Abstract
Purpose of review
Review novel insights into the biology of proprotein convertase subtilisin/kexin 9 (PCSK9) that may explain the extreme efficiency of PCSK9 inhibition and the unexpected metabolic effects resulting from PCSK9 monoclonal antibody therapy, and may identify additional patients as target of therapy.
Recent findings
For over 20 years, the practical knowledge of cholesterol metabolism has centered around cellular mechanisms, and around the idea that statin therapy is the essential step to control metabolic abnormalities for cardiovascular risk management. This view has been embraced by the recent AHA/ACC guidelines, but is being challenged by recent studies including nonstatin medications and by the development of a new class of cholesterol-lowering agents that seems destined to early US Food and Drug Administration approval. The discovery of PCSK9 – a circulating protein that regulates hepatic low-density lipoprotein (LDL) receptor and serum LDL cholesterol levels – has led to a race for its therapeutic inhibition. Recent findings on PCSK9 regulation and pleiotropic effects will help identify additional patient groups likely to benefit from the inhibitory therapy and unravel the full potential of PCSK9 inhibition therapy.
Summary
Injectable human monoclonal antibodies to block the interaction between PCSK9 and LDL receptor are demonstrating extraordinary efficacy (LDL reductions of up to 70%) and almost the absence of any side-effects. A more moderate effect is seen on other lipoprotein parameters, with the exception of lipoprotein(a) levels. We describe mechanisms that can explain the effect on lipoprotein(a), predict a potential effect on postprandial triglyderides, and suggest a new category of patients for anti-PCSK9 therapy.
Keywords: LDL-cholesterol, LDL-receptor, lipoprotein(a), PCSK9 inhibition, triglycerides
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in all countries [1]. Among the risk factors, hypercholesterolemia is directly linked to premature development of atherosclerosis, the pathology underlying coronary heart disease (CHD) and the most common form of stroke [1]. Familial hypercholesterolemia, commonly due to mutations in the low-density lipoprotein (LDL) receptor (LDLR), results in elevated LDL-cholesterol (LDL-c) levels and is known to cause premature atherosclerosis and aortic calcification in humans [2]. Statins and ezetimibe are currently the most common drugs used for reaching target LDL-c levels in patients with hypercholesterolemia, a strategy that improves CVD outcomes [3–5]. However, current lipid-lowering therapies have several limitations, including the fact that: approximately 50% of familial hypercholesterolemia patients still cannot reach desired LDL-c levels [6]; intolerance to statin is becoming increasingly common [7]; and increased incidence of new-onset diabetes reduces drug acceptance and compliance in some patient groups [8,9]. These unmet needs warrant the continuing search for new, potent, and safe cholesterol-lowering therapies.
Proprotein convertase subtilisin/kexin 9 (PCSK9) – a circulating serine protease – binds LDLR and leads to its intracellular degradation [10]. Mutations in the human PCSK9 gene can lead to hypercholesterolemia [11] or low cholesterol syndromes [12], depending on whether the mutation causes a gain or a loss of function, respectively. The impact of PCSK9 on LDLR degradation is being exploited with the development of PCSK9 inhibition therapies aimed at lowering serum LDL-c levels [13,14]. Clinical trials have shown that PCSK9 inhibition using either monoclonal antibodies (mAbs) to block serum PCSK9 or RNA interference (RNAi) to reduce PCSK9 production produces a large drop (>50%) in LDL-c levels [15,16▪,17]. The mAbs against PCSK9 also significantly reduce lipoprotein (a) [Lp(a)] by up to 30% in a dose-dependent manner [18▪,19▪]. The effect of PCSK9 inhibition on serum triglyceride levels is less clear, as most studies show a moderate reduction that does not always reach statistical significance; the effect on high-density lipoprotein cholesterol (HDL-c) is positive but modest [20,21]. All three mAbs [Evolocumab by Amgen (formally Applied Molecular Genetics, Thousand Oaks, CA, USA), Alirocumab by Sanofi (Paris, France)/Regeneron (Tarrytown, NY, USA), and Bococizumab by Pfizer (Groton, CT, USA)] are currently at phase III clinical trials, whereas the PCSK9 RNAi (ALN-PCSSC by Alnylam Pharmaceuticals) is still in phase I trials. In this review, we will summarize the most recent findings on PCSK9 inhibition, regulation, and function, focusing on the PCSK9–LDLR interaction, and on the effect of PCSK9 on atherogenic lipoproteins apart from LDL.
CROSS-TALK BETWEEN LOW-DENSITY LIPOPROTEIN RECEPTOR AND PCSK9: FROM PHYSIOLOGY TO INHIBITION THERAPY
The PCSK9 gene is 25-kb long and lies on the short arm of chromosome 1 in humans (chromosome 4 in mice), comprised 12 exons and 11 introns [22,23]. The proximal promoter of the PCSK9 gene contains a highly conserved hepatocyte nuclear-factor 1 (HNF1) binding site residing 28 base pairs upstream of a sterol regulatory element (SRE) motif that responds to changes in intracellular cholesterol levels [24]. In-vivo studies show that the sterol-dependent regulation of PCSK9 is mediated predominantly by SRE-binding protein (SREBP) 2 [25]. Studies using primary mice hepatocytes or human hepatoma cell line (HepG2) showed that SREBP1c can also induce PCSK9 transcription [25,26]. Statins inhibit cholesterol synthesis, which in turn activates the SREBP pathway and up-regulates both LDLR and PCSK9 in hepatocytes [27]. Similarly, in patients taking statins, circulating PCSK9 levels are increased by up to 50% compared to controls [28–30]. Thus, in a state of cellular cholesterol deficiency, the cell increases production of LDLR to allow more internalization of cholesterol and at the same time also increases the synthesis of PCSK9, which degrades LDLR.
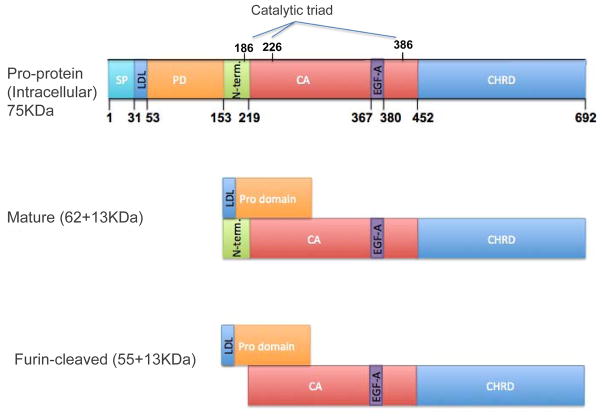
PCSK9 is synthesized as a precursor protein of 75 kDa that undergoes auto-catalytic cleavage to allow secretion of the mature product of 62 kDa [31,32]. It is important to mention that the catalytic activity of PCSK9 does not affect LDLR degradation [33]. The degradation of LDLR is mediated by the extracellular interaction between mature PCSK9 and the epidermal growth factor-like repeat A (EGF-A) of the LDLR, which leads to the internalization and degradation of both proteins [34]. In serum, mature PCSK9 can also undergo further cleavage by action of furin to generate a lower-molecular-weight form (55 kDa) that is less active than the mature form in causing LDLR degradation [35–37]. A detailed illustration of PCSK9 domains and their function is shown in Fig. 1. Of note, the mature form of PCSK9 was also shown to directly associate with the LDL compartment in the serum [38–41], although the clinical significance of this association remains to be determined.
FIGURE 1.
Schematic representation of PCSK9 domains and molecular forms. (a) Full-length PCSK9 (692 amino acids and 75 kDa) has a signal peptide (SP), a pro-domain (PD) which contains the binding site of PCSK9 to LDL, a catalytic activity domain (CA) in the N-terminal section (N-term), an active site (catalytic triad Asp186, His 226 and Ser386) which is responsible for intracellular autocatalysis, and an EGF-A binding site (amino acids 367 to 380), responsible for the effects on LDLR. (b) Mature PCSK9 – upon autocatalytic cleavage, PCSK9 is secreted as a catalytically inactive protein and the cleaved PD remains noncovalently bound to the PCSK9 to carry it in the secretory pathway. This 62 (+13) kDa protein is the main active molecular form (mature), responsible for LDLR-mediated degradation. The mature PCSK9 can bind LDL particles via amino acid 31–53 in the PD. (c) Furin-cleaved PCSK9 – in serum, PCSK9 can be cleaved by furin to generate a lower molecular weight form of PCSK9 (55 + 13 kDa) that is less active in terms of LDLR degradation ability. CHRD, C-terminal histidine rich domain; EGF-A, epidermal growth factor-like repeat A; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; N-term, fragment cleaved by furin; PCSK9, proprotein convertase subtilisin/kexin 9.
Blocking antibodies to PCSK9 inhibit the extra-cellular interaction between PCSK9 and the LDLR [42,43]. The inhibition of PCSK9 leads to higher levels of hepatic LDLR, which causes more efficient clearance of LDL from the circulation and drastic lowering of LDL-c levels. As shown in the most recent phase II clinical trials [44–46] and reports from the ongoing phase III trials [47,48▪,49–51], PCSK9 inhibition via mAb injection once every 2 or 4 weeks results in a 50–65% drop in LDL-c, with small positive effects on HDL-c and triglyceride levels. Similar results were also shown in a phase I trial using small interfering RNA (siRNA) to inhibit PCSK9 production [16▪]. On the basis of the established correlation between LDL-c reduction and CVD events, recently reinforced by the outstanding results of the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE IT) trial [4], it is anticipated that PCSK9 inhibition therapy will significantly improve CVD outcomes, although trial results will not be available until 2018. Table 1 summarizes the ongoing phase III clinical trials and expected completion dates. What is striking is that PCSK9 inhibition therapy does not show any adverse side-effects – a property that will facilitate the approval process for this new class of medications. Moreover, a prospective analysis of a single-nucleotide polymorphism (SNP) in PCSK9 associated with low LDL-c levels demonstrated no association with cognitive performance, functional status, or nonvascular clinical events [52].
Table 1.
Ongoing phase 3 clinical trials using monoclonal antibodies against proprotein convertase subtilisin/kexin 9
| Monoclonal antibody used | Evolocumab by Amgen | Alirocumab by Sanofi/Regeneron | Bococizumab by Pfizer |
|---|---|---|---|
| Phase 3 trial | FOURIER | ODYSSEY outcome | SPIRE I/II |
| Enrollment (n) | 22 500 | 18 000 | 17 000/9000 |
| Inclusion criteria | Age 40–85, History of CVD with high risk of recurrent event, LDL-c >70 mg/dl, triglycerides <400 mg/dl | Age >40, hospitalized of Acute Coronary Syndrome in the last 52 weeks before enrollment, LDL-c >70 mg/dl | I – Age >18, background of lipid-lowering treatment, at high risk of a cardiovascular event, LDL 70–100 mg/dl II – Age >18, background of lipid lowering treatment, at high risk of a cardiovascular event, LDL >100 mg/dl |
| Subcutaneous injection intervals | Once every 2 weeks | Once every 2 weeks | |
| End date | 02.2018 | 03.2018 | 06.2018/03.2018 |
FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; LDL, low-density lipoprotein; ODYSSEY; SPIRE, The Evaluation Of PF-04950615 (RN316), In Reducing The Occurrence Of Major Cardiovascular Events In High Risk Subjects.
LDLR–ligand interactions can be classified in three major categories [1]: type I, or the canonical interaction with apolipoprotein (apo)B on LDL, when the ligand is degraded in the lysosome, whereas the LDLR recycles back to the cell surface to capture more LDL particles [53,54]; type II, or the interaction with apoE on remnant lipoproteins and possibly HDL, when both receptor and ligand recycle to the surface [55]; and type III, or interaction with a noncanonical ligand such as PCSK9, when both receptor and ligand supposedly undergo degradation in the lysosome [10,56]. Type III receptor-mediated endocytosis suggests that not only does PCSK9 control surface LDLR levels but also that the LDLR acts as a key regulator of circulating PCSK9 levels [41,57]. Specifically, we were able to show that the deletion of one copy of the LDLR gene in mice results in a three-fold increase in circulating PCSK9 levels, whereas the complete loss of LDLR increases PCSK9 levels by 10-fold [41]. Similarly, transgenic expression of the inducible degrader of the LDLR, or IDOL [58], or of human PCSK9 [41] in mice reduced hepatic LDLR levels while increasing endogenous PCSK9 levels due to impaired clearance. In contrast, acute transgenic expression of the human LDLR in mouse liver causes an approximately 66% drop in serum PCSK9 levels [41]. This LDLR-mediated effect on PCSK9 levels also occurs in humans, where PCSK9 levels are lowest in normo-lipidemic patients, higher in hypercholesterolemic patients with heterozygous familial hypercholesterolemia, and highest in homozygous familial hypercholesterolemia (HoFH) patients [59].
There are over 1000 mutations identified in the LDLR gene [60], with a range of functional losses spanning the entire spectrum from slightly dysfunctional to completely nonfunctional (<2% of normal LDL uptake) [61]. Identification of the type of LDLR mutation (defective or negative) is of major interest in determining effectiveness of anti-PCSK9 therapies, as was shown in two recent clinical trials of patients with HoFH, when an average 30% drop in LDL-c levels was seen among patients with residual LDLR function, whereas there were no changes in LDL-c levels among the three homozygous familial hypercholesterolemia patients carrying two receptor-negative mutations [50,62]. These clinical studies suggest that an LDLR that is defective in LDL uptake can still bind (and be degraded by) PCSK9, but the complete absence of LDLR disallows the benefits of anti-PCSK9 therapy. By extrapolation, these results suggest the following scenarios in patients carrying hypothetical LDLR mutations with defective binding to PCSK9:
Hypercholesterolemia, caused by the presence of one mutant LDLR allele with impaired binding to both LDL and PCSK9: Here, the hypercholesterolemia caused by the reduced ability to bind and clear LDL from plasma is aggravated by the accumulation of PCSK9 in plasma and its increased degradation action on the normal LDLR allele product. It is likely that these patients will have a high ratio of PCSK9 to LDL-c, and may represent excellent candidates for PCSK9 inhibition therapy. Such a scenario was recently suggested to occur in patients carrying the rs688 polymorphism in the LDLR gene [63].
Hypocholesterolemia, caused by LDLR mutations with defective PCSK9 binding, but normal LDL binding: The effect of only one allele (heterozygous) with such a mutation is hard to predict, since the mutated receptor will be protected from degradation (and thus more efficiently clear LDL and lowering LDL-c levels) while causing a high circulating PCSK9 level that can degrade the normal LDLR allele product (and thus increasing LDL-c levels). A homozygous presentation, instead, is more likely to produce a low cholesterol syndrome, as both LDLR allele products will be virtually resistant to the action of PCSK9 and thus reproduce the phenotype seen both in patients with absent PCSK9 in the circulation and in patients treated with anti-PCSK9 antibodies.
Recently, Somanathan et al. [64] have generated such an artificial LDLR mutation (L318D) and were able to show reduced serum cholesterol levels in LDLR/APOBEC double knockout mice, a model of human-like severe hypercholesterolemia.
ADDITIONAL EFFECTS OF PCSK9 INHIBITION ON APOLIPOPROTEINB-CONTAINING LIPOPROTEINS
Apart from the massive reduction in LDL-c achieved by PCSK9 mAbs, phase II clinical trials and reports of the ongoing phase III trials have shown variations in the levels of other classes of lipoproteins, such as Lp(a) and triglyceride-containing particles. Lp(a) is an established risk factor for cardiovascular disease [65,66], which consists of an LDL particle in which case the apoB moiety is covalently linked to apo(a) by a disulfide bond [67]. Apo(a) shares structural similarities with plasminogen and exerts prothrombotic and antifibrinolytic effects through competition for removal of the complex between plasminogen activator inhibitor and tissue-type plasminogen activator. Lp(a) is found and accumulates in the atherosclerotic plaque; influencing lesion size through mechanisms that involve accelerated lipid oxidation with induction of inflammatory changes and macrophage cell death, favoring both plaque progression and rupture [68]. Two recent pooled analyses of phase II trials with PCSK9 mAbs highlighted their effectiveness in reducing Lp(a) levels [18▪,19▪]. In the first analysis, administration of evolocumab for 12 weeks lowered Lp(a) levels in a dose-dependent manner [18▪]. The highest efficacy was obtained after injection of either 140 mg every 2 weeks, or 420 mg every 4 weeks, which reduced Lp(a) levels by 29.4 and 25.5%, respectively, compared to placebo [18▪]. Similarly, administration of 150 mg of alirocumab biweekly for 8 and 12 weeks reduced Lp(a) levels by approximately 30%, with the greatest reduction in individuals with higher starting Lp(a) concentration [19▪]. On the contrary, a recent phase I clinical trial of siRNA to inhibit PCSK9 production did not show any effect on Lp(a) levels [16▪]. Thus, although the mechanism by which anti-PCSK9 mAb reduces Lp(a) is unknown, it can be assumed that this effect is antibody-specific and thus linked to events occurring in the extracellular milieu. Alternatively, the reduction in Lp(a) levels may be mediated by reduced apoB synthesis, as was recently shown in clinical data using the apoB synthesis inhibitor mipomersen [69].
The association between plasma triglyceride levels and the risk of CVD has been extensively studied [70]. In this context, a panel of experts reviewed the most recent epidemiological studies related to fasting and nonfasting triglyceride levels and established their role as a risk factor for ischemic cardiovascular disease [71]. Interest in plasma triglycerides as a biomarker and target of therapy has been aroused after the identification of loss-of-function mutations in ApoCIII related to low plasma triglyceride levels and lower incidence of CHD [72,73]. The administration of evolocumab at 420 mg every 4 weeks in individuals with hypercholesterolemia, in addition to atorvastatin alone, or atorvastatin and ezetimibe, reduced triglyceride levels by only 11.5% after 52 weeks [48▪]. In the LDL-C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-2 (LAPLACE-2) trial, evolocumab administration, in combination with moderate to high-dose statin, reduced triglyceride levels by 12–23% and 14–30% when administered every 2 and 4 weeks in hypercholesterolemic patients compared to placebo [47]. Triglyceride levels were also reduced by 20 and 12% after 12-week administration of 140 mg biweekly and 420 mg monthly in heterozygous familial hypercholesterolemia on stable lipid-lowering therapy, respectively [74]. However, other studies reported more modest reductions in triglyceride levels that did not reach statistical significance [20,21,51]. It is important to remember that measurements in all clinical trials are done under strict fasting conditions that highly affect the levels of both PCSK9 and triglycerides. Several in-vitro and in-vivo studies suggest a direct role for PCSK9 in the regulation of intestinal lipoprotein secretion and apoB48 production [75–78]. These studies should inform clinical investigations on the effect of PCSK9 mAbs specifically in the context of postprandial hypertriglyceridemia.
SUMMARY AND CONCLUSION
The discovery of PCSK9 as a circulating protein holding the power to regulate LDLR levels has changed our understanding of cholesterol metabolism, traditionally believed to be strictly under the control of cellular factors orchestrated by the SREBP transcriptional machinery [79,80]. Understanding the basic mechanism of PCSK9 action has led to a race for the therapeutic exploitation of this protein, whose inhibition produces safe and drastic lowering of LDL-c levels. A more refined knowledge of the complex interactions between PCSK9, LDL particle, and LDLR may help explain both the tremendous efficacy of anti-PCSK9 therapies and the unexpected effects on important predictors of CAD such as Lp(a) levels. Also, clarifying the inner secrets of PCSK9 biology will help identify additional patient groups likely to benefit from the inhibitory therapy, such as individuals with elevated PCSK9 levels and those prone to abnormal postprandial hypertriglyceridemia.
KEY POINTS.
Inhibition of PCSK9 achieves low LDL-c goals in individuals with severe hypercholesterolemia.
Reciprocity between PCSK9 and LDLR explains the extreme effect of inhibitory mAb on LDL-c levels.
Anti-PCSK9 mAb therapy also reduces the levels of Lp(a), through mechanisms not yet identified.
An effect on postprandial hypertriglyceridemia is suggested by novel data on PCSK9 regulation of intestinal lipoprotein assembly and secretion.
Acknowledgments
Financial support and sponsorship
The study was supported by the National Institutes of Health (National Heart, Lung, and Blood Institute) through grant R01-HL106845 to Dr Fazio.
Footnotes
Conflicts of interest
None.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Hunt KJ, Resendez RG, Williams K, et al. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 2.Ellison RC, Zhang Y, Wagenknecht LE, et al. Relation of the metabolic syndrome to calcified atherosclerotic plaque in the coronary arteries and aorta. Am J Cardiol. 2005;95:1180–1186. doi: 10.1016/j.amjcard.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 3.Hebert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. J Am Med Assoc. 1997;278:313–321. [PubMed] [Google Scholar]
- 4.Cannon Christopher P on behalf of the IMPROVE IT Investigators1 CD, Brigham and Women’s Hosp. Boston MA. IMPROVE-IT trial: a comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes after acute coronary syndrome. Circulation. 2014;130:2105–2126. [Google Scholar]
- 5.Stone NJ, Robinson GJ, Lichtenstein AH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 published online November 12, 2013. ACC/AHA Blood Cholesterol Guideline. [Google Scholar]
- 6.Avis HJ, Hutten BA, Gagne C, et al. Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J Am Coll Cardiol. 2010;55:1121–1126. doi: 10.1016/j.jacc.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Rallidis LS, Fountoulaki K, Anastasiou-Nana M. Managing the underestimated risk of statin-associated myopathy. Int J Cardiol. 2012;159:169–176. doi: 10.1016/j.ijcard.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 8.Bays H, Cohen DE, Chalasani N, Harrison SA. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8 (3 Suppl):S47–57. doi: 10.1016/j.jacl.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Brault M, Ray J, Gomez YH, et al. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism. 2014;63:735–745. doi: 10.1016/j.metabol.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Park SW, Moon YA, Horton JD. Posttranscriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279:50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 11.Benjannet S, Rhainds D, Essalmani R, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–48875. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J, Pertsemlidis A, Kotowski IK, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 13.Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 14.Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–1900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 15.Stein EA, Honarpour N, Wasserman SM, et al. Effect of the PCSK9 Monoclonal Antibody, AMG 145, in Homozygous Familial Hypercholesterolemia. Circulation. 2013;128:2113–2120. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- 16▪.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. The first and only clinical trial to show that PCKS9 inhibtion via siRNA can reduce cholesterol. All previous trials were done using mAb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 18▪.Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. This summary of four large phase II using evolocumab clarify that mAb against PCSK9 can be used to reduce Lp(a) [DOI] [PubMed] [Google Scholar]
- 19▪.Gaudet D, Kereiakes DJ, McKenney JM, et al. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials) Am J Cardiol. 2014;114:711–715. doi: 10.1016/j.amjcard.2014.05.060. This summary of four large phase II using alirocumab clarify that mAb against PCSK9 can be used to reduce Lp(a) [DOI] [PubMed] [Google Scholar]
- 20.Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 22.Dubuc G, Tremblay M, Pare G, et al. A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res. 2010;51:140–149. doi: 10.1194/jlr.M900273-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidah NG, Prat A. The proprotein convertases are potential targets in the treatment of dyslipidemia. J Mol Med. 2007;85:685–696. doi: 10.1007/s00109-007-0172-7. [DOI] [PubMed] [Google Scholar]
- 24.Ai D, Chen C, Han S, et al. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J Clin Invest. 2012;122:1262–1270. doi: 10.1172/JCI61919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong HJ, Lee HS, Kim KS, et al. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res. 2008;49:399–409. doi: 10.1194/jlr.M700443-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Costet P, Cariou B, Lambert G, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:6211–6218. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 27.Mayne J, Dewpura T, Raymond A, et al. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 2008;7:22. doi: 10.1186/1476-511X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Careskey HE, Davis RA, Alborn WE, et al. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–398. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Welder G, Zineh I, Pacanowski MA, et al. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51:2714–2721. doi: 10.1194/jlr.M008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davignon J, Dubuc G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans Am Clin Climatol Assoc. 2009;120:163–173. [PMC free article] [PubMed] [Google Scholar]
- 31.Seidah NG, Sadr MS, Chretien M, Mbikay M. The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J Biol Chem. 2013;288:21473–21481. doi: 10.1074/jbc.R113.481549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chorba JS, Shokat KM. The proprotein convertase subtilisin/kexin type 9 (PCSK9) active site and cleavage sequence differentially regulate protein secretion from proteolysis. J Biol Chem. 2014;289:29030–29043. doi: 10.1074/jbc.M114.594861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNutt MC, Lagace TA, Horton JD. Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem. 2007;282:20799–20803. doi: 10.1074/jbc.C700095200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang DW, Lagace TA, Garuti R, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–18612. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]
- 35.Lipari MT, Li W, Moran P, et al. Furin-cleaved proprotein convertase subtilisin/kexin type 9 (PCSK9) is active and modulates low density lipoprotein receptor and serum cholesterol levels. J Biol Chem. 2012;287:43482–43491. doi: 10.1074/jbc.M112.380618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjannet S, Rhainds D, Hamelin J, et al. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and posttranslational modifications. J Biol Chem. 2006;281:30561–30572. doi: 10.1074/jbc.M606495200. [DOI] [PubMed] [Google Scholar]
- 37.Han B, Eacho PI, Knierman MD, et al. Isolation and characterization of the circulating truncated form of PCSK9. J Lipid Res. 2014;55:1505–1514. doi: 10.1194/jlr.M049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan D, Yancey PG, Qiu S, et al. Self-association of human PCSK9 correlates with its LDLR-degrading activity. Biochemistry. 2008;47:1631–1639. doi: 10.1021/bi7016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosenko T, Golder M, Leblond G, et al. Low-density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated LDL receptor degradation. J Biol Chem. 2013;288:8279–8288. doi: 10.1074/jbc.M112.421370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavori H, Giunzioni I, Linton MF, Fazio S. Loss of plasma proprotein convertase subtilisin/kexin 9 (PCSK9) after lipoprotein apheresis. Circ Res. 2013;113:1290–1295. doi: 10.1161/CIRCRESAHA.113.302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavori H, Fan D, Blakemore JL, et al. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T, Lu C, Ryan RO. A two-step binding model of PCSK9 interaction with the low density lipoprotein receptor. J Biol Chem. 2011;286:5464–5470. doi: 10.1074/jbc.M110.199042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang DW, Garuti R, Fau-Tang W-J, et al. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc Natl Acad Sci U S A. 2008;105:13045–13050. doi: 10.1073/pnas.0806312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128:962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 45.Stein EA, Giugliano RP, Koren MJ, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J. 2014;35:2249–2259. doi: 10.1093/eurheartj/ehu085. [DOI] [PubMed] [Google Scholar]
- 46.Desai NR, Giugliano RP, Zhou J, et al. AMG 145, a monoclonal antibody against PCSK9, facilitates achievement of national cholesterol education program-adult treatment panel III low-density lipoprotein cholesterol goals among high-risk patients: an analysis from the LAPLACE-TIMI 57 trial (LDL-C assessment with PCSK9 monoclonal antibody inhibition combined with statin thErapy-thrombolysis in myocardial infarction 57) J Am Coll Cardiol. 2014;63:430–433. doi: 10.1016/j.jacc.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 47.Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. J Am Med Assoc. 2014;311:1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 48▪.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. The first study from phase III clinical trial with anti-PCSK9 mAb. The study shows the expected massive reduction in LDL-c levels. [DOI] [PubMed] [Google Scholar]
- 49.Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 51.Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 52.Postmus I, Trompet S, de Craen AJ, et al. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res. 2013;54:561–566. doi: 10.1194/jlr.M033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldstein JL, Brown MS. Regulation of low-density lipoprotein receptors: implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation. 1987;76:504–507. doi: 10.1161/01.cir.76.3.504. [DOI] [PubMed] [Google Scholar]
- 54.Fazio S, Linton MF, Hasty AH, Swift LL. Recycling of apolipoprotein E in mouse liver. J Biol Chem. 1999;274:8247–8253. doi: 10.1074/jbc.274.12.8247. [DOI] [PubMed] [Google Scholar]
- 55.Farkas MH, Swift LL, Hasty AH, et al. The recycling of apolipoprotein E in primary cultures of mouse hepatocytes. Evidence for a physiologic connection to high density lipoprotein metabolism. J Biol Chem. 2003;278:9412–9417. doi: 10.1074/jbc.M208026200. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denis M, Marcinkiewicz J, Zaid A, et al. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012;125:894–901. doi: 10.1161/CIRCULATIONAHA.111.057406. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki M, Terao Y, Ayaori M, et al. Hepatic overexpression of idol increases circulating protein convertase subtilisin/kexin type 9 in mice and hamsters via dual mechanisms: sterol regulatory element-binding protein 2 and low-density lipoprotein receptor-dependent pathways. Arterioscler Thromb Vasc Biol. 2014;34:1171–1178. doi: 10.1161/ATVBAHA.113.302670. [DOI] [PubMed] [Google Scholar]
- 59.Raal F, Panz V, Immelman A, Pilcher G. Elevated PCSK9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J Am Heart Assoc. 2013;2:e000028. doi: 10.1161/JAHA.112.000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guardamagna O, Restagno G, Rolfo E, et al. The type of LDLR gene mutation predicts cardiovascular risk in children with familial hypercholesterolemia. J Pediatr. 2009;155:199–204. e2. doi: 10.1016/j.jpeds.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Sly WS, Childs B, et al., editors. The metabolic and molecular bases of inherited disease. 8. McGraw-Hill; 2001. pp. 2863–2913. [Google Scholar]
- 62.Stein EA, Honarpour N, Wasserman SM, et al. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–2120. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- 63.Gao F, Ihn HE, Medina MW, Krauss RM. A common polymorphism in the LDL receptor gene has multiple effects on LDL receptor function. Hum Mol Genet. 2012;22:1424–1431. doi: 10.1093/hmg/dds559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Somanathan S, Jacobs F, Wang Q, et al. AAV vectors expressing LDLR gain-of-function variants demonstrate increased efficacy in mouse models of familial hypercholesterolemia. Circ Res. 2014;115:591–599. doi: 10.1161/CIRCRESAHA.115.304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 66.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 67.Gaubatz JW, Heideman C, Gotto AM, Jr, et al. Human plasma lipoprotein [a]. Structural properties. J Biol Chem. 1983;258:4582–4589. [PubMed] [Google Scholar]
- 68.van Dijk RA, Kolodgie F, Ravandi A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53:2773–2790. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein EA, Dufour R, Gagne C, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126:2283–2292. doi: 10.1161/CIRCULATIONAHA.112.104125. [DOI] [PubMed] [Google Scholar]
- 70.Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Genetic, epidemio-logic and clinical data strongly suggest that fasting or nonfasting triglycerides are independent cardiovascular risk factors. Curr Med Res Opin. 2014 doi: 10.1185/03007995.2014.958147. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Kolovou GD, Mikhailidis DP, Kovar J, et al. Assessment and clinical relevance of nonfasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9:258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 72.TG and HDL Working Group of the Exome Sequencing Project, National Heart Lung and Blood Institute. Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J MedicineV. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 74.Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–340. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 75.Levy E, Ben Djoudi Ouadda A, Spahis S, et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 2013;227:297–306. doi: 10.1016/j.atherosclerosis.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 76.Rashid S, Tavori H, Brown P, et al. PCSK9 promotes intestinal overproductin of triglyceride-rich apolipoprotien-B liporptoein through both LDL-receptor dependent and independent mechanisms. Circulation. 2014;130:431–441. doi: 10.1161/CIRCULATIONAHA.113.006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rashid S, Curtis DE, Garuti R, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le May C, Kourimate S, Langhi C, et al. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler Thromb Vasc Biol. 2009;29:684–690. doi: 10.1161/ATVBAHA.108.181586. [DOI] [PubMed] [Google Scholar]
- 79.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 80.Poirier S, Mayer G, Benjannet S, et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]