Abstract
Background
Cellulases and xylanases are the key enzymes involved in the conversion of lignocelluloses into fermentable sugars. Western Ghat region (India) has been recognized as an active hot spot for the isolation of new microorganisms. The aim of this work was to isolate new microorganisms producing cellulases and xylanases to be applied in brewer's spent grain saccharification.
Results
93 microorganisms were isolated from Western Ghat and screened for the production of cellulase and xylanase activities. Fourteen cellulolytic and seven xylanolytic microorganisms were further screened in liquid culture. Particular attention was focused on the new isolate Bacillus amyloliquefaciens XR44A, producing xylanase activity up to 10.5 U mL−1. A novel endo-1,4-beta xylanase was identified combining zymography and proteomics and recognized as the main enzyme responsible for B. amyloliquefaciens XR44A xylanase activity. The new xylanase activity was partially characterized and its application in saccharification of brewer's spent grain, pretreated by aqueous ammonia soaking, was investigated.
Conclusion
The culture supernatant of B. amyloliquefaciens XR44A with xylanase activity allowed a recovery of around 43% xylose during brewer's spent grain saccharification, similar to the value obtained with a commercial xylanase from Trichoderma viride, and a maximum arabinose yield of 92%, around 2-fold higher than that achieved with the commercial xylanase. © 2014 The Authors. Journal of Chemical Technology & Biotechnology published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Bacillus, xylanase, brewer's spent grain, saccharification
Introduction
The conversion of lignocelluloses into fermentable sugars for fuel production is preferentially performed by enzymatic hydrolysis of polysaccharides, resulting from biomass pretreatment, with cellulases and hemicellulases.1 Filamentous fungi are the major source of cellulases and hemicellulases.2 However, bacterial cellulases and hemicellulases are more often investigated due to the advantages presented by bacteria as a source of enzymes in comparison to fungi, such as higher growth rate, the possibility to be easily engineered, and the facility to be isolated from niches naturally driving them to produce enzymes resistant to environmental stresses.3
Cellulolytic and hemicellulolytic bacteria have been isolated from a wide diversity of composts, soils and habitats,4–16 and they represent one of the main enzyme sources currently exploited for the development of lignocellulose biorefinery.3,17
Huge amounts of lignocellulosic wastes are produced annually all around the world. They include agricultural residues, food farming wastes, green-grocer's wastes, tree pruning residues, and organic and paper fractions of urban solid wastes. A wide range of high added value products, such as biofuels, organic acids, biopolymers, bioelectricity and molecules for food and pharmaceutical industries,18 can be obtained by upgrading solid wastes using biotechnological processes.
Among the lignocellulosic wastes, brewer's spent grain (BSG), the most abundant by-product generated during the beer-brewing process, has generated scientific interest for its application in energy production, due to its composition rich in cellulose and xylan.19
BSG has been exploited for ethanol production in simultaneous saccharification and fermentation using commercial enzymes belonging to the classes glucoamylase, cellulase and hemicellulase,20 and in consolidate bioprocessing, exploiting the ability of the fungus Fusarium oxysporium to concomitantly produce hydrolytic enzymes and ferment sugars into ethanol.21
In this study, the Western Ghat region of Kerala was explored for the isolation of new microorganisms with cellulolytic and xylanolytic abilities. An endo-1,4-beta xylanase was identified from the new isolate Bacillus amyloliquefaciens XR44A and recognized as the main enzyme responsible for xylanase activity. The xylanase activity produced by B. amyloliquefaciens XR44A was investigated for its ability to hydrolyse brewer's spent grain pre-treated by aqueous ammonia soaking. The new xylanase activity was shown able to yield an amount of xylose comparable with the level obtained using a commercial enzyme applied to the pre-treated BSG, in the same reaction conditions. Interestingly, application of the new xylanase activity gave a maximum arabinose yield around 2-fold higher than that achieved with the commercial enzyme.
Materials and Methods
Isolation of samples
Samples used in this study were obtained from three areas of Western Ghat region (Arikan para, Puchi, Neelikal), Kerala, India. Samples, mainly collected from humic soil, the inner part of rock, rock surface and grass land, were aseptically stored in sterilized plastic bags at 4 °C.
Isolation of bacteria
Serial dilutions (10−1–10-5) of the samples were used to inoculate Petri dishes containing LB medium (10 g L−1 bactotryptone, 10 g L−1 NaCl, and 5 g L−1 yeast extract). Plates were incubated at 37 °C for a period varying from 2 to 4 days, depending on the speed of growth of the isolates. The isolates were purified, grown in liquid media and preserved in a 20% glycerol solution at –80 °C.
Screening of bacterial isolates for cellulolytic/xylanolytic activity production on solid and liquid media
In order to determine the production of cellulases and xylanases by the isolates, pure cultures of microorganisms were individually transferred onto solid medium (NaNO3 1 g L−1, K2HPO4 1 g L−1, KCl 1 g L−1, MgSO4 0.5 g L−1, yeast extract 0.5 g L−1, glucose 1 g L−1, agar 17 g L−1) containing 5 g L−1 of carboxymethylcellulose (CMC, Sigma) or Xylan (Sigma), respectively. After incubation for 2–4 days, depending on the speed of growth of the isolates, the cellulolytic/xylanolytic strains were assayed for their ability to degrade CMC/xylan by incubation with 0.1% Congo Red solution for 30 min, followed by washing with 5 mol L−1 NaCl as reported in Amore et al.6 All the strains with a clear halo around the colonies were chosen as positive.
The liquid medium adopted for analysis of cellulase and xylanase production levels contained 1% CMC or xylan, respectively, 0.7% yeast extract, 4 g L−1 KH2PO4, 4 g L−1 Na2HPO4, 0.2 g L−1 MgSO4.7H2O, 0.001 g L−1 CaCl2.2H2O, 0.004 g L−1 FeSO4.7H2O.13
Phenotypic characterization of microbial isolates
Morphological analysis of the colony of each bacterial strain was carried out observing shape (regular/irregular/rhizoid/punctiform/filamentous), edge (entire/undulate), surface (dry/viscid/powdery), elevation (flat/raised) and color of colony.
Inoculum preparation and incubation
The bacterial strains were pre-inoculated dissolving a single colony in 3 mL of liquid medium having the composition described in the paragraph ‘Screening of bacterial isolates for cellulolytic/xylanolytic activity production on solid and liquid media’, and incubated over night at 37 °C. Fermentation was carried out in 100 mL plugged Erlenmeyer flasks, each containing 20 mL of medium, and inoculated with volumes of pre-inoculum corresponding to 0.1 OD. Fermentations were incubated at 37 °C on a rotary shaker at 125 rpm. At the 6th or 24th hour, samples of liquid cultures were withdrawn and used to measure optical density (OD600nm) and extracellular cellulase and xylanase activity. The results of the analytical determinations reported in the figures correspond to mean values of three independent experiments, each one performed in three replicates.
Azo-CMCase assay
endo-1,4-ß-glucanase activity produced in liquid or submerged culture was assayed using Azo-CMC (Megazyme, Ireland) as substrate, following supplier's instructions, and determined by referring to a standard curve.
Xylanase assay
Xylanase assay was performed according to Bailey et al.22 The reaction mixture consisting of 1.8 mL of a 1.0% (w/v) suspension of birch-wood xylan in 50 mmol L−1 Na citrate at pH 5.3 and 0.2 mL of enzyme dilution (in 50 mmol L−1 sodium citrate at pH 5.3) was incubated at 50 °C for 5 min. Released reducing sugars were determined by adding 3 mL of 3,5-dinitrosalicylic acid solution and then incubating the mixture at 95 °C for 5 min. Absorbance was measured at 540 nm. One unit of enzyme is defined as the amount of enzyme catalyzing the release of 1 µmol of xylose equivalent per min.
Arabinofuranosidase activity assay
α-L-arabinofuranosidase activity was measured spectrophotometrically with p-nitrophenyl α-L-arabinofuranoside (pNPA) (Gold Biotechnology, St Louis, MO, USA) as substrate as described in Amore et al.23 One unit of α-L-arabinofuranosidase activity was defined as the amount of enzyme releasing 1 µmol of p-nitrophenol per minute in the reaction mixture under these assay conditions.
16S rRNA gene partial sequence
Genomic DNA extraction, 16S rRNA gene amplification and sequencing of PCR amplification fragments were performed according to Rameshkumar and Nair.24 The purified PCR product was used directly for DNA sequencing, using the dideoxy chain termination method with Big Dye Terminator kit (Applied Biosystems). The reaction products were analysed using capillary electrophoresis on an ABI 310 Genetic Analyser (Applied Biosystems).
The sequences were compared with the GenBank nucleotide data library using the Blast software at the National Centre of Biotechnology Information website (http://www.ncbi.nlm.nih.gov), in order to determine their closest phylogenetic relatives.
The partial 16S rDNA sequences of the isolates selected for cellulase and xylanase activity have been submitted to EMBL, and the accession numbers are reported in Table1.
Table 1.
Similarity analyses and accession number to EMBL nucleotide sequence database of the new isolates by BLAST analyses
| Microorganism code | Percentage identity with other sequences | Accession number to EMBL nucleotide sequence database |
|---|---|---|
| Cellulolytic microorganisms | ||
| R7A | Streptomyces sp. (100%) | HG975526 |
| R10B | Bacillus sp. (100%) | HG975527 |
| R13A | Bacillus sp. (100%) | HG975528 |
| R13B | Bacillus sp. (100%) | HG975529 |
| R14B | Bacillus sp. (100%) | HG975530 |
| R21A | Bacillus sp. (100%) | HG975531 |
| R43B | Bacillus sp. (100%) | HG975532 |
| R77A | Bacillus sp. (100%) | HG975533 |
| R79A | Streptomyces griseoruber (100%) | HG975534 |
| R79C | Streptomyces costaricanus (100%) | HG975535 |
| R79A | Bacillus sp. (100%) | HG975536 |
| R103A | Bacillus sp. (100%) | HG975537 |
| R5A | Bacillus sp. (100%) | HG975538 |
| R55B | Paenibacillus sp. (100%) | HG975539 |
| Xylanolytic microorganisms | ||
| XR13A | Bacillus sp. (100%) | HG975540 |
| XR18B | Bacillus sp. (100%) | HG975541 |
| XR24A | Bacillus sp. (100%) | HG975542 |
| XR44A | Bacillus sp. (100%) | HG975543 |
| XR84A | Lysinibacillus xylanilyticus (100%) | HG975544 |
| XR84A | Bacillus sp. (100%) | HG975545 |
| XR80B | Streptomyces olivochromogenes (100%) | HG975546 |
Strain identification and deposition in culture collection
The major strain XR44A used in this study was identified at the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, GERMANY).
Determination of protein concentration
Protein concentration of crude enzyme preparation was determined by Bradford method using Biorad reactive (München, Germany) and following the procedure suggested by the supplier. Bovin serum albumin (BSA) was used to set up the standard curve.
Protein fractionation
Secreted proteins produced by the strains XR18B and XR44A were precipitated from the cultures corresponding to the maximum xylanase production by the addition of ammonium sulphate up to 80% saturation, after removing cells by centrifugation. Precipitated proteins were recovered by centrifugation at 7500 rpm for 45 min at 4 °C and brought in 20 mmol L−1 Tris-HCl pH 7, by extensive dialysis.
Zymogram analyses
Semi-denaturing gel electrophoresis was carried out loading non-denatured and not-reduced samples on a SDS polyacrylamide gel, performed as described by Laemmli.25 Proteins showing xilanolytic activity were visualized following a modified version of the method reported by Béguin.26 After electrophoresis, the gel was soaked in the same buffer used for dissolving proteins and gently shaken to remove SDS and renature the proteins in the gel. The gel was then laid on the top of a thin sheet of 1.5% agar containing 1% xylan. After 1 h incubation at 40 °C, zones of xylan hydrolysis were revealed by staining the agar replica with 0.1% Congo red.
Protein identification by mass spectrometry
SDS polyacrylamide gel (SDS-PAGE) and zymogram analyses were aligned to select bands of interest, which were then cut from the SDS-PAGE, destained by washes with 0.1 mol L−1 NH4HCO3 pH 7.5 and acetonitrile, reduced for 45 min in 100 µL of 10 mmol L−1 dithiothreitol, 0.1 mol L−1 NH4HCO3, pH 7.5, and carboxyamidomethylated for 30 min in the dark by the addition of 100 µL of 55 mmol L−1 iodoacetamide dissolved in the same buffer. Enzymatic digestion was performed by adding to each slice 100 ng of proteomic-grade trypsin (SIGMA) in 10 µL of 10 mmol L−1 NH4HCO3 pH 7.5 for 2 h at 4 °C. The buffer solution was then removed and 50 µL of 10 mmol L−1 NH4HCO3 pH 7.5 were added and incubated for 18 h at 37 °C. Peptides were extracted with 20 µL of 10 mmol L−1 NH4HCO3, 1% formic acid, 50% acetonitrile at room temperature.
Peptide mixtures were filtered on 0.22 µm cellulose acetate spin filter membranes (Agilent Technologies) and analysed by LC-MSMS on a 6520 Accurate-Mass Q-TOF LC/MS System (Agilent Technologies, Palo Alto, CA) equipped with a 1200 HPLC system and a chip cube (Agilent Technologies). After loading, the peptide mixture was first concentrated and washed in 40 nL enrichment column (Agilent Technologies chip), with 0.1% formic acid in 2% acetonitrile as the eluent. The sample was then fractionated on a C18 reverse-phase capillary column (Agilent Technologies chip) at a flow rate of 400 nL min−1, with a linear gradient of eluent B (0.1% formic acid in 95% acetonitrile) in A (0.1% formic acid in 2% acetonitrile) from 7 to 80% in 50 min. Peptides analysis was performed using data-dependent acquisition of one MS scan (mass range from 300 to 1800 m/z) followed by MS/MS scans of the five most abundant ions in each MS scan. MS/MS spectra were measured automatically when the MS signal surpassed the threshold of 50 000 counts. Double and triple charged ions were preferably isolated and fragmented. The acquired MS/MS spectra were transformed in Mascot generic format (.mgf) and used for protein identification in the unreviewed set of protein entries that are present in the NCBInr database for all bacteria, with a licensed version of MASCOT software (http://www.matrixscience.com) version 2.4.0.
Additional MASCOT search parameters were: peptide mass tolerance 10 ppm, fragment mass tolerance 0.6 Da, allowed missed cleavages up to 3, carbamidomethylation of cysteines as fixed modification, oxidation of methionine, pyro-Glu N-term Q, as variable modifications. Only doubly and triply charge ions were considered. Ions score was –10 log(P), where P is the probability that the observed match is a random event. Individual ion scores >45 indicated identity or extensive homology (P < 0.05). Protein scores were derived from ion scores as a nonprobabilistic basis for ranking protein hits (http://www.matrixscience.com/help/interpretation_help.html).
Acrylamide/bis-acrylamide, ammonium persulfate, trypsin, dithiothreitol, iodoacetamide, spin filters and NH4HCO3 were purchased from SIGMA; Temed was purchased from AllChem. Trifluoroacetic acid (TFA)-HPLC grade was from Carlo Erba (Milan, Italy). All other reagents and solvents were of the highest purity available from Baker.
Optimum temperature and thermo-resistance
Supernatant of the strain XR44A containing xylanase activity after 24 h incubation was assayed in order to study the optimum temperature and thermo-resistance.
To determine the optimum temperature, the substrate of the activity assay (birch-wood xylan) was dissolved in 50 mmol L−1 Na citrate at pH 5.3 and the incubation (5 min) was performed at 40 °C, 50 °C, 60 °C and 70 °C. The thermo-resistance of the xylanase activity was studied by incubating the supernatant of the strain XR44A containing xylanase activity in 50 mmol L−1, Na citrate at pH 5.3, at 30 °C, 40 °C, 50 °C, 60 °C and 70 °C. The samples withdrawn were assayed for residual xylanase activity as described above.
The reported results are mean values of the three independent experiments, each one performed in three replicates.
Optimum pH and pH resistance
To determine the optimum pH of XR44A xylanase activity, xylanase activity assay was performed using the substrate birch-wood xylan dissolved in 50 mmol L−1 citrate phosphate buffers,27 with pH values between 3.0 and 9.0 and performing the incubation (5 min) at 50 °C.
The pH resistance of the supernatant of the strain XR44A containing xylanase activity was studied by diluting it in citrate phosphate buffers, pH 3–9 and incubating at 25 °C. From time to time, samples were withdrawn and immediately assayed for residual xylanase activity as described above.
The reported results correspond to mean values of the three independent experiments, each one performed in three replicates.
Pretreatments and determination of chemical composition
The brewer's spent grain (BSG) provided by the micro-brewery Maneba (Striano, Naples, Italy) was subjected to pretreatment with an aqueous ammonia solution at lab-scale, following conditions described by Maurelli et al. for chestnut shell.28 The biomass reduced to a fine powder, was soaked in 5% (v/v) aqueous ammonia solution at a solid loading of 10%, then incubated at 70 °C for 22 h in screw-capped 25 mL bottles to reduce evaporation. The alkaline mixtures were centrifuged at 800 × g, and the residues were washed extensively with 50 mmol L−1 sodium acetate buffer until obtaining the requested pH value for the subsequent enzymatic saccharification process (pH 5.0).
Carbohydrate compositions of the biomass samples untreated and pretreated were determined according to the method of Davis.29 This procedure involved an acid hydrolysis carried out in two steps to fractionate the polysaccharides into their corresponding monomers. First, the samples were soaked in 72% v/v H2SO4, at a solid loading of 10%, at 30 °C, for 1 h; then the mixtures were diluted to 4% (v/v) H2SO4 with distilled water, and the secondary hydrolysis was performed for 1 h at 120 °C, after adding fucose as an internal standard. After filtration through 0.45 µm Teflon syringe filters (National Scientific, Lawrenceville, GA), the hydrolysis products were analysed via HPLC as described below. The acid insoluble lignin (Klason lignin) was determined by weighing the dried residue after total removal of the sugars.
Enzymatic hydrolysis
The saccharification experiments were carried out at 30 °C for 72 h in a total volume of 5 mL (50 mmol L−1 sodium acetate buffer pH 5.0 plus enzyme cocktail), at a solid loading of 5%. The hydrolysis of pretreated lignocellulosic materials was carried out with enzyme cocktails prepared with the following commercial and non commercial products at the indicated amounts expressed as units per gram of pretreated biomass: 5.4 U g−1 of cellulase from Trichoderma reesei ATCC26921 (Sigma), 145 U g−1 of cellobiase from Aspergillus niger (Sigma), 1.5 U g−1 of xylanase from Trichoderma viride (Sigma) or 1.5 U g−1 of xylanase from the strain XR44A isolated in this study, 0.5 U g−1 of thermostable β-xylosidase (Sigma), and 145 U g−1 of cellobiase from Aspergillus niger (Sigma). The saccharification mixtures together with blanks (pretreated lignocellulosic materials without enzyme cocktail) were incubated in a shaking ThermoMixer C (Eppendorf) at 30 °C and 800 rpm, for 72 h. Samples were withdrawn at different times, chilled on ice and centrifuged at 16 500 × g for 30 min at 4 °C. The supernatants were analysed to quantify the amount of sugars released as described below. The saccharification yield was expressed as percentage of sugar production calculated with respect to the amount of sugars present in the dry weight pretreated materials before the hydrolysis processes. Each saccharification experiment was run in triplicate.
Determination of sugar content
For estimation of the sugars released from pretreated biomasses, the cleared supernatants were opportunely diluted, and analysed by high-performance liquid chromatography (HPLC; Dionex, Sunnyvale, CA, USA) equipped with an anionic exchange column (Carbopac PA-100) and a pulsed electrochemical detector. Glucose and xylose were separated with 16 mmol L−1 sodium hydroxide at a flow rate of 0.25 mL min−1, and identified by the respective standards. Fucose was used as internal standard.
Results and Discussion
Isolation and screening of xylanolytic and cellulolytic microorganisms from Western Ghat region
The biodiversity of the Western Ghat regions of Kerala has been exploited for the isolation of new microorganisms able to produce cellulolytic and xylanolytic activities. Western Ghats region (India) has been so far described as an active biodiversity hot spot.30
Three areas of Western Ghat Region, namely Arikan Para, Puchi Para, Neelikal, were explored and 50 samples of different nature, such as humic soil, grass land, inner part of rock, rock surface, excrement, laterite, were used for isolation of bacteria and actinomycetes. 93 purified microorganisms were screened for xylanase and cellulase activity production by Congo Red assay on xylan- and CMC-containing plates, respectively. The screening led to the selection of seven xylanolytic microorganisms and 14 cellulolytic microorganisms showing activity halos on xylan-and CMC-containing plates, with a diameter greater than 5 mm. Based on morphological analyses, these microorganisms were divided into bacteria – mainly Bacillus spp. – and actinomycetes.
Sequencing of 16S rRNA genes of xylanolytic and cellulolytic isolates
The 21 microorganisms isolated and selected on solid medium were identified by 16S rRNA sequencing. Data derived from BLAST showed that the new isolated strains have high sequence similarities with different species (Table1): 15 belong to the genus Bacillus, four to the genus Streptomyces, one to the genus Lysinobacillus, and one to the genus Paenibacillus.
Screening of the isolates in liquid medium
A further screening of the seven xylanolytic and fourteen cellulolytic strains was carried out in liquid culture, using a 1% xylan- and 1% CMC-containing medium, respectively. Xylanase (Fig. 1(A)) and CMCase activity (Fig. 1(B)) were assayed after 6 and 24 h of incubation. Eight microorganisms (R5A, R9A, R10A, R10B, R13A, R13B, R14B, R21A) were identified as the best producers of the cellulase activity with values of AZO-CMCase activity ranging from 0.045 up to 0.075 U mL−1, after 6 h incubation. XR18B, XR44A, XR76A and XR108A produced the highest levels of xylanase activity, ranging from 0.4 to 0.6 UmL−1, similarly to other xylanase-producing Bacillus strains.10,13
Figure 1.

Screening of cellulolytic (A) and xylanolytic (B) strains in liquid medium.
In order to increase the level of activity production, the volume culture of the selected strains was increased to 50 mL, and the temperature and shaking rotation adjusted to 40 °C and 250 rpm, respectively. Interestingly, these conditions increased xylanase activity production to 9.4 and 10.5 U mL−1, for XR18B and XR44A, respectively.
Zymogram analyses of xylanase in different selected isolates
Proteins responsible for xylanase activity of the most productive xylanolytic strains XR18B and XR44A were analysed by fractionation on a semi-denaturing SDS-PAGE. Samples from the supernatant of the cell cultures after 6 h were loaded on SDS-PAGE without boiling them and treating with reducing agents. The resulting gel was laid over another thin gel containing 1% xylan as substrate for xylanase activity detection. SDS-PAGE and zymogram were aligned to select bands of interest. An activity protein band, detected by Congo red staining as described in the section Materials and methods, was visualized only for the strain XR44A (Fig. 2, lane A), and the corresponding band in the colloidal Comassie blue stained SDS-PAGE gel (Fig. 2, lane B) was excised and subjected to protein identification by in situ digestion and LC-MS/MS analysis of the peptide mixtures. The acquired MS/MS spectra were transformed in Mascot generic (.mgf) format and used for protein identification with a licensed version of MASCOT software (http://www.matrixscience.com) version 2.4.0. against the whole unreviewed set of protein entries that are present in the NCBInr database for all bacteria. Protein identifications in the selected band are reported in Table2.
Figure 2.
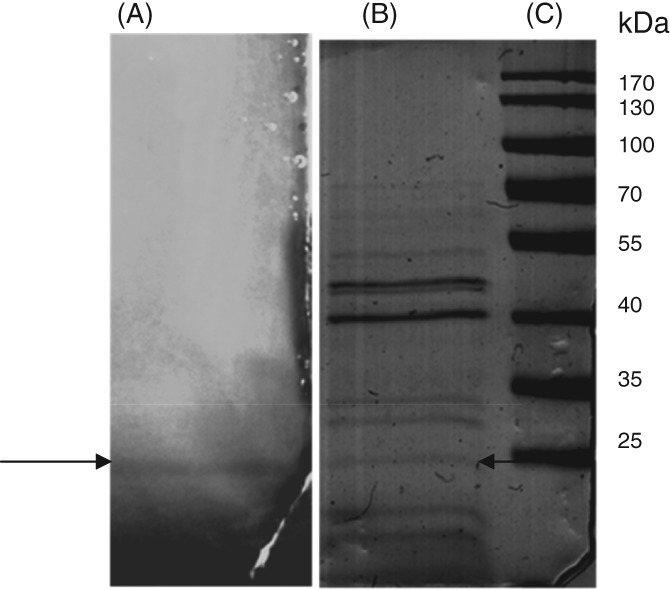
Detection of xylanase activity produced by XR44A after SDS-PAGE. Lane A: XR44A (0.25 U total xylanase activity); Lane B: XR44A (10 γ of total protein loaded); Lane C: Markers.
Table 2.
Mascot search results of LC-MS/MS data against NCBInr database showing identified proteins' nominal mass, peptide ion score, peptide sequence and protein sequence coverage
| Mascot | |||||
|---|---|---|---|---|---|
| NCBInr ID | Protein | nominal mass (Mr) | Score | Peptide | Sequence coverage (%) |
| gi|70998142 | endo-1,4-beta-xylanase [Paenibacillus macerans] | 23264 | 24 | GWTTGSPFR | 20 |
| 65 | SDGGTYDIYTTTR | ||||
| 61 | YNAPSIDGDNTTFTQYWSVR | ||||
| gi|14278871 | flagellin [Bacillus subtilis] | 35615 | 25 | LGAVQNR | 14 |
| 27 | QLNAGSNSAAK | ||||
| 39 | TAIDTVSSER | ||||
| 41 | LEHTINNLGTSSENLTSAESR | ||||
| gi|154686187 | chitin binding protein [Bacillus amyloliquefaciens FZB42] | 22437 | 31 | ADTNLTHK | 22 |
| 31 | YGSVIDNPQSVEGPK | ||||
| 28 | DEFELIGTVNHDGSK | ||||
It is worth noting that among the identified proteins, an endo-1,4-beta-xylanase (NCBInr entry: gi70998142) attributed to Paenibacillus macerans was identified (three peptides, 20% sequence coverage).
Strain identification
XR44A was identified as a Bacillus amyloliquefaciens by means of cellular fatty acids analysis, physiological study and partial sequencing of 16SrDNA.
Partial characterization of the xylanase activity to define the enzymatic properties useful for its application
The optimum pH of B. amyloliquefaciens XR44A xylanase activity (assayed in the range 3–9) is in the range 7–9 (Fig. 3(A)). Other xylanases from Bacillus strains so far characterized generally show wide differences in their optimal pH, going from acidic values, such as 4 for the glycosyl hydrolase family 11 xylanase from B. amyloliquefaciens CH51 strain,32 5 for the xylanase activity produced by Bacillus subtilis GN156,33 5.8 for the xylanase from B. subtilis str. CXJZ,34 up to 9 as in the case of the endoxylanase activity from B. halodurans TSEV1.31
Figure 3.

Optimum pH (A) and temperature (B) of xylanase activity produced by B. amyloliquefaciens XR44A.
The optimal temperature of B. amyloliquefaciens XR44A xylanase activity (assayed in the range 40–70 °C) is 70 °C (Fig. 3(b)), near to that of the xylanases from Bacillus halodurans TSEV1 (80 °C)31 and B. subtilis str. CXJZ isolated from the degumming line (60 °C),34 but distant from that of the xylanases produced by Bacillus subtilis GN156 (40 °C)33 and B. amyloliquefaciens CH51 strain(25 °C).32
The novel B. amyloliquefaciens XR44A xylanase activity shows a half life of 5 min at 70 °C, 15 min at both 50 °C and 60 °C, and 2 h at 40 °C. Interestingly, it retains 90% of activity for at least 2 days at 30 °C, with a half-life of 7 days. The enzyme immediately loses activity at temperatures higher than 70 °C, differently from the xylanase produced by Bacillus aerophilus KGJ2, which retained more than 90% activity after incubation at 80–90 °C for 60 min.35
Bacillus amyloliquefaciens XR44A xylanase activity shows very high stability over a broad range of pHs, with a half-life of 7 days at pH3, pH 5, pH 6, pH 7, pH 8 and pH 9.
Saccharification of pre-treated brewer's spent grain using new xylanase
The culture supernatant of B. amyloliquefaciens XR44A with xylanase activity was utilized after ammonium sulfate precipitation and dialysis for the saccharification of brewer's spent grains (BSG), which is considered a valuable low-cost feedstock, with an increasing attractiveness for energy production. BSG represents the major by-product of the brewing industry accounting for about 85% of the total residues generated after mashing and lautering processes19 and is available in large quantities throughout the year. This lignocellulosic residue has an elevated potential as feedstock for the production of ethanol, since it is characterized by a high (hemi)cellulose content. The chemical composition of BSG varies according to several factors such as the barley variety, the harvest time, the malting and mashing conditions, and also the quality and type of additives added in the brewing process.36,37 It generally consists of about 16.8–25.4% cellulose, 21.8–28.4% hemicellulose (mostly arabinoxylans) and 11.9–27.8% lignin.38,39 The composition of the raw material utilized in this work (Table3) shows very high amounts of cellulose and xylan (in the form of arabinoxylan), and very low lignin content, in comparison to the composition of other types of brewer's spent grain reported in the literature. In Table3 the composition of the material after pretreatment is also reported. As witnessed by a huge amount of scientific literature, pretreatment is a key step for effective enzymatic lignocellulose hydrolysis, having the important function of removing the lignin fraction that shields the cell wall polysaccharides from enzymatic attack.40 In this work, pretreatment by aqueous ammonia soaking was set up for BSG adapting the conditions reported by Kim et al.41 and Maurelli et al.28 for barley hull and chestnut shell, respectively. This method was chosen due to its high efficacy in breaking the ester bonds between lignin, hemicellulose and cellulose without degrading the hemicellulose polymers.42 As shown in Table3, the delignification percentage was 78% after 22 h incubation, and the glucan and xylan contents were only marginally modified (1.63 and 5.57% of glucan and xylan dissolution, respectively, were reported). Furthermore, it was shown that by prolonging the pretreatment time no significant improvements were achieved (data not shown).
Table 3.
Macromolecular composition of BSG before and after aqueous ammonia soaking (AAS) pretreatment
| Solid compositionb |
|||||
|---|---|---|---|---|---|
| SRa (%) | Lignin (%) | Glucan (%) | Xylan (%) | Arabinan (%) | |
| BSG untreated | - | 12.8 | 27.5 | 28.8 | 4.32 |
| BSG AAS | 59.96 | 2.09 | 25.9 | 23.3 | 3.26 |
Solid remaining after reaction.
Solid percentage composition based on oven dry untreated biomass.
Table4 reports the results regarding the saccharification of the pretreated BSG with B. amyloliquefaciens XR44A protein preparation endowed with xylanase activity (1.5 U g−1 pretreated biomass). The new enzymatic activity developed in this work, or alternatively, the same dosage of a commercial xylanase from Trichoderma viride (Sigma), were used in combination with different commercial enzymes (Sigma) – cellulase from Trichoderma reesei ATCC 26921 (5.4 U g−1), cellobiase from Aspergillus niger (145 U g−1) and β − xylosidase (0.5 U g−1). It is worth noting that the utilization of the enzyme preparation from B. amyloliquefaciens XR44A led to results similar to those obtained with the commercial xylanase from Trichoderma viride (Sigma). In both cases, the maximum xylose recovery was around 43%, after 48 h of hydrolysis reaction, and it did not change over prolonged incubation times. Interestingly, the utilization of B. amyloliquefaciens XR44A xylanase preparation improved the glucan fraction degradation, which was enhanced from 51.5 to 79.8% after 72 h incubation. However, also in this case most of the cellulose conversion was completed in 48 h.
Table 4.
Results of saccharification with sigma enzymes – cellulase from Trichoderma reesei ATCC 26921 (5.4 U g−1 of pretreated biomass); cellobiase from Aspergillus niger (145 U g−1 of pretreated biomass); xylanase from Trichoderma viride (1.5 U g−1 of pretreated biomass); β-xylosidase, thermostable (0.5 U g−1 of pretreated biomass) – and xylanase from Bacillus amyloliquefaciens XR44A (1.5 U g−1 of pretreated biomass)
| Glucose yield (%) |
Xylose yield (%) |
Arabinose yield (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Composition | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|
Cellulase Cellobiase Xylanase β-Xylosidase |
35.3 | 47.9 | 51.5 | 30.5 | 40.0 | 43.8 | 52.4 | 56.1 | 56.4 |
|
Cellulase Cellobiase Xylanase (Bacillus amyloliquefaciens XR44A) β-Xylosidase |
35.1 | 74.3 | 79.8 | 24.2 | 43.2 | 43.7 | 48.1 | 70.0 | 92.3 |
A potentially better synergism between the xylanase activity from B. amyloliquefaciens XR44A and the commercial cellulase could be at the basis of the easier cellulose deconstruction.
As regards the arabinose liberation, due to the lack of arabinofuranosidase activity in both the B. amyloliquefaciens XR44A enzyme preparation and the commercial xylanase (as demonstrated by assaying it towards the substrate p-nitrophenyl α − L-arabinofuranoside), the release of this monosaccharide must be ascribed to the presence of arabinofuranosidase activities in the other commercial enzymes. As a matter of fact, values of 0.11, 1.22 and 0.69 Um L−1 of arabinofuranosidase activity were detected for arabinofuranosidase activity in the commercial cellulase, cellobiase and xylosidase, respectively.
Moreover, a better arabinose yield, that exceeded by up to 38.9% the result reached with the commercial xylanase (56.4% after 72 h), was obtained in the presence of B. amyloliquefaciens XR44A enzyme preparation. This revealed that the arabinofuranosidases worked better in combination with the new xylanase than in the presence of its commercial counterpart. As already reported by Marcolongo et al.,43 the diverse values of arabinose recovery in the presence of the two different xylanase activities can be explained by their different specificities towards the same substrate, which account also for their different level of synergism reached with the xylan debranching enzymes.
These results, arising from the synergistic behavior of the various enzymes involved in the hydrolysis of (hemi)cellulosic substrates, are in agreement with other works described in the literature.44,45
Conclusion
In this work, 93 microorganisms were isolated from Western Ghat region (Kerale, Indi) and screened for cellulase and xylanase activity. 21 microorganisms were selected by screening on solid medium, and further analysed for the production of enzymes of interest in liquid medium. The xylanolytic strain Bacillus amyloliquefaciens XR44A was selected as the best producer of xylanase activity, with a value up to 10.5 U mL−1 under optimized growth conditions. The enzyme responsible for the xylanase activity produced by Bacillus amyloliquefaciens XR44A was identified as an endo-1,4-beta-xylanase using an approach based on a combination of zymography and mass spectrometral analyses. The novel enzyme in the crude supernatant was partially characterized in order to define the properties useful for its application, and it was tested in brewer's spent grain saccharification, this being recognized as an attractive lignocellulose feedstock for fermentable sugars production. Interestingly, the novel xylanolytic activity showed performances similar to a commercial xylanase from Trichoderma viride, with a xylose yield of 43%. Moreover, the presence of the new xylanase in the commercial enzymatic cocktail favoured an increase of arabinose recovery by up to 38% due to better synergism than the commercial counterpart, established in association with the arabinofuranosidases activities present in the commercial preparations.
Acknowledgments
This research was supported by a Marie Curie International Research Staff Exchange Scheme Fellowship within the 7th European Community Framework Programme: ‘Improvement of technologies and tools, e.g. biosystems and biocatalysts, for waste conversion to develop an assortment of high added value eco-friendly and cost-effective bio-products’ BIOASSORT (grant number 318931).
References
- 1.Yang B, Dai Z, Ding SY, Wyman CE. Enzymatic hydrolysis of cellulosic biomass. Biofuels. 2011;2:421–450. [Google Scholar]
- 2.Amore A, Giacobbe S, Faraco V. Regulation of cellulase and hemicellulase gene expression in fungi. Current Genomics. 2013;14:1–20. doi: 10.2174/1389202911314040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amore A, Pepe O, Ventorino V, Aliberti A, Faraco V. Cellulolytic Bacillus strains from natural habitats - a review. Chimica Oggi - Chem Today. 2013;31:6–9. [Google Scholar]
- 4.Eida MF, Nagaoka T, Wasaki J, Kouno K. Isolation and characterization of cellulose-decomposing bacteria inhabiting sawdust and coffee residue composts. Microbes Environ. 2012;3:226–233. doi: 10.1264/jsme2.ME11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Taleb KAA, Mashhoor WA, Nasr SA, Sharaf MS, Abdel-Azeem HHM. Nutritional and environmental factors affecting cellulase production by two strains of cellulolytic bacilli. J Basic Appl Sci. 2009;3:2429–2436. [Google Scholar]
- 6.Amore A, Pepe O, Ventorino V, Birolo L, Giangrande C, Faraco V. Industrial waste based compost as a source of novel cellulolytic strains and enzymes. FEMS Microbiol Lett. 2013;339:93–101. doi: 10.1111/1574-6968.12057. [DOI] [PubMed] [Google Scholar]
- 7.Mayende L, Wilhelmi BS, Pletschke BI. Cellulases (CMCases) and polyphenol oxidases from thermophilic Bacillus spp. isolated from compost. Soil Biol Biochem. 2006;38:2963–2966. [Google Scholar]
- 8.Heck JX, Hertz PF, Ayub MAZ. Cellulase and xylanase production by isolated amazon Bacillus strains using soybean industrial residue based solid-state cultivation. Braz J Microbiol. 2002;33:213–218. [Google Scholar]
- 9.Lee YJ1, Kim BK, Lee BH, Jo KI, Lee NK, Chung CH, Lee YC, Lee JW. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresource Technol. 2008;99:378–386. doi: 10.1016/j.biortech.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y-K, Lee S-C, Cho Y-Y, Oh H-J, Ko YH. Isolation of cellulolytic Bacillus subtilis strains from agricultural environments. ISRN Microbiol. 2012;2012:1–9. doi: 10.5402/2012/650563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayaraghavan P, Prakash Vincent SG. Purification and characterization of carboxymethyl cellulase from Bacillus sp. isolated from a paddy field. Polish J Microbiol. 2012;61:51–55. [PubMed] [Google Scholar]
- 12.Jones SM, van Dyk JS, Pletschke BI. Bacillus Subtilis SJ01 produces hemicellulose degrading multi-enzyme complexes. BioResources. 2012;7:1294–1309. [Google Scholar]
- 13.El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AM, Ibrahim IH, Al-Talhi HA. Solid fermentation of wheat bran for hydrolytic enzymes production and saccharification content by a local isolate Bacillus megatherium. BMC Biotechnol. 2014;14:29–36. doi: 10.1186/1472-6750-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma V, Verma A, Kushwaha A. Isolation and production of cellulase enzyme from bacteria isolated from agricultural fields in district Hardoi, Uttar Pradesh, India. Adv Appl Sci Res. 2012;3:171–174. [Google Scholar]
- 15.Singh J, Batra N, Sobti RC. Purification and characterisation of alkaline cellulase produced by a novel isolate, Bacillus sphaericus JS1J. Ind Microbiol Biotechnol. 2004;31:51–56. doi: 10.1007/s10295-004-0114-0. [DOI] [PubMed] [Google Scholar]
- 16.Shu-bin L, Ren-chao Z, Xia L, Chu-yi C, Ai-lin Y. Solid-state fermentation with okara for production of cellobiase-rich cellulases preparation by a selected Bacillus subtilis Pa5. African J Biotechnol. 2012;11:2720–2730. [Google Scholar]
- 17.Tamaru Y, Miyake H, Kuroda K, Ueda M, Doi RH. Comparative genomics of the mesophilic cellulosome-producing Clostridium cellulovorans and its application to biofuel production via consolidated bioprocessing. Environ Technol. 2010;31:889–903. doi: 10.1080/09593330.2010.490856. [DOI] [PubMed] [Google Scholar]
- 18.Liguori R, Amore A, Faraco V. Waste valorization by biotechnological conversion into added value products. Appl Microbiol Biotechnol. 2013;97:6129–6147. doi: 10.1007/s00253-013-5014-7. [DOI] [PubMed] [Google Scholar]
- 19.Mussatto SI. Brewer's spent grain: a valuable feedstock for industrial applications. J Sci Food Agric. 2014;94:1264–1275. doi: 10.1002/jsfa.6486. [DOI] [PubMed] [Google Scholar]
- 20.White JS, Yohannan BK, Walker GM. Bioconversion of brewer's spent grains to bioethanol. FEMS Yeast Res. 2008;8:1175–1184. doi: 10.1111/j.1567-1364.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- 21.Xiros C, Christakopoulos P. Enhanced ethanol production from brewer's spent grain by a Fusarium oxysporum consolidated system. Biotechnol Biofuels. 2009;2:4. doi: 10.1186/1754-6834-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. [Google Scholar]
- 23.Amore A, Amoresano A, Birolo L, Henrissat B, Leo G, Palmese A, Faraco V. A family GH51 alfa-L-arabinofuranosidase from Pleurotus ostreatus: identification, recombinant expression and characterization. Appl Microbiol Biotechnol. 2012;94:995–1006. doi: 10.1007/s00253-011-3678-4. [DOI] [PubMed] [Google Scholar]
- 24.Rameshkumar N, Nair S. Isolation and molecular characterization of genetically diverse antagonistic, diazotrophic red-pigmented vibrios from different mangrove rhizospheres. FEMS Microbiol Ecol. 2009;67:455–467. doi: 10.1111/j.1574-6941.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Beguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983;131:333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- 27.McIlvaine TC. A buffer solution for colorimetric comparison. J Biol Chem. 1921;49:183–186. [Google Scholar]
- 28.Maurelli L, Ionata E, La Cara F, Morana A. Chestnut shell as unexploited source of fermentable sugars: effect of different pretreatment methods on enzymatic saccharification. Appl Biochem Biotechnol. 2013;170:1104–1118. doi: 10.1007/s12010-013-0264-5. [DOI] [PubMed] [Google Scholar]
- 29.Davis MW. A rapid modified method for compositional carbohydrate analysis of lignocellulosics by high pH anion-exchange chromatography with pulsed amperometric detection (HPAEC/PAD) J Wood Chem Technol. 1998;18:235–252. [Google Scholar]
- 30.Nampoothiri KM, Ramkumar B, Pandey A. Western Ghats of India: rich source of microbial biodiversity. J Sci Ind Res. 2013;72:617–623. [Google Scholar]
- 31.Kumar V, Satyanarayana T. Production of thermo-alkali-stable xylanase by a novel polyextremophilic Bacillus halodurans TSEV1 in cane molasses medium and its applicability in making whole wheat bread. Bioprocess Biosyst Eng. 2013;37:1043–1053. doi: 10.1007/s00449-013-1075-3. [DOI] [PubMed] [Google Scholar]
- 32.Baek CU, Lee SG, Chung YR, Cho I, Kim JH. Cloning of a family 11 xylanase gene from Bacillus amyloliquefaciens CH51 isolated from Cheonggukjang. Indian J Microbiol. 2012;52:695–700. doi: 10.1007/s12088-012-0260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratumteep J, Sansernsuk J, Nitisinprasert S, Apiraksakorn J. Production, characterization and hydrolysation products of xylanase from Bacillus subtilis GN156KKU. Res J. 2010;15:343–350. [Google Scholar]
- 34.Gang G, Liu Z, Xu J, Liu J, Dai X, Xie D, Peng K, Feng X, Duan S, Zheng K, Cheng L, Fu Y. Purification and characterization of a xylanase from Bacillus subtilis isolated from the degumming line. J Basic Microbiol. 2012;52:419–428. doi: 10.1002/jobm.201100262. [DOI] [PubMed] [Google Scholar]
- 35.Gowdhaman D, Manaswini VS, Jayanthi V, Dhanasri M, Jeyalakshmi G, Gunasekar V, Sugumaran KR, Ponnusami V. Xylanase production from Bacillus aerophilus KGJ2 and its applicationin xylooligosaccharides preparation. Int J Biol Macromol. 64:90–98. doi: 10.1016/j.ijbiomac.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Santos M, Jimenez JJ, Bartolome B, Gomez-Cordoves C, Del Nozal MJ. Variability of brewers' spent grain within a brewery. Food Chem. 2014;80:17–21. 2003. [Google Scholar]
- 37.Robertson JAI, Anson KJA, Treimo J, Faulds CB, Brocklehurst TF, Eijsink VGH, Waldron KW. Profiling brewers' spent grain for composition and microbial ecology at the site of production LWT. Food Sci Technol. 2010;43:890–896. [Google Scholar]
- 38.Sežun M, Grilc V, Zupančič GD, Marinšek-Logar R. Anaerobic digestion of Brewery Spent Grain in a semi-continuous bioreactor: inhibition by phenolic degradation products. Acta Chim Slov. 2011;58:158–166. [PubMed] [Google Scholar]
- 39.Aliyu S, Bala M. Brewer's spent grain: a review of its potentials and applications. African J Biotechnol. 2011;10:324–331. [Google Scholar]
- 40.Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB. Biomass pretreatment: fundamentals toward application. Biotechnol Adv. 2011;29:675–685. doi: 10.1016/j.biotechadv.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim TH, Taylor F, Hicks KB. Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresource Technol. 2008;99:5694–5702. doi: 10.1016/j.biortech.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 42.Chang V, Holtzapple M. Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol. 2000;84–86:5–37. doi: 10.1385/abab:84-86:1-9:5. [DOI] [PubMed] [Google Scholar]
- 43.Marcolongo L, Ionata E, La Cara F, Amore A, Giacobbe S, Pepe O, Faraco V. The effect of Pleurotus ostreatus arabinofuranosidase and its evolved variant in lignocellulosic biomasses conversion. Fungal Genet Biol. 2014;72:162–167. doi: 10.1016/j.fgb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto T, Nakata Y. Synergistic degradation of arabinoxylan with alpha-l arabinofuranosidase, xylanase and beta-xylosidase from soy sauce koji mold, Aspergillus oryzae, in high salt condition. J Biosci Bioeng. 2003;95:164–169. doi: 10.1016/s1389-1723(03)80123-8. [DOI] [PubMed] [Google Scholar]
- 45.Gao D, Uppugundla N, Chundawat SP, Yu X, Hermanson S, Gowda K, Brumm P, Mead D, Balan V, Dale BE. Hemicellulases and auxiliary enzymes for improved conversion of lignocellulosic biomass to monosaccharides. Biotechnol Biofuels. 2011;22:5–15. doi: 10.1186/1754-6834-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


