Abstract
Aim:
Main the goal of the research is to analyze the occurrence of glaucoma in patients with diabetes mellitus type 1 (DM type 1) and diabetes mellitus type 2 (DM type 2).
Patients and methods:
The study involved 140 patients, 34 with DM type 1 and 106 with DM type2. In relation to the type of glaucoma to the patients are divided into two groups: Primary and Secondary glaucoma. According to the stage of diabetic retinopathy (DR) patients were analyzed in three groups: non-proliferative, preproliferative and proliferative DR. Since ophthalmological parameters were analyzed: best corrected visual acuity (BCVA), intraocular pressure (IOP), visual field (VF) of computerized perimetry, excavatio optic nerve (E/D) by optic coherent tomography (OCT).
Results:
Applying the test of quotient chance found that subjects with DM type 1 have a 5.94 times greater chance of developing secondary glaucoma, but is of primary (P <0.0001). In patients with DM type 2, where the chance of getting the subjects of secondary glaucoma 4.43 times larger than that of the primary (P = 0.0002).
Conclusion:
Patients with DM type have great chance of developing secondary glaucoma of the primary. Primary glaucoma more common in NPDR but secondary glaucoma more common in PDR.
Keywords: Diabetes mellitus (DM), diabetic retinopathy (DR), primary and secondary glaucoma (Gl) Introduction
1. INTRODUCTION
Diabetes mellitus is the metabolic disease of carbohydrates, fats and proteins, which is caused by reduced secretion or insulin resistance. It differs Diabetes mellitus type 1 (type 1 DM) and Diabetes mellitus type 2 (type 2 DM). Basically type 1 DM is an autoimmune process that destroys the islets of Langerhans, in genetically susceptible individuals. According to recent studies infection enteroviruses is a precipitating factor for developing the disease (1). A 2011 report from the US Centers for Disease Control and Prevention (CDC) estimated that approximately 1 million Americans have type 1 DM. Type 1 DM is the most common metabolic disease of childhood. Every 400-600 children and adolescents has type 1 DM (2).
Type 2 diabetes mellitus consists of an array of dysfunctions characterized by hyperglycemia and resulting from the combination of resistance to insulin action, inadequate insulin secretion, and excessive or inappropriate glucagon secretion. In an update to its 2008 diabetes screening guidelines, the US Preventive Services Task Force (USPSTF) has issued draft guidelines recommending that all adults aged 45 or older be screened for abnormal glucose and type 2 diabetes. The new guidelines also recommend screening in younger adults with risk factors, including those with overweight or obesity or with a first-degree relative with diabetes, as well as women with a history of gestational diabetes or polycystic ovarian (3-11). Hyperglycemia-inducing damage to the endothelium of blood vessels, ischemia horioretine and significant changes in it with clinical signs: microaneurysms, dot and blot hemorrhages, flame shaped hemorrhages, retinal edema and hard exudates, cotton-wool spots, venous beading, intraretinal microvascular abnormalities, macular edema, preretinal hemorrhages, neovascularization, hemorrhage into the vitreous, fibrovascular tissue proliferation, traction retinal detachments. Classified as nonproliferative diabetic retinopathy (NPDR), Perpoliferative diabetic retinopathy (PreDR)) and Proliferative diabetic retinopathy (PDR). Approximately 700,000 persons in the United States have proliferative diabetic retinopathy, with an annual incidence of 65,000. A recent estimate of the prevalence of diabetic retinopathy in the United States showed a high prevalence of 28.5% among those with diabetes aged 40 years and older (12).
In diabetic eye may develop primary glaucoma (open or closed chamber angle), ocular hypertension and secondary glaucoma (uveal, post-traumatic, neovascular, postoperative, iatrogenic, phakic, at intabulbarnih tumor). Primary Open-angleglaucom (POAG) is a multifactorial disease characterized by progressive retinal ganglion cell death and visual field loss. Focal and systemic vascular abnormalities have also been well documented in diabetic patients. The relationship between diabetes mellitus and POAG remains enigmatic in the literature. Although current studies support the role of vascular contributions to both diseases, the association between glaucoma and diabetes yields contrasting results (5). Although there are some hints for a correlation between diabetes and primary open angle glaucoma (POAG), it remains unclear in which way diabetes influences eye pressure (IOP) and glaucoma. Despite this, the main reason for neovascular glaucoma in diabetes is proven to be retinal ischemia due to diabetic vessel damage. Primary open angle glaucoma is more frequent than neovascular glaucoma, but neovascular glaucoma is very aggressive and difficult to treat (6).
The main purpose of the research is to analyze the occurrence of glaucoma in patients with diabetes mellitus type 1 (DM type 1) and diabetes mellitus type 2 (DM type 2).
2. PATIENTS AND METHODS
The research was conducted at the Eye Clinic, Clinical University Center Sarajevo in the period May 2012 - 2014. A prospective study included 140 patients with diabetes mellitus of that 34 patients had DM type 1 and 106 patients with DM type 2. In the study included patients insulin dependent and patients on oral antidiabetic. The patients according to the type of glaucoma (GL) divided into two groups: Primary and Secondary glaucoma. According to the stage of diabetic retinopathy patients were analyzed in three groups (non-proliferative, preprolifertive, proliferative) diabetic retinopathy. Since ophthalmological parameters were analyzed: best corrected visual acuity (BCVA), intraocular pressure (IOP), visual field (VF) of computerized perimetry, excavatio optic nerve (E/D) by optic coherent tomography (OCT). In addition ophthalmology examination is implied binocular slit-lamp direct and indirect ophthalmoscopes and gonioscopy.
3. RESULTS
DM type 2 was predominant in male and female. There is statistically significant difference in the age structure of respondents with diabetes type 1 and type 2.
By using chi-square test was not statistically significant difference in gender structure of respondents in relation to the type of DM. Type 2 DM was more frequent in both sexes.
The analysis of the age structure of the respondents found that there is a statistically significant difference in the age structure of respondents with DM type 1 and DM type 2.
Applying the test quotient chances (odds ratio) found that subjects with DM type 1 have 5.94 times higher chance to have secondary glaucoma than the primary. Similar results were obtained in patients with diabetes type 2, and the chance to have a secondary glaucoma 4.43 times higher than primary glaucoma. The analysis of visual acuity patients studied groups was no significant difference in the number of respondents in relation to the category of visual acuity. Most of the respondents had IOP <20mmHg. OCT findings in the total sample.
4. DISCUSSION
A sample of 140 patients, 34 patients with DM type 1 and 106 patients with DM type 2. The Poland epidemiologic study aimed to analyze the changes in incidence rates of DM type among children ages 0-14 years from 1989 to 2012 in this region. The overall incidence rate increased 3.8 times, confirm that Poland currently has one of the highest incidence rates of pediatric DM type 1 in Europe (9). A 2011. Centers for Disease Control and Prevention (CDC) report estimated that nearly 26 million Americans have diabetes. Additionally, an estimated 79 million Americans have prediabetes. In 2014, the CDC reported that about 40% of US adults will develop diabetes, primarily type 2, in their lifetime. The central reason for the increase is obesity (11, 12). Table 1. shows the results of the gender structure of the sample. By using chi-square test we had not statistically significant difference (X2 = 0.666, p = 0.414) in the gender structure of the respondents in relation to the DM type. DM type was more frequent in both sexes. Schmidl D. et al. amount that although little data is available, estrogen, progesterone and testosterone are most likely important regulators of blood flow in the retina and choroid, because they are key regulators of vascular tone in other organs. Estrogen seems to play a protective role since it decreases vascular resistance in large ocular vessels. Some studies indicate that hormone therapy is beneficial for ocular vascular disease in post-menopausal women (13). DM type 1 was most common in the age group of 36-45 years, and DM type 2 in the age group of 46-55 years (Table 2). Chi square test was found statistically significant difference - ss (Χ2 = 27.134, p = 0.001) between the analyzed groups. The CDC estimated that in 2010 year, 79 million Americans aged 20 years or older had prediabetes - 35% and 50% of those aged 65 years or older (11, 12). The patient with DM type 1 NPDR developed in 11 eyes, PreDR in 14 eyes, and PDR in 27 eyes. In this groups we had a primary glaucoma in 4 cases in the context of NPDR, and 12 eyes with PreDR, secondary glaucoma we had in 15 eyes with PreDR and 24 eyes with PDR (Table 3). In patients with DM type, NPDR developed at 40 eyes, PreDR at 61 and PDR in 89 eyes. Primary glaucoma is registered in 12 eyes with NPDR, and 7 eyes with PreDR and Secondary glaucoma was found in 13 eyes with PreDR and 27 cases in the PDR (Table 4).
Table 1.
Gender structure of the sample

Table 2.
Age structure of the sample

Table 3.
The frequencies of the Primary and Secondary glaucoma in various stages of DR in patients with type 1 DM

Table 4.
The frequencies of the Primary and Secondary glaucoma in various stages of DR in patients with type 2 DM

Figure 1.
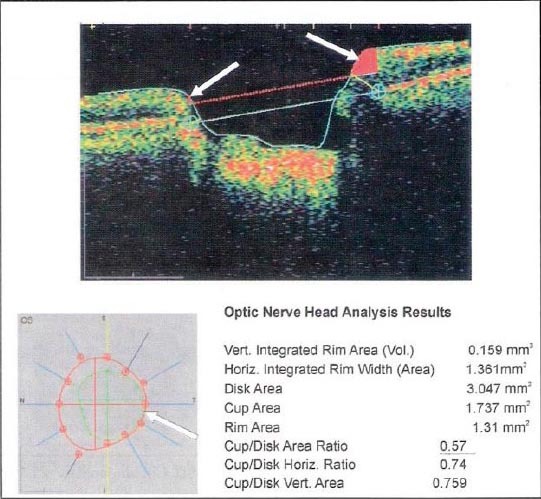
The excavatio of the optic nerve - Cup/Disc Area Ratio OCT findings
Figure 2.
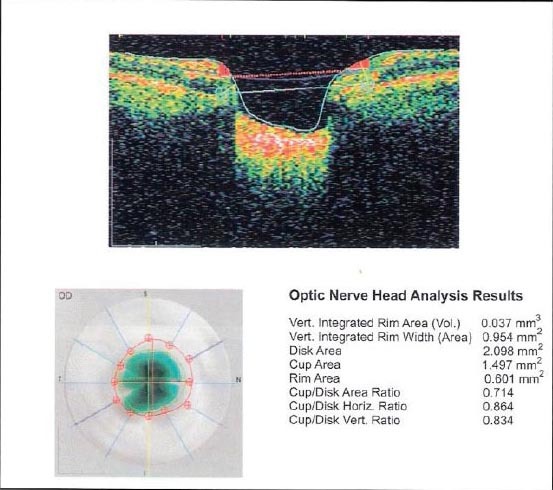
The excavatio of the optic nerve - Cup/Disc Area Ratio OCT findings
Primary glaucoma is more common answer at nonproliferetive diabetic retinopathy, while the secondary glaucoma more common in poliferative diabetic retinopathy (Table 3 and 4). Cruz-Ingo Y et.al. suggest that Puerto Rico patients between 40 to 79 years of age with diabetic retinopathy have an increased risk of developing open-angle glaucoma with each subsequent decade (8). Klemm M, Gesser C. In his article communicated that Primary open angle glaucoma is more frequent than neovascular glaucoma, but neovascular glaucoma is very aggressive and difficult to treated (6, 8). Applying the test of refraction chances (odds ratio) found that subjects with DM type 1 have a 5.94 times greater chance of developing secondary glaucoma, but is of primary (P <0.0001). Similar results were obtained in patients with DM type 2, where the chance of getting the subjects of secondary glaucoma 4.43 times larger than that of the primary (P = 0.0002) (Table 5).
Table 5.
The frequency of different types of glaucoma in the total sample tested

Apreutesei NA et al. in the study “Glaucoma evolution in patients with diabetes”, the presence that of non-proliferative diabetic retinopathy influenced (only marginally statistically) the glaucomatous disease progression (14). Noma H. et.al. amounts that was also a significant correlation between the vitreous levels of sVEGFR-1 and sVEGFR-2. These results suggest that the vitreous levels of sVEGFR-1 and sVEGFR-2 are dependent on VEGF in patients who have iris neovascularisatio (INV) with or without neovascular glaucoma(NVG) (15).
In patients with DM type 1, no clinically significant retinopathy can be seen in the first 5 years after the initial diagnosis of diabetes is made. After 10-15 years, 25-50% of patients show some signs of retinopathy. This prevalence increases to 75-95% after 15 years and approaches 100% after 30 years of diabetes. Proliferative diabetic retinopathy (PDR) is rare within the first decade of DM type 1 diagnosis but increases to 14-17% by 15 years, rising steadily thereafter. In patients with DM type 2, the incidence of diabetic retinopathy increases with the disease duration. Of patients with type 2 diabetes, 23% have nonproliferative diabetic retinopathy (NPDR) after 11-13 years, 41% have NPDR after 14-16 years, and 60% have NPDR after 16 years (16). As part of a complete ophthalmological examination is followed by visual acuity patients, who are the VO divided into four groups. There were no statistically significant (ss) in the number of respondents by category VO (Table 6). Were analyzed and values of IOP in the total sample. IOP was usually below 20 mmHg, which was statistically significant (Χ2 = 135.08, p = 0.001) (Table 7).
Table 6.
Best corrected visual acuity patients

Table 7.
Intraocular pressure (IOP) patients in the total sample

We analyzed excavatio of the optic nerve, on the basis of objective indicators Cup/Disc Area Ratio. OCT finding in the total sample recorded 29.6% of cases with the excavation of the optic nerve greater than 0.5, and in 13.9% of cases had the excavation of 0.4-0.5. These data indicate a significant damage of the optic nerve in patients with DM for DR and GL (Table 8). Results of computer perimetry point to falling sensibilities in 29.6% of patients, the occurrence of paracentral scotoma 14.6% and 11.42% patients had centrocecal scotoma. These findings also point to the obvious damaged visual field in patients with DM, DR IGL (Table 9).
Table 8.
The excavatio of the optic nerve, on the basis of objective indicators Cup/Disc Area Ratio

Table 9.
Results of computerized perimetry patients total sample

5. CONCLUSION
The analysis of changes in the eye in 140 patients with diabetes mellitus, we conclude that there was no statistically significant difference in relation to the sex of respondents. DM type 1 was the most common in the age group of 36-45 years, and DM type 2 in the age group of 46-55 years. Primary glaucoma was more common in NPDR and secondary glaucoma occurs more frequently in the PDR. Applying the test of quotient chance (odds ratio) found that subjects with DM type 1 have a 5.94 times greater chance of developing secondary glaucoma, but is of primary. Similar results were obtained in patients with DM type 2 where the chance of getting the subjects of secondary glaucoma 4.43 times larger than that of the primary.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Hsiao-Chuan L, et al. Enterovirus infection is associated with an increased risk of childhood type 1 diabetes in Taiwan: A nationwide population-based cohort study. Diabetologia. 2014 doi: 10.1007/s00125-014-3400-z. DOI 10.1007/s00125-014-3400. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011. [Accessed January 28 2011]. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf .
- 3.USPSTF. Public comment on draft recommendation statement and draft evidence review: screening for abnormal glucose and type 2 diabetes mellitus. US Preventive Services Task Force. [Accessed Oct 14 2014]. Available at http://www.uspreventiveservicestaskforce.org/Announcements/News/Item/publiccomment-on-draft-recommendation-statement-and-draft-evidence-review-screening-forabnormal-glucose-and-type-2-diabetes-mellitus .
- 4.Tucker ME. USPSTF: Screen everyone 45 and older for abnormal glucose. Medscape Medical News [serial online]. Oct 6. 2014. [Accessed Oct 14 2014]. Available at http://www.medscape.com/viewarticle/832850 .
- 5.Gerber AL, Harris A, Siesky B, Lee E, Schaab TJ, Huck A. Vascular Dysfunction in Diabetes and Glaucoma: A Complex Relationship Reviewed. J Glaucoma. 2014 Sep 26; doi: 10.1097/IJG.0000000000000137. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Klemm M, Gesser C. The relevance of diabetes for patients with glaucoma. Klin Monbl Augenheilkd. 2014;231(2):116–20. doi: 10.1055/s-0033-1360143. [DOI] [PubMed] [Google Scholar]
- 7.Pavlenko TA, Chesnokova NB, Davydova HG, Okhotsimskaia TD, Beznos OV, Grigor’ev AV. Level of tear endothelin-1 and plasminogen in patients with glaucoma and proliferative diabetic retinopathy. Vestn Oftalmol. 2013;129(4):20–3. [PubMed] [Google Scholar]
- 8.Cruz-lñigo Y, Izquierdo NJ, García O, Pérez Rl, Asoc Med PR. Open-angle glaucoma in patients with diabetic retinopathy at the Puerto Rico Medical Center. 2012;104(4):10–3. [PubMed] [Google Scholar]
- 9.Chobot A, Polanska J, Deja G, Jarosz-Chobot P. Incidence of type 1 diabetes among Polish children ages 0-14 years from 1989-2012. Acta Diabetol. 2014 Nov 8; doi: 10.1007/s00592-014-0682-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.U.S Department of Health and Human Services, Centers for Disease Control and Prevention. 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Hackethal V. 2 in 5 American Adults Will Develop Diabetes. Medscape Medical News. 2011. [Accessed January 5 2012, Accessed August 13, 2014]. Available at http://www.cdc.gov/ diabetes/pubs/pdf/ndfs_2011.pdf. Available at http://www.medscape.com/viewarticle/829833 .
- 11.Gregg EW, Zhuo X, Albright AL, et al. Trends in lifetime risk and years of life lost due to diabetes in the USA 1985—2011: a modelling study. The Lancet Diabetes & Endocrinology. [Accessed August 13 2014]. Available at http://www.thelancet.com/journals/landia/article/PIIS22138587(14)701615/fulltext . [DOI] [PubMed]
- 12.Zhang X, Sadine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidl D, Schmetterer L, Garhöfer G, Popa-Cherecheanu A Gender Differences in Ocular Blood Flow. Curr Eye Res. 2014:1–12. doi: 10.3109/02713683.2014.906625. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apreutesei NA, Chiselita D, Motas OI. Glaucoma evolution in patients with diabetes. Rev Med Chir Soc Med Nat Iasi. 2014;118(3):667–74. [PubMed] [Google Scholar]
- 15.Noma H, Mimura T, Yasuda K, Shimura M. Vascular endothelial growth factor and its soluble receptors-1 and -2 in iris neovascularization and neovascular glaucoma. Ophthalmologica. 2014;232(2):10. doi: 10.1159/000360303. [DOI] [PubMed] [Google Scholar]
- 16.Bragge P, Gruen RL, Chau M, Forbes A, Taylor HR. Screening for Presence or Absence of Diabetic Retinopathy: A Meta-analysis. Arch Ophthalmol. 2011;129(4):435–44. doi: 10.1001/archophthalmol.2010.319. Grandmultiparity: Risk factors and. [DOI] [PubMed] [Google Scholar]


