Abstract
Therapies for macular degeneration and diabetic retinopathy require intravitreal injections every 4-8 weeks. Injections are uncomfortable, time-consuming, and carry risks of infection and retinal damage. However, drug delivery via noninvasive methods to the posterior segment of the eye has been a major challenge due to the eye's unique anatomy and physiology. Here we present a novel nanoparticle depot platform for on-demand drug delivery using a far ultraviolet (UV) light-degradable polymer, which allows noninvasively triggered drug release using brief, low-power light exposure. Nanoparticles stably retain encapsulated molecules in the vitreous, and can release cargo in response to UV exposure up to 30 weeks post-injection. Light-triggered release of nintedanib (BIBF 1120), a small molecule angiogenesis inhibitor, 10 weeks post-injection suppresses choroidal neovascularization (CNV) in rats. Light-sensitive nanoparticles are biocompatible and cause no adverse effects on the eye as assessed by electroretinograms (ERG), corneal and retinal tomography, and histology.
Keywords: Ocular, triggered release, light-triggered, nanoparticle, polymer, anti-angiogenic
Introduction
Neovascular age-related macular degeneration (AMD) is one of the most common eye disorders that impair vision and is the leading cause of blindness in the elderly [1]. The majority of treatments for AMD require monthly or bimonthly intravitreal injection of anti-angiogenic drugs, such as bevacizumab (Avastin), ranibizumab (Lucentis), and more recently, aflibercept (VEGF-Trap/Eylea) [2]. Though the risk of adverse effects, such as cataracts or retinal detachment, with each injection is rare, it increases with the number of intravitreal injections [3]. Thus, strategies that reduce the frequency of injections while maintaining the therapeutic efficacy of these drugs are highly sought after. An ideal solution would also preserve ophthalmologist control over dosages to allow adjustment for each patient's response, which would maximize the efficacy of each injection.
While several systems have been developed to extend the lifetime of anti-angiogenics following intravitreal injection, including biodegradable implants [4, 5], liposomes [6, 7], micro- [8, 9] and nanoparticles [10, 11], none allow ophthalmologist control over the timing of release. One of the most commonly used materials for this purpose is poly(lactic-co-glycolic acid) (PLGA), which can be tuned to release at different rates by varying its composition and molecular weight. This reliance on a very simple and widely-used material does not take full advantage of recently developed technologies in the drug delivery field, which could allow on-demand [12, 13] or disease-triggered [14, 15] intravitreal release of AMD drugs. Here we propose a nanoparticulate drug delivery depot formulated from a light-degradable polymer for on-demand light-triggered release of drugs post-implantation. This polymer, containing an onitrobenzyl moiety in each monomer, responds to absorption of UV by degrading into fragments and small molecules through quinone-methide rearrangements [16]. These nanoparticles rapidly release encapsulated small molecules upon exposure to 365 nm light.
To assess the efficacy of this system in vivo, we delivered nintedanib (BIBF 1120), a small molecule inhibitor of the receptors for VEGF, PDGF, and FGF [17], and demonstrate that UV light-triggered release attenuates laser-induced choroidal neovascularization (CNV) in rats. To our knowledge, this is the first report of in vivo light-triggered release in the eye.
Methods
Nanoparticle formulation
Light-sensitive polymer was synthesized as previously published (MW 7.6 kDa, PDI 1.3 by gel permeation chromatography (Figure S1) [16]. 10 mg polymer was dissolved in 270 μL dichloromethane, and 30 μL dimethyl sulfoxide (DMSO) containing 2 mg payload (fluorescein diacetate (FDA), calcein AM, BIBF1120) was added. The resulting solution was added to 6 mL of sterile-filtered 1% polyvinyl alcohol (PVA) in water, and probe sonicated for 4 min at 9-10 W (S-4000, Misonix Sonicators). Organic solvents were removed by evaporation under light vacuum conditions while stirring at 600 RPM for 3 h. Remaining PVA was removed by concentrated mode tangential flow filtration (Pellicon XL, 500kDa, Millipore) with 250 mL cell-culture grade water (HyClone) at 45 RPM. The retentiate was then freeze-dried with 100 mg trehalose as cryoprotectant. The size and distribution of particles were confirmed by dynamic light scattering (DLS, Zeta Nanosizer, Malvern Instruments), and scanning electron microscopy (SEM, FE-SEM 8500, Agilent). Loading and encapsulation efficiency were measured using UV-vis spectroscopy (UV-3600, Shimadzu) and fluorescence spectrophotometry (Jobin Yvon FL-1000, Horiba).
In vitro release studies
Raw 264.7 mouse macrophages or retinal progenitor cells were seeded at 20,000 cells/well on a 96-well plate 12 h before the experiment. Cells were washed twice with 100 μL warm Dulbecco's phosphate buffered saline (DPBS) and incubated with 100 μL of 2 mg/mL suspension of FDA-containing nanoparticles in media or media alone as controls for 3 h at 37 °C under 5% CO2. Cells were again washed twice with 100 μL warm DPBS and replenished with fresh media, and free FDA was added to wells without particles as a positive control. Half of the particle-containing wells were irradiated with 8 mW/cm2 of 365 nm UV light for 5 min (OmniCure S2000, Lumen Dynamics) to induce particle degradation. Green fluorescence from wells was measured using a plate reader (SpectraMax M5, Molecular Devices), and images were collected using a fluorescence microscope (TS100F, Nikon).
UV calibration using lens explants
Adult Sprague-Dawley rats were euthanized by CO2 asphyxiation followed by exsanguination, and their eyes were enucleated and stored at 4 °C in Belzer UW cold preservation media (Bridge to Life), supplemented with 100 μg/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen), and 200 μg/mL d-glutamine (Invitrogen) following published protocols [26, 27]. Lenses were then carefully dissected from the globes and incubated in 4 mL Media 199 (Invitrogen) supplemented with 1% w/v of penicillin (100 μg/mL) and streptomycin (100 μg/mL) pre-incubated in 5% CO2 atmosphere at 37 °C for 24 h. Non-cloudy lenses were either exposed to 10 min of UV light (365 nm, 12 mW/cm2) or left unirradiated. The cloudiness was assessed visually and quantified through histograms in Adobe Photoshop CS2.
In vivo release of fluorescent dye
Sprague-Dawley rats (male, 4-8 weeks old) were anesthetized using 100 mg/kg ketamine and 10 mg/kg xylazine administered intraperitoneally. Both eyes were injected with 3 μL of a 200 mg/mL suspension of dry nanoparticle powder in DPBS by first piercing the inferotemporal quadrant with a 31 G insulin needle (BD Products), then inserting a 33 G syringe (Hamilton) through the puncture directly into the vitreous cavity. Both eyes were lubricated using a lubricant eye gel (GenTeal Severe, Novartis). The irradiation protocol to induce drug release from light-sensitive nanoparticles was as follows: one eye was dilated by corneal application of 0.5% propacaine hydrochloride (ophthalmic solution, Bausch & Lomb), followed by a drop of 0.5% tropicamide (ophthalmic solution , Bausch & Lomb). After 5 min, the rat was anesthetized with ketamine/xylazine intraperitoneally, set on one side, and covered with 2 layers of nitrile gloves to protect the body from UV light. The dilated eye was irradiated for 5 min with 365 nm UV light at 8 mW/cm2 (OmniCure S2000, Lumen Dynamics). After 45 min, rats were euthanized, and eyes were enucleated and fixed in 4% paraformaldehyde for 45 min. Retinas were then extracted, flat-mounted and imaged under a fluorescence microscope (Biorevo BZ-9000, Keyence).
In vitro cytotoxicity assay
Ultraviolet-sensitive polymer (UVSP) (5 mg) was dissolved in sterile DMSO (10 μL), and the solution was added to clear Dulbecco's Modified Eagle's Medium (DMEM) (990 μL). The resulting suspension was sonicated until uniform and further diluted to appropriate concentrations in DMEM/fetal bovine serum (FBS). Lyophilized particles containing FDA (5 mg) were resuspended in sterile media (1 mL) and half of the volume was irradiated for 5 min with UV light (10 mW/cm2, λex = 365 nm, OmniCure S2000 Curing System). Solutions were then diluted to appropriate concentrations in cell culture media. Raw 264.7 cells, seeded 24 h prior to incubation on a tissue culture treated 96-well plate (Corning) at 20,000 cells/well in DMEM, were washed twice with PBS at 37 °C, incubated with polymer/particle suspensions in triplicate for 24 h at 37 °C in 5% CO2, then washed twice again with PBS. Mitochondrial activity was then measured according to MTT assay kit instructions (Sigma-Aldrich). Triton-X (1% w/v, Sigma-Aldrich) was used as a positive apoptosis control. Absorbance at 570 nm normalized to background absorbance at 690 nm was measured using a plate reader (SpectraMax M5, Molecular Devices).
In vivo biocompatibility of materials
Sprague-Dawley rats (4-8 weeks, male) were anesthetized with ketamine/xylazine and injected intravitreously with 3 μL of a 200 mg/mL suspension of empty UVSP or PLGA nanoparticles in DPBS or 3 μL DPBS. Uninjected rats served as controls. Intraocular pressure (IOP) was measured in non-anesthetized animals at the same time of day using a veterinary tonometer (TONOVET, Icare) at 1, 5, and 7 days post-injection. Electroretinograms (ERGs) were also performed at 1 and 8 days post-injection following a previously reported protocol [28]. Briefly, rats were dark-adapted for 12 h, anesthetized, and given pupil-dilating solutions as described in the irradiation protocol above. Rats were examined within a Ganzfeld bowl (Diagnosys LLC), and electrodes were placed on each cornea, with a subcutaneously placed ground needle electrode in the tail. For scotopic ERG, the retina was stimulated with a xenon lamp at 0.01 and 0.3 cd.s/m2. For photopic ERG, rats were adapted to a background light of 10 cd*s/m2, and light stimulation was set at 30 cd.s/m2. Recordings were processed in Matlab and Excel.
mRNA extraction and reverse transcription
Sprague-Dawley rats (4-8 weeks, male) injected with UVSP, PLGA nanoparticles or DPBS, as well as controls (no injection) were euthanized 7 days post-injection, and their eyes were enucleated and retinas extracted. Tissue was homogenized by trituration in lysis buffer (QIAGEN), followed by centrifugation through QIAshredder homogenization columns (QIAGEN). mRNA was then extracted using an RNEasy Kit (QIAGEN). RNA concentration was determined by spectrophotometric optical density ratio (OD260 nm/OD280 nm, NanoDrop 2000, Thermo Scientific). Reverse transcription was carried out using Superscript III First-Strand Synthesis System (Invitrogen) with 800 ng RNA/reaction, primed with random hexamers.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Rat primer sequences for interleukin-1β (IL-1β), tumor necrosis factor- α (TNF-α), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from literature [29, 30], and confirmed using Primer-BLAST (NCBI, Table S1). qRT-PCR was carried out using Power SYBR® Green PCR Master Mix (PE Applied Biosystems) with 400 nmol/L of primers in a 7500 Fast Real-Time PCR System (PE Applied Biosystems). Each cDNA sample was run in duplicate and quantified by comparative CT algorithm. The average of two values is reported. All gene expression levels were normalized to GAPDH.
Histology
Enucleated eyes were pierced at the cornea with a 27½ gauge needle (BD Products) and fixed overnight in 4% PFA in PBS, followed by 24 h fixation in 30% sucrose in PBS. The ocular globe was then embedded in Tissue-Tek® OCT Compound (Sakura® Finetek) and stored at -80 °C until cryosectioning. Frozen sections of 20 μm thickness were cut using a microtome-cryostat and stained with hematoxylin and eosin (H&E). For paraffin sections, tissues were fixed for 24 h in 4% PFA in PBS, followed by 24 h fixation in 70% ethanol before embedding in paraffin blocks. Sections of 20 μm thickness were cut using a microtome and stained with H&E.
Laser-induced choroidal neovascularization (CNV)
Brown Norway rats (6-8 weeks, male) were subjected to laser-induced disruption of Bruch's membrane in both eyes using an Iridex OcuLight GL 532 nm laser photocoagulator (Iridex) with a slit lamp delivery system following pupil dilation with tropicamide eye drops and anesthesia with ketamine/xylazine. Five spots in the posterior pole of the retina were irradiated through the dilated pupil (150 mW, 75 μm spot, 0.1 s). Rats were sacrificed 2 weeks following laser photocoagulation, and their eyes were enucleated and fixed in 4% PFA. Choroids were then flat-mounted and stained with Alexa Fluor 594-conjugated isolectin IB4 (Life Technologies). Mounted slides were imaged by fluorescence microscopy at 10X magnification (Biorevo, Keyence), and images were analyzed in Adobe Photoshop CS2.
Retinal and corneal tomography
Sprague-Dawley rats were irradiated in one eye for 5 min at 8 mW/cm2 of 365 nm light, and the contralateral eye was left as control. Corneal volume confocal tomography scans were taken with HRT3 Cornea Module (Heidelberg) before, after procedure, and followed until 6 weeks post-irradiation. Endothelial cell counts were obtained from section scans of the corneal endothelium analyzed in Heidelberg software. Retinal optical coherence tomographs (OCT) were taken using HRT3 Retina Module (Heidelberg) after procedure. The acquisition software was used to quantify outer nuclear layer (ONL) thickness. Eyes were then enucleated, cryosectioned, stained and imaged.
Results
Light-triggered release in cells
To test whether our UV-sensitive nanoparticles release encapsulated molecules upon biocompatible irradiation, we formulated nanoparticles encapsulating fluorescein diacetate (FDA) from the light-sensitive polymer (UVSP) using a single emulsion-evaporation procedure. FDA allows detection of release in cells because only the cleaved product, formed by hydrolytic activity of intracellular esterases, is fluorescent [18]. Particles were 178 nm, with a polydispersity index (PDI) of 0.06 measured by dynamic light scattering (DLS, Figure S2A); this size was confirmed by scanning electron microscopy (SEM, Figure S2B). Particles contained 0.5 μg FDA/ mg lyophilized powder as determined by dissolution in dichloromethane (DCM) and fluorescence measurements of the hydrolyzed FDA extracted into 0.1 N NaOH.
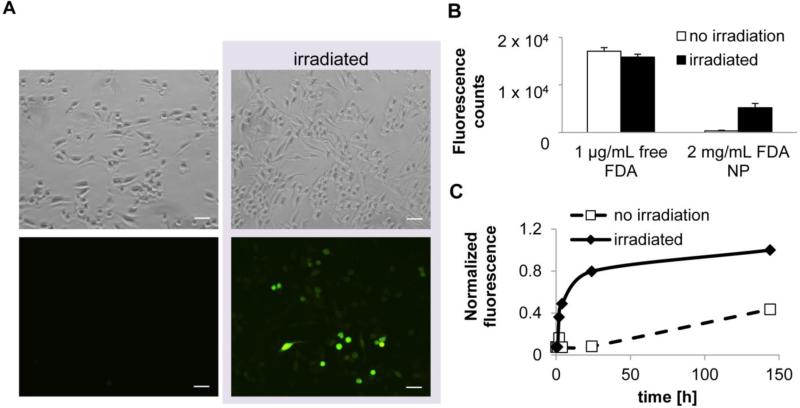
Raw 264.7 mouse macrophage cells were incubated with FDA-containing nanoparticles suspended in growth media. To eliminate the contribution of FDA released upon resuspension, cells were incubated with the nanoparticle suspension for 3 h, as fluorescein's fluorescence lasts a shorter period. Cells were then either irradiated with 365 nm UV light for 5 min at 8 mW/cm2 or left untreated and incubated 30 min. Fluorescence increased over the incubation period only in irradiated cells, suggesting that FDA was released upon light exposure, allowing esterase activation of the dye (Figure 1A). The difference in fluorescence between irradiated and non-irradiated wells containing cells and FDA nanoparticles was 16 ± 2.7 fold as quantified by a plate reader (Figure 1B). Results in non-phagocytosing retinal progenitor cell lines were also consistent with light-triggered release; fluorescence in irradiated cells was 2.5-fold greater than in non-irradiated (Figure S3).
Figure 1. UV light triggers release of encapsulated molecules in cultured macrophages.
A, Raw264.7 macrophages incubated with fluorescein diacetate-containing nanoparticles (FDA NP) were irradiated with UV light (365 nm, 5 min, 10 mW/cm2). Scale bar = 100 μm. B, Quantification of fluorescence (n= 3 wells, p < 0.001). C. FDA release in phosphate buffer at 37 °C measured by fluorescence upon irradiation (Ex/Em = 490/514 nm).
Light-sensitive nanoparticles are biocompatible
To evaluate the safety of light-sensitive nanoparticles for use in live animals, we first examined the effect of irradiated and non-irradiated polymer and nanoparticles on mitochondrial activity by MTT assay. All materials were well-tolerated by Raw 264.7 cells at concentrations up to 500 μg/mL (Figure S4). We then tested their safety in the eye in live Sprague-Dawley rats by assessing their effects on intraocular pressure (IOP), retinal activity (by electroretinogram (ERG)), and retinal ONL (outer nuclear layer) thickness using the commonly used polymer PLGA as a standard for safety and PBS as a negative control (Figure 2). To assess effects of acute exposure, we analyzed retinal thickness a week after injection with light-sensitive nanoparticles. Tissue histology sections stained with hematoxylin and eosin (H&E) reveal no difference in outer nuclear layer (ONL) thickness between eyes injected with light-sensitive nanoparticles compared to PBS and PLGA (Figure 2A, B). Nanoparticles caused no significant change in IOP compared to healthy animals either directly after injection with nanoparticles or a week after the procedure, similar to the effect of PBS (Figure 2C). Similarly, the effects of light-sensitive nanoparticles on ERG activity were similar to those of PLGA and PBS (Figure 2D); all injections caused a procedural drop in the ERG scotopic b-wave in both experimental and control groups, but all readings returned to normal within a week. Finally, we examined whether light-sensitive nanoparticles trigger an inflammatory response in the eye by measuring the expression of the inflammatory cytokines IL-1β and TNF-α post-injection by quantitative real-time polymerase chain reaction (qRT-PCR). No change in expression of any of these cytokines was detected; this lack of effect was similar to that of PBS and PLGA (Figure S5).
Figure 2. Light-sensitive nanoparticles are biocompatible.
A, Micrographs of H&E stained retinas 8 days after injections. Scale bar = 100 μm. B, Quantification of outer nuclear layer (ONL) thickness from histology sections (S2 – superior quadrant, I2 – inferior quadrant, ora – vicinity of ora serrata). C, Intraocular pressure measurements following the injection of nanoparticles. D, Electroretinograms (ERG) of rod scotopic response after injection of nanoparticles (n = 3).
Irradiation required for release does not damage the eye
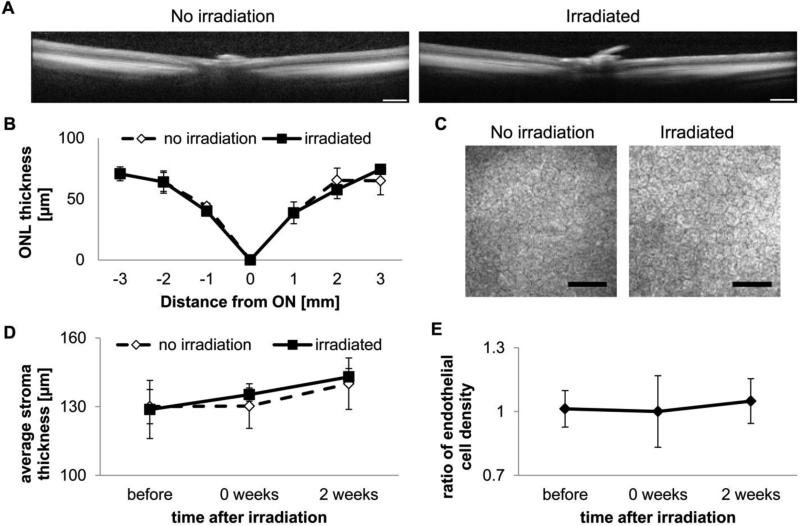
To address likely concerns about the light necessary to release drugs from our system, preliminary studies on irradiation conditions were carried out using cultured explanted rat lenses. By starting with 1 min at 40 mW/cm2, and stepwise decreasing the irradiance, we found that exposing Sprague-Dawley rat lenses to 10 min of 365 nm UV light at 12 mW/cm2 does not cause cloudiness within 48 h of exposure (Figure S6). To ensure that we were well within the safety threshold for lenses, in vivo experiments all employed irradiation of 5 min at 8 mW/cm2. Optical coherence tomography revealed no acute effects of irradiation on the retina (Figure 3A, B). Moreover, comparison of corneal tomography scans of irradiated and nonirradiated eyes showed no significant effect of irradiation on endothelial cell count (Figure 3C, E). The central corneal stroma thickness in the experimental animals did not differ from that in controls. No abnormalities such as corneal clouding or signs of cataracts were observed. Analysis of H&E-stained sections of whole eyes 8 weeks post-irradiation reveals no damage to the corneal endothelium, lens, or photoreceptor layers (Figure S7).
Figure 3. Irradiation required for release does not damage the eye.
A, Representative optical coherence tomography (OCT) scans through the retina (scale bar = 0.5 mm). B, Quantification of outer nuclear layer (ONL) thickness (ON – optical nerve, positive – towards temporal quadrant, negative – towards nasal quadrant; n = 3). C, Representative confocal tomographs of the corneal endothelium 2 weeks post-irradiation (scale bar = 50 μm). D, Quantification of stromal thickness from volume cornea tomographs (n = 4). E, Quantification of endothelial cell count ratio (n = 4).
Light-triggered release in vivo
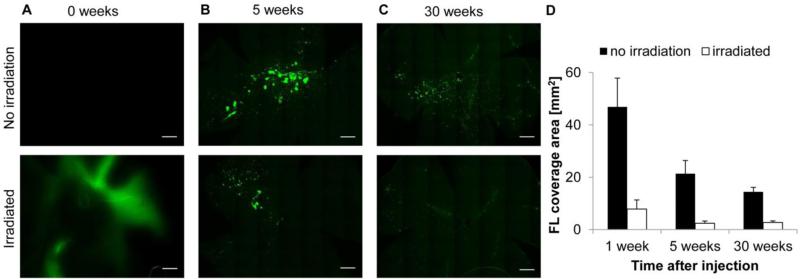
To determine whether the brief, low-power UV irradiation protocol developed above could trigger release of cargo in the eye, anesthetized Sprague-Dawley rats were injected in both eyes with FDA-containing nanoparticles. One eye of each rat was irradiated 3 h later (to allow fluorescence from FDA released upon resuspension of particles to fade) with UV light (365 nm, 8 mW/cm2) and retinas were collected 45 min later and flat-mounted. Green fluorescence was significantly greater in irradiated eyes than non-irradiated controls, indicating light-triggered release and activation of FDA upon entering retinal cells (Figure 4A).
Figure 4. Light-sensitive particles can release payload up to 30 weeks post-intravitreal injection.
A-C, Fluorescent microscopy of retinal flat-mounts. Scale bar = 1 mm. D, Quantification of area of fluorescence coverage on retinal flat-mounts. Error bars represent SEM.
To determine the lifetime of non-irradiated particles in the eye, we used the same assay. Fortuitously, because FDA within nanoparticles is hydrolyzed after an extended period of time to form non-cell permeable fluorescein, non-irradiated eyes exhibit a positive, particulate fluorescence signal. Irradiation would thus release fluorescein, which is removed during flat-mounting. Particulate fluorescence that could be turned off upon irradiation was observed up to 7 months after injection (Figure 4 B, C).
Light-released nintedanib inhibits CNV
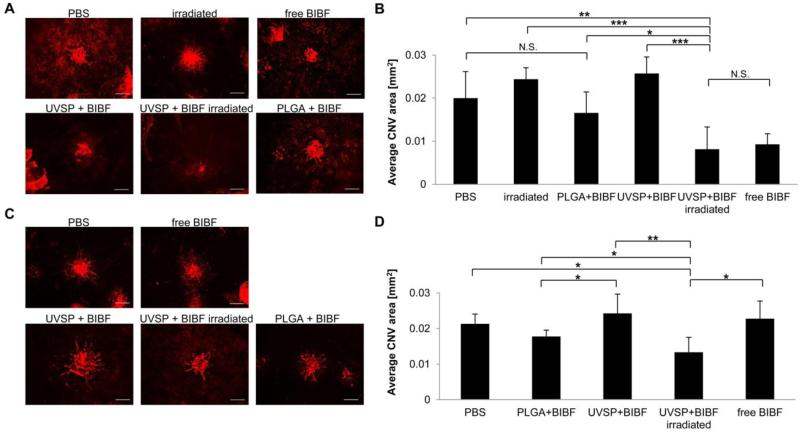
To explore the potential of light-sensitive nanoparticles as a delivery vehicle for therapeutic compounds, nanoparticles encapsulating the small molecule angiogenesis inhibitor nintedanib [16] were injected intravitreally into Brown Norway rats prior to laser induction of choroidal neovascularization. Inhibition of vessel growth was compared to rats receiving PLGA particles containing nintedanib, free drug, or saline. To assess the stability of nintedanib encapsulation, CNV was induced in the animals either immediately or 10 weeks after injections. Light-released nintedanib inhibited angiogenesis significantly (as assessed by isolectin staining; p = 0.012), as observed in rats treated with free nintedanib (p = 0.009; Figure 5A, B). Importantly, CNV areas in rats receiving UV light-released nintedanib were significantly smaller than those in rats receiving PLGA-encapsulated nintedanib (p = 0.028), suggesting that light-triggered release delivers drug more effectively than slowly hydrolyzing PLGA nanoparticles. Importantly, UVSP nanoparticles retained their ability to release drug and inhibit angiogenesis when CNV was induced 10 weeks after the implantation. CNV areas following light-triggered release were significantly smaller than in rats receiving PLGA-encapsuated nintedanib (Figure 5C, D).
Figure 5. Light-triggered release of nintedanib (BIBF) post-injection inhibits CNV.
A, Fluorescent microscope images of isolectin B4-Alexa Fluor 594 stained choroidal flat-mounts 2 weeks after CNV induction. Eyes were irradiated immediately post-injection (scale bar = 100 μm). B, Quantification of CNV spot size (n = 4-6). C, Choroidal flat-mounts from eyes irradiated 10 weeks post-injection, 2 weeks after CNV induction (scale bar = 100 μm). D, Quantification of CNV spot size (n = 4-6).
Discussion
Because most current anti-angiogenics are administered monthly (Avastin, Lucentis) or bimonthly (Eylea), delivery systems for these drugs must allow drug release at time points later than two months post-injection to offer a clinical advantage. The light-sensitive nanoparticles employed here retain and release therapeutically relevant amounts of drug after 10 weeks in the eye. Further, despite polymer hydrolysis, both nanoparticles and encapsulated dye are present in the intravitreous space 30 weeks after injection. Our system therefore has potential to improve both of the above approaches by allowing noninvasive dosing long after injection.
The requirement for UV irradiation will likely raise concerns about the safety of our approach to control anti-angiogenic dosing, as UV causes both acute and prolonged adverse effects in the cornea (where it is primarily absorbed) as well as in the lens and retina [19]. A major concern is damage to the corneal endothelium, which cannot repair itself [20, 21]. The UV wavelength used in our studies is 365 nm, which is in the lower energy end of UVA, close to the visible spectrum and within the range of ambient UV radiation. The effect of this wavelength of light on the eye has been extensively studied as cornea crosslinking (CXL) treatments for keratoconus require 30 min of irradiation at 3 mW/cm2 of 365 nm light [20, 22]. Though CXL therapy can cause cytotoxic effects in rabbits with thin corneas, and therefore in patients with advanced keratoconus [23], without the UV absorber the dose cytotoxic to the endothelium has been reported to be 42.5 J/cm2 at the surface of the cornea, equivalent to 270 min irradiation at 3 mW/cm2, or 90 min irradiation at 8 mW/cm2 [24, 25]. In contrast, the total energy density delivered by our light trigger is 2.4 J/cm2 over 5 min, which is well within the reported safety regime for this wavelength, and is well-tolerated by the rat endothelium over 4 weeks post-irradiation. Our studies also show that 10 min exposure to UVA at 12 mW/cm2, equivalent to 7.2 J/cm2, is safe for the lens as it is well within the reported damage threshold of >70 J/cm2 [24]. Therefore, the irradiation protocol employed here is within the safety thresholds for the eye.
Conclusion
To our knowledge, this is the first report of in vivo light-triggered release of drug in the eye. The period for which carriers and encapsulated drug remain present in the intravitreous space and retain their ability to degrade upon irradiation, up to 30 weeks, is the longest yet reported for a nanoparticulate depot and far exceeds the usual 4-8 week interval between injections of anti-angiogenics. These results, combined with our demonstration of therapeutic efficacy following light-triggered release at 10 weeks post-injection and the safety of the light trigger, promise clinical relevance of this platform for intravitreal drug delivery. We are currently examining whether light can trigger release of multiple therapeutic doses of BIBF1120.
Supplementary Material
Acknowledgements
NMR spectra were collected at the UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences NMR Facility. OCT and HRT tomographs were acquired at UCSD School of Medicine Core Imaging Facility, supported by the National Eye Institute (P30EY022589). This work was supported by an NIH New Innovator Award (DP 2OD006499) and King Abdulaziz City for Science and Technology (through the KACST-UCSD Center for Excellence in Nanomedicine and Engineering).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11:541–559. doi: 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DH, Luo J, Zhang K, Zhang M. Current therapeutic approaches in neovascular age-related macular degeneration. Discov Med. 2013;15:343–348. [PubMed] [Google Scholar]
- 3.Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv Drug Deliv Rev. 2001;52:5–16. doi: 10.1016/s0169-409x(01)00200-9. [DOI] [PubMed] [Google Scholar]
- 4.Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Ali Y, Lehmussaari K. Industrial perspective in ocular drug delivery. Adv Drug Deliv Rev. 2006;58:1258–1268. doi: 10.1016/j.addr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Honda M, Asai T, Umemoto T, Araki Y, Oku N, Tanaka M. Suppression of choroidal neovascularization by intravitreal injection of liposomal su5416. Arch Ophthalmol. 2011;129:317–321. doi: 10.1001/archophthalmol.2011.12. [DOI] [PubMed] [Google Scholar]
- 7.Wang J.-l., Liu Y.-l., Li Y, Dai W.-b., Guo Z.-m., Wang Z.-h., Zhang Q. EphA2 Targeted Doxorubicin stealth liposomes as a therapy system for choroidal neovascularization in rats. Invest Ophthalmol & Vis Sci. 2012;53:7348–7357. doi: 10.1167/iovs.12-9955. [DOI] [PubMed] [Google Scholar]
- 8.Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol & Vis Sci. 2006;47:1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paganelli F, Cardillo JA, Dare AR, Melo LA, Jr., Lucena DR, Silva AA, Jr., Oliveira AG, Pizzolitto AC, Lavinsky D, Skaf M, Souza-Filho AA, Hofling-Lima AL, Nguyen QD, Kuppermann BD, Herrero-Vanrell R, Belfort R, Jr., Brazilian Ocular P, Pharmaceutical Technology Research G. Controlled transscleral drug delivery formulations to the eye: establishing new concepts and paradigms in ocular anti-inflammatory therapeutics and antibacterial prophylaxis. Exp Opin Drug Deliv. 2010;7:955–965. doi: 10.1517/17425247.2010.498817. [DOI] [PubMed] [Google Scholar]
- 10.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 11.Iwase T, Fu J, Yoshida T, Muramatsu D, Miki A, Hashida N, Lu L, Oveson B, Lima e Silva R, Seidel C, Yang M, Connelly S, Shen J, Han B, Wu M, Semenza GL, Hanes J, Campochiaro PA. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J Controlled Release. 2013;172:625–633. doi: 10.1016/j.jconrel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliv Rev. 2012;64:1005–1020. doi: 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timko BP, Kohane DS. Materials to clinical devices: technologies for remotely triggered drug delivery. Clin Ther. 2012;34:S25–35. doi: 10.1016/j.clinthera.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi-Barr S, de Gracia Lux C, Mahmoud E, Almutairi A. Exploiting oxidative microenvironments in the body as triggers for drug delivery systems. Antioxid & Redox Signal. 2014;21:730–754. doi: 10.1089/ars.2013.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2012;64(Supplement):327–341. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 16.Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and near-IR triggered release from polymeric nanoparticles. J Am Chem Soc. 2010;132:9540–9542. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 18.Clarke JM, Gillings MR, Altavilla N, Beattie AJ. Potential problems with fluorescein diacetate assays of cell viability when testing natural products for antimicrobial activity. J Microbiol Methods. 2001;46:261–267. doi: 10.1016/s0167-7012(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 19.Young AR. Acute effects of UVR on human eyes and skin. Prog Biophys MolBiol. 2006;92:80–85. doi: 10.1016/j.pbiomolbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Wollensak G, Sporl E, Seiler T. [Treatment of keratoconus by collagen cross linking] Ophthalmologe. 2003;100:44–49. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- 21.Zavala J, Jaime GRL, Barrientos CAR, Valdez-Garcia J. Corneal endothelium: developmental strategies for regeneration. Eye. 2013;27:579–588. doi: 10.1038/eye.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wollensak G. Crosslinking treatment of progressive keratoconus new hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 23.Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refr Surg. 2003;29:1786–1790. doi: 10.1016/s0886-3350(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 24.Pitts DG, Cullen AP, Hacker PD. Ocular Effects of near Ultraviolet-Radiation -Literature-Review. Am J Optom Phys Opt. 1977;54:542–549. doi: 10.1097/00006324-197708000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Ringvold A, Davanger M, Olsen EG. Changes of the Cornea Endothelium after Ultraviolet-Radiation. Acta Ophthalmol. 1982;60:41–53. doi: 10.1111/j.1755-3768.1982.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 26.Sampath S, McLean LA, Buono C, Moulin P, Wolf A, Chibout SD, Pognan F, Busch S, Shangari N, Cruz E, Gurnani M, Patel P, Reising A. The use of rat lens explant cultures to study the mechanism of drug-induced cataractogenesis. Toxicol Sci. 2012;126:128–139. doi: 10.1093/toxsci/kfr344. [DOI] [PubMed] [Google Scholar]
- 27.Aleo MD, Doshna CM, Navetta KA. Ciglitazone-induced lenticular opacities in rats: in vivo and whole lens explant culture evaluation. J Pharmacol Exp Ther. 2005;312:1027–1033. doi: 10.1124/jpet.104.076950. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, Qiu A, Luo H, Hicks C, Zeng J, Zhu J, Lu J, Sfeir N, Wen C, Zhang M, Reade V, Patel S, Sinden J, Sun X, Shaw P, Young M, Zhang K. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289:6362–6371. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berti R, Williams AJ, Moffett JR, Hale SL, Velarde LC, Elliott PJ, Yao CP, Dave JR, Tortella FC. Quantitative real-time RT-PCR analysis of inflammatory gene expression associated with ischemia-reperfusion brain injury. J Cerebr Blood F Met. 2002;22:1068–1079. doi: 10.1097/00004647-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K, Utsumi H, Okada M, Sakairi T, Ikeda I, Kusakabe M, Takagi S. One-step RT PCR without initial RNA isolation step for laser-microdissected tissue sample. J Vet Med Sci. 2003;65:917–919. doi: 10.1292/jvms.65.917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.