Abstract
This study was conducted to investigate the life history, morphology, and maturation of larval stages and adult worms of Fasciola gigantica in experimental mice. Lymnaea auricularia rubiginosa was used as the intermediate host, and Oryza sativa was used for encystment of the metacercariae, while Mus musculus was used as the definitive host for maturation study. Fresh eggs from the gall bladder of water buffaloes fully developed into embryonated ones and hatched out at days 11-12 after incubation at about 29ºC. Free-swimming miracidia rapidly penetrated into the snail host, and gradually developed into the next larval stages; sporocyst, redia, and daughter redia with cercariae. Fully-developed cercariae were separated from the redia and shed from the snails on day 39 post-infection (PI). Free-swimming cercariae were immediately allowed to adhere to rice plants, and capsules were constructed to protect metacercariae on rice plants. Juvenile worms were detected in intestines of mice at days 3 and 6 PI, but they were found in the bile duct from day 9 PI. Juvenile and adult flukes were recovered from 16 mice experimentally infected with metacercariae, with the average recovery rate of 35.8%. Sexually mature adult flukes were recovered from day 42 PI. It could be confirmed that experimentally encysted metacercariae could infect and develop to maturity in the experimental host. The present study reports for the first time the complete life history of F. gigantica by an experimental study in Thailand. The obtained information can be used as a guide for prevention, elimination, and treatment of F. gigantica at environment and in other hosts.
Keywords: Fasciola gigantica, life history, biological characteristic, Digenea, Fasciolidae
INTRODUCTION
The giant liver fluke, Fasciola gigantica (Digenea: Fasciolidae), is important as a plant-borne trematode together with the sheep liver fluke, Fasciola hepatica. These parasites are the main cause of fascioliasis in ruminants and humans. They live in the liver of cattle, buffaloes, sheep, goats, and swines, which have a significant impact on growth rate, development, and productivity of ruminants, and therefore, are considered economically significant [1]. In some occasions, these flukes can be infected in humans, and it has been rarely reported that adult flukes have been recovered from the bile duct of people in Japan, northern Iran, and Thailand [2-4]. Most of the previous reports have focused on F. hepatica, whereas fewer studies have been conducted on F. gigantica. F. hepatica is widely distributed in temperate zones, whereas F. gigantica is typically found in tropical zones around the world [5,6].
The general life cycle of fasciolids is described in the following passage. Adults expel eggs and these are evacuated in the feces of the definitive host, usually cows or buffaloes. Miracidia are hatched from the eggs in water, and they penetrate into several lymnaeid snails (intermediate hosts), such as Lymnaea viridis, L. columella, L. cousin, L. ollula, L. natalensis, and L. auricularia rubiginosa [7-11], and then develop into the sporocysts, rediae, and cercariae. Cercariae separate from the snails and are encysted to become metacercariae on vegetation (infective stage). The metacercariae can then be found on vegetation, such as the rice plant, stubble, Japanese parsley, and water lilies [8,12]. These plants are important sources of fodder for definitive hosts. The life cycle completes itself when the definitive hosts eat vegetation containing the metacercariae.
Although studies on the life history of F. gigantica have been sometimes reported in several countries, those studies did not cover all life cycle stages. Dreyfuss and Rondeland [13] compared the productivity of infected F. gigantica and F. hepatica in Lymnaea tomentosa and found that the number of rediae of F. gigantica were substantially greater than that of F. hepatica. The patent period and the number of cercariae of both fasciolids were closely correlated with particular Lymnaea truncatula population and trematode species [14]. Moreover, Yadav and Gupta [15] reported that after infection of 2 rabbits with 50 F. gigantica metacercariae derived from L. truncatula, both rabbits died at days 83 and 87 post-infection (PI), and 8 and 10 immature adults were recovered from the liver, respectively. This aspect has not been studied in Thailand, and most reports were focused on the epidemiology and molecular detection of F. gigantica.
Fascioliasis in domestic ruminants of Thailand is mainly passed on by F. gigantica, and the adult stage has commonly recorded in cows and water buffaloes from different parts of the country. The highest prevalence of F. gigantica in cows and water buffaloes was found in the north and northeast, and the lowest incidence in the south [16,17]. However, there is sparse information on the life history of F. gigantica, especially with regard to the dynamics of the larval stage in Thailand. The larvae are transmitted to the intermediate hosts, which in many cases are vegetation, as well as to the definitive hosts. Understanding of these stages is needed to understand the epidemiology of fascioliasis in Thailand.
Thus, the present study was designed to investigate the complete life history of F. gigantica in Thailand. Previous studies have been only on 1 or 2 stages of the life history. Moreover, the previous studies focused on F. hepatica, although F. hepatica and F. gigantica are closely related species. The results can be applied for treatment, management, and control of these parasitic infections.
MATERIALS AND METHODS
Collection and culture of eggs
Fresh eggs of F. gigantica were recovered from bile of the gall bladder of water buffaloes (Bubalus bubalis) in the abattoir of Chiang Mai Province, Thailand. The eggs were washed several times with dechlorinated tap water and collected under a stereomicroscope.
A total of 1,000 F. gigantica eggs were placed in each well of multiple-well plates (6 wells) containing dechlorinated tap water, and were then incubated at room temperature (27-31˚C; av. 29˚C) under natural light to allow the development of miracidia. Notes on differentiation of the eggs were recorded daily under a stereomicroscope until miracidia hatched from the eggs.
Larval development in snail hosts
One-month-old non-parasitized snails, L. auricularia rubiginosa, were used for the experimental infection. One hundred snails were placed in 5 clay pots (20 snails/pot) with a holding capacity of 2 L of dechlorinated tap water. After then, 200 miracidia were placed in each clay pot. The exposed snails in the clay pots were supplied with fresh lettuce leaves for feeding the snails. One exposed snail was crushed daily to observe the larval stages until cercarial-shedding occurred. The cercarial shedding was investigated with the aid of a stereomicroscope.
One-month old cultured rice plants (Oryza sativa) were used for encystation of the cercariae to develop into the metacercariae. Mature cercariae were placed in rice plant pots containing dechlorinated tap water for the cercarial encystment. Rice plants were examined for the presence of metacercariae both by naked eyes and with a stereomicroscope.
Growth and development of worms in mice
Sixteen albino mice (Mus musculus domesticus) were used as the experimental definitive host. Thirty experimentally encysted metacercariae were fed to each mouse, and then they were sacrificed every 3 days post-infection (PI). Adult flukes were observed in the intestine and liver, and worm recovery and adult maturity were examined.
Ethical statements
All experimental hosts were managed according to the guidelines approved by the Animal Ethics Committee of the Faculty of Science, Chiang Mai University, and this document (no. RE 002/13) was approved by the committee. The guidelines for animal care were used according to the International Guiding Principles of Biomedical Research Involving Animals of Council for International Organizations of Medical Sciences (CIOMS). The gall bladders of the water buffaloes that were used for this study were processed as part of the work of the abattoir.
RESULTS
Experimental life cycle and biological characteristics
After incubation, the eggs developed from the unembryonate stage (Fig. 1A) to the embryonated stage (Fig. 1B) on day 3 post incubation, and then they fully developed into eggs containing miracidia (Fig. 1C). Hatching began to occur on day 11, while most eggs hatched on day 12. Fully-developed miracidia then protruded from the eggs by pushing through the operculum of the eggs (Fig. 1D).
Fig. 1.
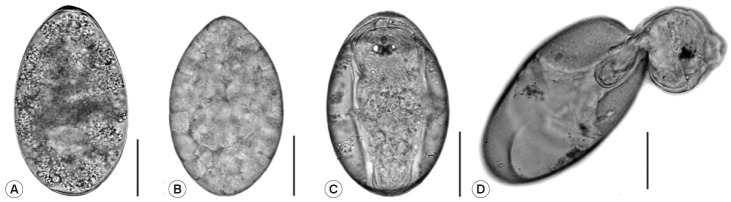
Photographs demonstrating the different stages of Fasciola gigantica eggs during the incubation period. (A) Unembryonated egg. (B) Embryonated egg. (C) Egg with fully matured miracidium. (D) Egg with escaping miracidium. Scale bar=0.05 mm.
Free-swimming miracidia encountered and penetrated the appropriate snail intermediate host (L. auricularia rubiginosa). Miracidia then attached themselves to the snails’ body via the apical papillae and lost its ciliated covering and transformed into the next stage. The miracidia that failed to find a snail host died within 24 hr. In infected snails, the miracidia transformed to the next 3 larval stages, referred to as the sporocyst, redia, and cercaria. The developmental stages in the snails are depicted in Fig. 2.
Fig. 2.
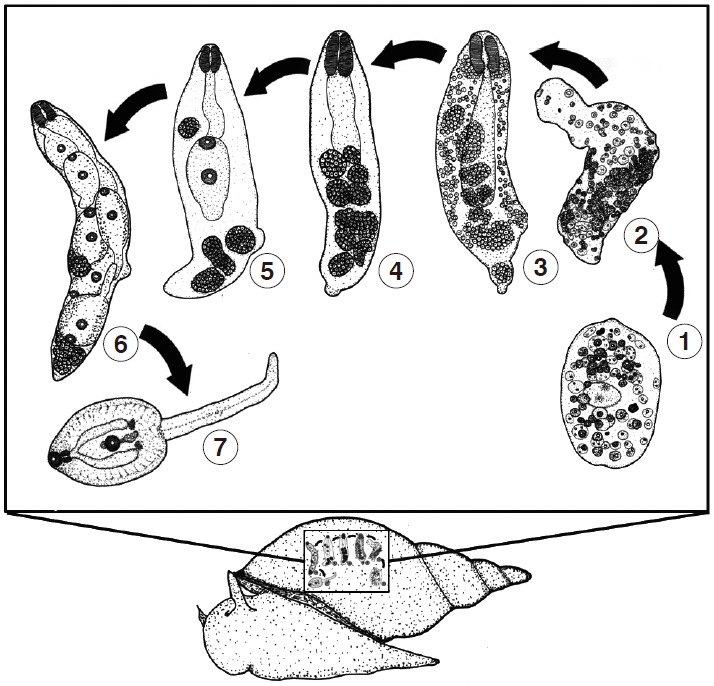
Illustration demonstrating the development of the larval stages of F. gigantica, as found in the experimental snail host, Lymnaea auricularia rubiginosa.
The miracidium lost its ciliated covering and became a young sporocyst on day 3. The young sporocyst had packed germinal cells and eyespots (no. 1 in Fig. 2). On day 7 post incubation, the young sporocyst transformed to become a mature sporocyst, redia-like in shape, but with no pharynx or primitive gut (no. 2). On day 10 post incubation, the mature sporocyst transformed to become a young redia, with the visible pharynx and primitive gut, the unique characteristics of the redia (no. 3). Each germinal cell in the sporocyst developed and formed into the germinal ball on day 14 post incubation (no. 4). Young daughter rediae and cercariae were present on day 21 (no. 5). On day 24, daughter rediae and young cercariae became fully developed (no. 6), and then the fully developed cercariae were separated from the rediae through a birth pore to the snail’s tissue and then shed to the water on day 39 post incubation (no. 7).
Active cercariae emerged from the snail and swam freely to search for the substrate for encystment. The cercariae came into contact with the rice plant or other substrate, adhered to the plant or substrate, released the outer layer of the cyst, and produced capsules to cover their bodies. At this point, the cercariae lost their tails and developed into the metacercarial stage (Fig. 3). The life cycle was completed when the metacercariae were eaten by a definitive host, such as a mammal.
Fig. 3.
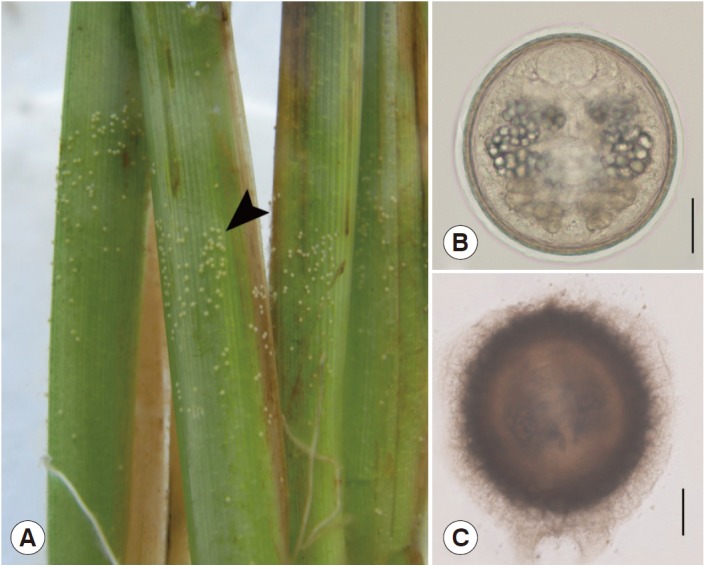
Photographs demonstrating the metacercariae of F. gigantica. (A) Metacercariae (arrowhead) adhered to the stem of the rice plant. (B) A metacercaria. (C) Capsule of a metacercaria. Scale bar=0.05 mm.
Morphology of larval stages
Eggs: The eggs are large, oval, yellowish-brown with a thin shell, and are flat and operculated. They are 0.12-0.18 (0.15) mm in length and 0.08-0.11 (0.09) mm in width. The outer surface of the eggs was smooth (Fig. 4A).
Fig. 4.
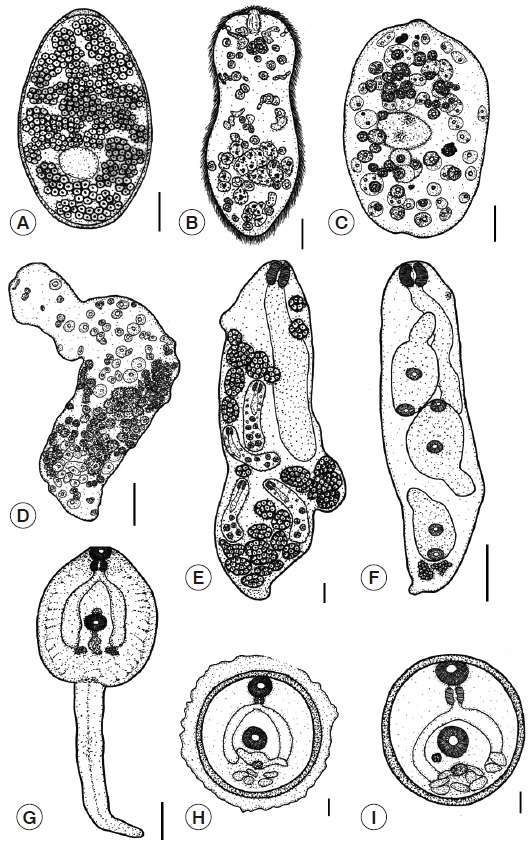
Illustration demonstrating the larval morphology of F. gigantica. (A) Egg. (B) Miracidium. (C) Young sporocyst. (D) Mature sporocyst. (E) Mother redia. (F) Daughter redia. (G) Cercaria. (H) Encapsulated metacercaria. (I) Metacercaria. Scale bars (A-D)=0.03 mm; (E-G)=0.1 mm; (H-I)=0.05 mm.
Miracidia: The body is elongated and conical and has a broad anterior part and a posterior part that tapers to a blunt end. It is 0.13-0.17 (0.15) mm long and 0.05-0.09 (0.07) mm wide. The surface is completely occupied with cilia. An apical papilla is seen in the middle of the anterior part, and there is a pair of darkly stained eyespots that are visible near the anterior part of the body. Germinal cells are scattered at the posterior segment (Fig. 4B).
Sporocysts: The young sporocyst is oval, 0.11-0.15 (0.13) mm in length and 0.10-0.11 (0.10) mm in width. The sporocyst consists initially of a minute ball of tightly packed germinal cells. At this stage, the eyespots can be seen (Fig. 4C). Each germinal cell gives rise to new germinal cells and these then multiplies to become germinal balls. After that, the bodies of mature sporocysts are elongated and formed to rediae (Fig. 4D).
Rediae: The redia is roughly cylindrical in shape, and the birth pore is located at the anterior end. The unique characteristics of this stage include 2 lateral projections at the posterior end. The redia stage consists of a mother redia and a daughter redia. The mother redia contain many daughter rediae and germinal balls (Fig. 4E), while the daughter redia contain many cercariae and germinal balls (Fig. 4F).
Cercariae: The cercaria is tadpole-like with a discoidal body and a long tail. They possess an oral sucker and a ventral sucker in the center of their bodies and have very conspicuous cystogenous glands and a forked intestine. A pharynx and prepharynx are present (Fig. 4G).
Metacercariae: The metacercariae of F. gigantica are covered with capsules, which serve to protect from environmental impacts. The diameters of the capsules range from 0.26 to 0.30 (0.28) mm (Fig. 4H). The metacercariae have a double thick wall that consists of an outer and inner cyst, which is 0.19-0.23 (0.20) mm in diameter. The cyst is white when laid and is almost immediately infective to the definitive host. After 1 or 2 days, the cyst gradually becomes yellow and darkens in color (Fig. 4I).
Growth and development of worms in mice
The morphological changes of the adult worms are shown in Fig. 5. The metacercariae excysted to become young adult worms and were then recovered in the intestine on days 3 and 6 PI, until day 9 PI when they were found in the liver of the host. The rate of parasitic incidence was 100%, and the average worm recovery rate was 35.8%. The genital pore was initially revealed on day 9 PI, while ceca were found on day 18 PI. However, the testes and ovary were discovered on day 27 PI, and they developed to maturity on day 39 PI. Immature eggs were discovered on day 42 PI, and these eggs were developed fully on day 48 PI, which indicated parasite maturation.
Fig. 5.
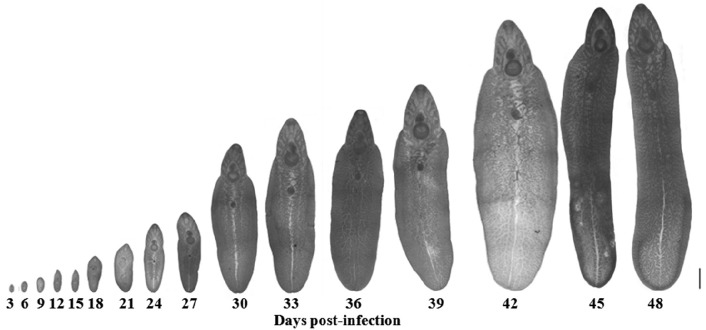
Different stages of F. gigantica recovered from albino mice. Scale bar=1 mm.
DISCUSSION
The present study is the first to report the complete life history of F. gigantica in Thailand. Our findings show that L. auricularia rubiginosa is an intermediate host of F. gigantica, which is distributed in all regions of Thailand. Moreover, the obtained metacercariae in this study developed to adults in mice, other than ruminants and humans. The pattern of the life history consisted of 7 stages involving the egg, miracidium, sporocyst, redia, cercaria, metacercaria, and adult stage, which is closely similar to the related species, F. hepatica [18].
In this study, the suitable hatching temperature ranged from 27-31˚C, at which the eggs successfully developed and hatched after 12 days, whereas the eggs of F. hepatica hatched within 2-4 weeks at the temperature range of 23-26˚C [18,19]. This indicated that the development of F. gigantica eggs in this study was more rapid than that of F. hepatica, and the high temperature may have affected the development and hatching of the eggs. A lower temperature resulted in a slower rate of development, while higher temperature results in a faster rate of development of the embryo in the eggs [18]. After the miracidia hatched, they actively swam to search for lymnaeid snail species, such as L. columella, L. cousin, L. natalensis, and L. truncatula [9,20]. The most frequently involved intermeadiate hosts were L. auricularia rubiginosa and L. natalensis [21]. In our study, L. auricularia rubiginosa were used as the experimental snail host, and miracidia successfully penetrated this snail host and developed to become sporocysts, rediae, and cercariae. When the miracidia (F. gigantica) failed to find a snail host, they died within 24 hr. This was also true for the miracidia of F. hepatica [19].
In infected snails, the larval stages of F. gigantica were completely developed, and the cercariae were separated from the snails within 42 days post incubation. This was different from the reports of Dreyfuss and Rondelaud [13] in which the cercarial shedding occurred at day 54 post incubation at 23˚C. The free-swimming cercariae contracted and successfully adhered to the rice plant or other substrate and suddenly formed the metacercarial cysts. The metacercarial encystment was suitable at high temperatures of above 24˚C [22]. The life cycle was completed when the metacercariae were eaten by a mammalian definitive host.
In this study, eggs of F. gigantica were found to be larger than those reported for F. hepatica [23]. The miracidia of F. gigantica were also bigger than those of F. hepatica [18]. The sporocyst was oval, the redia was roughly cylindrical, and the cercaria was tadpole-like with a long tail. The metacercarial cysts were protected with capsules, which had a double thick wall that consisted of an outer cyst and an inner cyst, all of which were of equal size to the cyst of F. hepatica [21].
The present study dealt with the completion of the full life history of F. gigantica using experimental hosts in the laboratory. Our findings indicate that F. gigantica can be completely developed in nature, and ruminants and humans living in Thailand may be at the risk of infection with F. gigantica.
Acknowledgments
We would like to thank Ms. Waraporn Noikong and Mr. Suksan Chuboon for their laboratory assistance. We also thank Mr. Russell Kirk Hollis for approving English grammar. Grateful thanks are extended to the Department of Biology, Faculty of Science, Chiang Mai University, Applied Technology for Biodiversity Research Unit, Institute for Science and Technology, and the Graduate School, Chiang Mai University, Thailand for provision of the facilities.
Footnotes
We have no conflict of interest related to this study.
REFERENCES
- 1.Kuchai JA, Chishti MZ, Zaki MM. Some epidemiological aspects of fascioliasis among cattle of Ladakh. Global Vet. 2011;7:342–346. [Google Scholar]
- 2.Inoue K, Kanemasa H, Inoue K, Matsumoto M, Kajita Y, Mitsufuji S, Kataoka K, Okanoue T, Yamada M, Uchikawa R, Tegoshi T, Arizono N. A case of human fasciolosis: discrepancy between egg size and genotype of Fasciola sp. Parasitol Res. 2007;100:665–667. doi: 10.1007/s00436-006-0370-1. [DOI] [PubMed] [Google Scholar]
- 3.Sharifiyazdi H, Moazeni M, Rabbani F. Molecular characterization of human Fasciola samples in Gilan province, Northern Iran on the basis of DNA sequences of ribosomal and mitochondrial DNA genes. Comp Clin Path. 2012;21:889–894. [Google Scholar]
- 4.Kanoksil W, Wattanatranon D, Wilasrusmee C, Mingphruedh S, Bunyaratvej S. Endoscopic removal of one live biliary Fasciola gigantica. J Med Assoc Thai. 2006;89:2150–2154. [PubMed] [Google Scholar]
- 5.Mage C, Bourgne H, Toullieu JM, Rondelaud D, Dreyfuss G. Fasciola hepatica and Paramphistomum daubneyi: changes in prevalences of natural infections in cattle and in Lymnaea truncatula from central France over the past 12 years. Vet Res. 2002;33:439–447. doi: 10.1051/vetres:2002030. [DOI] [PubMed] [Google Scholar]
- 6.Mas-Coma S, Barques MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Lee CG, Cho SH, Lee CY. Metacercarial production of Lymnaea viridis experimentally infected with Fasciola hepatica. Vet Parasitol. 1995;58:313–318. doi: 10.1016/0304-4017(94)00725-r. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara S, Ueno H. Ingestion of Fasciola gigantica metacercariae by the intermediate host snail, Lymnaea ollula, and infectivity of discharged metacercariae. Southeast Asian J Trop Med Public Health. 2004;35:535–539. [PubMed] [Google Scholar]
- 9.Salazar L, Estrada VE, Velásquez LE. Effect of the exposure to Fasciola hepatica (Trematoda: Digenea) on life history traits of Lymnaea cousini and Lymnaea columella (Gastropoda: Lymnaeidae) Exp Parasitol. 2006;114:77–83. doi: 10.1016/j.exppara.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Magalhães KG, Jannotti-Passos LK, Caldeira RL, Berne ME, Muller G, Carvalho OS, Lenzi HL. Isolation and detection of Fasciola hepatica DNA in Lymnaea viatrix from formalin-fixed and paraffin-embedded tissues through multiplex-PCR. Vet Parasitol. 2008;152:333–338. doi: 10.1016/j.vetpar.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Hussein AN, Khalifa RM. Experimental infections with Fasciola in snails, mice and rabbits. Parasitol Res. 2008;102:1165–1170. doi: 10.1007/s00436-008-0888-5. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JA, Copeman DB. Distribution of metacercariae of Fasciola gigantica on rice straw. Trop Anim Health Prod. 2006;38:117–119. doi: 10.1007/s11250-006-4312-9. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfuss G, Rondelaud D. Comparative studies on the productivity of Fasciola gigantica and F. hepatica sporocysts in Lymnaea tomentosa that died after a cercarial shedding or without emission. Vet Res. 1995;81:531–536. doi: 10.1007/BF00931798. [DOI] [PubMed] [Google Scholar]
- 14.Dreyfuss G, Rondelaud D. Fasciola gigantica and F. hepatica: a comparative study of some characteristics of Fasciola infection in Lymnaea truncatula infected by either of the two trematodes. Vet Res. 1997;28:123–130. [PubMed] [Google Scholar]
- 15.Yadav SC, Gupta SC. On the viability of Fasciola gigantica metacercariae ingested by Lymnaea auricularia. J. Helminthol. 1988;62:303–304. doi: 10.1017/s0022149x00011706. [DOI] [PubMed] [Google Scholar]
- 16.Sukhapesna V, Tantasuwan D, Sarataphan N, Imsup K. Economic impact of fascioliasis in buffalo production. J Med Assoc Thai. 1994;45:45–52. [Google Scholar]
- 17.Sobhon P, Anantavara S, Dangprasert T, Viyanant V, Krailas D, Upatham ES, Wanichanon C, Kusamran T. Fasciola gigantica: studies of the tegument as a basis for the developments of immunodiagnosis and vaccine. Southeast Asian J Trop Med Public Health. 1998;29:387–400. [PubMed] [Google Scholar]
- 18.Andrews SJ. The life cycle of Fasciola hepatica. In: Fasciolosis, Dalton JP, editor. Fasciolosis. Oxon, UK: CAB International; 1999. pp. 1–29. [Google Scholar]
- 19.Bowman DD. Georgis’ Parasitology for Veterinarians. 6th ed. Philadelphia, USA: WB Saunders Company; 1995. pp. 310–315. [Google Scholar]
- 20.Dinnik JA, Dinnik NN. Observations on the succession of redial generations of Fasciola gigantica Cobbold in a snail host. Z Tropenmed Parasitol. 1956;7:397–419. [PubMed] [Google Scholar]
- 21.Kaufmann J. Parasitic infections of domestic animals: a diagnosis manual. Basel, Switzerland: Birkhäuser Verlag; 1966. pp. 90–92. [Google Scholar]
- 22.Shalaby IM, Hassan MG, Soliman MFM, Sherif NE. Factors affecting dynamics of metacercarial productivity of Fasciola gigantica from its snail host. Pakistan J Biol Sci. 2004;7:393–398. [Google Scholar]
- 23.Thomas AP. The life history of the liver-fluke (Fasciola hepatica) Quart J Microscop Sci. 1883;2:99–133. [Google Scholar]


