice. Am J Respir Crit Care Med. 2013;187:206–211. doi: 10.1164/rccm.201203-0430OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwerling A, Benedetti A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, Schwartzman K, Menzies D, Pai M. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PLoS One. 2013;8:e54748. doi: 10.1371/journal.pone.0054748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med. 2013;188:1005–1010. doi: 10.1164/rccm.201305-0831OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai M, Joshi R, Dogra S, Zwerling AA, Gajalakshmi D, Goswami K, Reddy MV, Kalantri A, Hill PC, Menzies D, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis. 2009;13:84–92. [PMC free article] [PubMed] [Google Scholar]
- 15.Naseer A, Naqvi S, Kampmann B. Evidence for boosting Mycobacterium tuberculosis-specific IFN-γ responses at 6 weeks following tuberculin skin testing. Eur Respir J. 2007;29:1282–1283. doi: 10.1183/09031936.00017807. [DOI] [PubMed] [Google Scholar]
- 16.Vilaplana C, Ruiz-Manzano J, Gil O, Cuchillo F, Montané E, Singh M, Spallek R, Ausina V, Cardona PJ. The tuberculin skin test increases the responses measured by T cell interferon-γ release assays. Scand J Immunol. 2008;67:610–617. doi: 10.1111/j.1365-3083.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi JC, Shin JW, Kim JY, Park IW, Choi BW, Lee M-K. The effect of previous tuberculin skin test on the follow-up examination of whole-blood interferon-γ assay in the screening for latent tuberculosis infection. Chest. 2008;133:1415–1420. doi: 10.1378/chest.07-2193. [DOI] [PubMed] [Google Scholar]
- 18.Igari H, Watanabe A, Sato T. Booster phenomenon of QuantiFERON-TB Gold after prior intradermal PPD injection. Int J Tuberc Lung Dis. 2007;11:788–791. [PubMed] [Google Scholar]
- 19.Sauzullo I, Massetti AP, Mengoni F, Rossi R, Lichtner M, Ajassa C, Vullo V, Mastroianni CM. Influence of previous tuberculin skin test on serial IFN-γ release assays. Tuberculosis (Edinb) 2011;91:322–326. doi: 10.1016/j.tube.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Ritz N, Yau C, Connell TG, Tebruegge M, Leslie D, Curtis N. Absence of interferon-γ release assay conversion following tuberculin skin testing. Int J Tuberc Lung Dis. 2011;15:767–769. doi: 10.5588/ijtld.10.0339. [DOI] [PubMed] [Google Scholar]
- 21.Republic of South Africa Department of HealthNational tuberculosis management guidelines 2009 [accessed 2015 Jan 19]. Available from: http://familymedicine.ukzn.ac.za/Libraries/Guidelines_Protocols/TB_Guidelines_2009.sflb.ashx
- 22.Silverman BW. Using kernel density estimates to investigate multimodality. J R Stat Soc Series B Stat Methodol. 1981;43:97–99. [Google Scholar]
- 23.Middelkoop K, Bekker L-G, Liang H, Aquino LD, Sebastian E, Myer L, Wood R. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: a cross-sectional observation study. BMC Infect Dis. 2011;11:156. doi: 10.1186/1471-2334-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, Weinfurter P, Wang Y, Cronin W, Hirsch-Moverman Y, Teeter LD, et al. Tuberculosis Epidemiologic Studies Consortium. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77–87. doi: 10.1164/rccm.201302-0365OC. [DOI] [PubMed] [Google Scholar]
- 26.Joshi M, Monson TP, Joshi A, Woods GL. IFN-γ release assay conversions and reversions: challenges with serial testing in U.S. health care workers. Ann Am Thorac Soc. 2014;11:296–302. doi: 10.1513/AnnalsATS.201310-378OC. [DOI] [PubMed] [Google Scholar]
- 27.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, Fielding K, Wilkinson RJ, Pai M. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon-γ release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63–75. doi: 10.1378/chest.11-3157. [DOI] [PubMed] [Google Scholar]
- 29.Dahlstrom AW. The instability of the tuberculin reaction: observations on dispensary patients with special reference to the existence of demonstrable tuberculous lesions and the degree of exposure to tubercle bacilli. Am Rev Tuberc Pulm Dis. 1940;42:471–487. [Google Scholar]
- 30.Adams JM, Kalajan VA, Mork BO, Rosenblatt M, Rothrock WJ, O’Loughlin BJ. Reversal of tuberculin reaction in early tuberculosis. Dis Chest. 1959;35:348–356. doi: 10.1378/chest.35.4.348. [DOI] [PubMed] [Google Scholar]
- 31.Pai M, O’Brien R. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med. 2007;4:e208. doi: 10.1371/journal.pmed.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill PC, Brookes RH, Fox A, Jackson-Sillah D, Jeffries DJ, Lugos MD, Donkor SA, Adetifa IM, de Jong BC, Aiken AM, et al. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Med. 2007;4:e192. doi: 10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slater M, Parsonnet J, Banaei N. Investigation of false-positive results given by the QuantiFERON-TB Gold In-Tube assay. J Clin Microbiol. 2012;50:3105–3107. doi: 10.1128/JCM.00730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doberne D, Gaur RL, Banaei N. Preanalytical delay reduces sensitivity of QuantiFERON-TB Gold In-Tube assay for detection of latent tuberculosis infection. J Clin Microbiol. 2011;49:3061–3064. doi: 10.1128/JCM.01136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanaube K, De Haas P, Schaap A, Moyo M, Kosloff B, Devendra A, Raby E, Godfrey-Faussett P, Ayles H. Intra-assay reliability and robustness of QuantiFERON®-TB Gold In-Tube test in Zambia. Int J Tuberc Lung Dis. 2010;14:828–833. [PubMed] [Google Scholar]
- 36.Whitworth WC, Hamilton LR, Goodwin DJ, Barrera C, West KB, Racster L, Daniels LJ, Chuke SO, Campbell BH, Bohanon J, et al. Within-subject interlaboratory variability of QuantiFERON-TB Gold In-Tube tests. PLoS One. 2012;7:e43790. doi: 10.1371/journal.pone.0043790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitworth WC, Goodwin DJ, Racster L, West KB, Chuke SO, Daniels LJ, Campbell BH, Bohanon J, Jaffar AT, Drane W, et al. Variability of the QuantiFERON®-TB Gold In-Tube test using automated and manual methods. PLoS One. 2014;9:e86721. doi: 10.1371/journal.pone.0086721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kritzinger FE, den Boon S, Verver S, Enarson DA, Lombard CJ, Borgdorff MW, Gie RP, Beyers N. No decrease in annual risk of tuberculosis infection in endemic area in Cape Town, South Africa. Trop Med Int Health. 2009;14:136–142. doi: 10.1111/j.1365-3156.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 39.Wood R, Liang H, Wu H, Middelkoop K, Oni T, Rangaka MX, Wilkinson RJ, Bekker LG, Lawn SD. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–412. [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland I. The effect of tuberculin reversion upon the estimate of the annual risk of infection. Bull Int Union Tuberc. 1971;45:115–118. [PubMed] [Google Scholar]
- 41.Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–975. [PubMed] [Google Scholar]
- 42.Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG world atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012. doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis. 2014;210:597–603. doi: 10.1093/infdis/jiu138. [DOI] [PMC free article] [PubMed] [Google Scholar]