Idiopathic pulmonary fibrosis (IPF) and the related idiopathic interstitial pneumonias remain some of the most devastating disorders in medicine. As many as one in five patients with IPF report an affected family member; this clustering in families has given promise for understanding IPF’s root genetic cause with the hope of advancing its treatment. Mutations in two surfactant genes, SFTPC and SFTPA2, are found in 1 to 2% of families (1), supporting a role for epithelial dysfunction in at least this subset. In 2007, mutations in genes encoding the telomerase enzyme were found in up to 15% of families with pulmonary fibrosis (PF) (2). Telomerase has two essential components, and mutations in TERT, the telomerase reverse transcriptase, and TR, the telomerase RNA, explain the inheritance in the largest subset of cases. Mutations in the telomerase component, DKC1, and the telomere binding protein, TINF2, account for another 1 to 2% of cases (Figure 1A) (3, 4). Relevant to clinical practice, patients with telomere-mediated lung disease are at risk for syndromic comorbidities such as bone marrow failure, liver disease, and enteropathy (2). In the lung transplant setting, decreased bone marrow reserves frequently complicate the clinical course because of prolonged cytopenias related to immunosuppression (5). Even in the absence of mutations in these four genes, familial PF may be associated with short telomeres, suggesting other genes important for telomere function may explain the remaining susceptibility (2).
Figure 1.
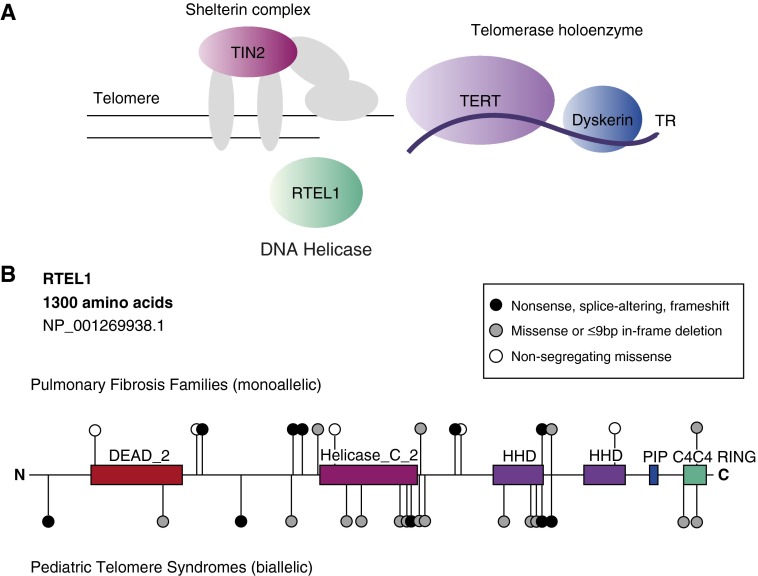
(A) Schematic depicting telomere genes found to be mutated in pulmonary fibrosis and their role at the telomere. Dyskerin is encoded by DKC1, and TIN2 is encoded by TINF2. (B) Map of regulator of telomere length 1 (RTEL1) mutations and variants relative to predicted conserved domains. The 1,300-amino-acid isoform is the longest curated isoform in the National Center for Biotechnology Information Reference Sequences (RefSeq). The variants identified in families with pulmonary fibrosis (heterozygous) are illustrated above, and those in pediatric telomere syndromes are below (biallelic). Black circles indicate changes predicted to disrupt protein stability (nonsense, splice-altering, or frameshift variants), gray circles are predicted to preserve protein stability (missense variants or short, in-frame deletions), and white circles are nonsegregating missense variants. Splice-altering and frameshift variants are positioned at the first amino acid predicted to be altered. C4C4 RING = C4C4 RING-finger motif; DEAD_2 = a conserved region within RAD3-like DNA-binding helicases; Helicase_C_2 = helicase C-terminal domain; HHD = harmonin homology domain; PIP = PCNA-interacting peptide motif; TERT = telomerase reverse transcriptase catalytic subunit; TIN2 = TRF1-interacting nuclear factor 1; TR = telomerase RNA.
In this issue of the Journal, Cogan and colleagues (pp. 646–655) add to this evolving story (6). They report mutations in the regulator of telomere length 1 gene, RTEL1, in familial PF. The authors analyzed exome sequence data from 25 families. They then used controls of convenience from the 1,000 Genome and Exome Sequencing Project databases to ensure identifying rare variants. They examined shared rare variants within families while prioritizing those in telomere genes. In one large autosomal dominant family, they identified heterozygous RTEL1 mutations that segregated with PF. Through a multi-institutional collaborative effort, they screened 163 additional cases by exome sequencing and found 4.7% of probands carried rare RTEL1 variants that also segregated with the PF phenotype. In some cases, telomere length was documented to be short, similar to telomerase mutation carriers. These findings identify RTEL1 as a fifth telomere gene in familial PF (Figure 1A).
Mutations in RTEL1 were initially identified in pediatric telomere syndromes (7–9). These children generally present with telomere-mediated disease before age 10 years and are often diagnosed with Hoyeraal-Hreidarsson syndrome, a rare disorder characterized by cerebellar hypoplasia, developmental delay, and a propensity to bone marrow failure. In contrast to the cases in the study by Cogan and colleagues, in whom heterozygous RTEL1 mutations were found, children with pediatric telomere syndromes carry two mutant alleles of RTEL1 (Figure 1B). These observations point to a dosage effect, wherein heterozygous mutations predispose to adult-onset disease, whereas biallelic mutation carriers manifest in early childhood. The dosage of RTEL1 likely causes variable degrees of telomere shortening, with the pulmonary fibrosis phenotype reflecting milder telomere defects (2). Interestingly, in earlier reports of children with Hoyeraal-Hreidarsson syndrome, there were no documented cases of lung disease in the parents. Because these parents carry heterozygous mutations similar to the ones reported in the accompanying study (Figure 1B), this observation suggests either these mutations are incompletely penetrant (i.e., not all carriers develop disease) or that these individuals may be at risk for PF as they age. More work will be needed to determine the clinical significance of heterozygous RTEL1 variants in asymptomatic individuals.
RTEL1 was first discovered as a regulator of telomere length in mice (10). It is an essential helicase related to a family of proteins that unwind G-rich DNA secondary structures. Human RTEL1 encodes four isoforms, but only the longest isoform includes all the reported mutations (Figure 1B). The most common mutations clearly disrupt RTEL1 stability (i.e., nonsense, splice-altering, frameshift). There are also missense and short in-frame deletions, but their consequences on protein function are less clear. The latter mutations fall in conserved as well as uncharacterized domains (Figure 1B). Even though RTEL1 is known to regulate telomere length, the exact mechanism by which specific mutations cause telomere shortening is the topic of ongoing research. One proposed hypothesis is that RTEL1’s helicase activity facilitates DNA replication through G-rich sequences at the telomere. Failure to resolve these secondary structures may result in the accumulation of extrachromosomal telomeric DNA known as “T-circles,” which were seen in some, but not all, mutation carriers in this study.
The work by Cogan and colleagues raises important questions. For example, are some of the patients who carry RTEL1 mutations susceptible to telomere syndrome complications such as bone marrow failure? Based on what we know so far about these disorders, one would expect that would be the case in a subset. Another important question is how to interpret the significance of RTEL1 variants in clinical settings. Clinical evaluation of TERT, TR, DKC1, and TINF2 may be offered after genetic counseling to determine disease risk. However, in contrast to these genes, RTEL1 has a highly variable sequence, and there are many rare variants in control populations that would have satisfied the filtering criteria used by Cogan and colleagues. However, as the authors found, not all rare RTEL1 variants segregate with the PF phenotype, suggesting they are likely benign and may not affect disease risk (Figure 1B). The strength of the accompanying study is that the authors tested segregation of RTEL1 variants in large, multigeneration families. Such evidence may not be readily available at the bedside when interpreting the significance of sequence changes in a single patient or in a small family. Robust functional assays and careful catalogs of nonsegregating RTEL1 variants and rare polymorphisms (such as the Telomerase Database [www.telomerase.asu.edu]) will be needed before the interpretation of RTEL1 sequence data can be fully used in a clinical setting.
Telomere dysfunction causes stem cell failure in the bone marrow and has been linked to alveolar epithelial senescence in the lung (2). It may be that the telomere-mediated PF phenotype represents a regenerative defect. A deeper understanding of this biology will be needed before genetic clues can be translated to targeted therapies. The evidence is clear, however, that telomere dysfunction underlies PF susceptibility in a sizable portion of cases. There is also evidence that telomeres will shed light on the genetics of lung disease beyond PF. Telomerase mutations were recently linked to emphysema/chronic obstructive pulmonary disease susceptibility; their frequency rivals that of α1-antitrypsin deficiency (11). The accompanying study is another step forward and represents the dedicated efforts of many families who volunteered for research with the hope they can pave a path for generations to come.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Garcia CK. Idiopathic pulmonary fibrosis: update on genetic discoveries. Proc Am Thorac Soc. 2011;8:158–162. doi: 10.1513/pats.201008-056MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest. 2013;123:996–1002. doi: 10.1172/JCI66370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, Auerbach AD, Wheelan SJ, Armanios M. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum Mutat. 2013;34:1481–1485. doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest. [online ahead of print] 24 Dec 2014; DOI: 10.1378/chest.14-1947. [DOI] [PMC free article] [PubMed]
- 5.Silhan LL, Shah PD, Chambers DC, Snyder LD, Riise GC, Wagner CL, Hellström-Lindberg E, Orens JB, Mewton JF, Danoff SK, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J. 2014;44:178–187. doi: 10.1183/09031936.00060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, Garnett ET, Montgomery KH, Mason WR, McKean DF, et al. University of Washington Center for Mendelian Genomics. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191:646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, Alter BP, Savage SA. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in dyskeratosis congenita. Hum Genet. 2013;132:473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, Carpentier W, Nitschke P, Picard C, Couillault G, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013;22:3239–3249. doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- 9.Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. Am J Hum Genet. 2013;92:448–453. doi: 10.1016/j.ajhg.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding H, Schertzer M, Wu X, Gertsenstein M, Selig S, Kammori M, Pourvali R, Poon S, Vulto I, Chavez E, et al. Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell. 2004;117:873–886. doi: 10.1016/j.cell.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Stanley SE, Chen JJ, Podlevsky JD, Alder JK, Hansel NN, Mathias RA, Qi X, Rafaels NM, Wise RA, Silverman EK, et al. Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–570. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]