In 1989, David Strachan showed that hay fever risk varied by the number of siblings in the family, noting that the risk for hay fever decreased as the number of children in the family increased (1). This epidemiologic finding was interpreted to mean that the risk for asthma and allergies varied as a function of the number of infections: the greater the number of infections, the lower the risk for asthma and allergies. Left unstated was the assumption that infections in early life were necessary for the proper development of the immune system and the exact immunologic mechanism by which this occurred. As epidemiologists investigated the home environment, and discovered that levels of biomarkers of microbes (e.g., endotoxin) were inversely associated with atopy, some suggested that excess cleanliness was the cause of “decreased stimulation of the immune system,” and hence the cause of asthma and allergies (2).
This cursory review of the hygiene hypothesis in some of its many incarnations sets the stage for an evaluation of an article published in this issue of the Journal, by Weber and colleagues (pp. 522–529) (3), that investigates the relationships among cleanliness assessed via questionnaire, microbial markers in home house and mattress dust, and the occurrence of asthma and allergies. Using participants in the PAULA (Perinatale Asthma Umwelt Langzeit Allergie Studie) birth cohort, personal and home indices of cleanliness were related to both muramic acid and endotoxin levels in house dust and mattress, and both cleanliness and dust measures were assessed relative to asthma. Cleanliness was weakly related to muramic acid and endotoxin levels but was not related to asthma or atopic sensitization. Only higher levels of muramic acid in house dust and endotoxin in mattress dust were protective for asthma and atopic sensitization. The authors conclude that personal and home cleanliness were not risk factors for allergies or asthma.
The authors readily acknowledge some methodologic deficiencies of their study, including lack of a true longitudinal design, missing dust samples on 25% of their children, and an urban population that might limit generalizability, but in our view, none of these methodologic concerns invalidate their results. A more serious concern is the relevance of the “hygiene hypothesis,” in its current variations, to the development of asthma.
The fundamental question here is not the results of Weber and colleagues, but whether the hygiene hypothesis is a useful paradigm to explain the origins of asthma as a disease, and its increase in prevalence that began in the 1970s. We believe not, as it explains neither the rise in prevalence of other autoimmune diseases with asthma nor the susceptibility to the disease.
Susceptibility, not only exposure, is key to understanding asthma’s origins. There are two essential features of asthma as a disease: first, it is a developmental disease, with 50% of cases diagnosed by age 3 years and 90% by age 6 years (4). Second, it has two developmental phenotypes: structural abnormalities of the lung that are expressed as increased airway responsiveness and increased response to bronchodilator, and an abnormal immune/inflammatory response that is related to a Th2 immune phenotype of CD4 cells. Both of these intermediate phenotypes are present at birth, before any overt clinical infection.
Although it is clear that viral respiratory illness (predominantly respiratory syncytial virus and rhinovirus) in the first 2 years of life trigger the onset of overt clinical disease, especially in the presence of home allergens, predominantly house dust mite, the attack rates for these viruses are 100%, so all children, not just the susceptible, are infected, but it is only the susceptible that go on to manifest the asthma phenotype. Viewed in this way, finding out what leads to susceptibility is as critical as looking at exposure.
In 2004, Graham Rook put forth the “old friends and neighbors hypothesis” to counter the hygiene hypothesis, suggesting it was contact with ancient bacteria in the human gut that was critical to preventing autoimmune disease generally, not just asthma (5). Most immunologists believe humans tolerize to foreign antigens primarily through the gut microbiome (6). Although all body sites contribute to at least local tolerization, the gut microbiome is the largest collection of bacteria that interacts with the human immune system: 1014 bacteria, 1,000 species with more than 2,000–6,000 antigenic determinants/bacterium. Even small changes in this ecosystem could have major effects on human tolerization and immune system ontology.
There is emerging evidence that the fetus may be exposed to microbial elements in utero, and that these microbial elements may represent the maternal oral and gut microbiome (7). Thus, microbial stimulation of the fetal immune system may begin in utero and continue during the birth process (especially with a vaginal delivery) and postnatally, and may determine responses to antigenic stimulation in later life. Although some research is focused on how the infant gut microbiome is established, there are preliminary data from twin studies indicating that this may have, in part, a genetic basis (8) or may be a result of the priming that occurs in utero. There are also intriguing new data that the home microbiome, indirectly measured in Weber and colleagues (via endotoxin and muramic acid), may be a function of the family’s collective microbiome (9) and is transportable wherever the family moves. Thus, what we measure in home dust may be determined by the individuals living in the home. Prebirth cohort studies of the maternal and infant gut microbiome are urgently needed to confirm preliminary results, suggesting that ancient anaerobes, such as Bacteroides fragilis, are critical to normal immune tolerization.
Finally, we have written extensively about vitamin D, which we believe is a critical link between the human gut microbiome and the developing fetal lung and immune system (10). First, vitamin D deficiency is recognized worldwide, and there are data indicating that levels have decreased over time, coincident with the rise in autoimmunity and asthma (11). Next, vitamin D has effects on the developing lung (12), and we have shown that vitamin D-related developmental genes are up-regulated in early lung development and that these same genes are linked to asthma (13). Third, vitamin D has effects on a variety of immune processes and cells that are critical to normal immune functioning (14). Finally, vitamin D is critical to the function of the gut microbiome (15). It controls the development of gut-associated lymphoid tissue, trafficking between gut dendritic cells, and gut Tregs and Treg function (Figure 1). Further study of how vitamin D influences the developing immune system is urgently needed.
Figure 1.
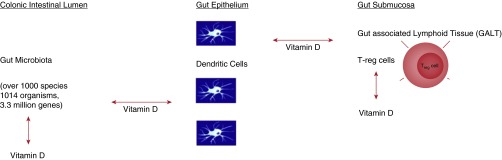
Interactions of vitamin D with the gut microbiome and the fetal immune system. Vitamin D up-regulates transforming growth factor β1 and IL-10, which will enhance Treg function and down-regulate CD4 T cells, reducing both Th1 and Th2 CD4+ cell inflammation. Vitamin D also enhances antigenic traffic between dendritic cells and Tregs. The gut microbiome is the primary source of antigen for Treg cell processing and may also influence the development of immunity in the fetus. Vitamin D controls the development of gut-associated lymphoid tissue and dendritic cell trafficking from the gut dendritic cells in the gut epithelium to the Tregs. Vitamin D may also control antigenic traffic from the gut microbiome to the gut dendritic cell and the protective organisms in the gut. Modified by permission from Reference 15.
Although it is comforting to know that cleanliness will not cause asthma, a sharper focus on scientifically based immunologic theories about how humans tolerize to antigen, the similarity of asthma to other autoimmune diseases, the role of infections in the tolerization process, and most important, a greater emphasis on fetal developmental susceptibility to asthma will lead to greater advances in understanding, and ultimately preventing, asthma specifically and autoimmunity more broadly.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013;381:861–873. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 3.Weber J, Illi S, Nowak D, Schierl R, Holst O, von Mutius E, Ege MJ. Asthma and the hygiene hypothesis: does cleanliness matter? Am J Respir Crit Care Med. 2015;191:522–529. doi: 10.1164/rccm.201410-1899OC. [DOI] [PubMed] [Google Scholar]
- 4.Yunginger JW, Reed CE, O’Connell EJ, Melton LJ, III, O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis. 1992;146:888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 5.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. 2004;25:237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- 6.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345:1048–1052. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183:1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 13.Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, Anderson C, Leeder JS, Weiss ST, Tantisira KG. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics. 2013;6:47. doi: 10.1186/1755-8794-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann EH, Chambers ES, Pfeffer PE, Hawrylowicz CM. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Ann N Y Acad Sci. 2014;1317:57–69. doi: 10.1111/nyas.12410. [DOI] [PubMed] [Google Scholar]
- 15.Weiss ST. Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic? J Allergy Clin Immunol. 2011;127:1128–1130. doi: 10.1016/j.jaci.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


